Evaluation of Electroosmotic Permeability Using Different Models and Investigation of Its Effect on Chromium Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Cr6+-Contaminated Soil
2.2. Experimental Setup
2.3. Experimental Procedures
2.4. Analytical Methods
3. Results and Discussion
3.1. Electroosmotic Permeability Determination with Different Models and Experiments
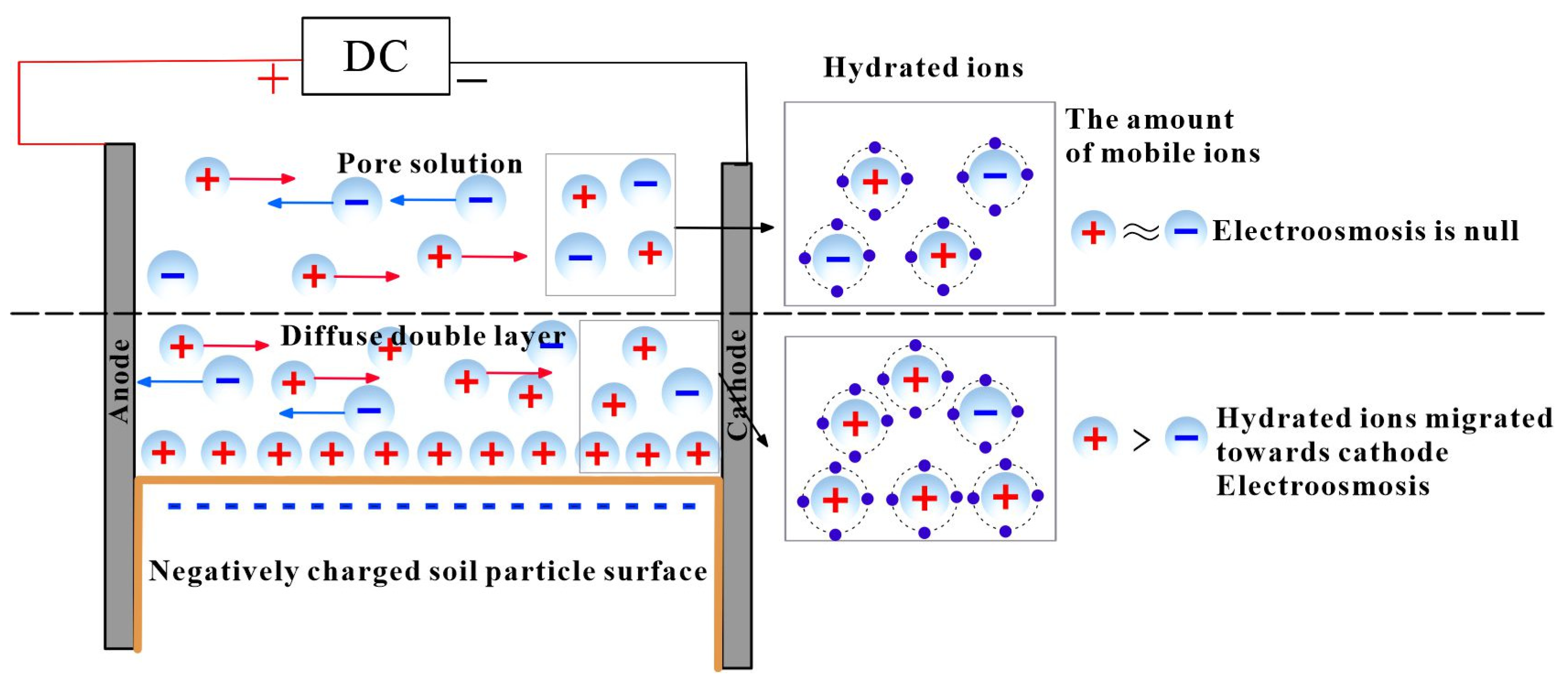
3.1.1. Ion Hydration Model
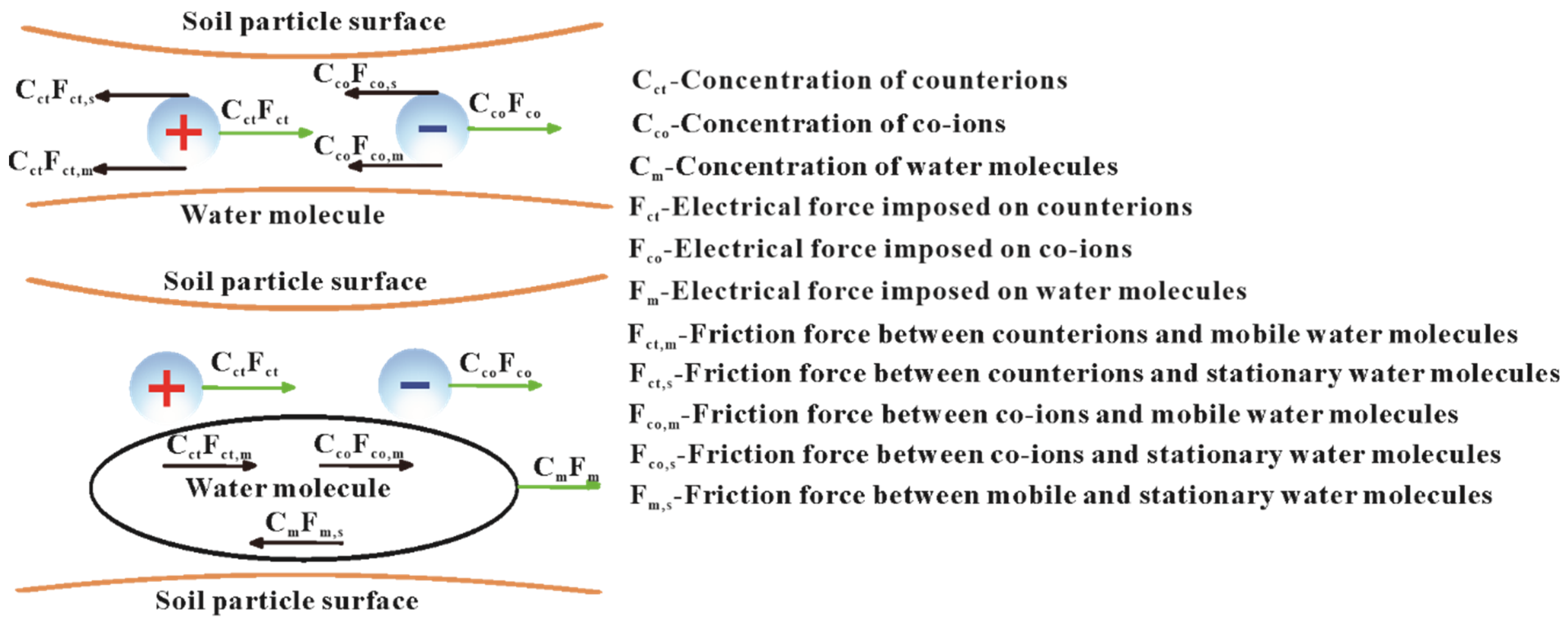
3.1.2. Ion Hydration–Friction Model
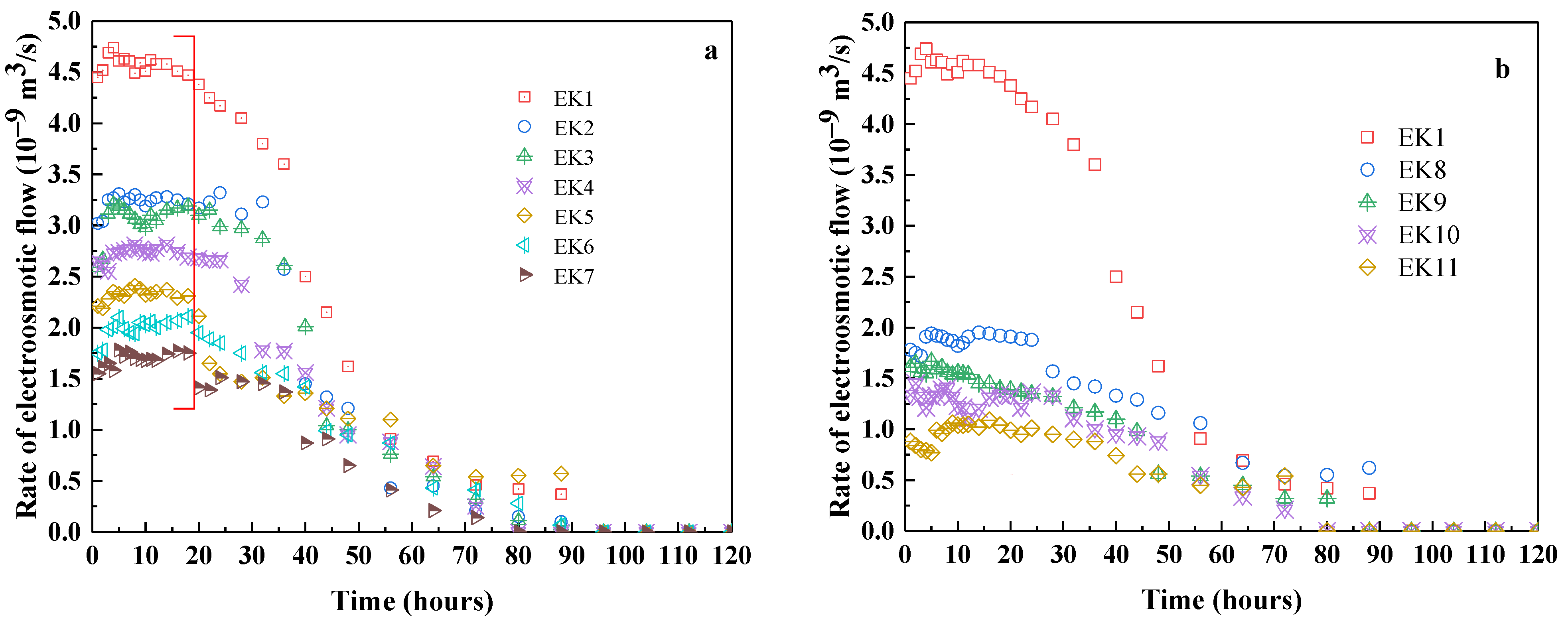
3.1.3. Electroosmotic Permeability Measured by Experiments
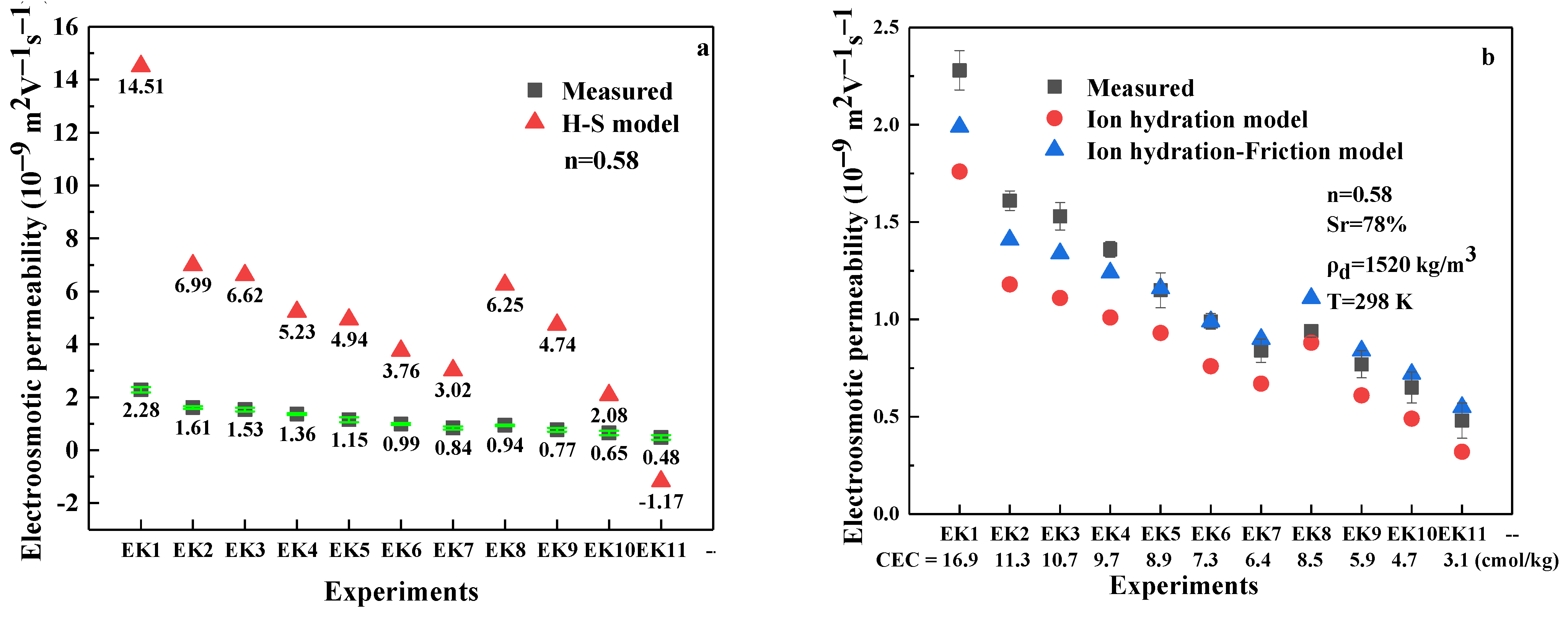
3.1.4. Calculation Verification
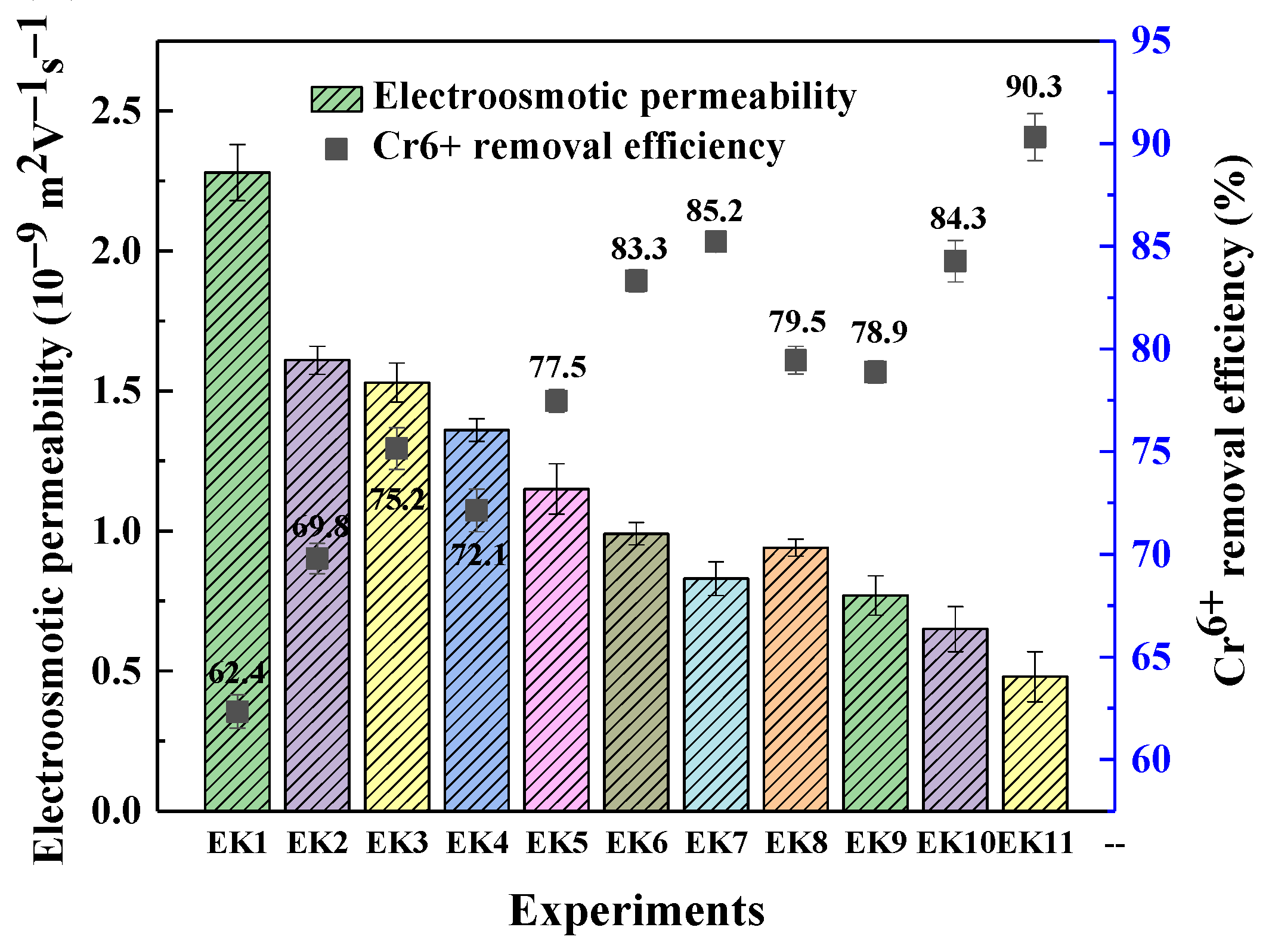
3.2. The Role of Electroosmotic Permeability for Cr6+ Removal
4. Conclusions
- (1)
- The electroosmotic permeability estimated by the H-S model is 3–7 times larger than the measured value. The ion hydration model and ion hydration–friction model are deduced from the principle of electroosmotic flow, and the value estimated by these two models is closer to the measured value compared to the value from the H-S model. However, the ion hydration–friction model has several drawbacks, such as too many parameters having to be determined and not being concise enough compared to the H-S model.
- (2)
- The lower the electroosmotic flow, the higher the Cr6+ removal efficiency. The zeta potential and CEC of soil were changed by adding acid, and the Cr6+ removal efficiency was improved.
- (3)
- No hazardous material was added or produced when using the EKR method to remediate heavy-metal-contaminated soil, meaning that it is a sustainable technology to use for this purpose. This study revealed the relationship between electroosmotic flow and ion migration, contributing towards an improvement in the heavy metal removal efficiency and in the environment. Moreover, the ion hydration and ion hydration–friction model are of great significance to the theory of ion migration in EKR.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigo, M.A.; Oturan, N.; Oturan, M.A. Electrochemically assisted remediation of pesticides in soils and water: A review. Chem. Rev. 2014, 114, 8720–8745. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.K.; Schwab, A.P.; Henderson, C. Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 2006, 62, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xiong, Z.; Nie, Y.; Niu, Y.-Y.; Wang, L.; Liu, Y.-Y. Near-anode focusing phenomenon caused by the high anolyte concentration in the electrokinetic remediation of chromium (VI)-contaminated soil. J. Hazard. Mater. 2012, 229, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Gouveia, S. Electrokinetic remediation for the removal of organic contaminants in soils. Curr. Opin. Electrochem. 2018, 11, 41–47. [Google Scholar] [CrossRef]
- Yeung, A.T.; Gu, Y.Y. A review on techniques to enhance electrochemical remediation of contaminated soil. J. Hazard Mater. 2011, 195, 11–29. [Google Scholar] [CrossRef]
- Sawada, A.; Mori, K.I.; Tanaka, S.; Fukushima, M.; Tatsumi, K. Removal of Cr(VI) from contaminated soil by electrokinetic remediation. Waste Manag. 2004, 24, 483–490. [Google Scholar] [CrossRef]
- Alshawabkeh, A.N.; Acar, Y.B. Electrokinetic remediation. II: Theoretical model. J. Geotech. Eng. 1996, 122, 186–196. [Google Scholar] [CrossRef]
- Zhuang, Y.F.; Wang, Z. Interface electric resistance of electroosmotic consolidation. J. Geotech. Geoenviron. Eng. 2007, 133, 1617–1621. [Google Scholar] [CrossRef]
- He, J.Y.; He, C.Q.; Chen, X.P.; Liang, X.; Huang, T.L.; Yang, X.C.; Shang, H. Comparative study of remediation of Cr(VI)-contaminated soil using electrokinetics combined with bioremediation. Environ. Sci. Pollut. Res. 2018, 25, 17682–17689. [Google Scholar] [CrossRef]
- Zhou, J.; Gan, Q.Y.; Tao, Y.L. Electro-osmotic permeability model based on ions migration. Acta Geotech. 2021, 17, 2379–2393. [Google Scholar] [CrossRef]
- Gimmi, T.; Alt-Epping, P. Simulating Donnan equilibria based on the Nernst-Planck equation. Geochim. Et Cosmochim. Acta 2018, 232, 1–13. [Google Scholar] [CrossRef]
- Yustres, A.; Lopez-Vizcaıno, R.; Cabrera, V.; Rodrigo, M.A.; Navarro, V. Donnan-ion hydration model to estimate the electroosmotic permeability of clays. Electrochim. Acta 2020, 355, 136758. [Google Scholar] [CrossRef]
- Wang, L.S.; Huang, L.H. Application of a multi-electrode system with polyaniline auxiliary electrodes for electrokinetic remediation of chromium-contaminated soil. Sep. Purif. Technol. 2019, 224, 106–112. [Google Scholar] [CrossRef]
- Yan, Y.J.; Xue, F.; Muhammad, F.; Yu, L.; Xu, F.; Jiao, B.; Shiau, Y.; Li, D. Application of iron-loaded activated carbon electrodes for electrokinetic remediation of chromium-contaminated soil in a three-dimensional electrode system. Sci. Rep. 2018, 8, 8753. [Google Scholar] [CrossRef]
- Yan, Y.J.; Ling, Z.; Shu, W.; Huang, T.; Crane, R. Chromium removal from contaminated soil using a novel FeOx/granular activated carbon-based three-dimensional electrokinetic system. Chem. Eng. J. 2023, 455, 140613. [Google Scholar] [CrossRef]
- Xu, J.W.; Liu, C.; Hsu, P.C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 2440. [Google Scholar] [CrossRef]
- Xie, X.Y.; Liu, Y.M.; Zheng, L.W. Experimental study on the effect of soil saturation on the electric permeability coefficient during electroosmosis process. Mar. Georesources Geotechnol. 2018, 37, 1188–1195. [Google Scholar] [CrossRef]
- Hu, L.M.; Hong, H.Q.; Wu, W.L. Electro-osmosis tests on kaolin clay. J. Tsinghua Univ. 2010, 50, 1353–1356. [Google Scholar]
- Kaniraj, S.R.; Huong, H.L.; Yee, J.H.S. Electroosmotic consolidation studies on peat and clayey silt using electric vertical drain. Geotech. Geol. Eng. 2011, 29, 277–295. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, W.M. Helmholtz–Smoluchowski velocity for viscoelastic electroosmotic flows. J. Colloid Interface Sci. 2008, 317, 631–636. [Google Scholar] [CrossRef]
- Shang, J.Q. Zeta potential and electroosmotic permeability of clay soils. Can. Geotech. J. 1997, 34, 627–631. [Google Scholar] [CrossRef]
- Esrig, M.I.; Majtenyi, S. A new equation for electroosmotic flow and its implications for porous media. Highway Re. Rec. 1966, 111, 31–41. [Google Scholar]
- Leroy, P.; Tournassat, C.; Bernard, O.; Devau, N.; Azaroual, M. The electrophoretic mobility of montmorillonite. Zeta potential and surface conductivity effects. J. Colloid Interface Sci. 2015, 451, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Paz-Garcia, J.M.; Johannesson, B.; Ottosen, L.M.; Ribeiro, A.B.; Rodriguez-Maroto, J.M. Modeling of electric double-layers including chemical reaction effects. Electrochim. Acta 2014, 150, 263–268. [Google Scholar] [CrossRef]
- Friedman, S.P. Soil properties influencing apparent electrical conductivity: A review. Comput. Electron. Agric. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Ye, Z.B.; Xu, J.T.; Yin, F.J.; Shi, L.T.; Chen, H.; Li, Y.L. Effect of Injected Water Composition on Zeta Potential of Liquid-Solid Interface in Reservoir. Fine Chem. 2018, 35, 1765–1771. [Google Scholar] [CrossRef]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metals. Rev. Can. De Ge’Otechnique 2011, 42, 1280–1289. [Google Scholar] [CrossRef]
- Averjanov, S.F. About permeability of subsurface soils in case of incomplete saturation. Engl. Collect. 1950, 7, 19–21. [Google Scholar]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior; Wiley: New York, NY, USA, 1976; pp. 75–164. [Google Scholar]
- Mattson, E.D.; Bowman, R.S.; Lindgren, E.R. Electrokinetic ion transport through unsaturated soil: 1 Theory, model development, and testing. J. Contam. Hydrol. 2002, 54, 99–120. [Google Scholar] [CrossRef]
- Xue, Z.J.; Tang, X.W.; Yang, Q.; Tian, Z.F.; Zhang, Y. Influence of salt content on clay electro-dewatering with copper and stainless steel anodes. Dry. Technol. 2019, 37, 2005–2019. [Google Scholar] [CrossRef]
- Zhou, H.J.; Fang, Y.G.; Chen, M.H.; Gu, R.G.; Li, W. Experimental and analytical study on electro-osmosis in low-permeability soil considering the pore size effect. Geotechnique 2019, 71, 141–152. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Chen, M.; Wang, J.G.; Deng, Y.R.; Li, Z.F. A new method for assessment of electro-osmotic permeability through the integration of theoretical and experimental ion flux in electrokinetic processes. J. Hazard. Mater. 2023, 459, 132049. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zeng, F.J.; Tang, F.; Wang, K.D.; Xu, X.Y.; Tian, G.M. An increasing Cr recovery from soil with catholyte-enhanced electrokinetic remediation: Effects on voltage redistribution throughout soil sections. Sep. Purif. Technol. 2020, 253, 117553. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, L.H.; Zhuang, Y.F. Effect of electrolyte, potential gradient and treatment time on remediation of hexavalent chromium contaminated soil by electrokinetic remediation and adsorption. Environ. Earth Sci. 2023, 82, 40. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Soil and Sediment—Determination of Cr(VI)—Alkaline Digestion/Flame Atomic Absorption Spectrometry; HJ 1082-2019; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2019.
- Ministry of Ecology and Environment of the People’s Republic of China. Soil Quality—Determination of Cation Exchange Capacity (CEC)—Hexamminecobalt Trichloride Solution-Spectrophotometric Mehod; HJ 889-2017; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2017.
- GB/T 50123-2019; Standard for Geotechnical Testing Method. China Planning Press: Beijing, China, 2019.
- Gray, D.H.; Mitchell, J.K. Fundamental aspects of electroosmosis in soils. J. Soil Mech. Found. Div. 1967, 6, 39–51. [Google Scholar]
- Spiegler, K.S. Transport processes in ionic membranes. Trans. Faraday Soc. 1958, 54, 1408–1428. [Google Scholar] [CrossRef]
- Karampinos, D.C.; Georgiadis, J.G. Ab initio study of the energetics of ionic hydration with the polarizable continuum model. Nanoscale Microscale Thermophys. Eng. 2007, 11, 363–378. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Meng, X.F.; Zhao, D.X.; Gong, L.D. Theoretical Study on Hydronium Ion Clusters by ab initio Calculation and ABEEM/MM Model. Acta Chim. Sin. 2009, 67, 2074–2080. [Google Scholar]
- Liu, Z.T.; Zhuang, Y.F. Electrokinetic transport and removal of lead contaminants from kaolin. Chin. J. Geotech. Eng. 2020, 42, 1359–1366. [Google Scholar] [CrossRef]
- Tournassat, C.; Bourg, I.C.; Steefel, C.I.; Bergaya, F. Chapter 1-surface properties of clay minerals. Dev. Clay Sci. 2015, 6, 5–31. [Google Scholar] [CrossRef]
- Liu, J.G.; Wang, H.T.; Nie, Y.F. Fractal model for predicting effective diffusion coefficient of solute in porous media. Adv. Water Sci. 2004, 15, 458–462. [Google Scholar]

| Parameters | Value | |
|---|---|---|
| Cr6+-contaminated soil | Water content (%) | 29.8 |
| Cr6+ concentration (mg/kg) | 498.5 | |
| Porosity (n) | 0.58 | |
| pH | 7.85 | |
| Specific surface area (m2/g) | 4.41 | |
| Dry density | ||
| Saturation (Sr) | 78% | |
| Plastic limit/liquid limit | 18.59/33.26 | |
| Particle size distribution | Clay | 12.89% |
| Silt | 34.00% | |
| Sand | 53.11% |
| Experiments | Pore Solution | Electrolyte in Anode and Cathode | Potential Gradient | Zeta Potential | Cation Exchange Capacity |
|---|---|---|---|---|---|
| EK1 | Deionized water | 0.5 mol/L citric acid | 2 V/cm | −35.5 mV | 16.9 cmol/kg |
| EK2 | 0.1 mol/L acetic acid | 0.5 mol/L citric acid | 2 V/cm | −17.1 mV | 11.3 cmol/kg |
| EK3 | 0.2 mol/L acetic acid | 0.5 mol/L citric acid | 2 V/cm | −16.2 mV | 10.7 cmol/kg |
| EK4 | 0.4 mol/L acetic acid | 0.5 mol/L citric acid | 2 V/cm | −12.8 mV | 9.7 cmol/kg |
| EK5 | 0.5 mol/L acetic acid | 0.5 mol/L citric acid | 2 V/cm | −12.1 mV | 8.9 cmol/kg |
| EK6 | 0.8 mol/L acetic acid | 0.5 mol/L citric acid | 2 V/cm | −9.2 mV | 7.3 cmol/kg |
| EK7 | 1.0 mol/L acetic acid | 0.5 mol/L citric acid | 2 V/cm | −7.4 mV | 6.4 cmol/kg |
| EK8 | 0.1 mol/L citric acid | 0.5 mol/L citric acid | 2 V/cm | −15.3 mV | 8.5 cmol/kg |
| EK9 | 0.2 mol/L citric acid | 0.5 mol/L citric acid | 2 V/cm | −11.6 mV | 5.9 cmol/kg |
| EK10 | 0.5 mol/L citric acid | 0.5 mol/L citric acid | 2 V/cm | −5.1 mV | 4.7 cmol/kg |
| EK11 | 1.0 mol/L citric acid | 0.5 mol/L citric acid | 2 V/cm | 10.2 mV | 3.1 cmol/kg |
| Parameters | Value |
|---|---|
| H+ diffusion coefficient | |
| Faraday constant (F) | |
| Temperature (T) | 298 K |
| Universal gas constant (R) | |
| Molar mass of water (Mw) | |
| Avogadro constant (NA) | |
| Permittivity of pore water | |
| Dynamic viscosity of pore water | |
| Coulomb constant (k) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-Y.; Zhuang, Y.-F. Evaluation of Electroosmotic Permeability Using Different Models and Investigation of Its Effect on Chromium Removal. Sustainability 2023, 15, 12706. https://doi.org/10.3390/su151712706
Liu X-Y, Zhuang Y-F. Evaluation of Electroosmotic Permeability Using Different Models and Investigation of Its Effect on Chromium Removal. Sustainability. 2023; 15(17):12706. https://doi.org/10.3390/su151712706
Chicago/Turabian StyleLiu, Xi-Yin, and Yan-Feng Zhuang. 2023. "Evaluation of Electroosmotic Permeability Using Different Models and Investigation of Its Effect on Chromium Removal" Sustainability 15, no. 17: 12706. https://doi.org/10.3390/su151712706
APA StyleLiu, X.-Y., & Zhuang, Y.-F. (2023). Evaluation of Electroosmotic Permeability Using Different Models and Investigation of Its Effect on Chromium Removal. Sustainability, 15(17), 12706. https://doi.org/10.3390/su151712706





