A Novel Biomimetic Lung-Shaped Flow Field for All-Vanadium Redox Flow Battery
Abstract
:1. Introduction
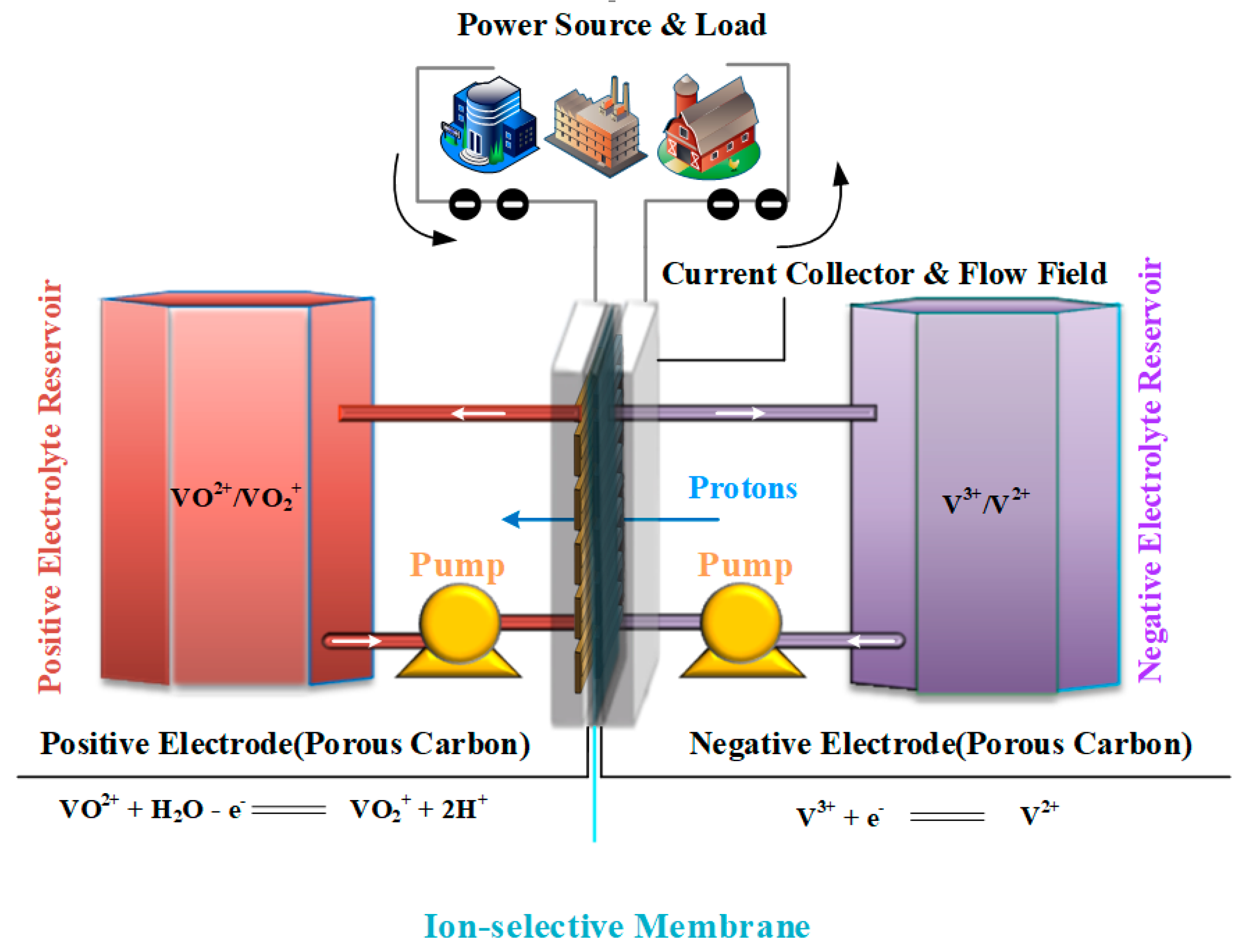
2. Model
- (1)
- The electrolyte was treated as an incompressible fluid.
- (2)
- The mass transport process in the porous electrode was assumed to be a dilute species transport.
- (3)
- The electrodes, electrolyte, and ion exchange membrane were regarded to be homogeneous and isotropic.
- (4)
- The state of charge (SOC) of the electrolyte is introduced to define the concentrations of reactants and products in the electrolyte at any given moment, simplifying the dynamic model into a steady-state model.
- (5)
- The ion exchange membrane only allowed the protons to pass through.
- (6)
- The intrusion of the felt electrode into the channel was ignored.
2.1. Governing Equations
2.1.1. Transport in the Flow Field
2.1.2. Transport in the Electrodes
2.1.3. Transport in the Membrane
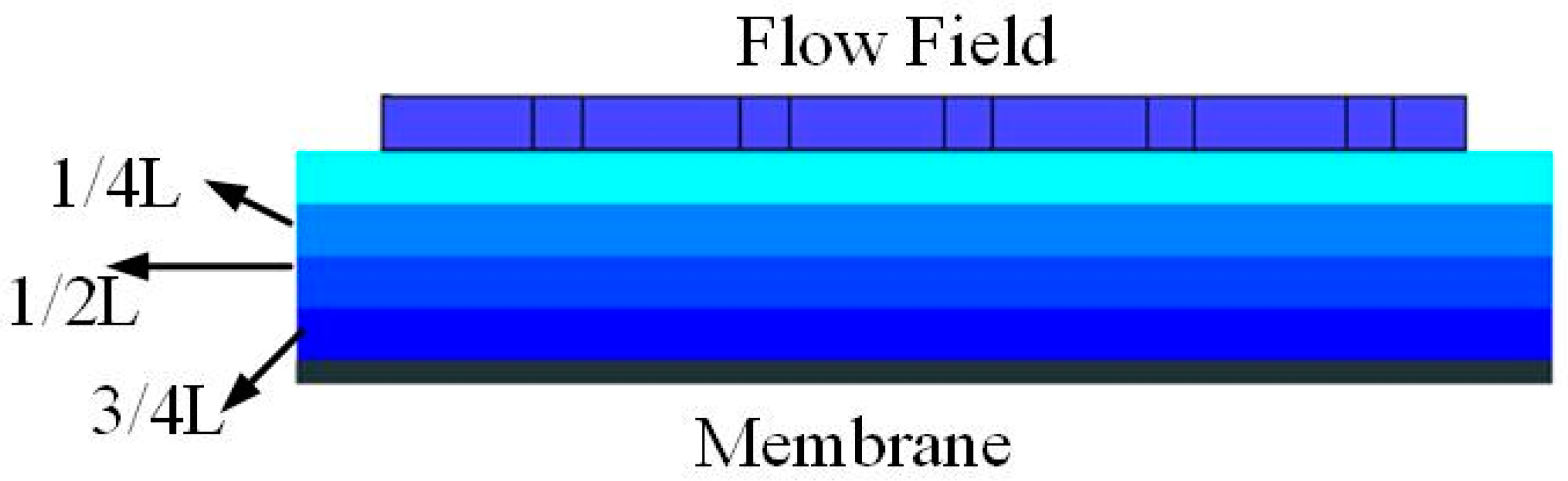
2.2. Boundary Condition
2.3. Performance Parameters
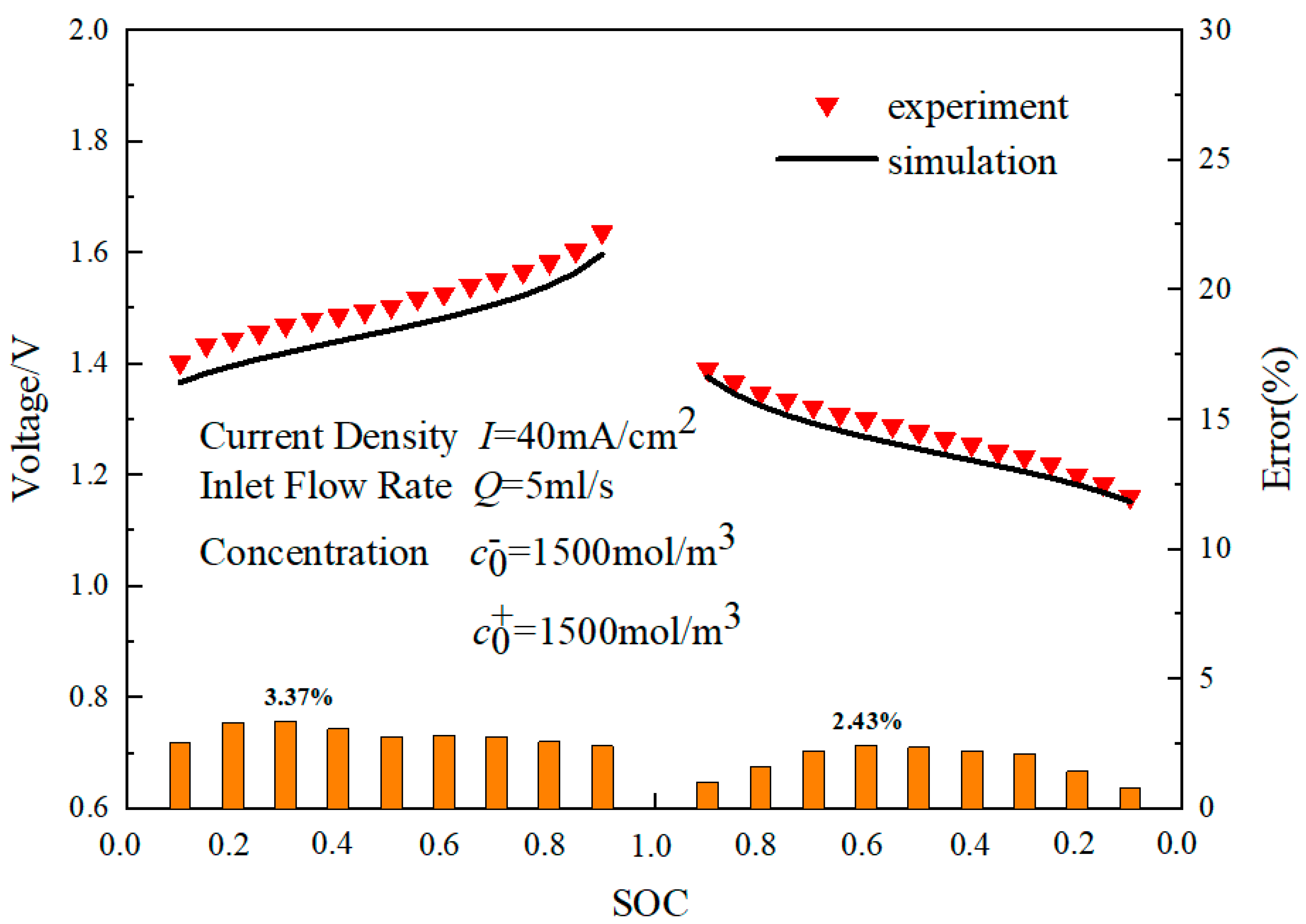
2.4. Numerical Details and Model Validation
3. Results and Discussion
3.1. Battery Performance
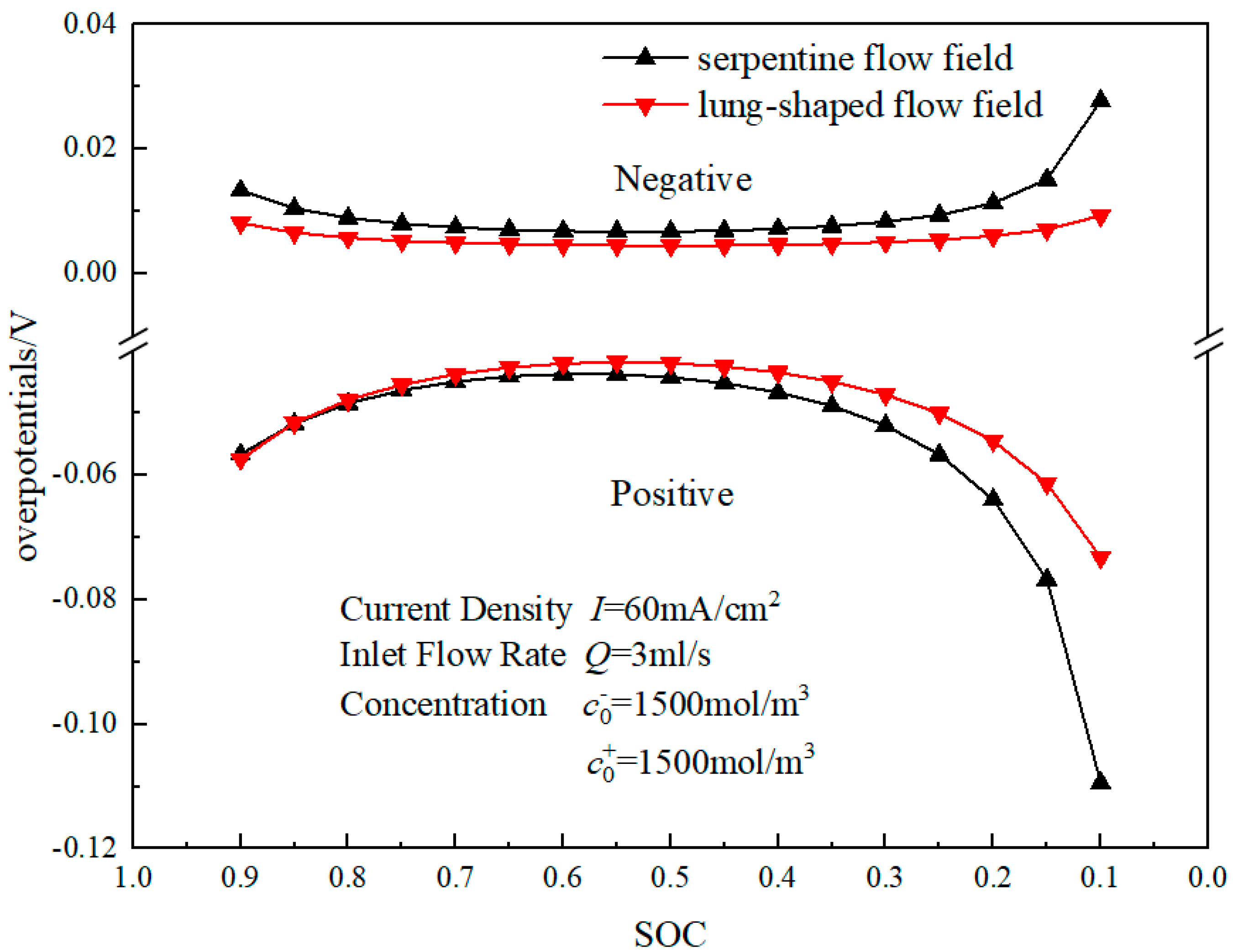
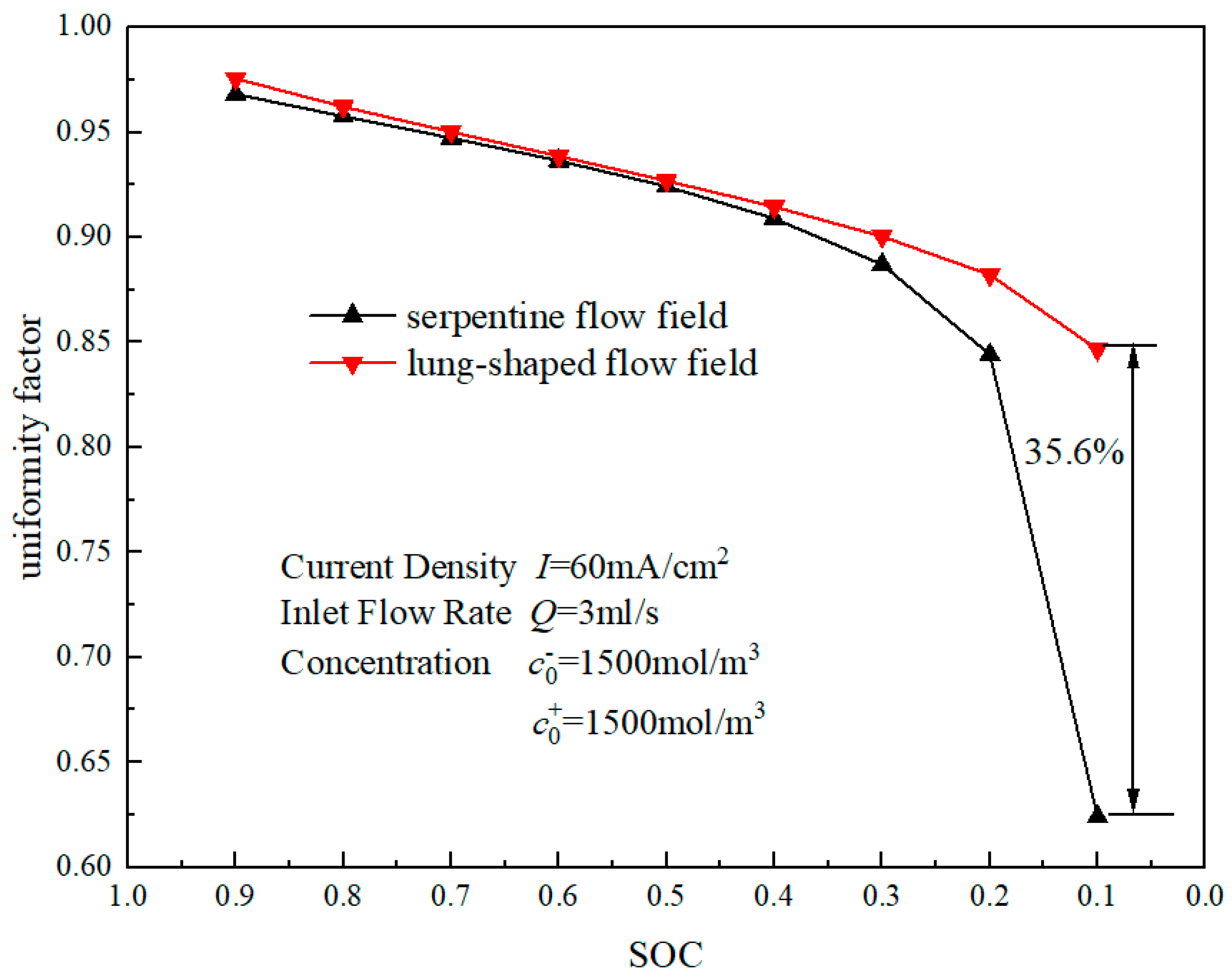
3.2. Mass Transfer Performance
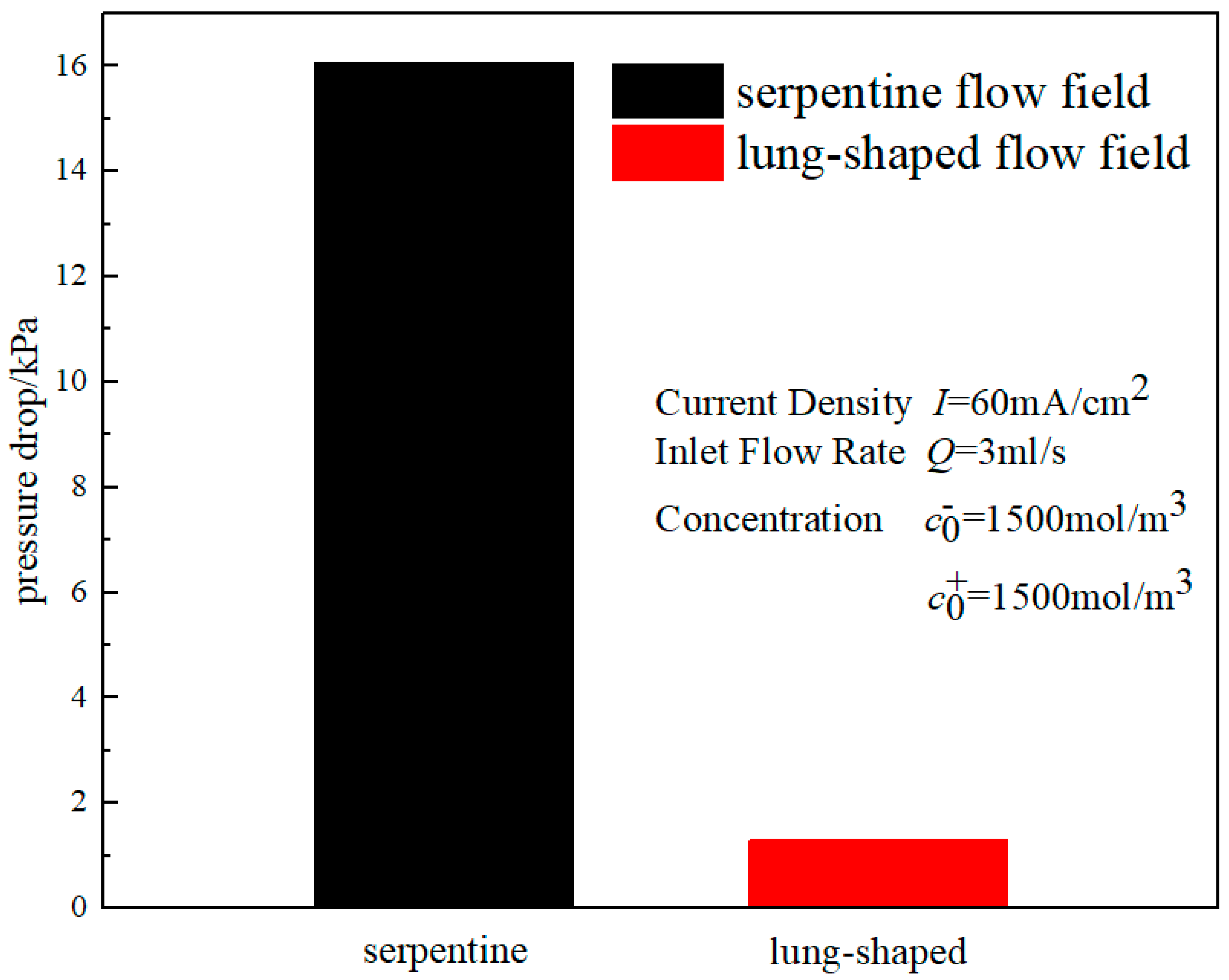
3.3. Output Characteristic
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Zhang, P.F.; Yang, J.L.; Li, J.; Chu, F.M. Effect of variable cross-section electrode on the battery performance of all-vanadium redox flow battery. Int. J. Heat Mass Transf. 2023, 215, 124382. [Google Scholar] [CrossRef]
- Ning, Y.; Fu, K.; Ke, Z.; Yunlong, Z.; Wen-Feng, L. A strategy for CO2 capture and utilization towards methanol production at industrial scale: An integrated highly efficient process based on multi-criteria assessment. Energy Convers. Manag. 2023, 293, 117516. [Google Scholar]
- Chuanyu, S.; Enrico, N.; Angeloclaudio, N.; Gioele, P.; Keti, V.; Thomas, A.Z.; Laura, M.; Chiara, G.; Vito, D.N. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar]
- Huan, Z.; Yi, T.; Xu-Dong, L.; Chuan-Yu, S.; Na, C. Polarization Effects of a Rayon and Polyacrylonitrile Based Graphite Felt for Iron-Chromium Redox Flow Batteries. ChemElectroChem 2019, 6, 3175–3188. [Google Scholar]
- Jungmyung, K.; Heesung, P. Mass transfer in flow batteries characterized by comparison of electrical potentials according to regions of porous electrodes. J. Energy Storage 2022, 55, 105556. [Google Scholar]
- Skyllas-Kazacos, M.; Goh, L. Modeling of vanadium ion diffusion across the ion exchange membrane in the vanadium redox battery. J. Membr. Sci. 2012, 399–400, 43–48. [Google Scholar] [CrossRef]
- Pugach, M.; Kondratenko, M.; Briola, S.; Bischi, A. Zero dimensional dynamic model of vanadium redox flow battery cell incorporating all modes of vanadium ions crossover. Appl. Energy 2018, 226, 560–569. [Google Scholar] [CrossRef]
- Knehr, K.W.; Agar, E.; Dennison, C.R.; Kalidindi, A.R.; Kumbur, E.C. A transient vanadium flow battery model incorporating vanadium crossover and water transport through the membrane. J. Electrochem. Soc. 2012, 159, 1446–1459. [Google Scholar] [CrossRef]
- Ma, X.K.; Zhang, H.M.; Xing, F. A three-dimensional model for negative half cell of the vanadium redox flow battery. Electrochim. Acta 2011, 58, 238–246. [Google Scholar] [CrossRef]
- Kyeongmin, O.; Haneul, Y.; Johan, K.; Seongyeon, W.; Hyunchul, J. Three-dimensional, transient, nonisothermal model of all-vanadium redox flow batteries. Energy 2015, 81, 3–14. [Google Scholar]
- Wang, Y.; Cho, S.C. Analysis and three-dimensional modeling of vanadium flow batteries. J. Electrochem. Soc. 2014, 161, 1200–1212. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Lin, L.; Chen, L.; Wang, M.; Zhou, J.J.J.o.E.S. A two-dimensional porous electrode model for designing pore structure in a quinone-based flow cell. J. Energy Storage 2018, 18, 16–25. [Google Scholar] [CrossRef]
- Zhang, B.W.; Lei, Y.; Bai, B.F.; Zhao, T.S. A two-dimensional model for the design of flow fields in vanadium redox flow batteries. Int. J. Heat Mass Transf. 2019, 135, 460–469. [Google Scholar] [CrossRef]
- Aaron, D.S.; Liu, Q.; Tang, Z.; Grim, G.M.; Papandrew, A.B.; Turhan, A.; Zawodzinski, T.A.; Mench, M.M. Dramatic performance gains in vanadium redox flow batteries through modified cell architecture. J. Power Sources 2012, 206, 450–453. [Google Scholar] [CrossRef]
- Kumar, S.; Jayanti, S. Effect of flow field on the performance of an all-vanadium redox flow battery. J. Power Sources 2016, 307, 782–787. [Google Scholar] [CrossRef]
- Ravendra, G.; Sreenivas, J. Effect of channel dimensions of serpentine flow fields on the performance of a vanadium redox flow battery. J. Energy Storage 2019, 23, 148–158. [Google Scholar]
- Darling, R.M.; Perry, M.L. The Influence of Electrode and Channel Configurations on Flow Battery Performance. J. Electrochem. Soc. 2014, 161, 1381–1387. [Google Scholar] [CrossRef]
- Kumar, S.; Jayanti, S. Effect of electrode intrusion on pressure drop and electrochemical performance of an all-vanadium redox flow battery. J. Power Sources 2017, 360, 548–558. [Google Scholar] [CrossRef]
- Kloess, J.P.; Wang, X.; Liu, J.; Shi, Z.Y.; Guessous, L. Investigation of bio-inspired flow channel designs for bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2009, 188, 132–140. [Google Scholar] [CrossRef]
- Jessica, M.C. Biomimetic Design Applied to the Redesign of a PEM Fuel Cell Flow Field. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2010. [Google Scholar]
- Gutierrez, L.D.; Hernandez-Guerrero, A.; Alvarado, B.R.; Perez-Raya, I.; Alatorre-Ordaz, A. Performance analysis of a proton exchange membrane fuel cell using tree-shaped designs for flow distribution. Int. J. Hydrogen Energy 2013, 38, 14750–14763. [Google Scholar] [CrossRef]
- Jinchao, L.; Jun, L.; Wenheng, H.; Wenjie, X.; Jun, L.; Huan, L.; Yaping, Z. Novel branched sulfonated polyimide membrane with remarkable vanadium permeability resistance and proton selectivity for vanadium redox flow battery application. Int. J. Hydrogen Energy 2022, 47, 8883–8891. [Google Scholar]
- Xu, Z.Y.; Jing, M.H.; Fan, X.Z.; Liu, J.G.; Yan, C.W. Advanced dual-gradient carbon nanofibers/graphite felt composite electrode for the next-generation vanadium flow battery. J. Mater. Sci. Technol. 2022, 136, 32–42. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Leung, P.J.A.e. Numerical investigations of flow field designs for vanadium redox flow batteries. Appl. Energy 2013, 105, 47–56. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.G.; Jiang, Z.Y.; Yang, W.W. Numerical study on vanadium redox flow battery performance with nonuniformly compressed electrode and serpentine flow field. Appl. Energy 2018, 220, 106–116. [Google Scholar] [CrossRef]
- Yang, W.W.; He, Y.L.; Li, Y.S. Performance modeling of a vanadium redox flow battery during discharging. Electrochim. Acta 2015, 155, 279–287. [Google Scholar] [CrossRef]
- Lu, M.Y.; Yang, W.W.; Bai, X.S.; Deng, Y.M.; He, Y.L. Performance improvement of a vanadium redox flow battery with asymmetric electrode designs. Electrochim. Acta 2019, 319, 210–226. [Google Scholar] [CrossRef]
- Yang, W.W.; Bai, X.S.; Zhang, W.Y.; Lu, M.Y.; Xu, Q. Numerical examination of the performance of a vanadium redox flow battery under variable operating strategies. J. Power Sources 2020, 457, 228002. [Google Scholar] [CrossRef]
- Lu, M.Y.; Yang, W.W.; Deng, Y.M.; Li, W.Z.; Xu, Q.; He, Y.L. Mitigating capacity decay and improving charge-discharge performance of a vanadium redox flow battery with asymmetric operating conditions. Electrochim. Acta 2019, 309, 283–299. [Google Scholar] [CrossRef]
- Yue, M.; Zheng, Q.; Xing, F.; Zhang, H.M.; Li, X.F.; Ma, X.K. Flow field design and optimization of high power density vanadium flow batteries: A novel trapezoid flow battery. AIChE J. 2018, 64, 782–795. [Google Scholar] [CrossRef]
- Chu, F.M.; Xiao, G.Z.; Xia, L.X.; Yang, Y.; Yang, G.A.; Tan, Z.A. Analysis of battery performance and mass transfer behavior for organic redox flow battery with different flow fields. J. Electrochem. Soc. 2022, 169, 70529. [Google Scholar] [CrossRef]





| Parameter | Symbol | Value | Source |
|---|---|---|---|
| Specific surface area(m−1) | a | 2.5 × 105 | Ref. [24] |
| Diffusion coefficient of V2+(m2/s) | DV2+ | 2.4 × 10−10 | Ref. [25] |
| Diffusion coefficient of V3+(m2/s) | DV3+ | 2.4 × 10−10 | Ref. [25] |
| Diffusion coefficient of VO2+ | DVO2+ | 3.9 × 10−10 | Ref. [25] |
| Diffusion coefficient of | D | 3.9 × 10−10 | Ref. [25] |
| Diffusion coefficient of SO4(m2/s) | DSO42− | 1.065 × 10−9 | Ref. [25] |
| Diffusion coefficient of HSO4(m2/s) | D | 1.33 × 10−9 | Ref. [25] |
| Diffusion coefficient of H+(m2/s) | DH+ | 3.5 × 10−9 | Ref. [25] |
| Operating temperature (K) | T | 300 K | Ref. [24] |
| Electrode conductivity(S/m) | σs | 1000 | Ref. [24] |
| Membrane conductivity(S/m) | σm | 10 | Ref. [24] |
| Anodic transfer coefficients | α+ | 0.5 | Assumed |
| Cathodic transfer coefficient | α− | 0.5 | Assumed |
| Rate constant, negative reaction(m/s) | kneg | 1.7 × 10−7 | Ref. [26] |
| Rate constant, positive reaction(m/s) | kpos | 6.8 × 10−7 | Ref. [25] |
| Standard potential of negative reaction(V) | E0,neg | −0.255 | Ref. [27] |
| Standard potential of positive reaction(V) | E0,pos | 1.004 | Ref. [28] |
| Fiber diameter of the electrode (μm) | df | 17.6 | Ref. [29] |
| Kozeny-Carmen Coefficient | Kck | 4.89 | Ref. [30] |
| Pump efficiency | φ | 0.9 | Ref. [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Chu, F. A Novel Biomimetic Lung-Shaped Flow Field for All-Vanadium Redox Flow Battery. Sustainability 2023, 15, 13613. https://doi.org/10.3390/su151813613
Zhong L, Chu F. A Novel Biomimetic Lung-Shaped Flow Field for All-Vanadium Redox Flow Battery. Sustainability. 2023; 15(18):13613. https://doi.org/10.3390/su151813613
Chicago/Turabian StyleZhong, Longchun, and Fengming Chu. 2023. "A Novel Biomimetic Lung-Shaped Flow Field for All-Vanadium Redox Flow Battery" Sustainability 15, no. 18: 13613. https://doi.org/10.3390/su151813613
APA StyleZhong, L., & Chu, F. (2023). A Novel Biomimetic Lung-Shaped Flow Field for All-Vanadium Redox Flow Battery. Sustainability, 15(18), 13613. https://doi.org/10.3390/su151813613






