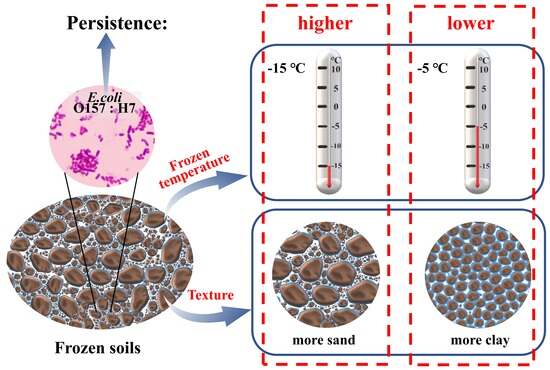
Persistence of E. coli O157:H7 in Frozen Soils: Role of Freezing Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Characterization
2.2. Soil DNA Extraction and Sequencing
2.3. Bacteria Strains
2.4. Persistence Experiment
2.5. Survival Data Modeling
2.6. Statistical Analysis
3. Results
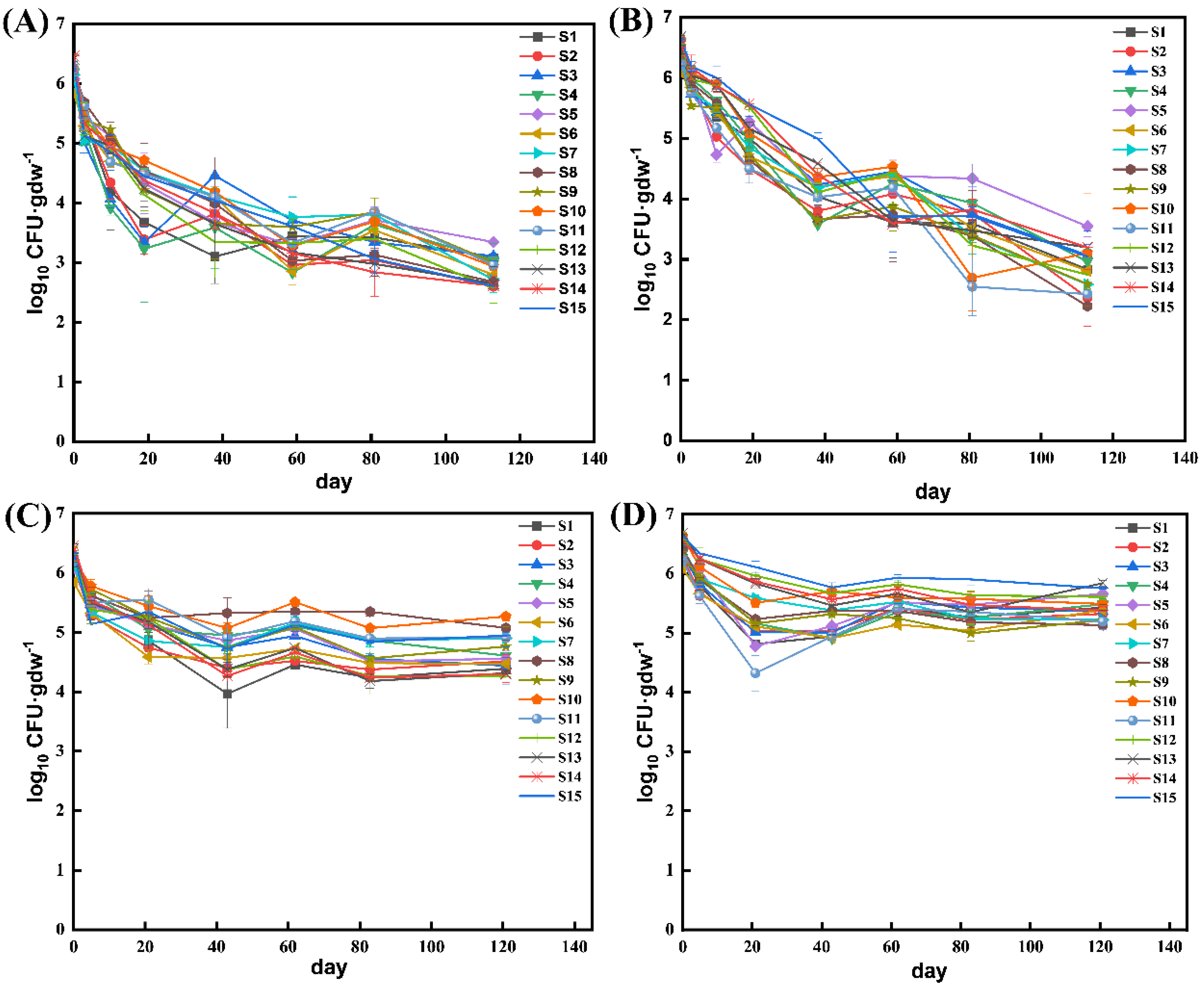
3.1. EcO157 Persistence in Frozen Soils
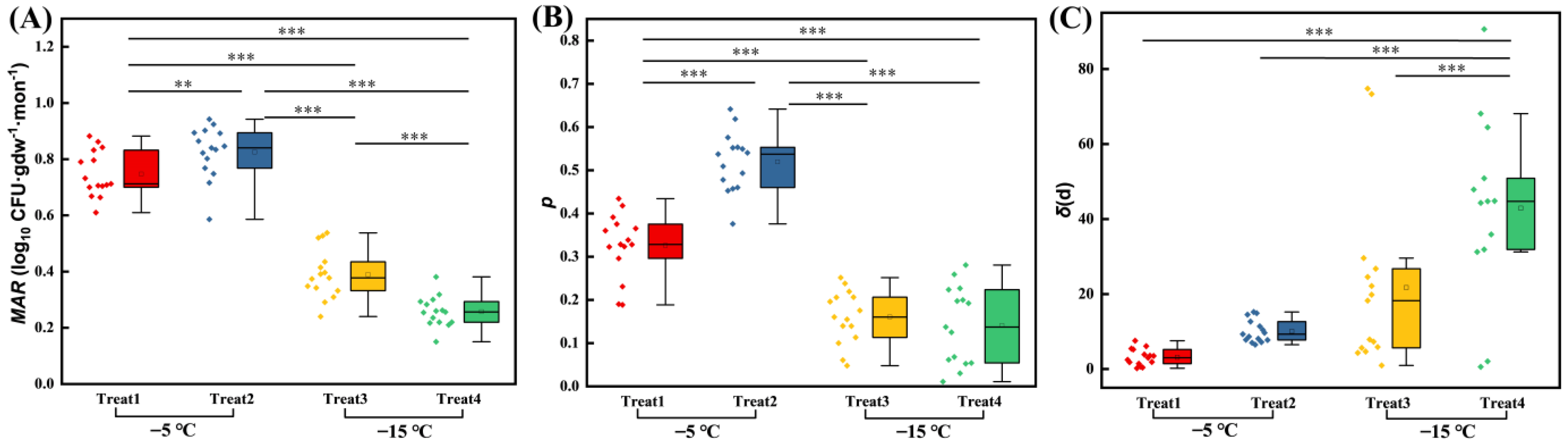
3.2. Effects of Soil Freezing Temperature and Moisture on the Persistence of EcO157
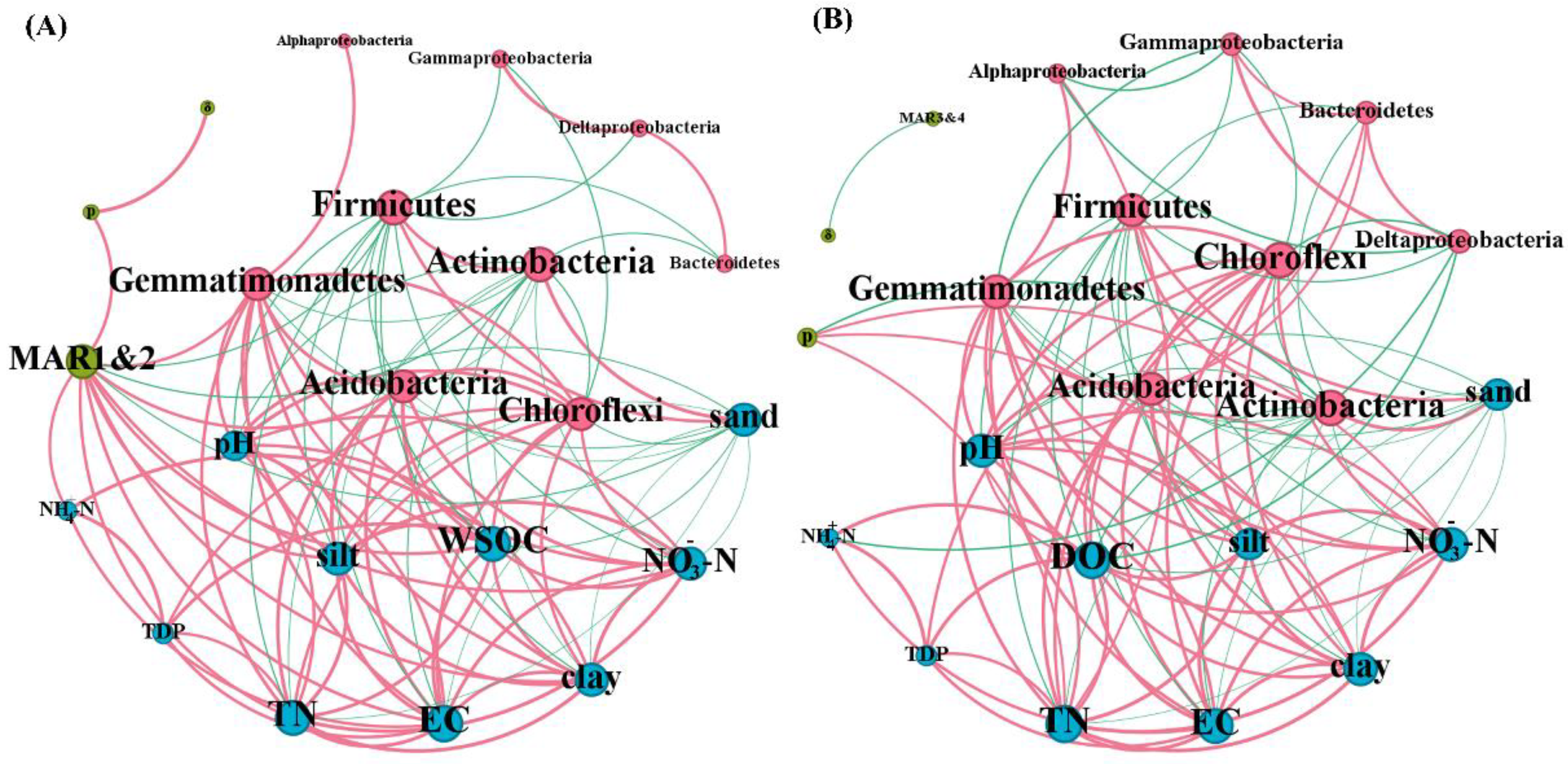
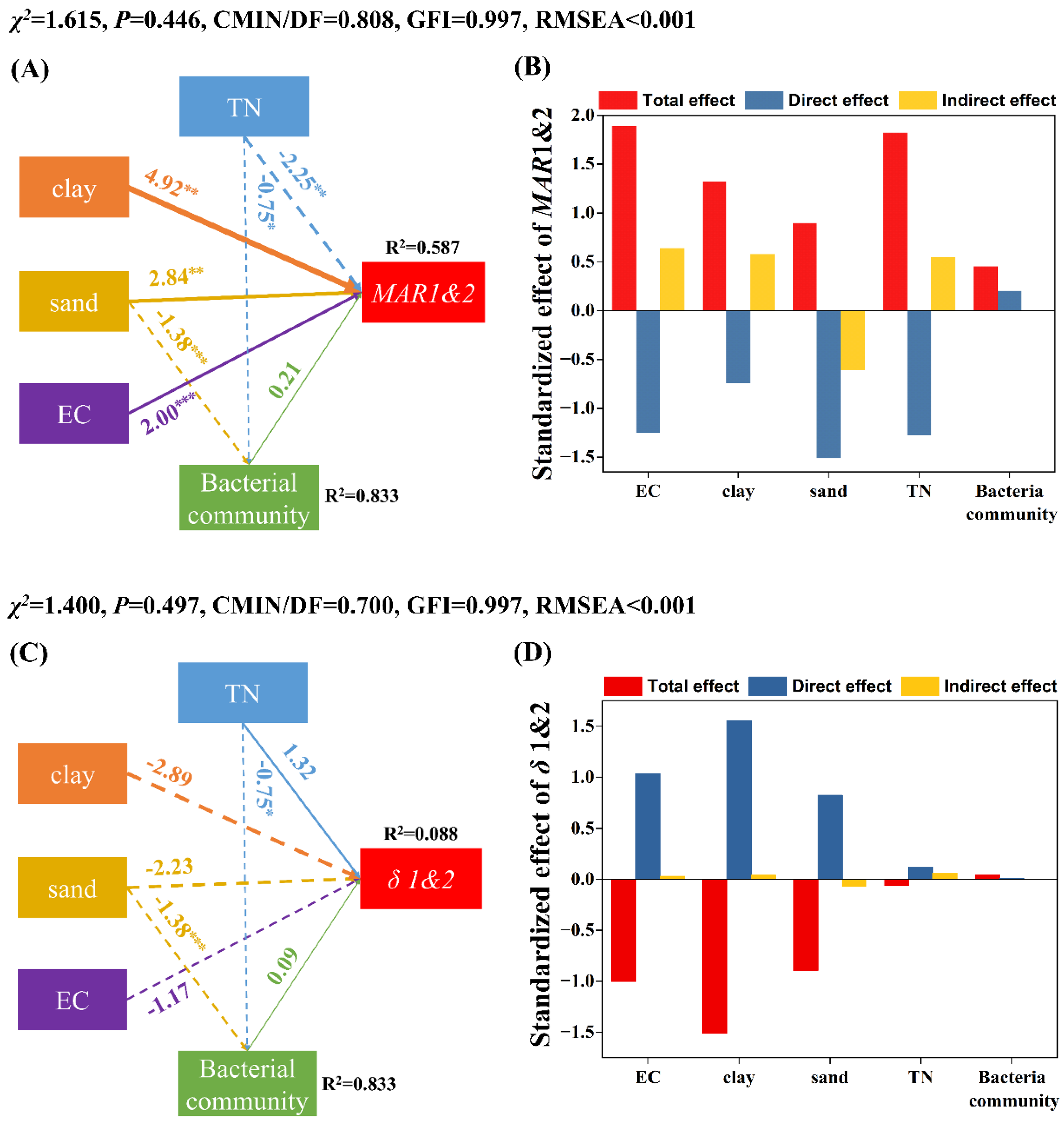
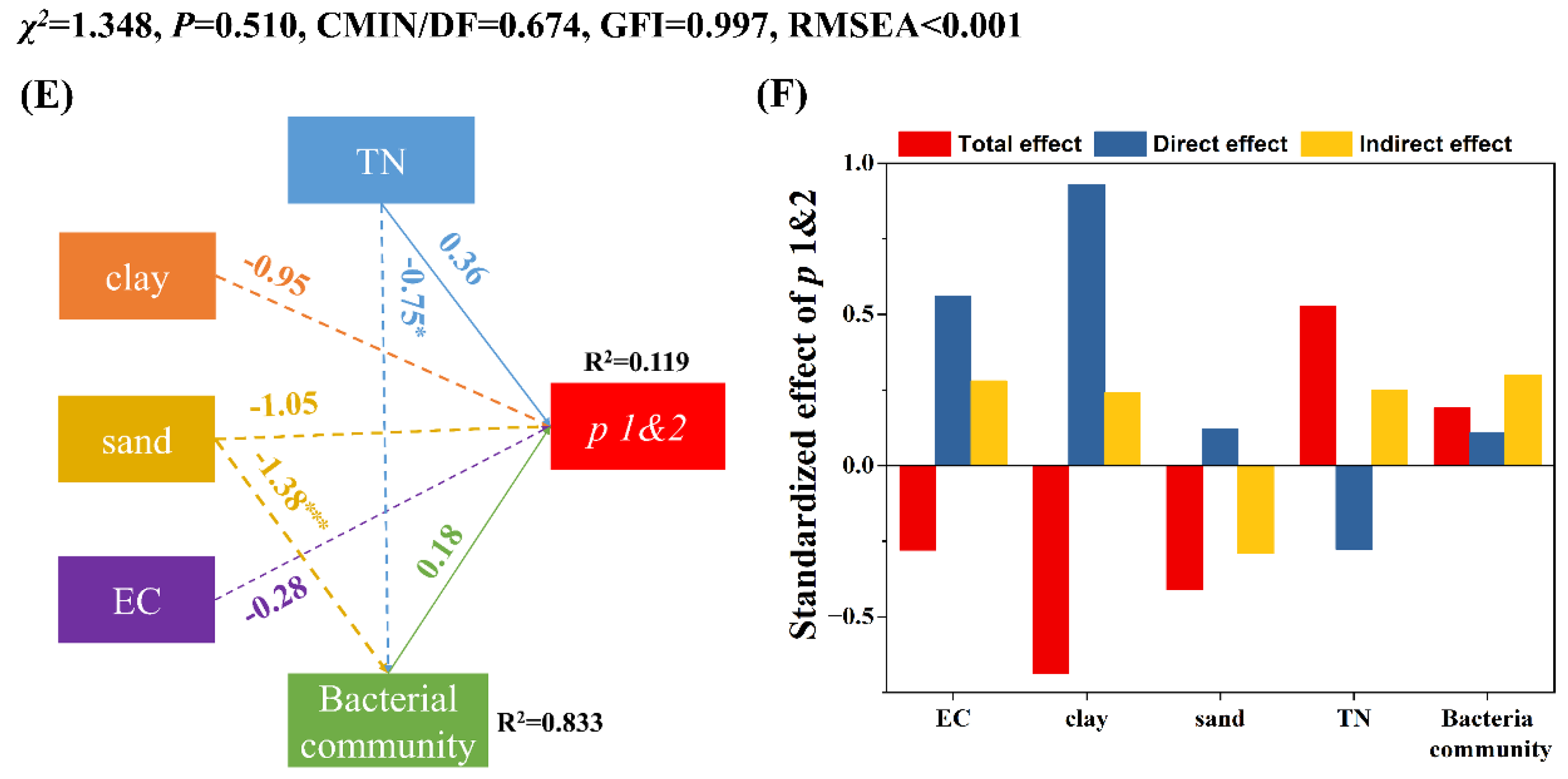
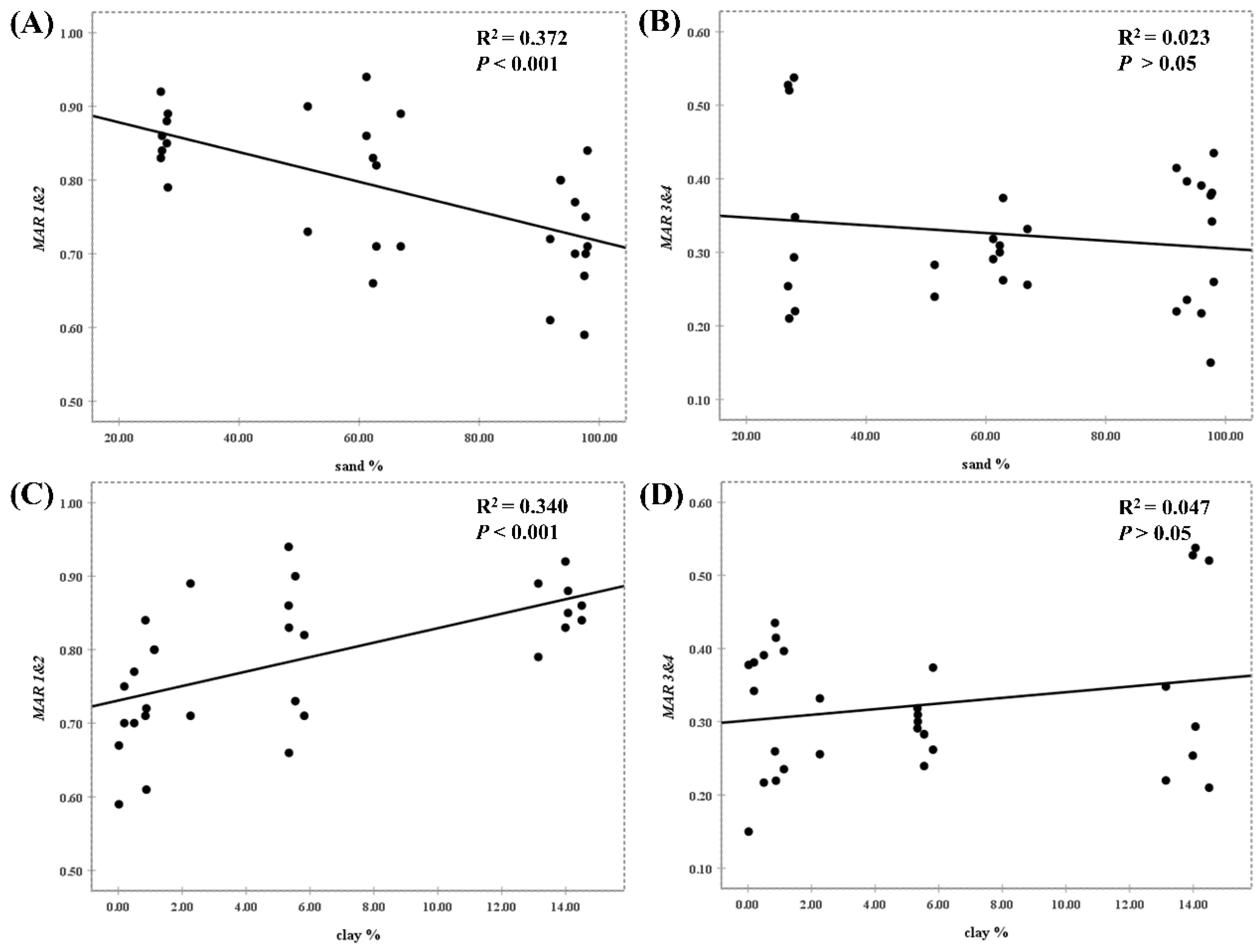
3.3. Factors Influencing the Persistence of EcO157 in Frozen Soils
4. Discussion
4.1. EcO157 Persists Longer at −15 °C Than at −5 °C under Freezing Conditions
4.2. Frozen Temperature Not Moisture Determines Persistence of EcO157 under Freezing Conditions
4.3. More Sand Favored the Persistence of EcO157 in Frozen Soils
4.4. Other Factors That Influence the Persistence of EcO157 under Freezing Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Y.Z.; Han, L.J.; Cheng, L.H.; Lv, Y.H.; Fu, B.J.; Feng, X.M.; Wu, X. Shortened duration and reduced area of frozen soil in the Northern Hemisphere. Innovation 2021, 2, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yanai, Y.; Okazaki, K. Effects of successive soil freeze-thaw cycles on soil microbial biomass and organic matter decomposition potential of soils. Soil Sci. Plant Nutr. 2004, 50, 821–829. [Google Scholar] [CrossRef]
- Ma, J.; Ibekwe, A.M.; Crowley, D.E.; Yang, C.H. Persistence of Escherichia coli O157:H7 in major leafy green producing soils. Environ. Sci. Technol. 2012, 46, 12154–12161. [Google Scholar] [CrossRef] [PubMed]
- Chase-Topping, M.; Gally, D.; Low, C.; Matthews, L.; Woolhouse, M. Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat. Rev. Microbiol. 2008, 6, 904–912. [Google Scholar] [CrossRef]
- Lee, W. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Li, C.; Cui, H. Inactivation mechanism of E. coli O157:H7 under ultrasonic sterilization. Ultrason. Sonochem. 2019, 59, 104751. [Google Scholar] [CrossRef]
- Fukushima, H.; Seki, R. High numbers of Shiga toxin-producing Escherichia coli found in bovine faeces collected at slaughter in Japan. FEMS Microbiol. Ecol. 2004, 238, 189–197. [Google Scholar] [CrossRef]
- Arthur, T.M.; Nou, X.; Kalchayanand, N.; Bosilevac, J.M.; Wheeler, T.; Koohmaraie, M. Escherichia coli O157:H7 survival on cattle hides. Appl. Environ. Microbiol. 2011, 77, 3002–3008. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Grieve, C.M. Changes in developing plant microbial community structure as affected by contaminated water. FEMS Microbiol. Ecol. 2004, 48, 239–248. [Google Scholar] [CrossRef]
- Williams, M.L.; Lejeune, J.T.; McSpadden Gardener, B. Soil conditions that can alter natural suppression of Escherichia coli O157:H7 in Ohio specialty crop soils. Appl. Environ. Microbiol. 2015, 81, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Fouché, J.; Christiansen, C.T.; Lafrenière, M.J.; Grogan, P.; Lamoureux, S.F. Canadian permafrost stores large pools of ammonium and optically distinct dissolved organic matter. Nat. Commun. 2020, 11, 4500. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Grosse, G.; Kuhry, P.; Hugelius, G.; Koven, C.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Lachenbruch, A.H.; Marshall, B.V. Changing climate: Geothermal evidence from permafrost in the alaskan arctic. Science 1986, 234, 689–696. [Google Scholar] [CrossRef]
- Nikrad, M.P.; Kerkhof, L.J.; Häggblom, M.M. The subzero microbiome: Microbial activity in frozen and thawing soils. FEMS Microbiol. Ecol. 2016, 92, fiw081. [Google Scholar] [CrossRef]
- Adhikari, H.; Barnes, D.L.; Schiewer, S.; White, D.M. Total coliform survival characteristics in frozen soils. J. Environ. Eng. 2007, 133, 1098–1105. [Google Scholar] [CrossRef]
- Panikov, N.S.; Flanagan, P.W.; Oechel, W.C.; Mastepanov, M.A.; Christensen, T.R. Microbial activity in soils frozen to below −39 °C. Soil Biol. Biochem. 2006, 38, 785–794. [Google Scholar] [CrossRef]
- Yamamoto, S.A.; Harris, L.J. Phosphate buffer increases recovery of Escherichia coli O157:H7 from frozen apple juice. J. Food Protect. 2001, 64, 1315–1319. [Google Scholar] [CrossRef]
- Xu, W.Q.; Chen, H.Q.; Wu, C.Q. Salmonella and Escherichia coli O157:H7 Inactivation, Color, and Bioactive Compounds Enhancement on Raspberries during Frozen Storage after Decontamination Using New Formula Sanitizer Washing or Pulsed Light. J. Food Protect. 2016, 79, 1107–1114. [Google Scholar] [CrossRef]
- Lowder, A.C.; Waite-Cusic, J.G.; DeWitt, C.A.M. High pressure-low temperature processing of beef: Effects on survival of internalized E-coli O157:H7 and quality characteristics. Innov. Food Sci. Emerg. 2014, 26, 18–25. [Google Scholar] [CrossRef]
- Manios, S.G.; Skandamis, P.N. Effect of frozen storage, different thawing methods and cooking processes on the survival of Salmonella spp. and Escherichia coli O157:H7 in commercially shaped beef patties. Meat Sci. 2015, 101, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Cheng, S.J.; Wang, Y.C.; Chung, K.T. Behavior of Escherichia coli O157:H7 and Listeria monocytogenes in Tryptic soy broth subjected to various low temperature treatments. Food Res. Int. 1999, 32, 1–6. [Google Scholar] [CrossRef]
- Ciftcioglu, G.; Arun, O.O.; Vural, A.; Aydin, A.; Aksu, H. Survival of Escherichia coli O157:H7 in minced meat and hamburger patties. J. Food Agric. Environ. 2008, 6, 24–27. [Google Scholar]
- Zhao, T.; Doyle, M.P.; Kemp, M.C.; Howell, R.S.; Zhao, P. Influence of freezing and freezing plus acidic calcium sulfate and lactic acid addition on thermal inactivation of Escherichia coli O157:H7 in ground beef. J. Food Protect. 2004, 67, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.D.A.; Racca, A.R.; Ribeiro, L.R.; Cruz, F.D.; Cap, M.; Mozgovoj, M.V.; Cristianini, M.; Vaudagna, S.R. High-pressure processing treatment of beef burgers: Effect on Escherichia coli O157 inactivation evaluated by plate count and PMA-qPCR. J. Food Sci. 2022, 87, 2324–2336. [Google Scholar] [CrossRef]
- Ansay, S.E.; Darling, K.A.; Kaspar, C.W. Survival of Escherichia coli O157:H7 in ground-beef patties during storage at 2, −2, 15 and then −2 °C, and −20 °C. J. Food Protect. 1999, 62, 1243–1247. [Google Scholar] [CrossRef]
- Bollman, J.; Ismond, A.; Blank, G. Survival of Escherichia coli O157:H7 in frozen foods: Impact of the cold shock response. Int. J. Food Microbiol. 2001, 64, 127–138. [Google Scholar] [CrossRef]
- Holliday, V.T. Methods of soil analysis, part 1, physical and mineralogical methods (2nd edition), A. Klute, Ed., 1986, American Society of Agronomy, Agronomy Monographs 9(1), Madison, Wisconsin, 1188 pp., $60.00. Geoarchaeology 1990, 5, 87–89. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Vadakedath, N.; Chandra, R. In-situ toxicity assessment of pulp and paper industry wastewater on Trigonella foenum-graecum L: Potential source of cytotoxicity and chromosomal damage. Environ. Technol. Innov. 2021, 21, 101251. [Google Scholar] [CrossRef]
- Liao, J.; Li, J.; Han, Z.; Lyu, G.; Ibekwe, A.M.; Ma, J. Persistence of Salmonella Typhimurium in apple-pear (Pyrus bretschneideri Rehd.) orchard soils influenced by bacterial communities and soil properties. Sci. Total Environ. 2021, 768, 144458. [Google Scholar] [CrossRef]
- Mackey, B.M. Lethal and sublethal effects of refrigeration, freezing and freeze-drying on micro-organisms. Soc. Appl. Bacteriol. Symp. Ser. 1984, 12, 45–75. [Google Scholar]
- Han, Z.; Huang, G.; Liao, J.; Li, J.; Lyu, G.; Ma, J. Disentangling survival of Escherichia coli O157:H7 in soils: From a subpopulation perspective. Sci. Total Environ. 2020, 749, 141649. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. B 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating net-works. In Proceedings of the International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Hopper, D.; Coughlan, J.; Mullen, M.R. Structural equation modeling: Guidelines for determining model fit. Electron. J. Bus. Res. Methods 2008, 6, 53–60. [Google Scholar] [CrossRef]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods Psychol. Res. Online 2003, 8, 23–74. [Google Scholar]
- Kim, J.; Ha, S.; Park, W. Expression and deletion analyses of cspE encoding cold-shock protein E in Acinetobacter oleivorans DR1. Res. Microbiol. 2018, 169, 244–253. [Google Scholar] [CrossRef]
- Meynell, G.G. The effect of sudden chilling on Escherichia coli. J. Gen. Microbiol. 1958, 19, 380–389. [Google Scholar] [CrossRef]
- Jefferies, R.L.; Walker, N.A.; Edwards, K.A.; Dainty, J. Is the decline of soil microbial biomass in late winter coupled to changes in the physical state of cold soils? Soil Biol. Biochem. 2010, 42, 129–135. [Google Scholar] [CrossRef]
- Mazur, P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J. Gen. Physiol. 1963, 47, 347–369. [Google Scholar] [CrossRef]
- Steven, B.; Léveillé, R.; Pollard, W.H.; Whyte, L.G. Microbial ecology and biodiversity in permafrost. Extremophiles 2006, 10, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Marechal, P.A.; Gervais, P. Influence of cooling rate on Saccharomyces cerevisiae destruction during freezing: Unexpected viability at ultra-rapid cooling rates. Cryobiology 2003, 46, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Z.; Wang, J. Unfrozen water content of permafrost during thawing by the capacitance technique. Cold Reg. Sci. Technol. 2018, 152, 15–22. [Google Scholar] [CrossRef]
- Peter, M. Physical-Chemical Basis of Injury from Intracellular Freezing in Yeast. Cryobiology 1967, 3, 171–189. [Google Scholar] [CrossRef]
- Ma, J.; Ibekwe, A.M.; Yang, C.-H.; Crowley, D.E. Influence of bacterial communities based on 454-pyrosequencing on the survival of Escherichia coli O157:H7 in soils. FEMS Microbiol. Ecol. 2013, 84, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Marechal, P.-A.; Gervais, P. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl. Environ. Microbiol. 2004, 70, 268–272. [Google Scholar] [CrossRef]
- Parker, L.V.; Martel, C.J. Long-Term Survival of Enteric Microorganisms in Frozen Wastewater; Technical Report TR-02-16; U.S. Army Corps of Engineers, Engineer Research and Development Center, Cold Regions Research and Engineering Laboratory (ERDC/CRREL): Hanover, NH, USA, 2002; pp. 1–65. [Google Scholar]
- Franks, F.; Mathias, S.F.; Hatley, R.H.M.; Baust, J.G.; Hvidt, A.; Chapman, D.; Jaenicke, R. Water, temperature and life [and discussion]. Philos. Trans. R. Soc. B Biol. Sci. 1990, 326, 517–533. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Physical principles of membrane damage due to dehydration and freezing. Mech. Swelling 1992, 64, 205–224. [Google Scholar] [CrossRef]
- Walker, V.K.; Palmer, G.R.; Voordouw, G. Freeze-thaw tolerance and clues to the winter survival of a soil community. Appl. Environ. Microbiol. 2006, 72, 1784–1792. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Chen, W.; Tao, J.; Xu, M.; Guo, P. Effects of fulvic acid and fulvic ions on Escherichia coli survival in river under repeated freeze-thaw cycles. Environ. Pollut. 2019, 247, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Chenu, C.; Heijnen, C.E. Micro-morphological studies on clay-amended and unamended loamy sand, relating survival of introduced bacteria and soil structure. Geoderma 1993, 56, 195–207. [Google Scholar] [CrossRef]
- Brennan, F.P.; Moynihan, E.; Griffiths, B.S.; Hillier, S.; Owen, J.; Pendlowski, H.; Avery, L.M. Clay mineral type effect on bacterial enteropathogen survival in soil. Sci. Total Environ. 2014, 468–469, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.M.; Clegg, C.D.; Heathwaite, A.L.; Haygarth, P.M. Preferential attachment of Escherichia coli to different particle size fractions of an agricultural grassland soil. Water Air Soil Poll. 2007, 185, 369–375. [Google Scholar] [CrossRef]
- Williams, P.J. Experimental determination of apparent specific heats of frozen soils. Géotechnique 1964, 14, 133–142. [Google Scholar] [CrossRef]
- Stähli, M.; Stadler, D. Measurement of water and solute dynamics in freezing soil columns with time domain reflectometry. J. Hydrol. 1997, 195, 352–369. [Google Scholar] [CrossRef]
- Trap, J.; Bonkowski, M.; Plassard, C.; Villenave, C.; Blanchart, E. Ecological importance of soil bacterivores for ecosystem functions. Plant Soil. 2016, 398, 1–24. [Google Scholar] [CrossRef]
- Ranjard, L.; Dequiedt, S.; Jolivet, C.; Saby, N.P.A.; Thioulouse, J.; Harmand, J.; Loisel, P.; Rapaport, A.; Fall, S.; Simonet, P.; et al. Biogeography of soil microbial communities: A review and a description of the ongoing french national initiative. Agron. Sustain. Dev. 2010, 30, 359–365. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef]

| Mean Square | F | p | ||
|---|---|---|---|---|
| MAR | R2 = 0.929 | |||
| Temperature | 4.02 | 715.86 | <0.001 | |
| Moisture | 0.00 | 0.34 | 0.565 | |
| Temperature × Moisture | 0.12 | 21.31 | <0.001 | |
| δ | R2 = 0.215 | |||
| Temperature | 115,853.96 | 7.33 | 0.009 | |
| Moisture | 69,987.01 | 4.43 | 0.040 | |
| Temperature × moisture | 56,505.20 | 3.58 | 0.064 | |
| p | R2 = 0.817 | |||
| Temperature | 1.11 | 199.39 | <0.001 | |
| Moisture | 0.11 | 20.24 | <0.001 | |
| Temperature × Moisture | 0.17 | 30.66 | <0.001 |
| MAR 1 and 2 | MAR 3 and 4 | |
|---|---|---|
| pH | 0.398 | 0.047 |
| EC | 0.643 *** | 0.077 |
| clay | 0.583 ** | 0.218 |
| silt | 0.610 ** | 0.134 |
| sand | −0.610 ** | −0.151 |
| NO3−-N | 0.590 ** | 0.234 |
| NH4+-N | 0.422 * | −0.048 |
| TDP | 0.357 | −0.032 |
| WSOC | 0.606 ** | 0.033 |
| TN | 0.554 ** | 0.175 |
| Actinobacteria | −0.466 * | −0.142 |
| Alphaproteobacteria | 0.169 | 0.060 |
| Deltaproteobacteria | −0.085 | 0.145 |
| Gammaproteobacteria | 0.082 | 0.194 |
| Acidobacteria | 0.531 ** | 0.073 |
| Gemmatimonadetes | 0.481 * | 0.022 |
| Chloroflexi | 0.349 | −0.049 |
| Firmicutes | −0.514 ** | −0.165 |
| Bacteroidetes | 0.237 | −0.040 |
| Persistence Parameters | Influencing Factor | Mantel | Partial Mantel | |||
|---|---|---|---|---|---|---|
| r | p | r | p | |||
| Treat1 and 2 (−5 °C) | MAR | env | 0.2501 | 0.002 | 0.1121 | 0.050 |
| bac | 0.2850 | 0.001 | 0.1796 | 0.002 | ||
| δ | env | −0.0779 | 0.928 | −0.1141 | 0.993 | |
| bac | 0.0288 | 0.278 | 0.0883 | 0.084 | ||
| p | env | −0.0563 | 0.829 | −0.1013 | 0.972 | |
| bac | 0.0477 | 0.192 | 0.0968 | 0.058 | ||
| Treat3 and 4 (−15 °C) | MAR | env | 0.0181 | 0.351 | −0.0207 | 0.572 |
| bac | 0.0620 | 0.157 | 0.0628 | 0.176 | ||
| δ | env | 0.0078 | 0.420 | −0.0075 | 0.482 | |
| bac | 0.0247 | 0.353 | 0.0246 | 0.322 | ||
| p | env | 0.0097 | 0.379 | −0.0198 | 0.592 | |
| bac | 0.0458 | 0.178 | 0.0490 | 0.182 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liao, J.; Ma, J.; Lyu, G.; Yang, X.; Ibekwe, A.M.; Ma, J. Persistence of E. coli O157:H7 in Frozen Soils: Role of Freezing Temperature. Sustainability 2023, 15, 13249. https://doi.org/10.3390/su151713249
Wang J, Liao J, Ma J, Lyu G, Yang X, Ibekwe AM, Ma J. Persistence of E. coli O157:H7 in Frozen Soils: Role of Freezing Temperature. Sustainability. 2023; 15(17):13249. https://doi.org/10.3390/su151713249
Chicago/Turabian StyleWang, Jiawei, Jiafen Liao, Jinhua Ma, Guangze Lyu, Xiaoyin Yang, Abasiofiok M. Ibekwe, and Jincai Ma. 2023. "Persistence of E. coli O157:H7 in Frozen Soils: Role of Freezing Temperature" Sustainability 15, no. 17: 13249. https://doi.org/10.3390/su151713249
APA StyleWang, J., Liao, J., Ma, J., Lyu, G., Yang, X., Ibekwe, A. M., & Ma, J. (2023). Persistence of E. coli O157:H7 in Frozen Soils: Role of Freezing Temperature. Sustainability, 15(17), 13249. https://doi.org/10.3390/su151713249