Recovery of Zn and Fe from Steelmaking By-Products by Ar Plasma Smelting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
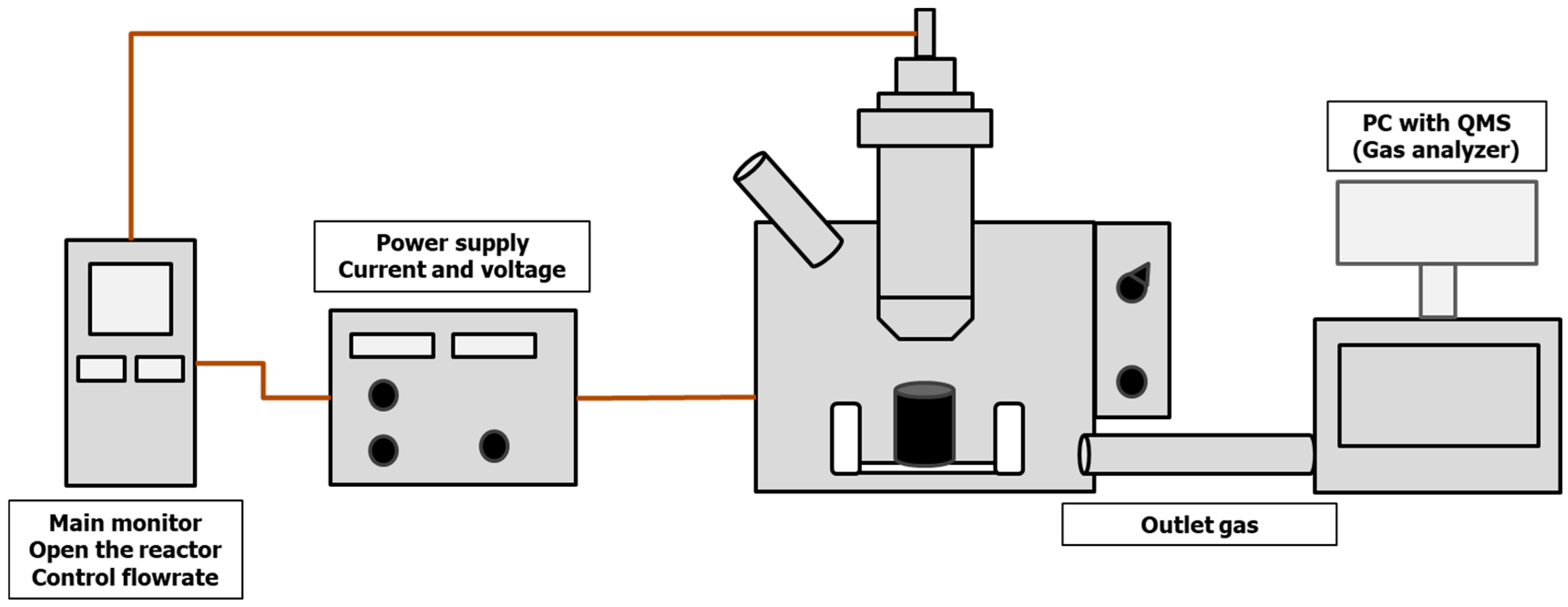
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
3.1. Removal and Recovery of Zn from By-Products
3.2. Recovery Evaluation of Zn and Fe from By-Products
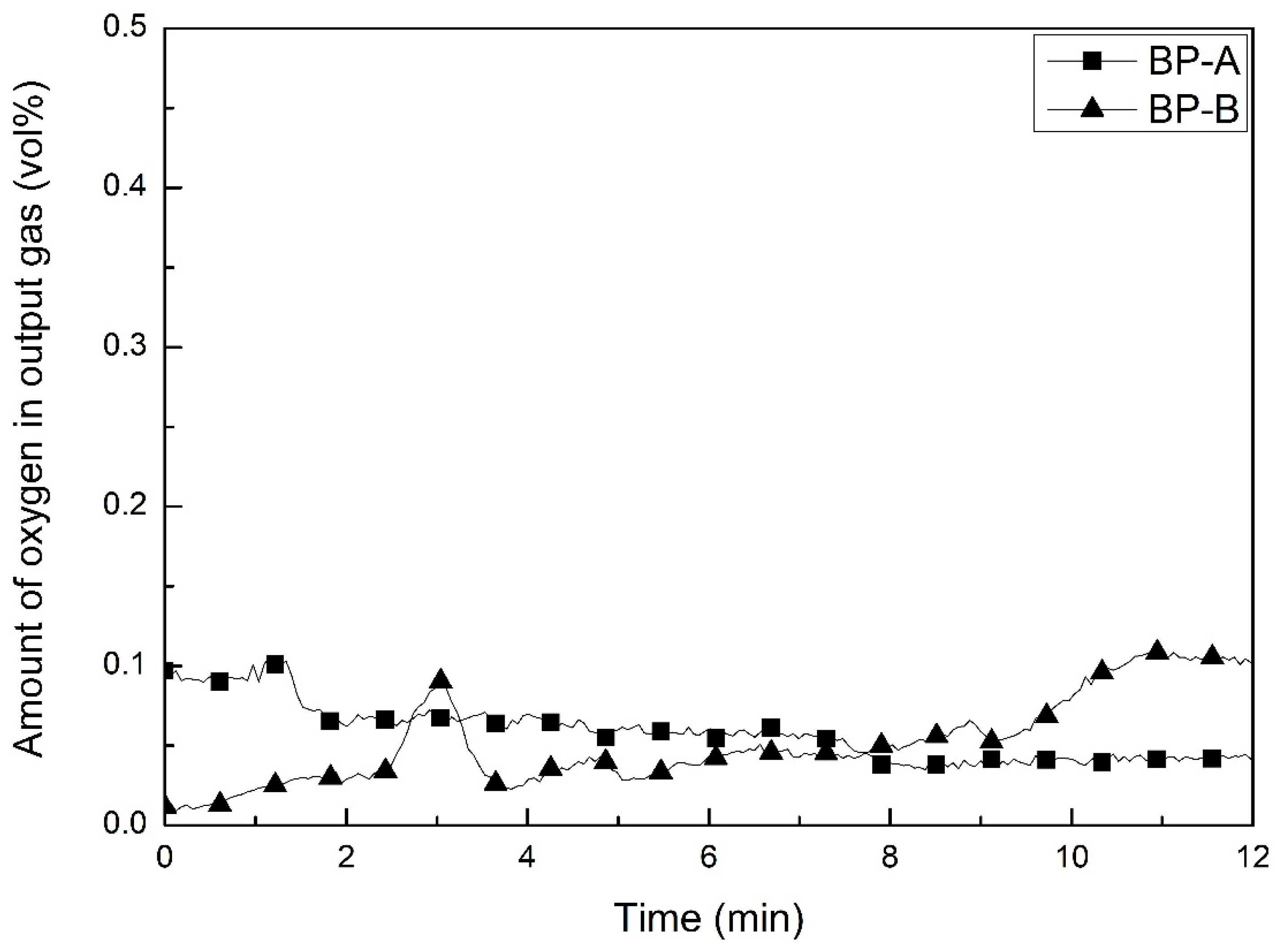
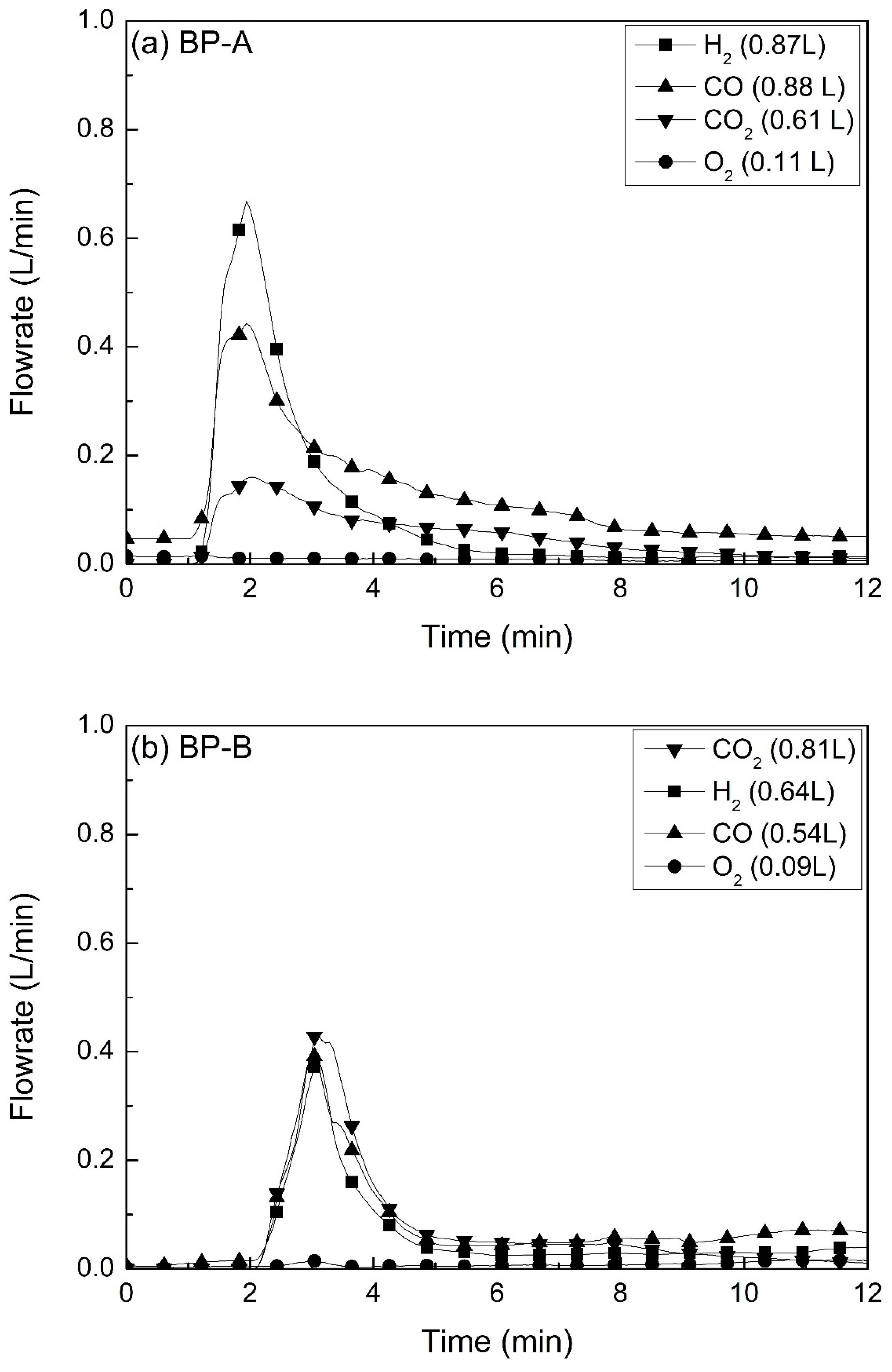
3.3. Evolution of Gases during Ar Plasma Smelting
4. Conclusions
- The first by-product, BP-A, contains metallic iron as the main phase along with 0.89 wt% of zinc oxide in the form of zinc ferrite (ZnO·Fe2O3). After Ar plasma smelting, the product obtained inside the crucible was identified to be metallic iron and FeO, where the removal ratio of zinc was evaluated to be 96% and recovery of iron was calculated to be 94%. The dust formed outside the crucible mainly consisted of zinc oxide and iron oxide where the recovery ratio of zinc was calculated to be 93%.
- The second by-product, BP-B contains Fe2O3 as the main phase along with 3.39 wt% of zinc oxide in the form of zinc silicate (2ZnO·SiO2). After the smelting process, the product obtained inside the crucible was mostly composed of FeO along with 0.18 wt% of zinc oxide where the removal ratio of zinc was evaluated to be 96%, and the recovery ratio of iron was calculated to be 83%. The dust formed outside the crucible was identified to contain 70 wt% of zinc oxide where the recovery ratio of zinc oxide was estimated to be 73%.
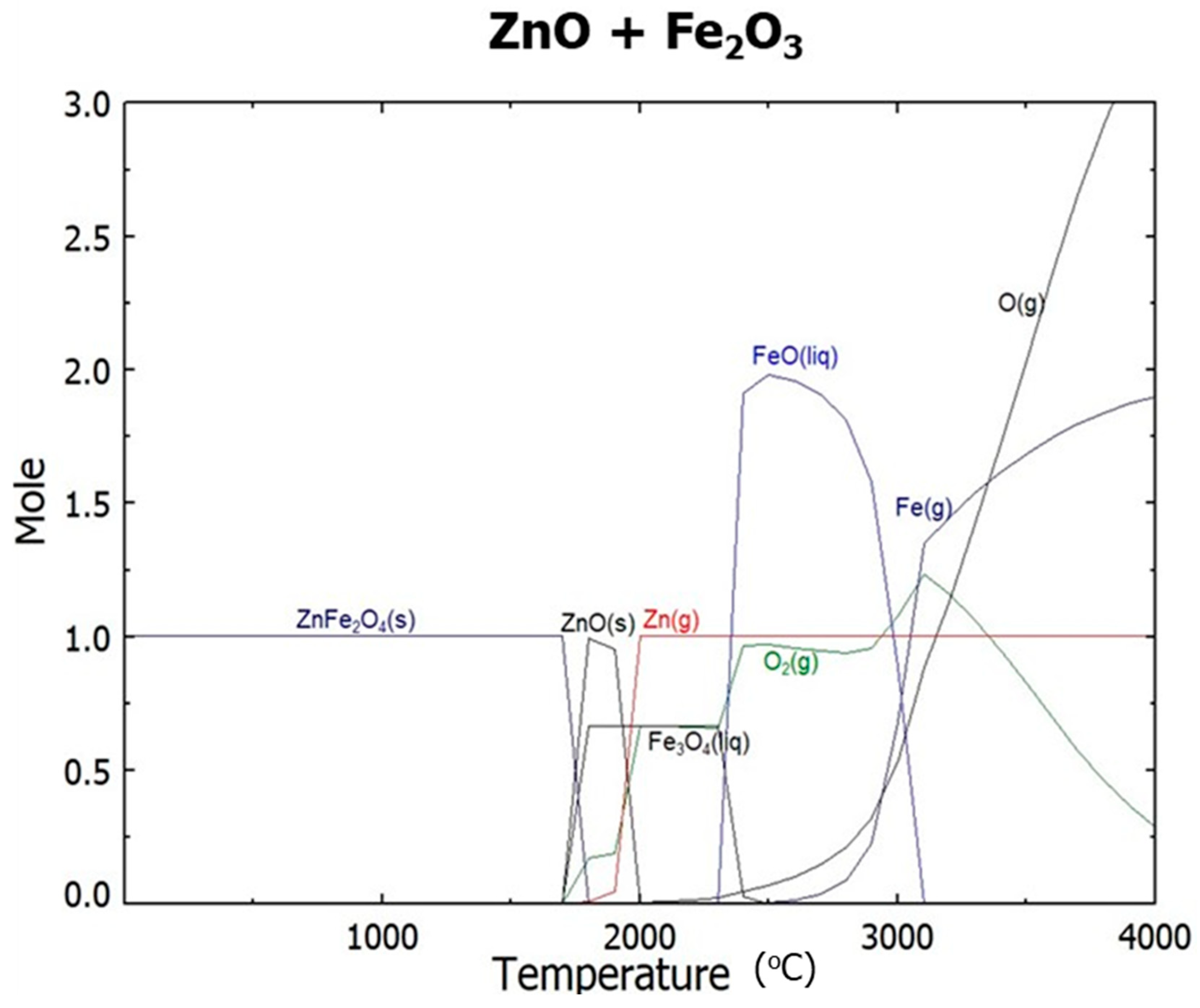
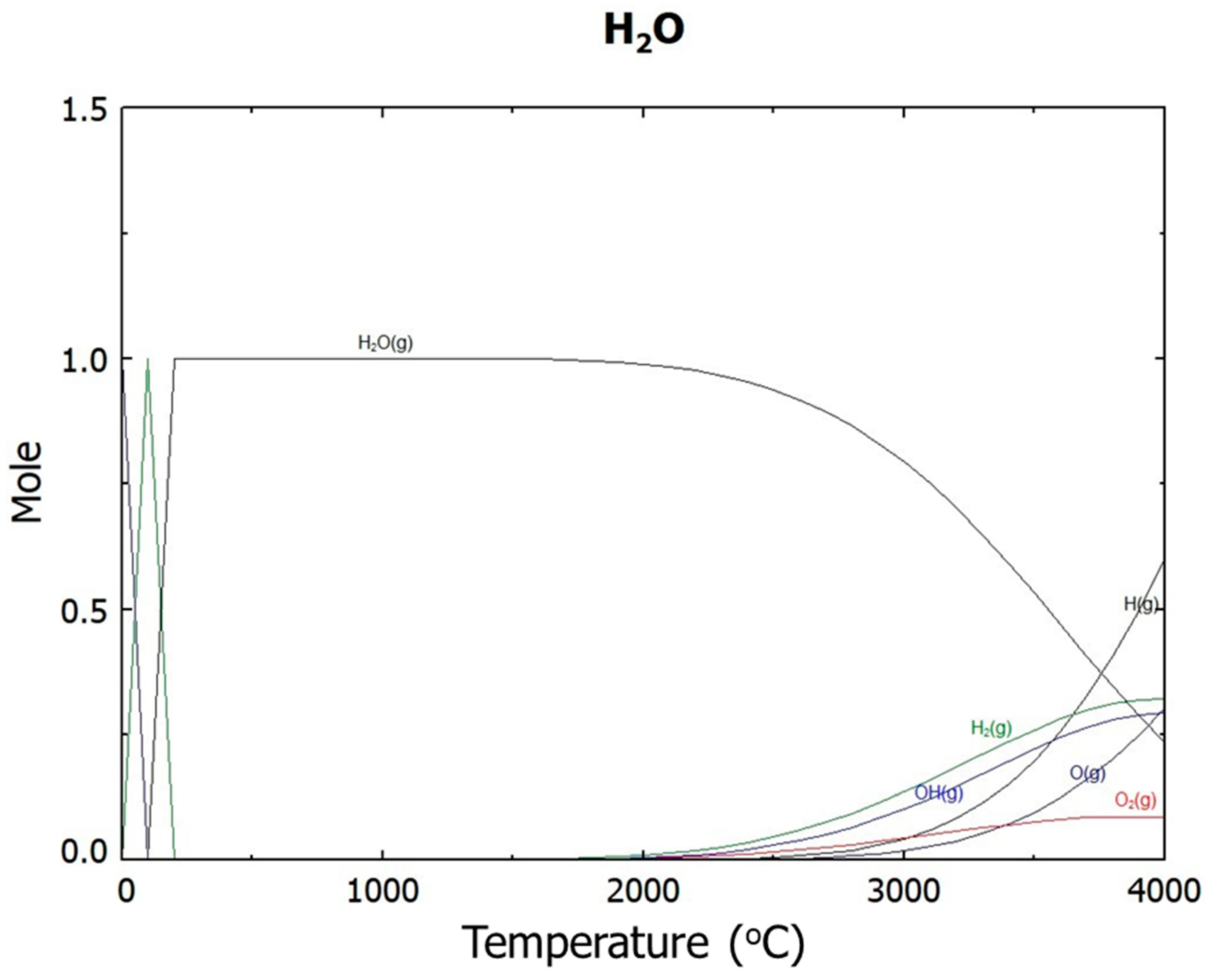
- Ar plasma smelting successfully removed zinc oxide whose phase in two kinds of by-products are different. In the thermodynamic calculation by FactSage, it was expected that zinc oxide would be reduced to zinc vapor at temperatures above 2000 °C and that Fe2O3 be reduced to FeO from 2500 °C. In actual experiments employing Ar plasma, zinc oxide was removed from by-products in terms of reduction and vaporization. One product consisting of metallic iron and FeO was obtained inside the crucible. The reduction of ZnO and Fe2O3 proceeded with Ar plasma smelting around 2500 °C.
- The main gases formed during Ar plasma smelting were H2, CO and CO2. Moisture and carbon in the by-products interactively reacted to produce H2, CO and CO2 by a high temperature of the plasma. The oxygen was originated from the reduction of Fe2O3 to FeO. Most of the gases were formed at the initial stage of experiments. Therefore, since the temperature of the sample did not reach equilibrium, the experimental results were different from those expected at a specific temperature by thermodynamic calculation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, W.; Gan, M.; Fan, X.; Ji, Z.; Chen, X.; Yao, J.; Jiang, T. Recycling utilization of zinc-bearing metallurgical dust by reductive sintering: Reaction behaviour of zinc oxide. J. Miner. Met. Mater. Soc. 2019, 38, 3173–3180. [Google Scholar] [CrossRef]
- Stewart, D.J.C.; Barron, A.R. Pyrometallurgical removal of zinc from basic oxygen steelmaking dust—A review of best available technology. Resour. Conserv. Recycl. 2020, 157, 104746. [Google Scholar] [CrossRef]
- Deng, Y.; Lyu, Q.; Zhang, J.; Jiao, K. Erosion of carbon brick by zinc in hearth of blast furnace. ISIJ Int. 2020, 60, 226–232. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Gijsemans, L.; Busse, J.; Roosen, J.; Onal, M.A.R.; Torres, V.M.; Fernandez, A.M.; Jones, P.T.; Binnemans, K. Selective removal of zinc from BOF sludge by leaching with mixtures of ammonia and ammonium carbonate. J. Sustain. Metall. 2020, 6, 680–890. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, M.; Fan, X.; Lv, W.; Ji, Z. Phase transformation and enhanced Zn removal technology during the iron ore sintering process. J. Miner. Met. Mater. Soc. 2023, 75, 310–320. [Google Scholar] [CrossRef]
- Lv, W.; Gan, M.; Fan, X.; Sun, Z.; Zhang, R.; Ji, Z.; Chen, X. Reaction behaviour and transformation path of heating-up zone during sintering process. Sustainability 2022, 14, 10147. [Google Scholar] [CrossRef]
- Oda, H.; Ibraraki, T.; Abe, Y. Dust recycling system by the rotary hearth furnace. Nippon. Steel Tech. Rep. 2006, 94, 147–152. [Google Scholar]
- Sohn, H. Status of pyrometallurgical treatment technology of EAF dust. J. Korean Inst. Resour. Recycl. 2018, 27, 68–76. [Google Scholar] [CrossRef]
- Ju, D.; Yao, H.; Ma, H.; Mao, R.; Qiu, J.; Chen, C. Removal process and mechanism of lead in Zn-containing rotary hearth furnace dust. Inorg. Chem. Commun. 2021, 127, 108496. [Google Scholar] [CrossRef]
- Choi, K.; Park, H. Kinetics of brass waste by hydrogen reduction for recovery of valuable metals. J. Miner. Met. Mater. Soc. 2022, 74, 878–884. [Google Scholar] [CrossRef]
- Murphy, A.B.; Tanaka, M.; Yamamoto, K.; Tashiro, S.; Sato, T.; Lowke, J.J. Modelling of thermal plasma for arc welding: The role of the shielding gas properties and of metal vapour. J. Phys. D Appl. Phys. 2009, 42, 194006. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, F.; Zhu, L.; Planche, M.P.; Liao, H.; Dong, C.; Coddet, C. Electron temperature and density of the plasma measured by optical emission spectroscopy in VLPPS conditions. J. Therm. Spray Technol. 2011, 20, 1321–1327. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, F.; Zhu, L.; Verdy, C.; Planche, M.P.; Liao, H.; Dong, C.; Coddet, C. Characteristics of Cu film deposited using VLPPS. J. Therm. Spray Technol. 2011, 20, 351–357. [Google Scholar] [CrossRef]
- Das, S.; Das, D.P.; Sarangi, C.K.; Bhoi, B.; Mishra, B.K.; Ghosh, J. Optical emission spectroscopy study of Ar-H2 plasma at atmospheric pressure. IEEE Trans. Plasma Sci. 2018, 46, 2909–2915. [Google Scholar] [CrossRef]
- Cho, Y.; Jeong, G.; Kim, C.; Kim, W.; Jeong, Y. Arc plasma flow variation by obstruction structures between anode and cathode. Metals 2021, 11, 1416. [Google Scholar] [CrossRef]
- Sabat, K.C.; Murphy, A.B. Hydrogen plasma processing of iron ore. Metall. Mater. Trans. B 2017, 48, 1561–1594. [Google Scholar] [CrossRef]
- Seftejani, M.N.; Schenk, J. Fundamental of hydrogen plasma smelting reduction (HPSR) of iron oxides, a new generation of steelmaking processes. Conf. Asiasteel 2018, 24, 26. [Google Scholar]
- Behera, P.B.; Bhoi, B.; Paramguru, R.K.; Mukherjee, P.S.; Mishra, B.K. Hydrogen plasma smelting reduction of Fe2O3. Metall. Mater. Trans. B 2019, 50, 262–270. [Google Scholar] [CrossRef]
- Sabat, K.C. Physics and chemistry of solid state direct reduction of iron ore by hydrogen plasma. Phys. Chem. Solid State 2021, 22, 292–300. [Google Scholar] [CrossRef]
- Mohai, I.; Szepvolgyi, J.; Karoly, Z.; Mohai, M.; Toth, M.; Babievskaya, I.Z.; Krenev, V.A. Reduction of metallurgical wastes in an RF thermal plasma reactor. Plasma Chem. Plasma Process. 2001, 21, 547–563. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chu, J.P.; Tzane, C.C.; Chen, Y.S. Treatment and recycling of incinerated ash using thermal plasma technology. Waste Manag. 2002, 22, 485–490. [Google Scholar] [CrossRef]
- Heberlein, J.; Murphy, A.B. Thermal plasma waste treatment. J. Phys. D Appl. Phys. 2008, 41, 053001. [Google Scholar] [CrossRef]
- Changming, D.; Chao, S.; Gong, X.; Ting, W.; Xiange, W. Plasma methods for metal recovery from metal-containing waste. Waste Manag. 2018, 77, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.; Blanco, I. An overview of thermal plasma arc system for treatment of various wastes in recovery of metals. Materials 2022, 15, 683. [Google Scholar] [CrossRef]
- Plaul, J.F.; Krieger, W.; Back, E. Reduction of fine ores argon-hydrogen plasma. Steel Res. Int. 2005, 76, 548–554. [Google Scholar] [CrossRef]
- Seftejani, M.N.; Schenk, J.; Zarl, M.A. Reduction of haematite using hydrogen thermal plasma. Materials 2019, 12, 1608. [Google Scholar] [CrossRef]









| By-Products | T.Fe | FeO | M.Fe | Fe2O3 | CaO | SiO2 | Al2O3 | ZnO |
|---|---|---|---|---|---|---|---|---|
| BP-A | 73.70 | 25.90 | 45.50 | 11.54 | 9.11 | 1.69 | 0.50 | 0.89 |
| BP-B | 62.10 | 22.40 | 11.80 | 47.02 | 6.68 | 1.25 | 0.2 | 3.39 |
| Element | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| Fe crucible | <0.25 | <0.45 | <1.5 | <0.05 | <0.05 |
| Sample | Chemical Composition (wt%) | |||
|---|---|---|---|---|
| Raw Material (90 g) | Product 1 (33.81 g) | Product 2 (40.05 g) | Dust 1 (2.07 g) | |
| Fe2O3 | 11.54 | - | 7.9 | 6.6 |
| FeO | 25.90 | 2.7 | 78.3 | 49.0 |
| M.Fe | 45.50 | 97.1 | 5.8 | 4.1 |
| ZnO | 0.89 | 0.01 | 0.08 | 35.7 |
| SiO2 | 1.69 | 0.02 | 1.4 | 0.4 |
| CaO | 9.11 | 0.02 | 6.5 | 0.9 |
| Sample | Chemical Composition (wt%) | |||
|---|---|---|---|---|
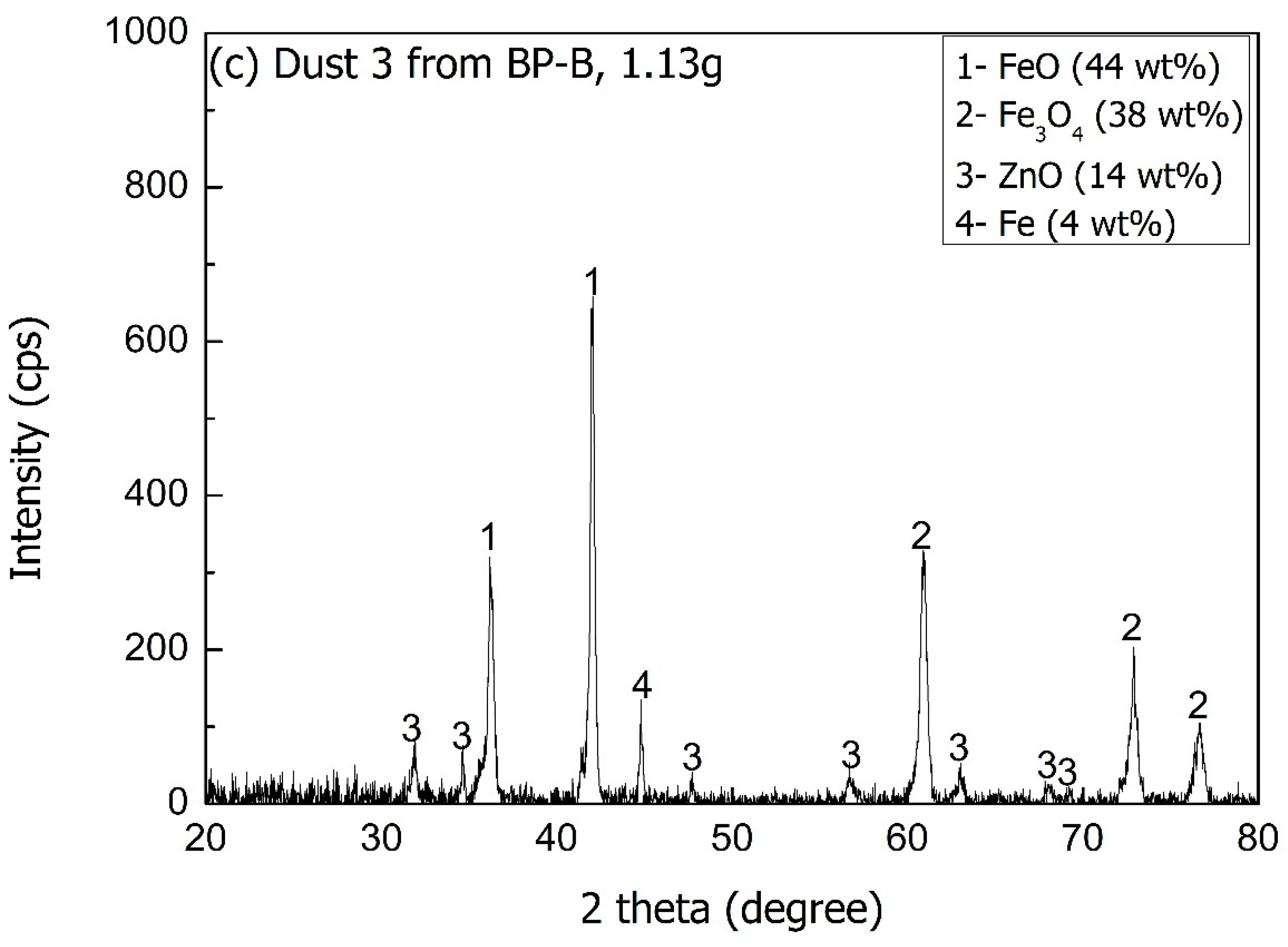
| Raw Material (90 g) | Product 3 (60.17 g) | Dust 2 (2.91 g) | Dust 3 (1.13 g) | |
| Fe2O3 | 47.02 | - | - | 24.3 |
| FeO | 22.40 | 90.6 | 26.6 | 56.3 |
| M.Fe | 11.80 | 6.3 | - | 3.7 |
| ZnO | 3.39 | 0.18 | 71.4 | 14.1 |
| SiO2 | 1.25 | 1.3 | 0.7 | 0.6 |
| CaO | 6.68 | 1.5 | 1.1 | 0.8 |
| Zinc Oxide | Iron | |
|---|---|---|
| Before experiment (g) | 0.80 | 66.33 |
| After experiment (g) | 0.74 | 62.47 |
| Recovery ratio (%) | 92.50 | 94.18 |
| Zinc Oxide | Iron | |
|---|---|---|
| Before experiment (g) | 3.05 | 55.89 |
| After experiment (g) | 2.23 | 46.19 |
| Recovery ratio (%) | 73.11 | 82.64 |
| Results | BP-A | BP-B |
|---|---|---|
| Removal ratio of zinc (%) | 95.63 | 96.41 |
| Recovery ratio of zinc (%) | 92.50 | 73.11 |
| Recovery ratio of iron (%) | 94.18 | 82.64 |
| Phase of recovered zinc | ZnO | |
| Phase of residues | Metallic Fe and FeO | FeO |
| Formation of gases | H2, CO2 and CO | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Tomas Da Rocha, L.; Kim, S.-W.; Jung, S.-M. Recovery of Zn and Fe from Steelmaking By-Products by Ar Plasma Smelting. Sustainability 2023, 15, 12890. https://doi.org/10.3390/su151712890
Cho S, Tomas Da Rocha L, Kim S-W, Jung S-M. Recovery of Zn and Fe from Steelmaking By-Products by Ar Plasma Smelting. Sustainability. 2023; 15(17):12890. https://doi.org/10.3390/su151712890
Chicago/Turabian StyleCho, Seongkyu, Leonardo Tomas Da Rocha, Sung-Wan Kim, and Sung-Mo Jung. 2023. "Recovery of Zn and Fe from Steelmaking By-Products by Ar Plasma Smelting" Sustainability 15, no. 17: 12890. https://doi.org/10.3390/su151712890
APA StyleCho, S., Tomas Da Rocha, L., Kim, S.-W., & Jung, S.-M. (2023). Recovery of Zn and Fe from Steelmaking By-Products by Ar Plasma Smelting. Sustainability, 15(17), 12890. https://doi.org/10.3390/su151712890







