Abstract
More than 80% of the energy from fossil fuels is utilized in homes and industries. Increased use of fossil fuels not only depletes them but also contributes to global warming. By 2050, the usage of fossil fuels will be approximately lower than 80% than it is today. There is no yearly variation in the amount of CO2 in the atmosphere due to soil and land plants. Therefore, an alternative source of energy is required to overcome these problems. Biohydrogen is considered to be a renewable source of energy, which is useful for electricity generation rather than relying on harmful fossil fuels. Hydrogen can be produced from a variety of sources and technologies and has numerous applications including electricity generation, being a clean energy carrier, and as an alternative fuel. In this review, a detailed elaboration about different kinds of sources involved in biohydrogen production, various biohydrogen production routes, and their applications in electricity generation is provided.
1. Introduction
Over the past decade, the growth of industries has been increasing enormously, which has resulted in the requirement for alternate energy sources. At the beginning of human history, wood biomass was used for heating, cooking, and shelter, which made it an ideal energy source for man. However, fossil fuels were exploited to meet the energy demands due to the growth of the human population []. Depletion and the inability to replenish the energy sources due to increasing industries resulted in the usage of fossil fuels. Increased usage of fossil fuels not only depletes them but also causes significant global warming by emitting harmful greenhouse gases []. In recent years, emissions of carbon dioxide and other harmful gases by human activities have been rising more recently than in previous years. Pollution due to fossil fuels can be controlled by the transition from fossil fuels to alternative renewable resources []. Sustainable development requires energy as a main component, which must be available constantly at an affordable range for a long period. The conversion of wastes into useful forms is the best way for sustainable development, for example, biohydrogen, biogas, and biofuel, which release less greenhouse gas than fossil fuels []. Electricity plays a major role in everyday life, of which 32.9% is produced from fossil fuels supplying approximately 213 Terawatt per hour (TWh) worldwide []. In India, the most contributing source of fossil fuel is coal, which contributes approximately 69.5% to power generation []. The balance between the preservation of the environment and economic growth is considered “sustainability” [].
The demand for hydrogen is increasing rapidly nowadays as hydrogen is considered a clean source of energy and a valuable gas. It is used as a feedstock in many industries [,]. The ionic form of hydrogen is present abundantly in the universe. It is odorless, colorless, tasteless, and non-toxic []. Hydrogen draws prominent attention as a future fuel because of its versatility and efficiency. It can be used as the best and most efficient fuel for transportation as the combustion of this fuel produces only water vapor and eliminates the release of hydrocarbons, carbon monoxide, carbon dioxide, and other micro particles that cause environmental pollution []. Hydrogen is used in the hydrogenation of coal, oil, petroleum, and shale oil and is also used in the production of ammonia. Hydrogen can be produced by oil and natural gas using the steam reforming process and other methods such as coal gasification and water electrolysis. However, these processes are considered non-renewable and do not draw much attention. Therefore, the eco-friendly production of bio hydrogen gas using renewable sources such as agricultural waste, inorganic waste, and microorganisms is highly encouraged []. The production of hydrogen becomes more interesting when produced from renewable sources because it can be operated at ambient pressure and temperature with a lower amount of energy consumption. Energy production from hydrogen is 122 kJ/g, which is 2.75 times greater than hydrocarbon fuels so it acts as a potential energy carrier []. Obtaining hydrogen from biomass is rather challenging as the amount of hydrogen present in biomass is nearly 6% versus 25% for methane, and the lower energy content is due to the 40% of oxygen present in biomass [].
2. Definition
The term biohydrogen in Greek refers to Bio- or life, hydro- or water, and gen- or genes, which indicates non-degradable organic fuel obtained from biological sources such as plants, microorganisms, animals, etc. []. Hydrogen produced biologically is termed “Biohydrogen”. It draws much attention because it is a clean, non-degradable, non-condensable fuel with higher efficiency, high energy density, and a lack of pollution []. Biohydrogen is a natural or transient byproduct of several microbial-mediated biochemical reactions. It can be produced either by a biological process or the thermochemical treatment of biomass []. Biohydrogen has the ability to be converted into usable power at a higher efficiency. However, the lower yields, storage, and rate of production remain barriers to biohydrogen production [].
3. Feedstocks of Biohydrogen Production
The sources selected for the production of hydrogen gas should be low cost and biodegradable and must have a high level of carbohydrate content with the presence of simple sugars such as glucose, lactose, and sucrose, which can be used as reliable biodegradable substrates for biohydrogen production []. The production of biohydrogen via bio photolysis of water using cyanobacteria, microalgae, and photosynthetic anoxygenic bacteria is most suitable as it utilizes major natural resources such as sunlight, water, etc. []. These microorganisms either supply electrons as an alternate source for the sake of survival in minimal optimum conditions or the need to prevent the reduction of the electron transport chain and act as a security valve. In addition to these biochemical reactions, hydrogen gas can also be produced during nitrogen fixation by the nitrogenases enzyme, which is a major mechanism in the heterocyst forming blue-green algae [].
3.1. Agricultural Waste
Over the last decade, many research works have been carried out focusing on the findings of alternate sources of green, clean, and renewable energy. However, the production of biofuels from food sources such as corn and sugar has served as an alternate source but has indirectly increased food prices, which has resulted in a global food crisis. Hence, nowadays, the production of biofuels from agricultural wastes has gained much attention from researchers []. The production of hydrogen gas from agricultural waste, which consists of lignocellulose material, contributes to the global energy conversion process. Agricultural waste is rich in hemicellulose and cellulose after conversion into mono or disaccharides and can be used in dark fermentation, photo fermentation, and bio photolysis (direct and indirect) [].
3.1.1. Lignocellulose Waste
Lignocellulose waste is considered a macromolecule consisting of lignin, cellulose, and hemicellulose. Lignin is a highly insoluble, irregular polymer that bonds with hemicellulose with a covalent bond, and in the cell wall, cellulose is enwrapped in a complex containing lignin and cellulose. This complex nature causes the barriers to transform into lignocellulosic waste. Waste such as residues of plants, agricultural waste, and the logging of wood is considered to be lignocellulosic waste and they are degraded slowly as they are difficult to degrade []. Around 180 million tons per year of lignocellulose materials are produced as byproducts or in the form of agricultural residues, which can be used as an inexpensive source for the production of biofuels [,]. These materials, due to their low fiber porosity, heterogeneity, and crystallinity are not readily fermentable, and pre-treatment is required for the process of forming fermentable sugars []. Nowadays, researchers are focused on the next-generation organic matter, which includes lignocellulose, rather than using first-generation products, as lignocellulose is a rich source of fermentable sugars and can be used for the production of biohydrogen. Some of the steps to be followed when lignocellulose materials are used for biohydrogen production are as follows:
- Lignocellulose materials consist of a hetero polymeric substance, and in order to break the complex, the raw materials must be pre-treated.
- A large number of monomeric sugars was obtained by hydrolysis of cellulose and hemicellulose.
- The obtained monomers liberated from the fractions were converted into the respective biofuel by the utilization of a microorganism using techniques involved in the bioprocess [].
High yields of biohydrogen are obtained by following the aforementioned steps []. The production of biohydrogen from lignocellulose waste has attracted the attention of many researchers due to its efficiency. Several researchers proved the efficiency and positive response of biohydrogen production by utilizing various lignocellulosic substrates and also identified the sources responsible for the inhibition []. The production of biohydrogen from lignocellulosic biomass after the pre-treatment, hydrolysis, and utilization of different microbial cultures via the process of dark fermentation has improved the yield and rates of biohydrogen production []. The production of biohydrogen from various substrates of lignocellulose via the process of dark fermentation is considered to be effective. The next most effective process used for the production of biohydrogen after dark fermentation is photo fermentation []. Taguchi et al. [] isolated Clostridium sp. strain no. 2 from termites and produced biohydrogen with 18.6 mmol/g of the substrate using xylan from oat spelts. Taguchi et al. [] used the same Clostridium sp. for the hydrolysis of cellulose and observed that the bacterium consumed 0.92 mmol of glucose per h and produced 4.1 mmol of hydrogen per h. The increase in the concentration of cellulose (12.5 g/L to 50 g/L) decreased the yield (2.18 mmol/g of cellulose to 0.42 mmol/g of cellulose). At high temperatures, high conversion of cellulose into hydrogen took place (43 mL of hydrogen/g of cellulose at 37 °C to 69 mL of hydrogen/g of cellulose at 55 °C; 567 mL of hydrogen was produced from 1 g of cellulose) []. The production of biohydrogen from lignocellulosic biomass is described in Figure 1. Some of the lignocellulose biomass used and its composition, types of monomers present, and the amount of hydrogen produced is tabulated below (Table 1).
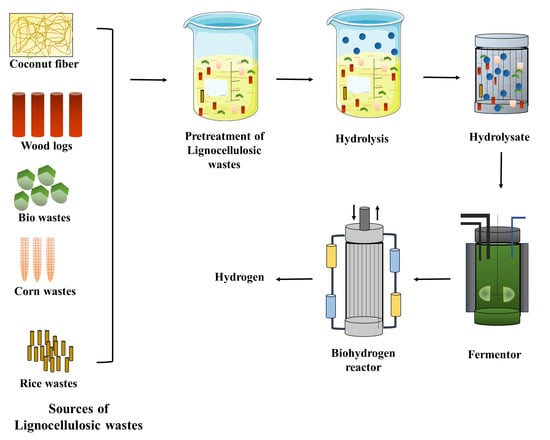
Figure 1.
Biohydrogen production from lignocellulosic wastes.

Table 1.
Lignocellulose biomass, its composition, and hydrogen production.
3.1.2. Livestock Waste
After the depletion of fossil fuels, solid wastes have become a promising factor for the production of renewable sources []. The terrestrial surface is occupied by livestock, and it plays a major role as a significant global asset. Livestock is considered to be an important provider of nutrition for growing crops in a small area. In recent days, in developing countries, livestock is considered to be the fastest-growing agricultural subsector []. Livestock serves as an important factor in increasing food security and contributing to rural and agricultural development []. Nowadays, the waste generated by livestock from cattle, swine buildings, and poultry is a major source of contamination of underground water systems due to its odor, gases, and dust. Due to the contamination caused by these wastes, many researchers proposed the idea of generating useful products from these wastes. These livestock wastes include fodder, manure, and slaughterhouse and poultry farm wastes. The improper maintenance of these wastes is harmful to both human health and the environment. From these polluting substances, a renewable non-polluting energy source is produced, named biohydrogen [,]. However, the production of biohydrogen is inhibited due to the presence of ammoniacal nitrogen (NH3-N) in chicken manure and the presence of high sulphate content in swine manure. In order to produce biohydrogen, the high sulphate content can be treated with a rich carbohydrate source such as lignocellulose materials, which provide the perfect C/N ratio and enhance the buffering capacity and provide nutritional manure []. Livestock waste can be used as a substrate along with the carbohydrate source for the efficient production of biohydrogen [].
Lateef et al. [] produced biohydrogen with cow manure as a source along with waste milk as a co-substrate. After adding the organic load, which is obtained from the co-digestion of cow manure, the production of biohydrogen increased. Tenca et al. [] produced biohydrogen with a yield of 126 ± 22 mL H2/g VS-added when swine manure was used, along with fruit and vegetable waste. Marone et al. [] produced biohydrogen with a maximum yield of 117 mL H2/g VS-added through the co-fermentation of buffalo slurry with cheese whey and crude glycerol using a mixed microbial culture. Bari et al. [] produced biohydrogen from organic waste by fermentation process and had various industrial applications like steel making, ammonia production, Glass making etc. Fan et al. [] produced biohydrogen with a yield of 68.6 mL H2/g TVS when beer-less wastes were converted into biohydrogen via cow dung compost. The hydrogen yield and hydrogen production rate were higher (30.00 mL/g VS-added and 1.00 L/L/d, respectively) when the biohydrogen was produced via the co-digestion of cattle manure and food wastes with an optimal mixing ratio of 47 to 51%, a hydraulic retention time of 2 days, and a substrate concentration of 76 to 86 g/L []. The production of biohydrogen using the co-digestion of cattle manure with specified risk materials has been reported by Gilroyed et al. []. The maximum hydrogen production rate and hydrogen yield was 109.55 mL H2/L per day and 0.84 mol H2/mol of total sugar consumed, respectively, when elephant dung was used as the inoculum for sugarcane bagasse hydrolysate []. The maximum hydrogen production rate and hydrogen yield were 215.4 (±62.1) mL H2/L/d 152.2 and (±43.9) mL H2/g VS-added, respectively, achieved at an organic loading rate of 2.1 g VS/L/d of cheese whey via the dark fermentation method using buffalo manure as a buffering agent []. The co-digestion of cassava wastewater along with buffalo dung for biohydrogen production gave a maximum hydrogen production rate and hydrogen yield of 839 mL H2/L/d and 16.90 mL H2/g COD-added, respectively []. Perera et al. [] produced a maximum hydrogen yield of 2.9–5.3 M hydrogen/M sucrose when sucrose along with dairy cattle manure was used for production. Biohydrogen was produced when the liquid swine manure was co-fermented with molassesm of which the hydrogen production rate and hydrogen yield of 31.9 L/d and 1.52 L/g sugar, respectively, was obtained []. Zhu et al. [] produced biohydrogen with swine manure co-fermented with glucose as a substrate. Biohydrogen production from livestock waste is illustrated and tabulated in Figure 2 and Table 2, respectively.
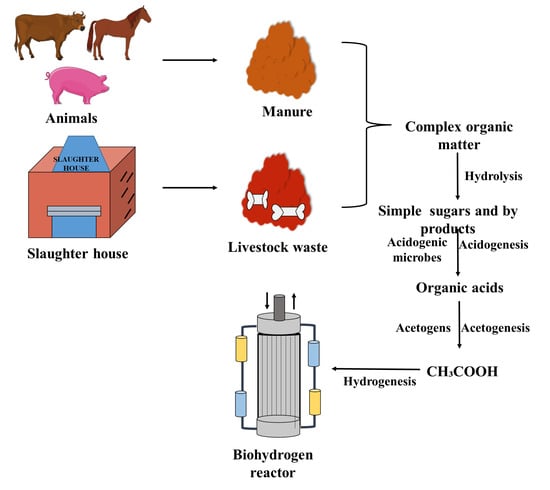
Figure 2.
Biohydrogen production from livestock wastes.

Table 2.
Livestock waste as a source for hydrogen production.
3.2. Industrial Waste
The growth of the world relies mostly on industrialization. Pollution is caused by these industries by utilizing more water and the excessive production of effluents []. Industrial wastes are substances that cause severe environmental pollution as they are non-biodegradable. The application of these industrial wastes in road construction has attracted many researchers in recent days []. The waste materials generated from these industries are renewable biomass and can be used for the production of biohydrogen. Industrial wastewater and biodiesel industry wastes are some examples of industrial wastes that can be used for biohydrogen production. Many reports on biohydrogen production from electrolysis and other chemical processes have been reported, but the biological conversion of wastes into hydrogen can be the best alternative method and also the most cost-efficient method []. Many starch- and cellulose-based materials are present in the waste products from the food and agricultural industries. These waste products are rich in carbohydrate content. It is easier to process the starch waste content by hydrolyzing it into maltose or glucose via enzymatic or acid hydrolysis followed by conversion into carbohydrates and then into hydrogen gas, but cellulose-containing wastes are difficult to process as they require the pre-treatment of wastes, and hydrolyses, followed by conversion into carbohydrates and then into biohydrogen production [,,].
Biohydrogen was produced using the waste from food industries by Alexandropoulou et al. [] using the continuous-type reactor under different pH and hydraulic retention times. The obtained hydrogen yield was 96.27 ± 3.36 and 101.75 ± 213.7 L H2/kg FIW for 12 and 6 h, respectively. Moreno-Andrade et al. [] produced hydrogen using different industrial wastes as feedstocks. The feedstocks used were tequila vinasses, sugar vinasses, wastewater from the plastic industry, aircraft wastewater, and physio-chemically treated wastewater from the plastic industry. The tequila vinasses produced the maximum amount of hydrogen followed by wastewater from the plastic industry, aircraft wastewater, physio-chemically treated wastewater from the plastic industry, and sugar vinasses, and it was observed that the hydrogen production in aircraft wastewater increased when an anaerobic sequencing batch reactor was used. Moreno-Dávila et al. [] produced hydrogen with 60.75 mmol/h∗g volatile solids when pre-treated wastes of paper industries were used as the source. The process followed for biohydrogen production was simultaneous saccharification and fermentation. Oceguera-Contreras et al. [] produced biohydrogen with a yield of 1246.36, 1571.81, and 232.72 mL H2/L from the bagasse, molasses, and vinasses agro-industry wastes when vermihumus-associated microorganisms as inoculum were used as a source and found that these microbes not only produce biohydrogen but also help in the degradation of lignocellulosic waste material.
Lopez-Hidalgo et al. [] produced hydrogen from agro-industrial wastes such as cheese whey and wheat straw hydrolysate. The authors reported that both the wheat straw hydrolysate and cheese whey produced hydrogen efficiently as both an individual substrate and even when mixed together. Lucas et al. [] produced biohydrogen using cassava wastewater, dairy wastewater, and citrus processing wastewater as sources and the production of hydrogen was found to be 31.41, 28.95, and 37.25 mL/g. Gomez-Romero et al. [] utilized fruit and vegetable wastes and crude cheese whey for the production of biohydrogen. The yield of produced hydrogen was 813.3 mL H2 g COD−1 and was determined at 17.5 h (Hydraulic Retention Time) with an organic loading rate of 80.02 g COD L−1 d−1. The usage of agro-industrial wastes such as starch wastes produces biohydrogen efficiently and is a cheaper process. A variety of raw materials from agro-industries can be used for the production of biohydrogen []. Biohydrogen production from industrial wastes is illustrated and tabulated in Figure 3 and Table 3, respectively.
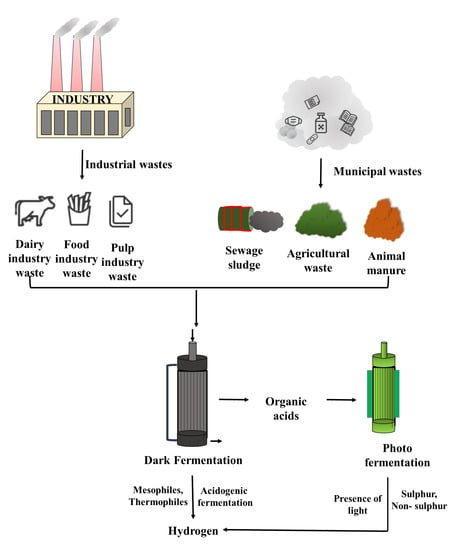
Figure 3.
Biohydrogen production from industrial wastes.

Table 3.
Production of biohydrogen from industrial wastes.
3.3. Municipal Wastes—Waste Sludge
The management and generation of waste products are becoming a global challenge and causing environmental problems. The management and recycling of waste are the best way to avoid pollution. Waste sludge causes much environmental pollution and also affects human health in many ways. Waste sludges are used for the generation of many renewable resources in order to maintain the quality of the environment, reduce many risk factors, produce sustainable energy, and serve as a reliable source of energy production [,]. Biohydrogen was produced via the co-digestion of food waste and sewage sludge, and the maximum hydrogen production rate was observed to be 111:2 mL H2/g VSS/h []. Cai et al. [] produced biohydrogen from sewage sludge and reported that the hydrogen yield of alkali pre-treated sludge was higher than dry sludge. The yield increased from 9.1 mL of H2/g of dry solids (DS) to 16.6 mL of H2/g of DS when alkali-pre-treated sludge was used. Yin and Wang [] produced hydrogen using waste sludge and reported that the irradiation and gamma irradiation combined with the alkali pretreatment was able to produce biohydrogen by dissolving the waste-activated sludge. The co-fermentation of sewage sludge and fallen leaves produced biohydrogen. The mixing ratio of 20:80 of fallen leaves and sewage sludge produced biohydrogen with a yield of 37.8 mL/g VS-added []. Natural sludge was used as an inoculum to produce biohydrogen using corn stalks via anaerobic fermentation, and the maximum hydrogen yield was observed to be 126.22 mL g−1-CS []. A Continuous Mixed Immobilized Sludge Reactor (CMISR) using activated carbon as a support carrier was used for hydrogen production via dark fermentation from enzymatically hydrolyzed food waste. The maximum hydrogen production rate of 353.9 mL/h/L was obtained under the conditions of a packing ratio of 15% and an organic loading rate of 40 kg/m3/d []. Yang and Wang [] reported that the combined sodium citrate and ultrasonic pretreatment disrupted the sludge floc structure and promoted biohydrogen fermentation performance. Yang and Wang [] produced biohydrogen from waste-activated sludge in which the sludge consisted of a complex structure due to the presence of an extracellular polymeric substance, which had to be pre-treated. The maximum hydrogen yield of 38.8 mL/g VS-added was obtained after the combined pre-treatment of sodium citrate pre-treatment and ultrasonic pre-treatment.
Yang and Wang [] produced biohydrogen from waste-activated sludge with the addition of a cationic binding agent (sodium citrate) to disintegrate the extracellular polymeric substance present in the sludge. The addition of the binding agent improved biohydrogen production from 3.7 to 18.8 mL/g VS-added when 0.3 g of sodium citrate/of SS was added. Biohydrogen was produced via the dark fermentation method by using waste-activated sludge from fructose processing manufacturing and the maximum hydrogen yield obtained was 7.8 mmol []. Biohydrogen production from municipal wastes is illustrated and tabulated in Figure 3 and Table 4, respectively.

Table 4.
Production of biohydrogen from municipal wastes.
3.4. Microbial Routes
The production of biohydrogen on a large scale came into thought after the rapid depletion of fossil fuels. It has been known for more than 70 years that algae can make bio-hydrogen under illumination. The evolution of hydrogen was induced in the cells when pre-incubation in the dark was performed on the cells. Hydrogen production is due to the hydrogenase enzyme expressed during the period of incubation []. The fermentative hydrogen production depends on the type of inoculum used, the reactor type, and its temperature settings. Many types of inoculums are used for hydrogen production and must be pure cultures of hydrogen-producing bacteria, mixed cultures of anaerobic bacteria obtained from compost piles, and anaerobic sludges [,,]. The metabolic shifts in pure cultures are easily visible, and the utilization of pure cultures enables us to understand the conditions that promote a high hydrogen production rate and yield []. Biohydrogen production from municipal wastes is tabulated in Table 5.

Table 5.
Production of biohydrogen from microbial routes wastes.
4. Biohydrogen Production
4.1. Bio Photolysis
Light-dependent production of hydrogen from water is a biological process that converts sunlight into chemical energy []. The enzymes are responsible for catalyzing chemical reactions such as nitrogenase, Ni-Fe- hydrogenase, and Fe- hydrogenase. The bio photolysis process makes use of the Fe- hydrogenase enzyme [].
The various routes of biohydrogen production are illustrated in Figure 4.
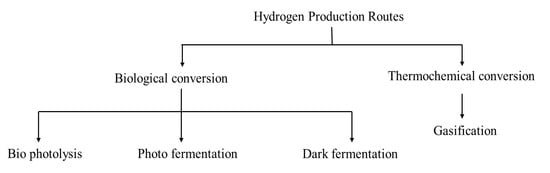
Figure 4.
Routes of hydrogen production.
4.1.1. Direct Bio Photolysis
There are many advantages of direct bio photolysis of hydrogen production. This reaction can be observed in laboratory conditions and is self-limited by the oxygen that builds up in the cellular environment and takes place during the initial transition to conventional photosynthesis. Photosystem I and Photosystem II are involved in photosynthesis, where photosystem I reduces carbon dioxide and photosystem II splits H2O and produces oxygen []. The photosynthetic apparatus absorbs sunlight directly and uses photoenergy for the splitting of water, and the resulting low-potential reductant reduces the hydrogenase enzyme system. Thus, photo energy could convert the readily available substrate and H2O into O and H molecules [].
Photoautotrophic organisms do produce hydrogen from water using the hydrogenase enzyme under anaerobic conditions in the presence of light energy. Cyanobacteria and green algae produce hydrogen via direct bio-photolysis through chlorophyll and other pigments that have the ability to absorb photons at a wavelength of less than 680 nm []. Green algae can produce hydrogen when exposed to light or uptake hydrogen via the CO2-fixation process when exposed to darkness in anaerobic conditions. The unicellular green algae Chlamydomonas reinhardtii has gained a great deal of attention in recent decades for its direct bio-photolysis production of hydrogen molecules. Cyanobacteria are prokaryotes that can perform oxygenic photosynthetic reactions [,].
When plants are used as a source for biohydrogen production, only CO2 reduction takes place as plants cannot undergo the process of producing hydrogen as it does not have the hydrogenase enzyme, but green macroalgae and cyanobacteria can produce hydrogen as they do have the hydrogenase enzyme []. Synechocystis sp. PCC 6803 was used for the production of hydrogen by direct bio photolysis, and 0.037 mmol H2/mg Chl/h of hydrogen was produced in the dark within 120 h [,,].
4.1.2. Indirect Bio Photolysis
Cyanobacteria and microalgae are employed to produce hydrogen from water, where photosynthesis occurs and solar energy is transformed into electrical energy []. In indirect bio photolysis, the hydrogen and oxygen evolution takes place at separate stages linked to carbon dioxide fixation, where CO2 is used for the production of the cellular substance, and these are used for the production of hydrogen. Primarily cyanobacteria are used during indirect bio photolysis as it has the property of using carbon dioxide in the air as a carbon source and the energy source is provided by solar energy [].
An alternate process for direct photolysis is indirect bio photolysis, where carbon dioxide acts as an electron carrier between photosynthesis and hydrogen production. The reason for the wide usage of nitrogen-fixing cyanobacteria in this process for hydrogen production is that it can produce hydrogen using the nitrogenase enzyme present in it, even in the absence of nitrogen, which is also possible under laboratory conditions []. The most commonly used cyanobacteria in indirect bio photolysis are Oscillatoria sp., Gloeocapsa sp., Anabaena sp., and Calothrix sp. []. Generally, four steps are involved in the production of biohydrogen via indirect bio photolysis []:
- i.
- Photosynthesis for the production of biomass.
- ii.
- The concentration of biomass.
- iii.
- Dark fermentation in aerobic conditions, which produces 4 mol hydrogen/mol glucose along with 2 mol of acetates.
- iv.
- The production of hydrogen.
Indirect bio photolysis is a two-step process that starts with photosynthesis and sugar reduction, followed by the induction of light. The maximum efficiency for the conversion of light is 16.3%. Better conversion of light takes place at the lowest illumination, and at the highest illumination, the efficiency is less []. To date, reports regarding indirect bio photolysis are fewer, and more studies must be conducted in order to obtain a better understanding of this process.
4.2. Dark Fermentation
The production of biohydrogen via dark fermentation involves the use of anaerobic or facultative anaerobic bacteria in anaerobic conditions. Even for the estimation of fermentative hydrogen production, various substances can be used such as carbohydrates, proteins, sugar molecules, and lipids. Glucose biotransformation toward acetate is widely preferred []. The bacteria are responsible for producing biohydrogen from organic waste during dark fermentation. The substrate primarily used is lignocellulose biomass, but other raw materials such as municipal waste and wastewater from industries are also able to be used as a substrate for the production of biohydrogen. Compared with photo fermentation, dark fermentation is considered to be the most promising method for biohydrogen production [].
Anaerobic bacteria are responsible for using the organic substance as the source of electrons and the energy required for converting it into hydrogen. The reactions taking place during dark fermentation occur as a rapid process as there are no requirements for solar radiation. Large quantities of biomass are treated using a large fermenter []. Under anaerobic conditions, protons can act as electron acceptors to accept the electrons generated and bacteria reduce the protons in hydrogen by using hydrogenase, which maintains the electrical neutrality for the uninterrupted and continuous supply of ATP []. This hydrogenase enzyme can be divided into many types depending on the metal-binding capacity, and microbial hydrogen metabolism greatly depends on the hydrogenase enzyme []. Dark fermentation can take place using both mixed and pure cultures, but there is an advantage of using a pure culture over a mixed culture as the metabolic changes can be monitored easily [].
C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2
C6H12O6 + 2H2O → 2H2 + CH3CH2CH2COOH + 2CO2
From Equations (2) and (3), it is evident that 4 mol H2/mol glucose can be produced if acetic acid is the volatile fatty acid (VFA) product. Moreover, 2 mol H2/mol glucose can be produced if butyric acid is the Volatile Fatty Acid (VFA) product []. The advantages of this method include that it can produce hydrogen even for a day without light, various carbon sources can be used as the substrate, there is no oxygen limitation problem as it is an anaerobic reaction, and the byproducts produced during dark fermentation are valuable byproducts such as acetic acid, lactic acid, etc. []. Dark fermentation for the production of biohydrogen is illustrated in Figure 3.
4.3. Photofermentation
Photosynthetic and Non-Sulfur (PNS) bacteria have the ability to convert the volatile fatty acid into carbon dioxide and hydrogen under anoxygenic conditions []. PNS bacteria is a non-taxonomic group that is capable of growing as photoautotrophs, photoheterotrophs, and chemoheterotrophs, depending on the availability of carbon, oxygen, and light sources []. The optimum growth conditions for PNS bacteria are pH 7 and temperatures ranging between 30 and 35 °C []. This method is considered to be an effective process for producing hydrogen without the generation of oxygen. Organic components are decomposed under the presence of light by anaerobic or photosynthetic bacteria via the nitrogenase-catalyzed reaction []. Rhodobacter capsulatus, Rhodobacter sphaeroides, Rhodopseudomonas palustris, and Rhodovulum sulfidophilum are some of the PNS bacteria responsible for photo fermentation. Photo fermentation can be performed in both batch and continuous systems by supplying an artificial light source or illumination. Various physical parameters such as the temperature, pH, medium composition, and intensity of light affect the productivity of hydrogen by bacteria [].
PNS bacteria have the ability to reduce H+ ions to hydrogen in the gaseous phase by extracting power from the oxidation of certain compounds such as fatty acids of low molecular weight and light energy []. For the PNS organism to grow and produce hydrogen, photo heterotrophy is generally preferred. This photo fermentation is carried out via the catalytic action of two enzymes involving hydrogenase and nitrogenase via the Tricarboxylic Acid (TCA) cycle [, ]. The production of hydrogen gas by PNS bacteria is possible as a result of one of the important enzymes: Nitrogenase. It is highly sensitive to oxygen as it is an iron sulfur molybdenum enzyme. The main source for photo fermentation is light, which is most required for developing a photobioreactor with a greater illumination facility for industrial purposes [].
The production of hydrogen under dark fermentation is usually lower compared to photo fermentation, but a 14 h light and 10 h dark cycle can improve the rate of hydrogen production [].
4.4. Gasification
After biological conversion, gasification became the most widely studied field. More studies on gasification have been performed by China and the United States of America, while the UK, Italy, Malaysia, Canada, and Japan have also contributed many findings in the field of producing hydrogen using gasification. At high temperatures and high pressures, organic feedstock undergoes partial oxidation, which is termed gasification. During this process, several byproducts can also be produced such as tar, biochar, light hydrocarbon, etc. []. Gasification is not a biological process but it is still used for the conversion of organic wastes into biohydrogen. The optimization of operating parameters helps in improving hydrogen production [].
2C + O2 → 2CO2C + O2 → 2CO
C + O2 → CO2C + O2 → CO2
C + H2O → CO + H2C + H2O → CO + H2
C + CO2 → 2COC + CO2 → 2CO
C + 2H2 → CH4C + 2H2 → CH4
CO + H2O → CO2 + H2CO + H2O → CO2 + H2
CH4 + H2O → CO + 3H2
Biomass is considered to be a very good source for gasification because of its low sulfur content, and if the moisture content is less than 35% for any kind of biomass, then it can be converted into fuel gas []. Gasification is considered to be a biological process that converts biomass into carbon monoxide, carbon dioxide, hydrogen, and methane with controlled amounts of steam and oxygen used at high temperatures []. Biomass gasification usually takes place between 700 and 1200 °C using oxygen, air, and other gasifying agents. Steam employment during gasification enhances the production of hydrogen and produces high-heating-value gas with no N2. Some of the major steps involved in steam gasification are pyrolysis, the homogenous reaction by volatiles produced during pyrolysis, and heterogenous char gasification []. For the production of hydrogen, a good-quality gas from the gasifier should consist of a low tar content and a high hydrogen content, but the gas quality can be affected by various parameters such as the pressure, temperature, equivalence ratio, gasifier design, and characteristics of biomass []. The advantages and disadvantages of the above-mentioned techniques are summarized in Table 6.

Table 6.
Advantages and disadvantages of various biohydrogen production techniques.
4.5. Applications of Biohydrogen in Fuel Cells
Fuel cells convert chemical energy for the production of electricity. It is considered an electrochemical conversion device. Hydrogen and microbial fuel cells, when coupled together, produce electricity without the emission of water and other toxic elements as byproducts. H2 is produced effectively in MFC and can also be used in the generation of electricity and aids in the purification of wastewater []. Hydrogen produced by the biohydrogen separation system is used as fuel in fuel cells. The Proton Exchange Membrane Fuel Cell (PEMFC) has received much attention due to its portable nature and ability to work in low-temperature conditions []. Wei et al. [] produced biohydrogen through anaerobic fermentation by using the starch in wastewater as a source, and it was transferred immediately to PMEFC for the effective generation of electricity.
Biohydrogen was produced from dairy wastewater and was transferred to PMEFC for electricity generation. Contaminants such as carbon monoxide, carbon dioxide, ammonia, and hydrogen sulfide present/produced in the fuel cells affect the performance of the fuel cells as CO2 poisons the surface of the catalyst by damaging the electrochemically active surface area and blocks the hydrogen from reaching the active platinum sites []. Biohydrogen is produced by a C. sorokiniana strain under sulphur-deprived conditions. This produced hydrogen is transferred into PEMFC and converted into electricity, and when 27.09 mL of hydrogen was injected, 8.9 mA of current was generated []. Hydrogen was produced photobiologically by Chlamydomonas reinhardtii and was integrated with PEMFC for electricity generation, and 1.81 mA cm−2 of current density was produced for approximately 50 h and 0.23 mA cm−2 for approximately 80 h []. Hydrogen was produced using the marine microalgae Tetraselmis subcordiformis and was coupled with an alkaline fuel cell for the production of electricity [].
The heat-treated microbial population (HTMP) and the Acid-Treated Microbial Population (ATMP) produced higher H2 yields at 35 °C, where HTMP was Clostridium sp. and ATMP was a mixed microbial population. With a flux of 0.9 L/h hydrogen, a PEMFC was operated successfully []. Using a single-chambered MFC and pre-fermented wastewater, biohydrogen was produced and simultaneously biohydrogen production was linked to electricity generation. MFC was used to treat wastewater and for bioenergy production [].
5. Discussion
From a green perspective, biohydrogen adheres to the green chemistry concept because the wastes produced by food, vegetables, and manure are not released into the environment but are instead treated and used to generate hydrogen gas. MFCs offer a cutting-edge, versatile alternative method of producing hydrogen. Over the past few decades, a number of technological developments have improved the yield of the product. However, this technology is still far from being able to serve as a profitable real-world application []. The idea of a hydrogen economy is gaining popularity, and technologists are working to obtain methods of producing H2 with a zero-emission plan. More studies on the sustainability of this process must be carried out to understand its efficiency.
6. Conclusions
The use of biohydrogen is an alternative source of energy, as it is a renewable source of energy. It can be produced by various sources such as agricultural waste, industrial waste, municipal waste, and microbial routes. Dark fermentation is considered the most effective method for producing biohydrogen with a higher yield and purity, even though it is not currently feasible for large-scale implementation. It is a very reliable source of energy for electricity generation around the world, but it has its own limitations. The greatest challenge is to ensure that the process is sustainable, considering the low level of substrate conversion, production rate, and yield. Cost-wise and yield-wise, current biohydrogen technologies are not yet competitive with conventional H2 production methods. It is essential to conduct extensive research in order to reduce costs and maximize H2 yield with the current production technologies. As a result, future research should focus on increasing the sustainability and measuring the economic feasibility of biohydrogen production in order to enable its scalability.
Author Contributions
A.V.S.: Conceptualization, writing—original draft, editing, supervision; D.R.: Writing—original draft, editing; M.S.: Writing—original draft, editing; S.S.: Writing—original draft, editing; K.K.: Writing—original draft, editing; N.V.: Writing—original draft, editing; N.J.: Writing—original draft, editing; P.P.: Writing—original draft, editing; N.S.: Writing—original draft, editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included in the article. Should further data or information be required, these are available from the corresponding author upon request.
Acknowledgments
The authors thank the Sathyabama Institute of Science and Technology, India and MAHSA University, Malaysia for providing facilities and support to complete this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen production from biomass and wastes via dark fermentation: A review. Waste Biomass Valorization 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Mohan, S.V.; Pandey, A. Biohydrogen production: An introduction. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–24. [Google Scholar]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Tyagi, V.V.; Pathak, A. Waste-to-energy: A way from renewable energy sources to sustainable development. Renew. Sustain. Energy Rev. 2010, 14, 3164–3170. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tan, J.H.; Fadzlita, M.T.; Muzammil, A.W.K. A Review on the development of Gravitational Water Vortex Power Plant as alternative renewable energy resources. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Miri, Malaysia, 2017; Volume 217, p. 012007. [Google Scholar]
- Jha, S.K.; Puppala, H. Prospects of renewable energy sources in India: Prioritization of alternative sources in terms of Energy Index. Energy 2017, 127, 116–127. [Google Scholar] [CrossRef]
- Demirtas, O. Evaluating the best renewable energy technology for sustainable energy planning. Int. J. Energy Econ. Policy 2013, 3, 23. [Google Scholar]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A. Food waste and food processing waste for biohydrogen production: A review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Demirbas, A. Biohydrogen; Springer: London, UK, 2009; pp. 163–219. [Google Scholar]
- Percival Zhang, Y.-H. Hydrogen Production from Carbohydrates: A Mini-Review. In Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; ACS Symposium Series; Oxford University Press: Oxford, UK, 2011; Volume 1067, pp. 203–216. [Google Scholar]
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production: The way forward? Trends Biotechnol. 2009, 27, 287–297. [Google Scholar] [CrossRef]
- Oh, Y.K.; Raj, S.M.; Jung, G.Y.; Park, S. Current status of the metabolic engineering of microorganisms for biohydrogen production. Bioresour. Technol. 2011, 102, 8357–8367. [Google Scholar] [CrossRef]
- Esper, B.; Badura, A.; Rögner, M. Photosynthesis as a power supply for (bio-)hydrogen production. Trends Plant Sci. 2006, 11, 543–549. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Li, Y.C.; Liu, Y.F.; Chu, C.Y.; Chang, P.L.; Hsu, C.W.; Lin, P.J.; Wu, S.Y. Techno-economic evaluation of biohydrogen production from wastewater and agricultural waste. Int. J. Hydrogen Energy 2012, 37, 15704–15710. [Google Scholar] [CrossRef]
- Cheng, C.L.; Lo, Y.C.; Lee, K.S.; Lee, D.J.; Lin, C.Y.; Chang, J.S. Biohydrogen production from lignocellulosic feedstock. Bioresour. Technol. 2011, 102, 8514–8523. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Chang, J.S. Biohydrogen from Renewable Resources; Pandey, A., Chang, J.S., Hallenbeck, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 185–221. [Google Scholar]
- Saratale, G.D.; Kshirsagar, S.D.; Sampange, V.T.; Saratale, R.G.; Oh, S.E.; Govindwar, S.P.; Oh, M.K. Cellulolytic enzymes production by utilizing agricultural wastes under solid state fermentation and its application for biohydrogen production. Appl. Biochem. Biotechnol. 2014, 174, 2801–2817. [Google Scholar] [CrossRef]
- Kang, S.W.; Park, Y.S.; Lee, J.S.; Hong, S.I.; Kim, S.W. Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour. Technol. 2004, 91, 153–156. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Periyasamy, S.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose biohydrogen: Practical challenges and recent progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrère, H. Lignocellulosic materials into biohydrogen and biomethane: Impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.-H.; Kim, D.-H. Fermentative hydrogen production using lignocellulose biomass: An overview of pre-treatment methods, inhibitor effects and detoxification experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen production from lignocellulosic biomass: Technology and sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Taguchi, F.; Mizukami, N.; Yamada, K.; Hasegawa, K.; Saito-Taki, T. Direct conversion of cellulosic materials to hydrogen by Clostridium sp. strain no. 2. Enzym. Microb. Technol. 1995, 17, 147–150. [Google Scholar] [CrossRef]
- Taguchi, F.; Yamada, K.; Hasegawa, K.; Taki-Saito, T.; Hara, K. Continuous hydrogen production by Clostridium sp. strain no. 2 from cellulose hydrolysate in an aqueous two-phase system. J. Ferment. Bioeng. 1996, 82, 80–83. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, T.; Fang, H.H. Thermophilic H2 production from a cellulose-containing wastewater. Biotechnol. Lett. 2003, 25, 365–369. [Google Scholar] [CrossRef]
- Fan, Y.T.; Zhang, G.S.; Guo, X.Y.; Xing, Y.; Fan, M.H. Biohydrogen-production from beer lees biomass by cow dung compost. Biomass Bioenergy. 2006, 30, 493–496. [Google Scholar] [CrossRef]
- Ren, N.Q.; Cao, G.L.; Guo, W.Q.; Wang, A.J.; Zhu, Y.H.; Liu, B.F.; Xu, J.F. Biological hydrogen production from corn stover by moderately thermophile Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrogen Energy 2010, 35, 2708–2712. [Google Scholar] [CrossRef]
- Sigurbjornsdottir, M.A.; Orlygsson, J. Combined hydrogen and ethanol production from sugars and lignocellulosic biomass by Thermoanaerobacterium AK54, isolated from hot spring. Appl. Energy. 2012, 97, 785–791. [Google Scholar] [CrossRef]
- Han, H.; Wei, L.; Liu, B.; Yang, H.; Shen, J. Optimization of biohydrogen production from soybean straw using anaerobic mixed bacteria. Int. J. Hydrogen Energy. 2012, 37, 13200–13208. [Google Scholar] [CrossRef]
- Pan, C.; Fan, Y.; Hou, H. Fermentative production of hydrogen from wheat bran by mixed anaerobic cultures. Ind. Eng. Chem. Res. 2008, 47, 5812–5818. [Google Scholar] [CrossRef]
- Keskin, T.; Abubackar, H.N.; Arslan, K.; Azbar, N. Biohydrogen production from solid wastes. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 321–346. [Google Scholar]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef]
- Solomon, A.; Authority, E.L.M. Livestock Marketing in Ethiopia: A Review of Structure, Performance, and Development Initiatives; ILRI (aka ILCA and ILRAD): Addis Ababa, Ethiopia, 2003; Volume 52. [Google Scholar]
- Xing, Y.; Li, Z.; Fan, Y.; Hou, H. Biohydrogen production from dairy manures with acidification pretreatment by anaerobic fermentation. Environ. Sci. Pollut. Res. 2010, 17, 392–399. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.S. Biohydrogen production from renewable biomass resources. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. [Google Scholar]
- Lateef, S.A.; Beneragama, N.; Yamashiro, T.; Iwasaki, M.; Ying, C.; Umetsu, K. Biohydrogen production from co-digestion of cow manure and waste milk under thermophilic temperature. Bioresour. Technol. 2012, 110, 251–257. [Google Scholar] [CrossRef]
- Tenca, A.; Schievano, A.; Perazzolo, F.; Adani, F.; Oberti, R. Biohydrogen from thermophilic co-fermentation of swine manure with fruit and vegetable waste: Maximizing stable production without pH control. Bioresour. Technol. 2011, 102, 8582–8588. [Google Scholar] [CrossRef]
- Marone, A.; Varrone, C.; Fiocchetti, F.; Giussani, B.; Izzo, G.; Mentuccia, L.; Rosa, S.; Signorini, A. Optimization of substrate composition for biohydrogen production from buffalo slurry co-fermented with cheese whey and crude glycerol, using microbial mixed culture. Int. J. Hydrogen Energy 2015, 40, 209–218. [Google Scholar] [CrossRef]
- El Bari, H.; Lahboubi, N.; Habchi, S.; Rachidi, S.; Bayssi, O.; Nabil, N.; Mortezaei, Y.; Villa, R. Biohydrogen production from fermentation of Organic Waste, storage and applications. Clean. Waste Syst. 2022, 3, 100043. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Zheng, G.; Yang, H.; Li, L. Optimization of Cattle Manure and Food Waste Co-Digestion for Biohydrogen Production in a Mesophilic Semi-Continuous Process. Energies 2020, 13, 3848. [Google Scholar] [CrossRef]
- Gilroyed, B.H.; Li, C.; Hao, X.; Chu, A.; McAllister, T.A. Biohydrogen production from specified risk materials co-digested with cattle manure. Int. J. Hydrogen Energy 2010, 35, 1099–1105. [Google Scholar] [CrossRef]
- Fangkum, A.; Reungsang, A. Biohydrogen production from sugarcane bagasse hydrolysate by elephant dung: Effects of initial pH and substrate concentration. Int. J. Hydrogen Energy 2011, 36, 8687–8696. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Pirozzi, F.; Lens, P.N.; Esposito, G. Continuous biohydrogen production by thermophilic dark fermentation of cheese whey: Use of buffalo manure as buffering agent. Int. J. Hydrogen Energy 2017, 42, 4861–4869. [Google Scholar] [CrossRef]
- Wadjeam, P.; Reungsang, A.; Imai, T.; Plangklang, P. Co-digestion of cassava starch wastewater with buffalo dung for bio-hydrogen production. Int. J. Hydrogen Energy 2019, 44, 14694–14706. [Google Scholar] [CrossRef]
- Perera, K.R.J.; Nirmalakhandan, N. Modeling fermentative hydrogen production from sucrose supplemented with dairy manure. Int. J. Hydrogen Energy 2011, 36, 2102–2110. [Google Scholar] [CrossRef]
- Wu, X.; Lin, H.; Zhu, J. Optimization of continuous hydrogen production from co-fermenting molasses with liquid swine manure in an anaerobic sequencing batch reactor. Bioresour. Technol. 2013, 136, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Wu, X.; Miller, C.; Chen, P.; Ruan, R. Swine manure fermentation for hydrogen production. Bioresour. Technol. 2009, 100, 5472–5477. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Dong, C.; Miller, C.; Li, Y.; Wang, L. Continuous biohydrogen production from liquid swine manure supplemented with glucose using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2009, 34, 6636–6645. [Google Scholar] [CrossRef]
- Kotsopoulos, T.A.; Fotidis, I.A.; Tsolakis, N.; Martzopoulos, G.G. Biohydrogen production from pig slurry in a CSTR reactor system with mixed cultures under hyper-thermophilic temperature (70 C). Biomass Bioenergy 2009, 33, 1168–1174. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, U. Usage of Industrial Waste Products in Village Road Construction. Int. J. Environ. Sci. Dev. 2010, 1, 122. [Google Scholar] [CrossRef]
- Chong, M.L.; Sabaratnam, V.; Shirai, Y.; Hassan, M.A. Biohydrogen production from biomass and industrial wastes by dark fermentation. Int. J. Hydrogen Energy 2009, 34, 3277–3328. [Google Scholar] [CrossRef]
- De Vrije, T.; De Haas, G.G.; Tan, G.B.; Keijsers, E.R.P.; Claassen, P.A.M. Pretreatment of Miscanthus for hydrogen production by Thermotoga elfii. Int. J. Hydrogen Energy 2002, 27, 1381–1390. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Trably, E.; Carrere, H.; Lyberatos, G. Continuous biohydrogen production from a food industry waste: Influence of operational parameters and microbial community analysis. J. Clean. Prod. 2018, 174, 1054–1063. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Moreno, G.; Kumar, G.; Buitrón, G. Biohydrogen production from industrial wastewaters. Water Sci. Technol. 2015, 71, 105–110. [Google Scholar] [CrossRef]
- Moreno-Dávila, I.M.M.; Ríos-González, L.J.; Rodríguez-de la Garza, J.A.; Morales-Martínez, T.K.; Garza-García, Y. Biohydrogen production from paper industry wastes by SSF: A study of the influence of temperature/enzyme loading. Int. J. Hydrogen Energy 2019, 44, 12333–12338. [Google Scholar] [CrossRef]
- Oceguera-Contreras, E.; Aguilar-Juárez, O.; Oseguera-Galindo, D.; Macías-Barragán, J.; Bolaños-Rosales, R.; Mena-Enríquez, M.; Arias-García, A.; Montoya-Buelna, M.; Graciano-Machuca, O.; De León-Rodríguez, A. Biohydrogen production by vermihumus-associated microorganisms using agro industrial wastes as substrate. Int. J. Hydrogen Energy 2019, 44, 9856–9865. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Alvarado-Cuevas, Z.D.; De Leon-Rodriguez, A. Biohydrogen production from mixtures of agro-industrial wastes: Chemometric analysis, optimization and scaling up. Energy 2018, 159, 32–41. [Google Scholar] [CrossRef]
- Lucas, S.D.M.; Peixoto, G.; Mockaitis, G.; Zaiat, M.; Gomes, S.D. Energy recovery from agro-industrial wastewaters through biohydrogen production: Kinetic evaluation and technological feasibility. Renew. Energy 2015, 75, 496–504. [Google Scholar] [CrossRef]
- Gomez-Romero, J.; Gonzalez-Garcia, R.A.; Chairez, I.; Torres, L.; García-Peña, E.I. Continuous two-staged co-digestion process for biohydrogen production from agro-industrial wastes. Int. J. Energy Res. 2016, 40, 257–272. [Google Scholar] [CrossRef]
- Vendruscolo, F. Starch: A potential substrate for biohydrogen production. Int. J. Energy Res. 2015, 39, 293–302. [Google Scholar] [CrossRef]
- Lu, L.; Ren, N.; Xing, D.; Logan, B.E. Hydrogen production with effluent from an ethanol–H2-coproducing fermentation reactor using a single-chamber microbial electrolysis cell. Biosens. Bioelectron. 2009, 24, 3055–3060. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Muthukumar, K. Enzymatic saccharification and fermentation of paper and pulp industry effluent for biohydrogen production. Int. J. Hydrogen Energy 2010, 35, 3389–3400. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Annuar, M.S.M.; Law, S. Effects of different pretreatment methods on anaerobic mixed microflora for hydrogen production and COD reduction from palm oil mill effluent. J. Clean. Prod. 2011, 19, 1654–1658. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chiang, C.C.; Nguyen, M.L.T.; Lay, C.H. Enhancement of fermentative biohydrogen production from textile desizing wastewater via coagulation-pretreatment. Int. J. Hydrogen Energy 2017, 42, 12153–12158. [Google Scholar] [CrossRef]
- Taifor, A.F.; Zakaria, M.R.; Yusoff, M.Z.M.; Toshinari, M.; Hassan, M.A.; Shirai, Y. Elucidating substrate utilization in biohydrogen production from palm oil mill effluent by Escherichia coli. Int. J. Hydrogen Energy 2017, 42, 5812–5819. [Google Scholar] [CrossRef]
- Li, Y.C.; Chu, C.Y.; Wu, S.Y.; Tsai, C.Y.; Wang, C.C.; Hung, C.H.; Lin, C.Y. Feasible pretreatment of textile wastewater for dark fermentative hydrogen production. Int. J. Hydrogen Energy 2012, 37, 15511–15517. [Google Scholar] [CrossRef]
- Ramprakash, B.; Muthukumar, K. Comparative study on the production of biohydrogen from rice mill wastewater. Int. J. Hydrogen Energy 2014, 39, 14613–14621. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Johnson, O.A.; Napiah, M.; Kamaruddin, I. Potential uses of waste sludge in construction industry: A review. Res. J. Appl. Sci. Eng. Technol. 2014, 8, 565–570. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Cai, M.; Liu, J.; Wei, Y. Enhanced biohydrogen production from sewage sludge with alkaline pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Biohydrogen production using waste activated sludge disintegrated by gamma irradiation. Appl. Energy 2015, 155, 434–439. [Google Scholar] [CrossRef]
- Yang, G.; Hu, Y.; Wang, J. Biohydrogen production from co-fermentation of fallen leaves and sewage sludge. Bioresour. Technol. 2019, 285, 121342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Feng, X.; Wang, X.; Huang, J. Biohydrogen production from cornstalk wastes by anaerobic fermentation with activated sludge. Int. J. Hydrogen Energy 2010, 35, 3092–3099. [Google Scholar] [CrossRef]
- Han, W.; Liu, D.N.; Shi, Y.W.; Tang, J.H.; Li, Y.F.; Ren, N.Q. Biohydrogen production from food waste hydrolysate using continuous mixed immobilized sludge reactors. Bioresour. Technol. 2015, 180, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J. Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: Energy conversion and microbial community. Energy Convers. Manag. 2020, 225, 113436. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Enhancing biohydrogen production from waste activated sludge disintegrated by sodium citrate. Fuel 2019, 258, 116177. [Google Scholar] [CrossRef]
- Lin, Y.H.; Zheng, H.X.; Juan, M.L. Biohydrogen production using waste activated sludge as a substrate from fructose-processing wastewater treatment. Process Saf. Environ. Prot. 2012, 90, 221–230. [Google Scholar] [CrossRef]
- Zhu, H.; Béland, M. Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int. J. Hydrogen Energy 2006, 31, 1980–1988. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F. Effects of sludge pre-treatment method on bio-hydrogen production by dark fermentation of waste ground wheat. Int. J. Hydrogen Energy 2009, 34, 8543–8548. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrogen Energy 2008, 33, 2934–2941. [Google Scholar] [CrossRef]
- Luo, G.; Xie, L.; Zou, Z.; Zhou, Q.; Wang, J.Y. Fermentative hydrogen production from cassava stillage by mixed anaerobic microflora: Effects of temperature and pH. Appl. Energy 2010, 87, 3710–3717. [Google Scholar] [CrossRef]
- Tang, G.L.; Huang, J.; Sun, Z.J.; Tang, Q.Q.; Yan, C.H.; Liu, G.Q. Biohydrogen production from cattle wastewater by enriched anaerobic mixed consortia: Influence of fermentation temperature and pH. J. Biosci. Bioeng. 2008, 106, 80–87. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, C.W.; Chu, C.P.; Lee, D.J.; Chang, B.V.; Liao, C.S. Producing hydrogen from wastewater sludge by Clostridium bifermentans. J. Biotechnol. 2003, 102, 83–92. [Google Scholar] [CrossRef]
- Kamalaskar, L.B.; Dhakephalkar, P.K.; Meher, K.K.; Ranade, D.R. High biohydrogen yielding Clostridium sp. DMHC-10 isolated from sludge of distillery waste treatment plant. Int. J. Hydrogen Energy 2010, 35, 10639–10644. [Google Scholar] [CrossRef]
- Shaishav, S.; Singh, R.N.; Satyendra, T. Biohydrogen from algae: Fuel of the future. Int. Res. J. Environ. Sci. 2013, 2, 44–47. [Google Scholar]
- Hafez, H.; Baghchehsaraee, B.; Nakhla, G.; Karamanev, D.; Margaritis, A.; El Naggar, H. Comparative assessment of decoupling of biomass and hydraulic retention times in hydrogen production bioreactors. Int. J. Hydrogen Energy 2009, 34, 7603–7611. [Google Scholar] [CrossRef]
- Hafez, H.; Nakhla, G.; El Naggar, H. An integrated system for hydrogen and methane production during landfill leachate treatment. Int. J. Hydrogen Energy 2010, 35, 5010–5014. [Google Scholar] [CrossRef]
- Elsharnouby, O.; Hafez, H.; Nakhla, G.; El Naggar, M.H. A critical literature review on biohydrogen production by pure cultures. Int. J. Hydrogen Energy 2013, 38, 4945–4966. [Google Scholar] [CrossRef]
- Hafez, H.; Nakhla, G.; El Naggar, H. Biological hydrogen production. In Handbook of Hydrogen Energy; Sherif, S.A., Ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 2012. [Google Scholar]
- Lin, P.; Whang, L.; Wu, Y.; Ren, W.; Hsiao, C.; Li, S.; Chang, J. Biological hydrogen production of the genus Clostridium: Metabolic study and mathematical model simulation. Int. J. Hydrogen Energy 2007, 32, 1728–1735. [Google Scholar] [CrossRef]
- Yokoi, H.; Maki, R.; Hirose, J.; Hayashi, S. Microbial production of hydrogen from starch-manufacturing wastes. Biomass Bioenerg 2002, 22, 389–395. [Google Scholar] [CrossRef]
- Seppälä, J.J.; Puhakka, J.A.; Yli-Harja, O.; Karp, M.T.; Santala, V. Fermentative hydrogen production by Clostridium butyricum and Escherichia coli in pure and cocultures. Int. J. Hydrogen Energy 2011, 36, 10701–10708. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C. Co-culture of Clostridium thermocellum and Clostridium thermosaccharolyticum for enhancing hydrogen production via thermophilic fermentation of cornstalk waste. Int. J. Hydrogen Energy 2012, 37, 10648–10654. [Google Scholar] [CrossRef]
- Liu, F.; Fang, B. Optimization of bio-hydrogen production from biodiesel wastes by Klebsiella pneumoniae. Biotechnol. J. 2007, 2, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Plangklang, P.; Reungsang, A.; Pattra, S. Enhanced biohydrogen production from sugarcane juice by immobilized Clostridium butyricum on sugarcane bagasse. Int. J. Hydrogen Energy 2012, 37, 15525–15532. [Google Scholar] [CrossRef]
- Wang, A.; Ren, N.; Shi, Y.; Lee, D. Bioaugmented hydrogen production from microcrystalline cellulose using cocultured Clostridium acetobutylicum and Ethanoigenens harbinense. Int. J. Hydrogen Energy 2008, 33, 912–917. [Google Scholar] [CrossRef]
- Cappelletti, B.M.; Reginatto, V.; Amante, E.R.; Antonio, R.V. Fermentative production of hydrogen from cassava processing wastewater by Clostridium acetobutylicum. Renew. Energy 2011, 36, 3367–3372. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process Biochem. 2000, 35, 589–593. [Google Scholar] [CrossRef]
- Mars, A.E.; Veuskens, T.; Budde, M.A.; Van Doeveren, P.F.; Lips, S.J.; Bakker, R.R.; de Vrije, T.; Claassen, P.A. Biohydrogen production from untreated and hydrolyzed potato steam peels by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy 2010, 35, 7730–7737. [Google Scholar] [CrossRef]
- Manish, S.; Banerjee, R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Benemann, J.R. Biological hydrogen production: Fundamentals and limiting processes. Int. J. Hydrogen. Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.; Leung, M.K.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Saifuddin, N.; Priatharsini, P. Developments in bio-hydrogen production from algae: A review. Res. J. Appl. Sci. Eng. Technol. 2016, 12, 968–982. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Yu, J.; Takahashi, P. Biophotolysis-based hydrogen production by cyanobacteria and green microalgae. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 79–89. [Google Scholar]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production; Academic Press: Cambridge, MA, USA, 2019; pp. 45–83. [Google Scholar]
- Kossalbayev, B.D.; Tomo, T.; Zayadan, B.K.; Sadvakasova, A.K.; Bolatkhan, K.; Alwasel, S.; Allakhverdiev, S.I. Determination of the potential of cyanobacterial strains for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 2627–2639. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Shahavi, M.H.; Neshat, S.A. Assessment of biological hydrogen production processes: A review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2016; Volume 36, p. 012068. [Google Scholar] [CrossRef]
- Kothari, R.; Tyagi, V.V.; Pathak, A. Renewable and sustainable energy reviews. Renew. Sustain. Energ. Rev. 2010, 14, 1744–1751. [Google Scholar]
- Benemann, J.R. Feasibility analysis of photobiological hydrogen production. Int. J. Hydrogen Energy 1997, 22, 979–987. [Google Scholar] [CrossRef]
- Sen, U.; Shakdwipee, M.; Banerjee, R. Status of biological hydrogen production. J. Sci. Ind. Res. 2008, 67, 980–993. [Google Scholar]
- Sarangi, P.K.; Nanda, S. Biohydrogen production through dark fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Saratale, G.D.; Chen, S.D.; Lo, Y.C.; Saratale, R.G.; Chang, J.S. Outlook of biohydrogen production from lignocellulosic feedstock using dark fermentation–a review. J. Sci. Ind. Res. 2008, 67, 11. [Google Scholar]
- Lee, D.J.; Show, K.Y.; Su, A. Dark fermentation on biohydrogen production: Pure culture. Bioresour. Technol. 2011, 102, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Malhotra, B.D.; Gupta, V.K.; Ramteke, P.W.; Silva, R.N.; Shukla, P.; Dubey, K.K.; Mishra, P.K. Nanoengineered cellulosic biohydrogen production via dark fermentation: A novel approach. Biotechnol. Adv. 2019, 37, 107384. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Venegas, E.; Ramirez-Morales, J.E.; Silva-Illanes, F.; Toledo-Alarcón, J.; Paillet, F.; Escudie, R.; Lay, C.H.; Chu, C.Y.; Leu, H.J.; Marone, A. Biohydrogen production by dark fermentation: Scaling-up and technologies integration for a sustainable system. Rev. Environ. Sci. Bio/Technol. 2015, 14, 761–785. [Google Scholar] [CrossRef]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Jahim, J.M. Biohydrogen production through photo fermentation or dark fermentation using waste as a substrate: Overview, economics, and future prospects of hydrogen usage. Biofuels Bioprod. Biorefining 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Khanna, N.; Das, D. Biohydrogen production by dark fermentation. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 401–421. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: An overview. Int. J. Hydrogen Energy 2011, 36, 7443–7459. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Role of chemicals addition in affecting biohydrogen production through photofermentation. Energy Convers. Manag. 2018, 165, 509–527. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo-fermentation of lignocellulosic biomass. Biomass Convers. Biorefinery 2020, 1–19. [Google Scholar] [CrossRef]
- Sampath, P.; Reddy, K.R.; Reddy, C.V.; Shetti, N.P.; Kulkarni, R.V.; Raghu, A.V. Biohydrogen production from organic waste–a review. Chem. Eng. Technol. 2020, 43, 1240–1248. [Google Scholar] [CrossRef]
- Adessi, A.; De Philippis, R. Hydrogen production: Photofermentation. In Microbial Technologies in Advanced Biofuels Production; Springer: Boston, MA, USA, 2012; pp. 53–75. [Google Scholar]
- Androga, D.D.; Özgür, E.; Eroglu, I.; Gündüz, U.; Yücel, M. Photofermentative hydrogen production in outdoor conditions. In Hydrogen Energy—Challenges and Perspectives; In Tech, 2012; pp. 77–120. [Google Scholar]
- Keskin, T.; Abo-Hashesh, M.; Hallenbeck, P.C. Photofermentative hydrogen production from wastes. Bioresour. Technol. 2011, 102, 8557–8568. [Google Scholar] [CrossRef]
- Tian, H.; Li, J.; Yan, M.; Tong, Y.W.; Wang, C.H.; Wang, X. Organic waste to biohydrogen: A critical review from technological development and environmental impact analysis perspective. Appl. Energy 2019, 256, 113961. [Google Scholar] [CrossRef]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefin. 2020, 1–19. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Fidalgo, B. Production of bio-syngas and bio-hydrogen via gasification. In Handbook of Biofuels Production, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–494. [Google Scholar]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen production from the thermochemical conversion of biomass: Issues and challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Ding, C.; Yang, K.L.; He, J. Biological and fermentative production of hydrogen. In Handbook of Biofuels Production, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 303–333. [Google Scholar]
- Dincer, I. Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 1–40. [Google Scholar]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen production from phototrophic microorganisms: Reality and perspectives. Int. J. Hydrogen Energy 2019, 44, 5799–5811. [Google Scholar] [CrossRef]
- Melitos, G.; Voulkopoulos, X.; Zabaniotou, A. Waste to Sustainable Biohydrogen Production Via Photo-Fermentation and Biophotolysis−A Systematic Review. Renew. Energy Environ. Sustain. 2021, 6, 45. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen production from biomass sources: Metabolic pathways and economic analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Pandu, K.; Joseph, S. Comparisons and limitations of biohydrogen production processes: A review. Int. J. Adv. Eng. Technol. 2012, 2, 342. [Google Scholar]
- Kasipandian, K.; Saigeetha, S.; Samrot, A.V.; Abirami, S.; Renitta, R.E.; Dhiva, S. Bioelectricity Production Using Microbial Fuel Cell—A review. Biointerface Res. Appl. Chem. 2021, 11, 9420–9431. [Google Scholar]
- Rahman, S.N.A.; Masdar, M.S.; Rosli, M.I.; Majlan, E.H.; Husaini, T. Overview of biohydrogen production technologies and application in fuel cell. Am. J. Chem. 2015, 5, 13–23. [Google Scholar]
- Wei, J.; Liu, Z.T.; Zhang, X. Biohydrogen production from starch wastewater and application in fuel cell. Int. J. Hydrogen Energy 2010, 35, 2949–2952. [Google Scholar] [CrossRef]
- Koroglu, E.O.; Ozdemir, O.K.; Ozkaya, B.; Demir, A. An integrated system development including PEM fuel cell/biogas purification during acidogenic biohydrogen production from dairy wastewater. Int. J. Hydrogen Energy 2019, 44, 17297–17303. [Google Scholar] [CrossRef]
- Chader, S.; Mahmah, B.; Chetehouna, K.; Amrouche, F.; Abdeladim, K. Biohydrogen production using green microalgae as an approach to operate a small proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2011, 36, 4089–4093. [Google Scholar] [CrossRef]
- Oncel, S.; Vardar-Sukan, F. Application of proton exchange membrane fuel cells for the monitoring and direct usage of biohydrogen produced by Chlamydomonas reinhardtii. J. Power Sources 2011, 196, 46–53. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Guo, H. Characterization of H 2 photoproduction by marine green alga Tetraselmis subcordiformis integrated with an alkaline fuel cell. Biotechnol. Lett. 2016, 38, 435–440. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Guerrero-Barajas, C.; Ramirez, D.; Arriaga-Hurtado, L.G. Semi-continuous biohydrogen production as an approach to generate electricity. Bioresour. Technol. 2009, 100, 6369–6377. [Google Scholar] [CrossRef]
- Oh, S.; Logan, B.E. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005, 39, 4673–4682. [Google Scholar] [CrossRef]
- Varanasi, J.L.; Veerubhotla, R.; Pandit, S.; Das, D. Biohydrogen production using microbial electrolysis cell: Recent advances and future prospects. Microb. Electrochem. Technol. 2019, 843–869. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).