Abstract
We first revealed the behavior and biochemical mechanism of high iron attapulgite (HIAP) and different dosages affecting sewage sludge (SS) composting. HS/TOC ratio increased, but HA/TOC and HA/FA ratios reduced with the increase in HIAP dose. High-dose HIAP promoted the formation of more HS by weak catalytic effect but could bind more FA than HA by strong adsorption effect to inhibit the polymerization of the adsorbed FA into HA. Mixing SS with HIAP and subsequent composting as two consecutive processes during HIAP-amended composting significantly influenced the species distribution of heavy metals (HMs) Cu, Zn, and Cr. Each process roughly contributed one-half to HMs passivation. The bioavailable fraction (BF) of HMs reduced with the increase of HIAP dose. HIAP dose greatly affected the microbial community. Both 1% and 5% HIAP treatments promoted Proteobacteria and Firmicutes, but 10% HIAP promoted Actinobacteriota and Bacteroidota. At the thermophilic phase, HIAP dose greatly affected core thermophilic microbial genera, which were significantly correlated to pile temperature and pH value. In the maturity stage, core microbial genera in different treatments were basically similar and closely correlated to the bioavailable fraction (BF) of HMs and HA, and the influence order was BF–Cr > BF–Cu > BF–Zn > HA. The optimal 5% HIAP dose was recommended.
1. Introduction
According to the “Chinese Statistical Yearbook of Urban and Rural Development-2022”, a total of 4602 sewage treatment plants were built and operated in cities and counties in 2021, with a daily sewage treatment capacity of 248 million cubic meters, the annual production of sewage sludge (SS) exceeds 70 million tons. It is expected that the annual production of SS will exceed 90 million tons by 2025 in China. Global sludge production is growing at an average annual rate of 4.6% [1]. Some advanced treatment and disposal technologies have been applied to SS resource recycling in recent years, including incineration power generation, anaerobic digestion, aerobic composting, pyrolysis carbonization, hydrothermal technology, etc. [2]. Among them, aerobic composting is regarded as an inexpensive, easily practical, safe, and sustainable strategy for recycling SS as soil amendments and fertilizers [3,4]. However, traditional aerobic composting is not very effective in the humification and passivation of heavy metals (HMs). The application of suitable biotic and abiotic additives or catalysts [5,6], including microbial inoculants, biochar, metal oxides, mineral materials, and combination additives, to aerobic composting of different biosolid wastes for promoting composting process, has quickly attracted the interest of researchers in recent years.
Attapulgite (AP) is a hydrous magnesium-aluminum silicate mineral with a chain-layered structure, large surface area, and excellent cation exchange capacity, which has the characteristics of low cost, good biology, and soil compatibility in soil application. AP has been demonstrated as a promising amendment to reduce the phytotoxicity of heavy metals (HMs) for direct SS soil utilization [7,8] and as a promising additive to reduce the bioavailability of HMs [9,10,11] and the emission of greenhouse gases [12,13], enhance the degradation of organic pollutants [10,13], promote composting process [14] during the composting of different biosolid wastes. To our best knowledge, research on the effect of AP and its dosage on SS composting humification is hitherto rare based on the heterogeneity of the SS composting matrix. The reported AP is a good passivator for heavy metals in direct soil utilization of sludge or sludge compost products, and the passivation capacity increases with the increase of AP dose [8,11]. However, during an AP-amended SS composting, first mixing SS with AP and subsequent composting are two consecutive processes, but few works have been conducted to clarify the speciation evolution of HMs as well as the contribution to passivating HMs for the two consecutive processes. Iron-bearing AP can catalyze the degradation of Rhodamine-B by heterogeneous Fenton reaction [15]. Hydroxyl radicals produced by magnetite additives can accelerate the degradation of organic matter (OM) and the formation of humus in sludge composting [16]. A high iron attapulgite (HIAP) maybe contributes more to the humification, HMs passivation, and influencing microbial community during HIAP-amended SS composting.
Therefore, the objectives of this study were as follows: (1) to comparatively investigate the effect of HIAP dosage on the humification and HMs passivation during SS composting; (2) to highlight the evolution of microbial community with HIAP dose; and (3) to reveal the potentially biochemical mechanism of HIAP dosage affecting SS composting. This study is not only helpful in understanding the behavior and mechanism of HIAP and its dose affecting SS composting but is also beneficial in selecting the appropriate HIAP dose to promote the HIAP-amended SS composting process.
2. Materials and Methods
2.1. Composting Materials and Trials
Dewatered SS was collected from Xinkaipu municipal wastewater treatment plant (Changsha, China). The waste mushroom residue (WMR) and sawdust were chosen as conditioners. HIAP, which contained 7.20% Fe2O3 and 0.12% MnO, was purchased from Attapulgite Mineral Limited Company (Lingshou, China). WMR and sawdust were crushed in a mill and sieved through a 2 mm mesh. Raw materials of SS, WMR, and sawdust were fully blended at a weight ratio of 6:4:1 to regulate the carbon/nitrogen ratio (C/N) of the mix feedstocks to around 25.8:1. Then, HIAP power was added at the ratio (w/w) of 1%, 5%, and 10% [9,13] into the feedstocks and then mixed for one hour each, and the initial moisture contents (MC) of composting piles were all regulated to approximate 65.0%. The four composting experiments in 50 L stainless steel solid fermentation tanks were simultaneously carried out, denoted CK (the control, no additive), T1 (1% HIAP), T2 (5% HIAP), and T3 (10% HIAP). Each pile was regularly turned and sampled. The collected sample was split into three parts. Part one, as a fresh sample, was used to measure the pH value. Part two was freeze-dried, ground, sieved (0.1 mm sieve), and stored at 4 °C to detect humic substances (HS), humic acid (HA), and fulvic acid (FA). Part three was stored at −80 °C for DNA extraction and sequencing analysis.
2.2. Analysis of Physiochemical Properties
The pH of extracting solution from the sample (1:10, w/V) was analyzed by pH meter (Phs-3C, Leici, Shanghai, China). The extracted Zn, Cu, and Cr by the BCR method [17] from a 0.5 g dried sample were determined by inductively coupled plasma (ICP-OES) (ICAP 7000, Singapore). Total carbon (TOC) was detected by a TOC analyzer (SHIMADZUTOC-LCPH/CPH, Kyoto, Japan). Total nitrogen (TN), total phosphorus (TP), and total potassium (TK) were measured as described by standard methods [18]. HS, FA, and HA were separated, extracted, and detected according to the reported method [18], expressed in TOC form. The humification ratio (HR), humification index (HI), percentage of humic acids (PHA), and degree of polymerization (DP) were calculated [19,20]. All tests were three replicates.
2.3. DNA Extraction, High-Throughput Sequencing, and Data Analysis
According to the manufacturer’s instructions, a PowerSoil DNA Isolation Kit (MO BIO, Carlsbad, CA, USA) was utilized to isolate DNA from the composting sample. The microbial community was assessed by the Illumina Miseq PE300 platform (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China) through the amplification of V3–V4 region of bacterial 16S rRNA using 515F/907 R (5′GTGCCAGCMGCCGCGG-3′/5′CCGTCAATTCMTTTRAGTTT-3′). The high-throughput sequencing data were filtered, modified, improved, and variously analyzed according to our earlier work [21].
3. Results and Discussion
3.1. Effect of HIAP Dosage on SS Composting Humification
3.1.1. Variations of Temperature and pH during Composting
As seen in Figure 1A, all composting temperatures rapidly increased within two days and reached the maximum values of 62.1 °C, 62.6 °C, 62.7 °C, and 61.6 °C for the control, 1%, 5%, and 10% HIAP treatments, respectively. The phenomenon of a rapid rise in pile temperature to the maximum value is very consistent with similar substrate composting without HIAP addition [22,23]. The thermophilic phases (≥55 °C) of four treatments have been maintained for more than four days, which were sufficient to meet the standard hygienic requirement for the destruction of pathogens and weed seeds [13,24]. Compared with the control, HIAP treatment slightly extends the cooling phases (55 °C > T > 35 °C), which is consistent with palygorskite (PG)-amended SS composting [13]. In the middle and late stages, T1 and T2 piles than CK and T3 piles had higher pile temperature, and T3 pile than CK pile had slightly lower pile temperature, which hints that high dosage HIAP likely influenced microbial activity and metabolism [9] through a varying core microbial community in the composting [25]. A detailed discussion is shown in the microbial community analysis below.
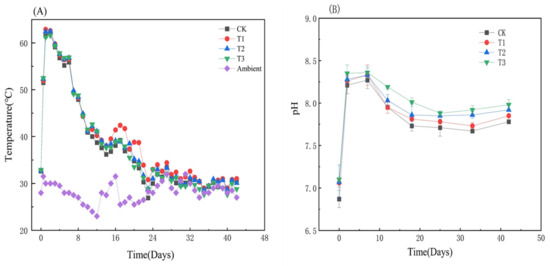
Figure 1.
Variations in temperature and pH with HIAP dose during composting. (A) The change of temperature with composting time; (B) The change of pH with composting time.
As seen in Figure 1B, the initial pH of three HIAP-amended piles was between 7.06 and 7.10, which was higher than the 6.87 of the control due to the weak alkalinity of HIAP powder [11]. The pH values of CK, T1, T2, and T3 all rapidly increase and reach their peak values on the 2nd day, which are 8.27, 8.33, 8.33, and 8.36, respectively, and then gradually decrease from day 2 to day 42. The initially rapid rise in pH is caused by the production of ammonia and the degradation of organic acids, whereas the subsequent decrease is attributed to ammonia volatilization and organic matter (OM) decomposition into low molecular organic acids [13,22,26] and also possibly due to the release of H+ during nitrification process [24]. The pH values in HIAP-amended piles are greater than that of the control pile due to the nitrogen retention function of HIAP [12,13] and alkaline HIAP neutralizing a portion of H+ and promoting OM biodegradation [11].
3.1.2. Variations of TOC, HS, HA, FA, and Humification Indexes with HIAP Dosage
As presented in Figure 2A, the initial TOC levels of CK, T1, T2, and T3 were 433.7, 425.8, 396.8, and 366.0 g/kg, respectively. Different HIAP dosages and negligible OM content in HIAP resulted in their initial difference. Generally, TOC loss reflects the mineralization of OM into carbon dioxide during composting [19,27,28]. The TOC contents in four treatments gradually decrease along the composting time due to OM decomposition, gases release, and ventilation [21,28]. OM mineralization mainly occurs at the thermal phase due to the rapid decrease of TOC and is very slow in the maturity stage due to a slight reduction of OM. At the end of composting, the TOC levels of CK, T1, T2, and T3 were 367.1, 345.5, 326.6, 311.5 g/kg, and corresponding TOC loss rates were 15.4%, 18.9%, 17.7%, 14.9%, respectively. Obviously, the OM mineralization decreased with the increase of HIAP dosage, which is very consistent with the findings in PG-amended SS composting [13]. Both 1% and 5% HIAP treatments accelerate OM mineralization, whereas 10% HIAP treatment slightly slows it down. This probably is due to the fact that high dosage HIAP reduces microbial activity and metabolism [9] by varying core bacterial community [25] and has more space to absorb OM, which is not easy to be mineralized [19]. The result implies that low-dose HIAP exhibited a stronger catalytic effect to promote OM degradation, especially in the thermophilic stage, while high-dose HIAP exhibited a stronger adsorption effect to inhibit OM degradation, especially in the maturity stage.
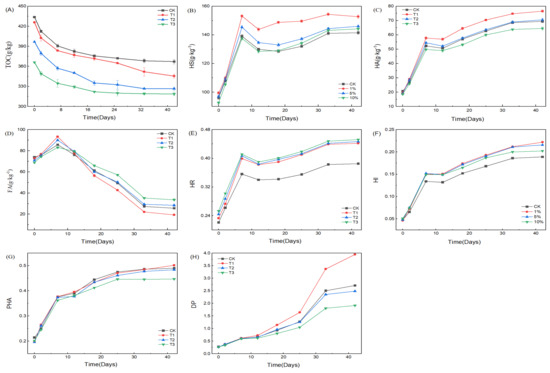
Figure 2.
Variations in TOC, HS, HA, and FA and humification indexes during composting. (A) The change of TOC with composting time; (B) The change of HS with composting time; (C) The change of HA with composting time; (D) The change of FA with composting time; (E) The change of HR with composting time; (F) The change of HI with composting time; (G) The change of PHA with composting time; (H) The change of DP with composting time.
Composting is a complicated biochemical process to form a humus-like compost product [29,30]. HA and FA are the dominant components of HS, reflecting the humification of biosolid waste composting [31,32]. FA can be utilized as an energetic source by microbes and can also be transformed into HA via condensation and polymerization reactions [33,34]. As seen from Figure 2B–D, the HS and HA in the four treatments rapidly increased at the thermophilic phase (>50 °C), slightly decreased in the cooling stage, and slowly increased until the end of composting. During composting, HS precursors and HS-like are mainly formed in the heating and thermophilic phases, but HS and FA are polymerized in the cooling and mature phases [34]. The change trends of HS and HA along composting time are similar to most of the previous works in the composting of other biosolid wastes [19,20,34]. However, the FA slightly increased at the thermophilic phase and then decreased continuously until the end of composting. Carbonated basic oxygen furnace steel slag accelerates the humification process of wood waste composting through the formation of humic-like acids [35]. MnO2 additive can improve the formation of highly humified components from simple components, which is beneficial for slowing down the degradation of FA products in the composting of chicken manure and rice straw [25]. Hyperthermophilic pretreatment composting significantly accelerates HS formation in the composting of pig manure and rice straw [20]. AP containing Fe can induce a Fenton reaction to produce hydroxyl radicals in the presence of O2 and catalyze OM degradation [15]. Hydroxyl radicals produced by magnetite additives can accelerate the degradation of organic matter (OM) and the formation of humus in sludge composting [16]. The organic substances in municipal SS are mainly microbial cells and extracellular polymers (EPS). Thus, the slight increase of FA at the thermophilic phase likely was caused by the rapid pyrolysis of microbial cells and EPS in the SS [36] and the rapid putrefaction of higher content WMR into HS [22] under the strong catalysis of HIAP. The changes of HS and HA with composting time indicate that the formation of more HS or HS-like mainly came from the catalytic cracking of organic macromolecule matter at the thermophilic phase, while in the maturity stage, HS-like or FA also was slowly converted into HA to obtain a stable compost product.
In terms of the effect of HIAP dosage on SS composting humification, it appeared to be very complex. During whole composting, the HS contents in three HIAP treatments in Figure 2B always are higher than that of the control and decrease as the HIAP dosage increase. The HA content in Figure 2C shows in the following order: 1% > 5% > CK > 10% HIAP treatment, but the FA content in Figure 2D showed in the following order: 1% > 5% > CK > 10% HIAP treatment at the thermophilic phase, 10% > 5% > CK > 1% HIAP treatment at the cooling and maturity stage phases. It seemed very difficult to find the law of HIAP dosage influencing HA and FA. During PG-amended chicken manure/corn stover composting [14], the change of HA and FA contents along the composting time always show in the following order: CK > 5% > 10% PG treatment. In terms of the effect of HIAP dose on HA and FA, the HA during whole SS composting and the FA at the thermophilic phase were similar to the reported results [14]. In this study, the HA and FA are much higher than the reported results [14], high-dose HIAP has a positive impact on FA, and HS and HA contents always are higher than those of the control (CK). These differences are likely related to the heterogeneity of composting substrate between SS and chicken manure and the greater catalytic effect of HIAP than normal PG to form lots of HS-like substances.
The humification ratio (HR) and humification index (HI) represent the conversion degree of OM into HS and HA, respectively, and the percentage of humic acid (PHA) and degree of polymerization (DP) represent the polymerization degree of FA into HA during composting process [20]. As presented in Figure 2E–H, HR and HI indexes rapidly increased in the thermophilic stage, slightly decreased in the cooling stage, and then continuously increased until the end of composting, were higher than those of the control. It clearly demonstrates that complex macromolecular OM is rapidly mineralized into HS or HS-like in the thermophilic stage, and at the same time, FA is slowly polymerized into HA in the maturity stages [20]. Surprisingly, the effect of HIAP dosage on HR, HI, PHA, and DP indexes became very clear, showing a very regular change rule. HR index increases as the HIAP dose increases, and it is always higher than that of the control. It indicates that high-dose HIAP promoted the formation of more HS or HS-like [31,37]. However, both HI and PHA indexes decrease with the increase in HIAP dose, and the HI index is always higher than that of the control, whereas the PHA is basically lower than that of the control. The result indicates that high-dose HIAP promoted the formation of more FA rather than HA and also confirmed his findings that the catalytic oxidation of additive is beneficial for slowing down the degradation of FA products in chicken manure composting due to the formation of more FA [25]. HI and DP are the most suitable indexes for characterizing the humification degree [19]. Compared with the control, the DP index, as shown in Figure 2H, clearly showed that 10% HIAP inhibits, but 1% HIAP promotes the polymerization of FA into HA, and the polymerization degree of FA into HA became weaker with the increase of HIAP dose. Awasthi et al. [28] found that the HA/FA ratio in the composting of SS and wheat straw increased with the addition of Ca-bentonite, which is different from our result. This likely was because HIAP than Ca-bentonite had a greater catalytic effect in the thermophilic stage. It also confirmed that Fe in clay minerals than Al and Si elements could contribute more to humification [38]. Pan et al. [19] reported that the thermally treated montmorillonite (M-) and illite (I-) than natural montmorillonite (M) and illite (I) have a lower increase rate in humus content that the absorbed OM does not to be mineralized during composting, and demonstrated that M- and I-treatments promote HS precursors to synthesize HA by coordinated regulation of biotic pathway and abiotic pathway, the increase of HA in the M and I treatments mainly through the abiotic pathway [6,19], which indicated that the catalytic effect of thermally treated montmorillonite and illite become weaker. HIAP easily adsorbed, and ions exchanged small molecular OM such as FA to disrupt the chemical equilibrium, thereby enhancing the formation of HS by the catalytic oxidation of HIAP, especially at the high-temperature phase. Low-dose HIAP is absorbed on macromolecular OM to form more HS-like through catalyzing degradation [31]. High-dose rather than low-dose HIAP could bind more small molecular FA by ion exchange and adsorption, resulting in the bound FA to be not easily polymerized into HA [19], and macromolecule OM also was not easily mineralized into HS or HS-like [19]. In other words, the adsorption effect of high-dose HIAP was greater than its catalytic effect. Because HA only adsorbed on the outer surface of HIAP [39], small-molecule FA easily entered the pores of HIAP and clogged the pores [40], resulting in the adsorbed FA not easily oxidized due to the difficult entry of oxygen. So, low-dose HIAP mainly exhibited a catalytic effect, whereas high-dose HIAP significantly exhibited an adsorption effect. In fact, during the reported PG or AP-amended SS composting, PG or AP can reduce the emission of greenhouse gases such as NH3, CH4, and N2O [12,13], and the reduction rate increase with PG dose [13]. These reported results have already hinted that a high dose of AP or PG was manifested as an adsorption effect because if a high dose of AP or PG was manifested as a stronger catalytic effect, more carbon dioxide and nitrogen oxides should be released with the increase of AP or PG dose. These previous results indirectly confirmed our findings that low-dose HIAP was greatly manifested as a catalytic effect, while high-dose HIAP was simultaneously exhibited as a weaker catalytic effect and a stronger adsorption effect.
At the end of composting, DP values were 2.71, 3.96, 2.49, and 1.91 for the control, and 1%, 5%, and 10% for the HIAP treatments, respectively, were higher than 1.9, indicating the maturity and stability of composting [20,41]. In final compost products, HS contents were 141.5, 152.7, 146.0, and 144.2 g/kg, HA contents were 69.4, 76.6, 70.6, and 64.5 g/kg, FA contents were 25.6, 19.4, 28.4, and 33.7 g/kg, total sums of HA and FA contents were 95.0, 96.0, 99.0, and 98.2 g/kg for the control, and 1%, 5%, and 10% for the HIAP treatments. Here, the values of HS and (HA+FA) were higher than those of the control, and the value of (HA+FA) increased as the HIAP dose increased and 10% HIAP treatment has basically tended to a stable (HA+FA) value, which showed that HIAP promoted the formation of more HS. The HS was higher than the (HA+FA) due to the presence of HS-like [25,38] besides HA and FA [6,14]. The HS and HA were reduced, but the FA increased with the increase of HIAP dose because high-dose HIAP could bind more FA than HA [39], and the adsorbed FA was difficultly polymerized or converted into HA, especially in the maturity stage.
Summarily, HS/TOC ratio increased, but HA/TOC and HA/FA ratios reduced with the increase in the HIAP dose. HIAP promoted the degradation of OM to form more HS. In the thermophilic stage, low-dose HIAP mainly exhibited a catalytic effect, but high-dose HIAP simultaneously manifested weaker catalytic and stronger adsorption effects. In the maturity stage, high-dose HIAP manifested a very strong adsorption effect to bind more FA and inhibited the polymerization of the adsorbed FA into HA.
3.2. Effect of HIAP Dose on Passivating HMs during Composting Process
The BCR extraction scheme classifies HM species into four forms [14]: (A) exchangeable fraction (Exc.) bound by carbonates, (B) reducible fraction (Red.) bound by amorphous Fe and Mn oxides and hydroxides, (C) oxidizable fraction (Oxi.) bound by organic matter and sulfides, and (D) residual fraction (Res.) embedded in the crystal lattice of the SS [41,42]. The Exc. and Red. forms are the bioavailability fraction (BF), and the Oxi. and Res. forms are the passivation fraction in compost [43]. Figure 3 illustrates the percentage distribution of Exc., Red., Oxi., and Res. fractions of Cu, Zn, and Cr during HIAP-amended SS composting. Obviously, adding HIAP to the mixture of raw materials and subsequent composting increased the Oxi. and Res. fractions while reducing the Exc. and Red. fractions, and the un-bioavailability fraction increased as the HIAP dose increased. However, direct treatments of the mixed raw materials with different HIAP doses prior to the composting, corresponding to samples D0T1, D0T2, and D0T3, significantly increased the Oxi. fractions and slightly increased the Res. fractions of Cu, Zn, and Cr in comparison to D0CK. Compared with D0T1, D0T2, and D0T3 samples, subsequent composting significantly increased the Res–Cu, Oxi–Cr, and slightly increased Oxi–Zn and Res–Zn. Total percentages of the Oxi. and Res. fractions of Cu, Zn, and Cr were 59.3%, 54.1%, and 78.0% in the D0CK sample, and 76.4%, 68.0%, and 93.3% in the D42CK sample, and 69.3%, 69.3%, and 88.0% in D0T1 sample, and 81.0%, 77.1%, and 97.1% in the D42T1 sample, respectively. The sum increments of the Oxi. and Res. fractions of Cu, Zn, and Cr were 17.1%, 13.9%, and 15.3% between D42CK and D0CK, and 10.0%, 15.2%, and 10.0% between D0T1 and D0CK, and 21.7%, 23.0%, 19.1% between D42T1 and D0CK, and 11.7%, 7.8%, 9.1% between D42T1 and D0T1, respectively. Thus, the Oxi. and Res. fractions enhanced with HIAP doses, the HM passivation efficiency was Cu > Cr > Zn for direct SS composting, and Zn > Cu ≈ Cr for direct treating SS by HIAP, and Zn > Cu > Cr for next HIAP-amended composting, but composting enhanced Oxi–Cr. Each process roughly contributed one-half to HMs passivation, and the first than the second process had a greater passivation effect on zinc.
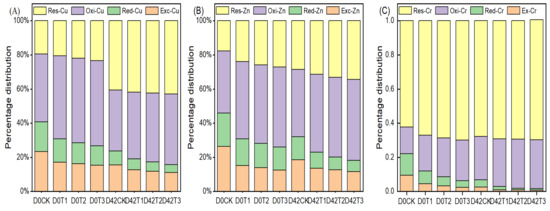
Figure 3.
Variations of HM fractions during composting. (A) Cu; (B) Zn; (C) Cr.
3.3. Succession of Microbial Community during Composting
3.3.1. Effect of HIAP Dosage on Microbial Community
As seen in Figure 4a, the top five dominant phyla in the whole composting process were Actinobacteriota, Proteobacteria, Firmicutes, Bacteroidota, and Chloroflexi, and their relative abundances were 16%~55%, 13%~49%, 15%~31%, 4%~18%, and 1%-7%, respectively. Dominant phyla were consistent with many previous studies on biosolid waste composting [21,44,45]. The relative abundances of Proteobacteria and Firmicutes basically increased, whereas Actinobacteriota decreased with composting time for the control, 1% (T1), and 5% (T2) HIAP treatments. However, 10% (T3) HIAP treatment did not show a similar change rule. Actinobacteriota and Bacteroidota significantly increased with composting time. Composting obviously had an inhibition for Actinobacteriota, but the 10% HIAP additive obviously had a protective effect on Actinobacteriota.
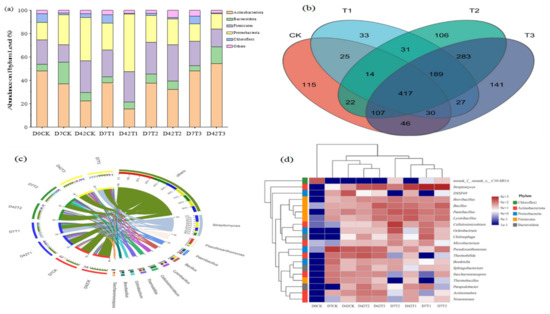
Figure 4.
Microbial community composition at phylum and genus levels. (a) At phylum level; (b) Veen diagram of OUT; (c) At genus level; (d) Heatmap at genus level.
Figure 4b shows the similarity and overlap on the genera level in CK, T1, T2, and T3 treatments. The OTU statistics of CK, T1, T2, and T3 groups are 776, 766, 1169, and 1240, respectively. With the increase of the HIAP dose, the abundance of microorganisms increases. There are 486 genera shared between CK and T1, 560 genera shared between CK and T2, 600 genera shared between CK and T3, 651 genera shared between T1 and T2, 996 genera shared between T2 and T3, and 417 genera shared among CK, T1, T2, and T3. Obviously, CK and T1 have a more similar community composition, while T2 and T3 have more similar community composition, and four composting treatments share more than one-third genera.
As seen in Figure 4c, the top 10 genera are Streptomyces, Pseudoxanthimonas, Paenibacillus, Bacillus, Lysinibacillus, Cellulosimicrobium, Thermobifida, Ochrobactrum, Brevibacillus, and Saccharomonospora, which are significantly influenced by composting temperature and HIAP dosage. As seen in Figure 4d, the top 20 genera are mainly grouped into two clusters except for Norank, Streptomyces, and DSSF69. Streptomyces are WMR-degrading microorganisms [46]. Cluster I is composed of genera Paenibacillus, Bacillus, Brevibacillus, Lysinibacillus (Firmicutes phylum), Cellulosimicrobium, Microbacterium (Actinobacteriota phylum), Ochrobactrum (Proteobacteria phylum), and Chitinophaga (Bacteroidota phylum), which mineralizes and degrades OM [34,45]. Cluster II includes genera Pseudoxanthimonas, Bordetella (Proteobacteria phylum), Thermobifida, Saccharomonospora, Actinomadura, Nonomuraea (Actinobacteriota phylum), Sphingobacterium, Parapedobacter (Bacteroidota phylum), and Thermobacillus (Firmicutes phylum), which degrades high-molecular-weight compounds and recalcitrant compounds [34,45]. Bacteroidota played a vital role in the mineralization and decomposition of lignocellulose in the cooling and maturity stages [47]. The top three genera are Pseudoxanthimonas, Thermobifida, and Bordetella in the D7CK sample, Chitinophaga, Ochrobactrum, and Cellulosimicrobium in the D7T1 sample, Cellulosimicrobium, Ochrobactrum, and Paenibacillus in the D7T2 sample, and Bacillus, Pseudoxanthimonas, and Lysinibacillus in the D7T3 sample. These genera, Cellulosimicrobium, Ochrobactrum, Bacillus, Pseudoxanthimonas, etc., degraded cellulose in the thermophilic and cooling stages [14]. Ochrobactrum, Chitinophaga, and Parapedobacter genera in the D7T1 and D7T2 samples than the D7CK sample have very higher relative abundance. However, Thermobifida, Thermobacillus, and Saccharomonospora genera in the D7CK sample than in the D7T1 and D7T2 samples have higher relative abundance, which participates in the formation of HA in composting [48]. Before composting (D0CK sample), the abundances of the genera above were relatively low, and during composting, their abundances became high. The results indicated that composting and HIAP promoted the growth of some core thermophilic bacteria, such as Streptomyces in all HIAP treatment groups. In the maturity stage, Pseudoxanthimona, Thermobifida, and Actinomadura were dominant genera in the final composts of four treatments (D42 samples), and Parapedobacter had higher relative abundances in the D42T1 and D42T2 samples than the D42T3 and D42CK samples, these genera converted recalcitrant lignocellulose to humus [34,45,49]. So, HIAP dosage significantly influenced the structure of the core thermophilic microbial community of degrading and converting WMR and cellulose [25] during composting.
3.3.2. Relationship between Microbial Community and Environmental Factors
Figure 5a,b illustrates the relationship between microbial community and environmental factors. As depicted in Figure 5a, Nonomuraea and Actinomadura, as biological indicators of compost maturity [22], are significantly negatively correlated with composting temperature because their abundance increased with compost maturity. Thermobifida, Thermobacillus, Pseudoxanthimonas, and Parapedobacter mainly occurred in the maturity stage also were negatively correlated with pile temperature. Cellulosimicrobium, Ochrobactrum, Chitinophaga, Brevibacillus, Sphingobacterium, and Paenibacillus mainly occurred in the thermophilic stage and were positively correlated with pile temperature, obviously. These genera mentioned above are greatly affected by pile temperature and HIAP dose in the D7 and D42 samples in Figure 4d. Nonomuraea, Parapedobacter, Thermobifida, etc., were positively correlated with TN and HA, were negatively correlated with pH value, and were inhibited at the thermophilic phase but had high abundance at the maturity phase, showing that these bacteria could effectively degrade high-molecular-weight OM such as cellulose and convert HS and FA into HA in the cooling and maturity stages. Microbacterium, Parapedobacter, Brevibacillus, etc., were positively correlated with HS, while Streptomyces, Paenibacillus, Cellulosimicrobium, Chitinophaga, flavobacterium, Ochrobactrum, etc., were positively correlated with HS and also positively correlated with FA, and had high abundance in the thermophilic stage and were significantly affected by HIAP dose. Thus, HIAP promoted the formation of HS and FA by enhancing core thermophilic microorganisms, such as Microbacterium, Parapedobacter, Brevibacillus, Streptomyces, Paenibacillus, Cellulosimicrobium, Chitinophaga, flavobacterium, and Ochrobactrum, and by inhibiting Thermobifida, Thermobacillus, Pseudoxanthimonas, and Saccharomonospora at the thermophilic phase, and the inhibition became weaker as HIAP dosage increased. The genera Sphingobacterium, Pseudoxanthimonas, DSSF69, Thermobacillus, Saccharomonospora, and Bordetella were positively related to the bioavailable fraction (BF) of HMs [14], which played an important role in HM resistance, while Bacillus were significantly negatively related to the BF of HMs.
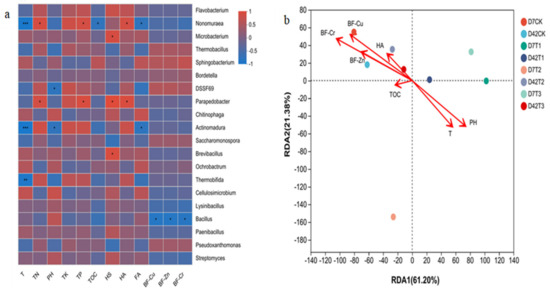
Figure 5.
The correlation between core microbial community and environmental factors. (a) Spearman correlation heatmap analysis (significance levels are * p < 0.05, ** p < 0.01, *** p < 0.001). (b) Redundancy analysis.
As seen in Figure 5b, the two ordination axes demonstrate that the selected parameters could explain 82.6% of total sample variation, and RDA1 and RDA2 explain 61.2% and 21.4% of core microbial community structure, respectively. Among them, pH and composting temperature were the most significant influencing factors in the thermophilic stage (D7 samples). In contrast, BF–Cr, BF–Cu, BF–Zn, and HA were the most significant impact factors in the maturity stage (D42 samples), and the influence order was BF–Cr > BF–Cu > BF–Zn > HA due to their different toxicity.
Summarily, there were more similar community compositions between 1% HIAP and the control groups and between 5% and 10% HIAP groups. Both 1% and 5% HIAP promoted Proteobacteria and Firmicutes, but 10% HIAP promoted Actinobacteriota and Bacteroidota. At the thermophilic phase, HIAP and its dose greatly affected core thermophilic microbial genera, which were significantly correlated to pile temperature and pH value. In the maturity stage, core microbial genera in the composting piles treated by different doses of HIAP basically tended to be similar and closely correlated to the bioavailable fraction (BF) of HMs and HA, and the influence order was BF–Cr > BF–Cu > BF–Zn > HA.
4. Conclusions
HS/TOC ratio increased, but HA/TOC and HA/FA ratios reduced with the increase in HIAP dose. HIAP promoted the formation of more HS. HIAP simultaneously had two functions: catalysis and adsorption effects. Low-dose HIAP was mainly manifested as a catalytic effect, but high-dose HIAP was simultaneously manifested as weaker catalytic and stronger adsorption effects in the thermophilic stage and as a very strong adsorption effect in the maturity stage. Because small-molecule FA easily entered the pores of HIAP and large-molecule HA was only adsorbed on the outer surface of HIAP, high-dose HIAP could bind more FA than HA to inhibit the polymerization or conversion of the adsorbed FA into HA, especially in the maturity stage.
Mixing SS with HIAP and subsequent composting were two consecutive processes during HIAP-amended composting, which significantly influenced the species distribution of Cu, Zn, and Cr, especially enhancing the oxidizable fraction of Cr. Each process roughly contributed one-half to HMs passivation, and the first than the second process had a greater passivation effect on zinc. The bioavailable fraction (BF) of HMs reduced with the increase of HIAP dose.
There was a more similar community composition between 1% HIAP and the control groups and between 5% and 10% HIAP groups. Both 1% and 5% HIAP promoted Proteobacteria and Firmicutes, but 10% HIAP promoted Actinobacteriota and Bacteroidota. At the thermophilic phase, HIAP and its dose greatly affected core thermophilic microbial genera, which was significantly correlated to pile temperature and pH value. In the maturity stage, core microbial genera in HIAP-amended composting were basically similar and closely correlated to the bioavailable fraction (BF) of HMs and HA, and the influence order was BF–Cr > BF–Cu > BF–Zn > HA.
The optimal condition of 5% HIAP addition was recommended by considering the effect of HIAP dose on the humification, heavy metal passivation as well as additive cost comprehensively.
Author Contributions
Conceptualization, Z.Y. and W.Z.; methodology, Z.Y. and W.Z; software, Y.L.; validation, R.Y. and W.Z.; formal analysis, Z.Y.; investigation, Z.Y., X.J. and B.W.; resources, W.Z.; data curation, Z.Y. and X.W. (Xiaoyan Wu).; writing—original draft preparation, Z.Y.; writing—review and editing, Z.Y., W.Z., R.Y., J.L. and X.W. (Xueling Wu).; supervision, W.Z., R.Y. and X.W. (Xueling Wu).; project administration, W.Z.; funding acquisition, W.Z., L.S. and R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (No. 2019YFC1803600), the National Natural Science Foundation of China (No. 51934009, No. 52074353, No. 52274289), the Central Government Guides Local Science and Technology Development Fund (No. 2022ZYC122), and the Natural Science Foundation of Hunan (No. 2021JJ30855).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiong, R.W.; Gao, X.F.; Tu, X.Y.; Mao, Y.L.; Jiang, L.; Zheng, L.; Du, Y.T. Heavy Metal Remediation in Sludge Compost: Recent Progress. J. Renew. Mater. 2022, 10, 469–486. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Shaheen, S.M.; Abdelrahman, H.; Ali, E.F.; Bolan, N.S.; Li, G.; Rinklebe, J. Microbial inoculants and struvite improved organic matter humification and stabilized phosphorus during swine manure composting: Multivariate and multiscale investigations. Bioresour. Technol. 2022, 351, 126976. [Google Scholar] [CrossRef] [PubMed]
- Borgulat, A.; Zgórska, A.; Głodniok, M. Comparison of different municipal sewage sludge products for potential ecotoxicity. Arch. Environ. Prot. 2022, 48, 92–99. [Google Scholar]
- Bruni, C.; Akyol, Ç.; Cipolletta, G.; Eusebi, A.L.; Caniani, D.; Masi, S.; Colón, J.; Fatone, F. Decentralized community composting: Past, present and future aspects of Italy. Sustainability 2020, 12, 3319. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Gao, Y.; Tan, W.; Xi, B. Additives for reducing nitrogen loss during composting: A Review. J. Clean. Prod. 2021, 307, 127308. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Truong, H.B.; Hong, S.J.; Li, X.W.; Hur, J. Biotic and abiotic catalysts for enhanced humification in composting: A comprehensive review. J. Clean. Prod. 2023, 402, 136832. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.L.; Ning, X.; Yang, M.; Liu, M.B.; Zang, F.; Nan, Z.R. A promising amendment for the immobilization of heavy metal(loid)s in agricultural soil, northwest China. J. Soil. Sediment. 2021, 21, 2273–2286. [Google Scholar] [CrossRef]
- Ren, J.; Dai, L.; Tao, L. Stabilization of heavy metals in sewage sludge by attapulgite. J. Air Waste Manag. Assoc. 2021, 71, 392–399. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, J.; Zhao, J.J.; Xia, D.S.; Pan, F.; Liu, C.; George, Z.K.; Fu, J. Palygorskite changes heavy metal bioavailability and microbial functional diversity in sewage sludge composting. Environ. Technol. 2015, 36, 2855–2862. [Google Scholar] [CrossRef]
- Lin, H.; Sun, W.C.; Yu, Y.J.; Ding, Y.Z.; Yang, Y.Y.; Zhang, Z.L.; Ma, J.W. Simultaneous reductions in antibiotics and heavy metal pollution during manure composting. Sci. Total Environ. 2021, 788, 147830. [Google Scholar] [CrossRef]
- Chen, Y.N.; Tang, P.; Li, Y.P.; Chen, L.; Jiang, H.J.; Liu, Y.H.; Luo, X.L. Effect of attapulgite on heavy metals passivation and microbial community during co-composting of river sediment with agricultural wastes. Chemosphere 2022, 299, 134347. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.Z.; Jia, X.S.; Xu, P.Z.; Huang, X.; Gu, W.J.; Zhang, F.B.; Yang, S.H.; Tang, S.H. The addition of modified attapulgite reduces the emission of nitrous oxide and ammonia from aerobically composted chicken manure. J. Air Waste Manag. 2012, 62, 1174–1181. [Google Scholar] [CrossRef]
- Pan, J.T.; Li, R.H.; Zhai, L.M.; Zhang, Z.Q.; Ma, J.Y.; Liu, H.B. Influence of palygorskite addition on biosolids composting process enhancement. J. Clean. Prod. 2019, 217, 371–379. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yu, C.X.; Wang, X.J.; Yin, S.Q.; Chang, X.Y. Additives improved saprotrophic fungi for the formation of humic acids in chicken manure and corn stover mix composting. Bioresour. Technol. 2022, 346, 126626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.G.; Yang, W.J.; Lu, T.Y.; Ru, X.; Dai, Z.B.; Meshack, A.O.; Hou, J.X. Heterogeneous Fenton for Removal Rhodamine B by Iron bearing Attapulgite Granular Catalysts. Water Air Soil Pollut. 2023, 234, 61. [Google Scholar] [CrossRef]
- Sun, H.Y.; Xing, R.Z.; Ye, X.Y.; Yin, K.; Zhang, Y.; Chen, Z.; Zhou, S. Reactive oxygen species accelerate humification process during iron mineral-amended sludge composting. Bioresour. Technol. 2023, 370, 128544. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef]
- Tmecc, T.W.; Leege, P.; Millner, P.; Watson, M.E. Test Methods for the Examination of Composts and Composting; The US Composting Council: Raleigh, NC, USA; US Government Printing Office: Washington, DC, USA, 2003. [Google Scholar]
- Pan, C.N.; Zhao, Y.; Zhao, L.; Wu, J.Q.; Zhang, X.; Xie, X.Y.; Kang, K.J.; Jia, L.M. Modified montmorillonite and illite adjusted the preference of biotic and abiotic pathways of humus formation during chicken manure composting. Bioresour. Technol. 2021, 319, 124121. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, Y.L.; Shah, G.M.; Chen, W.; Wang, W.; Xu, Y.D.; Huang, H.Y. Hyperthermophilic pretreatment composting significantly accelerates humic substance formation by regulating precursor production and microbial communities. Waste Manag. 2019, 92, 89–96. [Google Scholar] [CrossRef]
- Wu, X.Y.; Wang, J.S.; Yu, Z.J.; Charles, A.; Shen, L.; Wu, X.L.; Li, J.K.; Yu, R.L.; Liu, Y.D.; Zeng, W.M. Impact of bamboo sphere amendment on composting performance and microbial community succession in food waste composting. J. Environ. Manag. 2022, 303, 114144. [Google Scholar] [CrossRef]
- Yang, P.P.; Yin, H.; Peng, H.; Tang, S.Y.; Lu, M.; Liu, H. Effects of exogenous microorganism inoculation on efficiency and bacterial community structure of sludge composting. HuanjingKexue 2017, 38, 3536–3543. [Google Scholar]
- Liu, H.; Yin, H.; Tang, S.Y.; Wei, K.; Peng, H.; Lu, G.N.; Dang, Z. Effects of benzo [a] pyrene (BaP) on the composting and microbial community of sewage sludge. Chemosphere 2019, 222, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment- A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhai, W.; Du, Y.; Zhao, Y.; Wei, Z.; Wu, J.; Xie, X.; Yang, H.; Wu, D.; Guo, T. Core bacterial community driven the conversion of fulvic acid components during composting with adding manganese dioxide. Bioresour. Technol. 2021, 337, 125495. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Jiang, Y.; Li, R.; Ren, X.N.; Zhao, J.; Shen, F.; Wang, M.; Zhang, Z. Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresour. Technol. 2016, 220, 297–304. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.Y. Addition of seaweed and bentonite accelerates the two-stage composting of green waste. Bioresour. Technol. 2017, 243, 154–162. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Awasthi, S.K.; Wang, Q.; Awasthi, M.K.; Zhao, J.; Chen, H.; Ren, X.; Wang, M.; Zhang, Z. Role of Ca-bentonite to improve the humification, enzymatic activities, nutrient transformation and end product quality during sewage sludge composting. Bioresour. Technol. 2018, 262, 80–89. [Google Scholar] [CrossRef]
- Liu, L.; Ye, Q.; Wu, Q.; Liu, T.; Peng, S. Effect of biochar addition on sludge aerobic composting and greenbelt utilization. Environ. Technol. Innov. 2021, 21, 101279. [Google Scholar] [CrossRef]
- Akyol, C.; Ince, O.; Ince, B. Crop-based composting of lignocellulosic digesates: Focus on bacterial and fungal diversity. Bioresour. Technol. 2019, 288, 121549. [Google Scholar] [CrossRef]
- Qi, H.; Wei, Z.; Zhang, J.; Zhao, Y.; Wu, J.; Gao, X.; Liu, Z.; Li, Y. Effect of MnO2 on biotic and abiotic pathways of humic-like substance formation during composting of different raw materials. Waste Manag. 2019, 87, 326–334. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Li, R.; Chang, C.C.; Pan, J.; Zhang, Z. Effect of clay on greenhouse gas emissions and humification during pig manure composting as supported by spectroscopic evidence. Sci. Total Environ. 2020, 737, 139712. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Zhang, Z.H.; Zhang, D.W.; Tian, Y.; Nan, J.; Feng, Y.J. Hydrothermal pretreatment and compound microbial agents promoting high-quality kitchen waste compost: Superior humification degree and reduction of odor. Sci. Total Environ. 2023, 862, 160657. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, R.; Wu, S.; He, Q.; Ling, Z.; Liu, T.; Wang, Q.; Zhang, Z.Q.; Quan, F.S. Cattle manure compost humification process by inoculation ammonia-oxidizing bacteria. Bioresour. Technol. 2022, 344, 126314. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Yue, D.; Fukushima, M.; Fukuchi, S.; Nishimoto, R.; Nie, Y. Enhanced humification by carbonated basic oxygen furnace steel slag–II. Process characterization and the role of inorganic components in the formation of humic-like substances. Bioresour. Technol. 2012, 114, 637–643. [Google Scholar] [CrossRef]
- Wang, Q.H.; Hao, K.H.; Chelsea, B.; Kou, Y.; An, Z.X.; Mohamed, G.E.D.; Chen, C.M. The role and potential of attapulgite in catalytic pyrolysis of refinery waste activated sludge. Petrol. Sci. 2022, 19, 354–362. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Zhao, W.; Yang, T.; Zhang, X.; Xie, X.; Cui, H.; Wei, Z. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Technol. 2017, 226, 191–199. [Google Scholar] [CrossRef]
- Miura, A.; Okabe, R.; Izumo, K.; Fukushima, M. Influence of the physicochemical properties of clay minerals on the degree of darkening via polycondensation reactions between catechol and glycine. Appl. Clay Sci. 2009, 46, 277–282. [Google Scholar] [CrossRef]
- Chen, H.F.; Koopal, L.K.; Xiong, J.; Avena, M.; Tan, W.F. Mechanisms of soil humic acid adsorption onto montmorillonite and kaolinite. J. Colloid Interface Sci. 2017, 504, 457–467. [Google Scholar] [CrossRef]
- Zhang, L.C.; Luo, L.; Zhang, S.Z. Integrated investigations on the adsorption mechanisms of fulvic and humic acids on three clay minerals. Colloid Surf. A 2012, 406, 84–90. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.X.; Yan, B.X.; Li, Y.X.; Bao, M. Critical passivation mechanisms on heavy metals during aerobic composting with different grain-size zeolites. J. Hazard Mater. 2021, 406, 124313. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, L.; Zhan, J.; Guo, X.S. Variation and factors on heavy metal speciation during co-composting of rural sewage sludge and typical rural organic solid waste. J. Environ. Manag. 2022, 306, 114418. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, G.; Castellano-Hinojosa, A.; Correa-Galeote, D.; Bedmar, E.J. Evolution of bacterial diversity during two-phase olive mill waste ("alperujo") composting by 16S rRNA gene pyrosequencing. Bioresour. Technol. 2017, 224, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Z.; Li, X.X.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.P.; Chu, S.; Wang, B.; Zhang, D.; Zhou, P. Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting. Bioresour. Technol. 2020, 313, 123692. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.P.; Li, M.X.; Song, L.Y.; Wang, C.; Yang, S.; Yan, Z.Y.; Wang, Y.Q. Study on a nitrogen-retaining microbial agent to reduce nitrogen loss during chicken manure composting and nitrogen transformation mechanism. J. Clean. Prod. 2021, 285, 124813. [Google Scholar] [CrossRef]
- Song, Y.J.; Li, R.Y.; Wang, Y.X.; Hou, Y.; Chen, G.Y.; Yan, B.B.; Cheng, Z.J.; Mu, L. Co-composting of cattle manure and wheat straw covered with a semipermeable membrane: Organic matter humification and bacterial community succession. Environ. Sci. Pollut. Res. 2023, 30, 32776–32789. [Google Scholar] [CrossRef]
- Rastogi, G.; Bhalla, A.; Adhikari, A.; Bischoff, K.M.; Hughes, S.R.; Christopher, L.P.; Sani, R.K. Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour. Technol. 2010, 101, 8798–8806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).