Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Determination of Chemical Composition of Spent Coffee Grounds and Spent Coffee Ground Derived Cellulose-Based Carrier
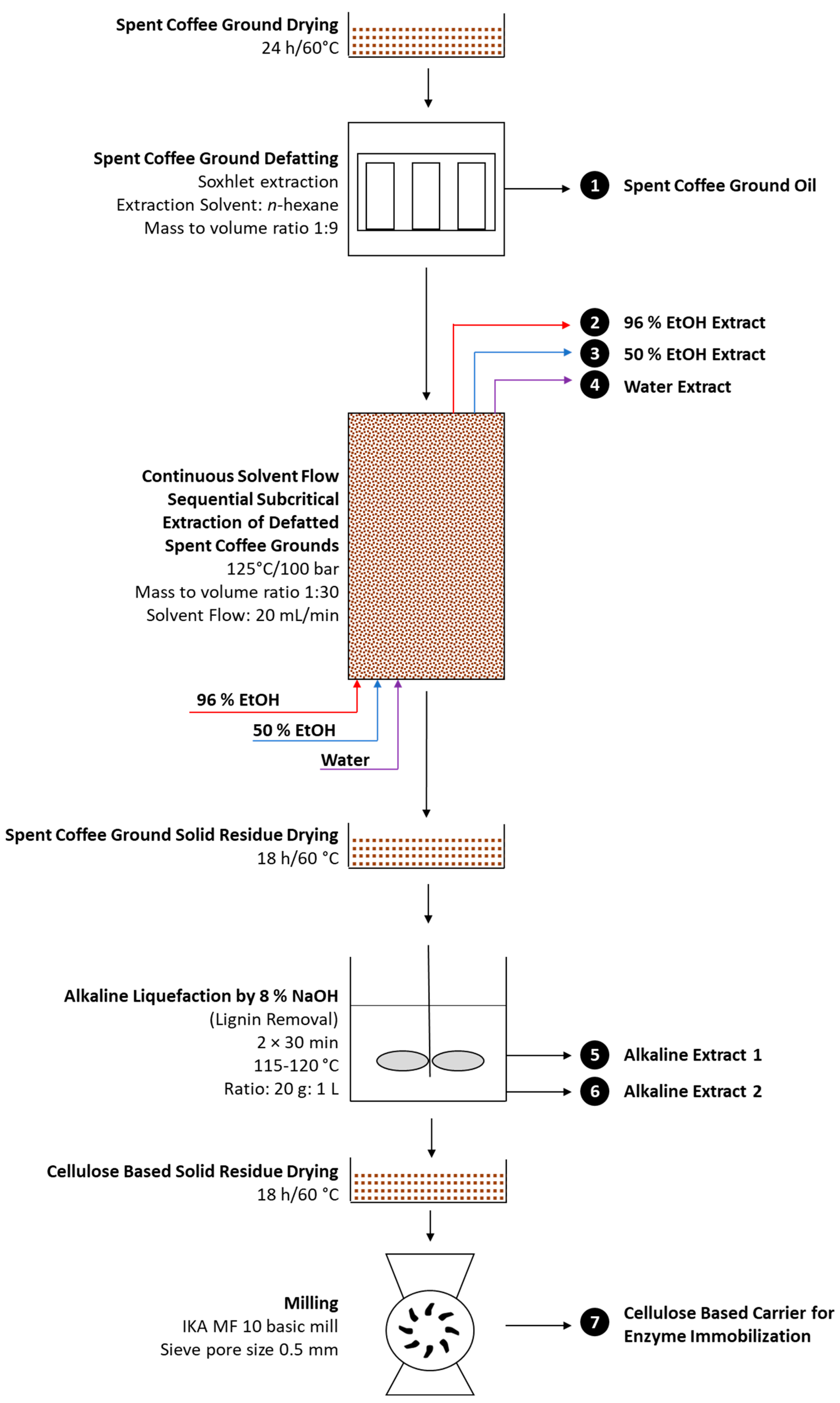
2.3. Multistep Extraction of Spent Coffee Grounds
2.4. Chemical Analysis of Obtained Extracts
2.5. Characterization of Spent Coffee Ground Derived Cellulose-Based Enzyme Immobilization Carrier
3. Results and Discussion
3.1. Chemical Composition of Spent Coffee Grounds
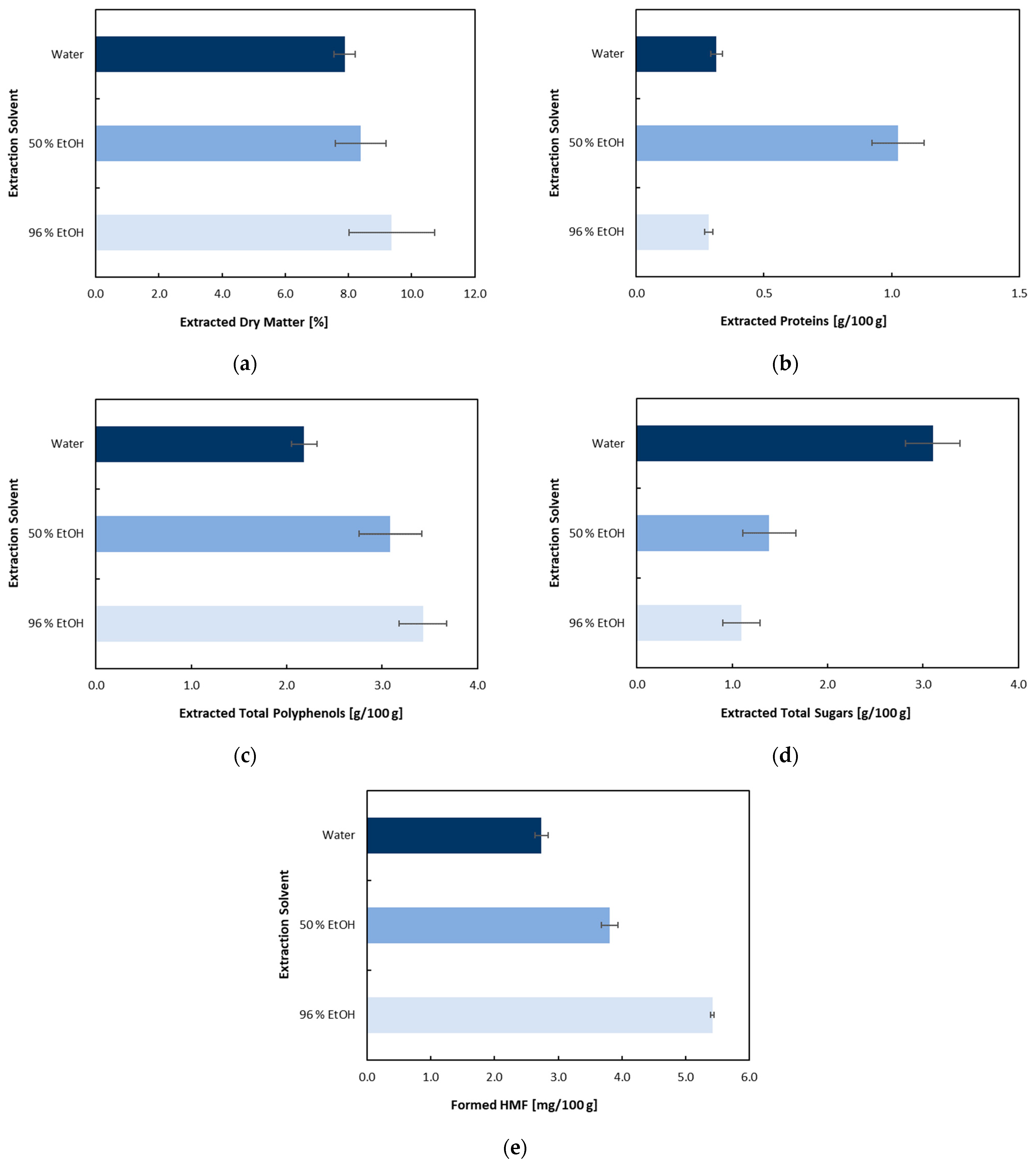
3.2. Multistep Extraction of Spent Coffee Grounds Oriented toward Production of Cellulose-Based Enzyme Immobilization Carrier
3.3. Characterization of the Produced SCG-Derived Cellulose-Based Enzyme Immobilization Carrier
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Determination of Crude Fiber, NDF, ADF and Lignin Contents by Van Soest Method Using ANKOM2000 Fiber Analyzer
References
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- de Bomfim, A.S.C.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. (Sophia) Spent Coffee Grounds: A Review on Current Utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Pedras, B.M.; Nascimento, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Semi-Continuous Extraction/Hydrolysis of Spent Coffee Grounds with Subcritical Water. J. Ind. Eng. Chem. 2019, 72, 453–456. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, L.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Palma, A.; Civitarese, V.; Gallucci, F. Spent Coffee Ground Characterization, Pelletization Test and Emissions Assessment in the Combustion Process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO) FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 3 August 2023).
- World Coffee Consumption. Available online: http://www.ico.org/trade_statistics.asp?section=Statistics (accessed on 3 August 2023).
- Budžaki, S.; Velić, N.; Ostojčić, M.; Stjepanović, M.; Rajs, B.B.; Šereš, Z.; Maravić, N.; Stanojev, J.; Hessel, V.; Strelec, I. Waste Management in the Agri-Food Industry: The Conversion of Eggshells, Spent Coffee Grounds, and Brown Onion Skins into Carriers for Lipase Immobilization. Foods 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent Coffee Grounds as a Valuable Source of Phenolic Compounds and Bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative Phenolics Obtained from Spent Coffee Grounds (Coffea arabica L.) by Subcritical Water Extraction. Ind. Crops Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Vu, D.C.; Vu, Q.T.; Huynh, L.; Lin, C.-H.; Alvarez, S.; Vo, X.T.; Nguyen, T.H.D. Evaluation of Fatty Acids, Phenolics and Bioactivities of Spent Coffee Grounds Prepared from Vietnamese Coffee. Int. J. Food Prop. 2021, 24, 1548–1558. [Google Scholar] [CrossRef]
- da Silva, M.F.; Pettinato, M.; Casazza, A.A.; Maciel, M.I.S.; Perego, P. Design and Evaluation of Non-Conventional Extraction for Bioactive Compounds Recovery from Spent Coffee (Coffea arabica L.) Grounds. Chem. Eng. Res. Des. 2022, 177, 418–430. [Google Scholar] [CrossRef]
- Scully, D.S.; Jaiswal, A.K.; Abu-Ghannam, N. An Investigation into Spent Coffee Waste as a Renewable Source of Bioactive Compounds and Industrially Important Sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Alcaraz, L.; Pérez-Ferreras, S.; León-González, M.E.; Rosales-Conrado, N.; López, F.A. Extraction of Polyphenols and Synthesis of New Activated Carbon from Spent Coffee Grounds. Sci. Rep. 2019, 9, 17706. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, M.; Casazza, A.A.; Ferrari, P.F.; Palombo, D.; Perego, P. Eco-Sustainable Recovery of Antioxidants from Spent Coffee Grounds by Microwave-Assisted Extraction: Process Optimization, Kinetic Modeling and Biological Validation. Food Bioprod. Process. 2019, 114, 31–42. [Google Scholar] [CrossRef]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso Coffee Residues: A Valuable Source of Unextracted Compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, V.; Solanki, K.; Mukherjee, J.; Gupta, M.N. Combi-Protein Coated Microcrystals of Lipases for Production of Biodiesel from Oil from Spent Coffee Grounds. Sustain. Chem. Process. 2013, 1, 14. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and Physicochemical Properties of Carboxymethyl Cellulose Films Enriched with Spent Coffee Grounds Polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-Value Molecules Recovery and Biofuels Production from Spent Coffee Grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A. Spent Coffee Grounds Alkaline Pre-Treatment as Biorefinery Option to Enhance Their Anaerobic Digestion Yield. Waste Biomass Valoriz. 2018, 9, 2565–2570. [Google Scholar] [CrossRef]
- Go, A.W.; Conag, A.T.; Cuizon, D.E.S. Recovery of Sugars and Lipids from Spent Coffee Grounds: A New Approach. Waste Biomass Valoriz. 2016, 7, 1047–1053. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent Coffee Grounds as a Versatile Source of Green Energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Passadis, K.; Fragoulis, V.; Stoumpou, V.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Study of Valorisation Routes of Spent Coffee Grounds. Waste Biomass Valoriz. 2020, 11, 5295–5306. [Google Scholar] [CrossRef]
- Pereira, A.P.; Woodman, T.J.; Chuck, C.J. An Integrated Biorefinery to Produce 5-(Hydroxymethyl)Furfural and Alternative Fuel Precursors from Macroalgae and Spent Coffee Grounds. Sustain. Energy Fuels 2021, 5, 6189–6196. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Park, J.-S.; Nkurunziza, D.; Roy, V.C.; Ho, T.C.; Kim, S.-Y.; Lee, S.-C.; Chun, B.-S. Pretreatment Processes Assisted Subcritical Water Hydrolysis for Valorisation of Spent Coffee Grounds. Int. J. Food Sci. Technol. 2022, 57, 5090–5101. [Google Scholar] [CrossRef]
- Mayanga-Torres, P.C.; Lachos-Perez, D.; Rezende, C.A.; Prado, J.M.; Ma, Z.; Tompsett, G.T.; Timko, M.T.; Forster-Carneiro, T. Valorization of Coffee Industry Residues by Subcritical Water Hydrolysis: Recovery of Sugars and Phenolic Compounds. J. Supercrit. Fluids 2017, 120, 75–85. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of Pretreatment and Modifiers on Subcritical Water Liquefaction of Spent Coffee Grounds: A Green Waste Valorization Approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Asl, A.H.; Khajenoori, M.; Asl, A.H.; Khajenoori, M. Subcritical Water Extraction. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; IntechOpen: London, UK, 2013; ISBN 978-953-51-1170-2. [Google Scholar]
- Shang, Y.-F.; Xu, J.-L.; Lee, W.-J.; Um, B.-H. Antioxidative Polyphenolics Obtained from Spent Coffee Grounds by Pressurized Liquid Extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- AOAC. Official Method 2001.11-2005 Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain, and Oilseeds. In AOAC Official Method; Wendt Thiex, N.J., Latimer, G.W., Jr., Eds.; Oxford University Press: New York, NY, USA, 2023. [Google Scholar]
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Standard Organization (ISO): Geneva, Switzerland, 2000.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The Chemical Composition of Exhausted Coffee Waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Menezes, E.G.T.; do Carmo, J.R.; Alves, J.G.L.F.; Menezes, A.G.T.; Guimarães, I.C.; Queiroz, F.; Pimenta, C.J. Optimization of Alkaline Pretreatment of Coffee Pulp for Production of Bioethanol. Biotechnol. Prog. 2014, 30, 451–462. [Google Scholar] [CrossRef]
- Ostojčić, M.; Budžaki, S.; Flanjak, I.; Bilić Rajs, B.; Barišić, I.; Tran, N.N.; Hessel, V.; Strelec, I. Production of Biodiesel by Burkholderia Cepacia Lipase as a Function of Process Parameters. Biotechnol. Prog. 2021, 37, e3109. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Food Science Texts Series; Springer: Boston, MA, USA, 2010; pp. 47–53. ISBN 978-1-4419-1463-7. [Google Scholar]
- White, J.W. Spectrophotometric Method for Hydroxymethylfurfural in Honey. J. Assoc. Off. Anal. Chem. 1979, 62, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Corici, L.; Ferrario, V.; Pellis, A.; Ebert, C.; Lotteria, S.; Cantone, S.; Voinovich, D.; Gardossi, L. Large Scale Applications of Immobilized Enzymes Call for Sustainable and Inexpensive Solutions: Rice Husks as Renewable Alternatives to Fossil-Based Organic Resins. RSC Adv. 2016, 6, 63256–63270. [Google Scholar] [CrossRef]
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.-W.; Verniquet, A.; Broeze, J.; et al. A Research Challenge Vision Regarding Management of Agricultural Waste in a Circular Bio-Based Economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of Oil Extracted from Spent Coffee Grounds for Sustainable Production of Polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical Fluid Extraction of Lipids from Spent Coffee Grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Mota, D.A.; Santos, J.C.B.; Faria, D.; Lima, Á.S.; Krause, L.C.; Soares, C.M.F.; Ferreira-Dias, S. Synthesis of Dietetic Structured Lipids from Spent Coffee Grounds Crude Oil Catalyzed by Commercial Immobilized Lipases and Immobilized Rhizopus Oryzae Lipase on Biochar and Hybrid Support. Processes 2020, 8, 1542. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shaik, L.; Gokhale, J.S. 2.38—Subcritical Water: An Innovative Processing Technology. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 552–566. ISBN 978-0-12-815782-4. [Google Scholar]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Getachew, A.T.; Lee, H.J.; Cho, Y.J.; Chae, S.J.; Chun, B.S. Optimization of Polysaccharides Extraction from Pacific Oyster (Crassostrea gigas) Using Subcritical Water: Structural Characterization and Biological Activities. Int. J. Biol. Macromol. 2019, 121, 852–861. [Google Scholar] [CrossRef]
- Choi, B.; Koh, E. Spent Coffee as a Rich Source of Antioxidative Compounds. Food Sci. Biotechnol. 2017, 26, 921–927. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, C.Y. Extraction and Isolation of Polyphenolics. In Pigments, Colorants, Flavors, Texture, and Bioactive Food Components; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2005; Volume 2, pp. 471–482. ISBN 978-0-471-66378-2. [Google Scholar]
- Kanai, N.; Honda, T.; Yoshihara, N.; Oyama, T.; Naito, A.; Ueda, K.; Kawamura, I. Structural Characterization of Cellulose Nanofibers Isolated from Spent Coffee Grounds and Their Composite Films with Poly(Vinyl Alcohol): A New Non-Wood Source. Cellulose 2020, 27, 5017–5028. [Google Scholar] [CrossRef]
- Ramos-Andrés, M.; Andrés-Iglesias, C.; García-Serna, J. Production of Molecular Weight Fractionated Hemicelluloses Hydrolyzates from Spent Coffee Grounds Combining Hydrothermal Extraction and a Multistep Ultrafiltration/Diafiltration. Bioresour. Technol. 2019, 292, 121940. [Google Scholar] [CrossRef] [PubMed]
- Raspolli Galletti, A.M.; D’Alessio, A.; Licursi, D.; Antonetti, C.; Valentini, G.; Galia, A.; Nassi o Di Nasso, N. Midinfrared FT-IR as a Tool for Monitoring Herbaceous Biomass Composition and Its Conversion to Furfural. J. Spectrosc. 2015, 2015, e719042. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, D.I. ur Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of Hemicellulose, Cellulose, Holocellulose and Lignin Content Using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. and Guazuma crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Wongsiridetchai, C.; Chiangkham, W.; Khlaihiran, N.; Sawangwan, T.; Wongwathanarat, P.; Charoenrat, T.; Chantorn, S. Alkaline Pretreatment of Spent Coffee Grounds for Oligosaccharides Production by Mannanase from Bacillus Sp. GA2(1). Agric. Nat. Resour. 2018, 52, 222–227. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Navarini, L.; Olivieri, G.; Russo, M.E.; Marzocchella, A. Coffee Silverskin as a Renewable Resource to Produce Butanol and Isopropanol. Chem. Eng. Trans. 2018, 64, 139–144. [Google Scholar] [CrossRef]
- Ittrat, P.; Chacho, T.; Pholprayoon, J.; Suttiwarayanon, N.; Charoenpanich, J. Application of Agriculture Waste as a Support for Lipase Immobilization. Biocatal. Agric. Biotechnol. 2014, 3, 77–82. [Google Scholar] [CrossRef]
- Cespugli, M.; Lotteria, S.; Navarini, L.; Lonzarich, V.; Del Terra, L.; Vita, F.; Zweyer, M.; Baldini, G.; Ferrario, V.; Ebert, C.; et al. Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors. Catalysts 2018, 8, 471. [Google Scholar] [CrossRef]
- Biró, E.; Németh, A.S.; Sisak, C.; Feczkó, T.; Gyenis, J. Preparation of Chitosan Particles Suitable for Enzyme Immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [Google Scholar] [CrossRef]
- Ferrario, V.; Veny, H.; De Angelis, E.; Navarini, L.; Ebert, C.; Gardossi, L. Lipases Immobilization for Effective Synthesis of Biodiesel Starting from Coffee Waste Oils. Biomolecules 2013, 3, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.N.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Tharanathan, R.N. Dietary Fiber from Coconut Residue: Effects of Different Treatments and Particle Size on the Hydration Properties. Eur. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- Femenia, A.; Lefebvre, A.-C.; Thebaudin, J.-Y.; Robertson, J.A.; Bourgeois, C.-M. Physical and Sensory Properties of Model Foods Supplemented with Cauliflower Fiber. J. Food Sci. 1997, 62, 635–639. [Google Scholar] [CrossRef]





| Parameter | Spent Coffee Grounds | Cellulose-Based SCG-Derived Enzyme Immobilization Carrier |
|---|---|---|
| Dry matter [g/100 g] | 39.88 ± 0.75 | 92.25 ± 0.18 |
| Proteins [g/100 g d.w.b.] 2 | 16.68 ± 0.06 | 1.12 ± 0.01 |
| Fats [g/100 g d.w.b.] | 10.58 ± 1.63 | n.d. 3 |
| Crude fiber [g/100 g d.w.b.] 4 | 29.86 ± 3.13 | 54.65 ± 1.31 |
| Crude fiber (NDF) [g/100 g d.w.b.] 5 | 58.73 ± 1.31 | 81.87 ± 0.04 |
| Cellulose (ADF-ADL) [g/100 g d.w.b.] | 23.59 ± 0.15 | 59.36 ± 1.74 |
| Hemicellulose (NDF-ADF) [g/100 g d.w.b.] | 23.35 ± 1.51 | 21.30 ± 1.67 |
| Lignin (ADL) [g/100 g d.w.b.] | 11.79 ± 0.35 | 1.21± 0.04 |
| Total polyphenols [g/100 g d.w.b.] | 2.88 ± 0.40 | n.d. |
| Ash [g/100 g d.w.b.] | 1.98 ± 0.13 | 1.21 ± 0.05 |
| Fatty Acid | Amount in SCG Oil [%] |
|---|---|
| Palmitic (C16:0) | 33.59 |
| Stearic (C18:0) | 7.02 |
| Oleic (C18:1) | 9.56 |
| Linoleic (C18:2) | 46.28 |
| α-Linolenic (C18:3) | 0.92 |
| Arachidic (C20:0) | 2.63 |
| NaOH [%] | Mass Yield [%] 1 |
|---|---|
| 2 | 83.25 ± 0.1 |
| 4 | 45.05 ± 0.3 |
| 6 | 33.98 ± 0.1 |
| 8 | 20.83 ± 0.1 |
| NaOH [%] | Polyphenols [%] | Proteins [%] | Sugars [%] |
|---|---|---|---|
| 0 2 | 0.30 ± 0.04 | 0.24 ± 0.04 | 0.96 ± 0.21 |
| 2 | 0.68 ± 0.07 | n.d. | 0.37 ± 0.06 |
| 4 | 0.36 ± 0.09 | n.d. | 0.37 ± 0.08 |
| 6 | 0.32 ± 0.06 | n.d. | 0.21 ± 0.05 |
| 8 | n.d. 3 | n.d. | n.d. |
| Fiber | SCG 2 | DSCG 3 | SEDSCG 4 | ALSEDSCG 5 |
|---|---|---|---|---|
| Crude fiber (NDF) [g/100 g d.w.b.] | 58.73 ± 1.3 | 60.31 ± 1.27 | 79.10 ± 1.04 | 81.87 ± 0.04 |
| Cellulose (ADF-ADL) [g/100 g d.w.b.] | 23.59 ± 0.15 | 24.77 ± 0.49 | 37.23 ± 0.56 | 59.36 ± 1.74 |
| Hemicellulose (NDF-ADF) [g/100 g d.w.b.] | 23.35 ± 1.51 | 23.48 ± 0.65 | 28.39 ± 0.10 | 21.30 ± 1.67 |
| Lignin (ADL) [g/100 g d.w.b.] | 11.79 ± 0.35 | 12.07 ± 0.14 | 13.48 ± 0.38 | 1.21 ± 0.04 |
| Component | 1st Alkaline Liquefaction | 2nd Alkaline Liquefaction |
|---|---|---|
| Proteins [g/100 g] | 7.33 ± 0.14 | 0.12 ± 0.04 |
| Total Polyphenols [g/100 g] | 8.26 ± 0.71 | 0.37 ± 0.06 |
| Total sugars [g/100 g] | 44.41 ± 3.14 | 6.32 ± 0.14 |
| Process | Product | Mass Yield [%] 1 |
|---|---|---|
| Initial drying at 60 °C | Spent Coffee Ground (SCG) | 100.00 |
| Soxhlet extraction | Defatted Spent Coffee Ground (DSCG) | 89.42 ± 0.01 |
| Continuous solvent flow sequential subcritical extraction | Sequentially Extracted Defatted Spent Coffee Grounds (SEDSCG) | 66.01 ± 0.57 |
| Alkaline liquefaction by 8% NaOH | Alkaline Liquefied Sequentially Extracted Defatted Spent Coffee Grounds (ALSEDSCG) | 13.75 ± 0.04 |
| Liquid Examined | Carrier Holding Capacity [mL/g] |
|---|---|
| Water | 7.55 ± 0.13 |
| Oil | 2.93 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brekalo, M.; Rajs, B.B.; Aladić, K.; Jakobek, L.; Šereš, Z.; Krstović, S.; Jokić, S.; Budžaki, S.; Strelec, I. Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier. Sustainability 2023, 15, 13142. https://doi.org/10.3390/su151713142
Brekalo M, Rajs BB, Aladić K, Jakobek L, Šereš Z, Krstović S, Jokić S, Budžaki S, Strelec I. Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier. Sustainability. 2023; 15(17):13142. https://doi.org/10.3390/su151713142
Chicago/Turabian StyleBrekalo, Mirna, Blanka Bilić Rajs, Krunoslav Aladić, Lidija Jakobek, Zita Šereš, Saša Krstović, Stela Jokić, Sandra Budžaki, and Ivica Strelec. 2023. "Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier" Sustainability 15, no. 17: 13142. https://doi.org/10.3390/su151713142
APA StyleBrekalo, M., Rajs, B. B., Aladić, K., Jakobek, L., Šereš, Z., Krstović, S., Jokić, S., Budžaki, S., & Strelec, I. (2023). Multistep Extraction Transformation of Spent Coffee Grounds to the Cellulose-Based Enzyme Immobilization Carrier. Sustainability, 15(17), 13142. https://doi.org/10.3390/su151713142










