Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth
Abstract
1. Introduction
2. Chemical Fertilizers and Their Environmental and Health Implications
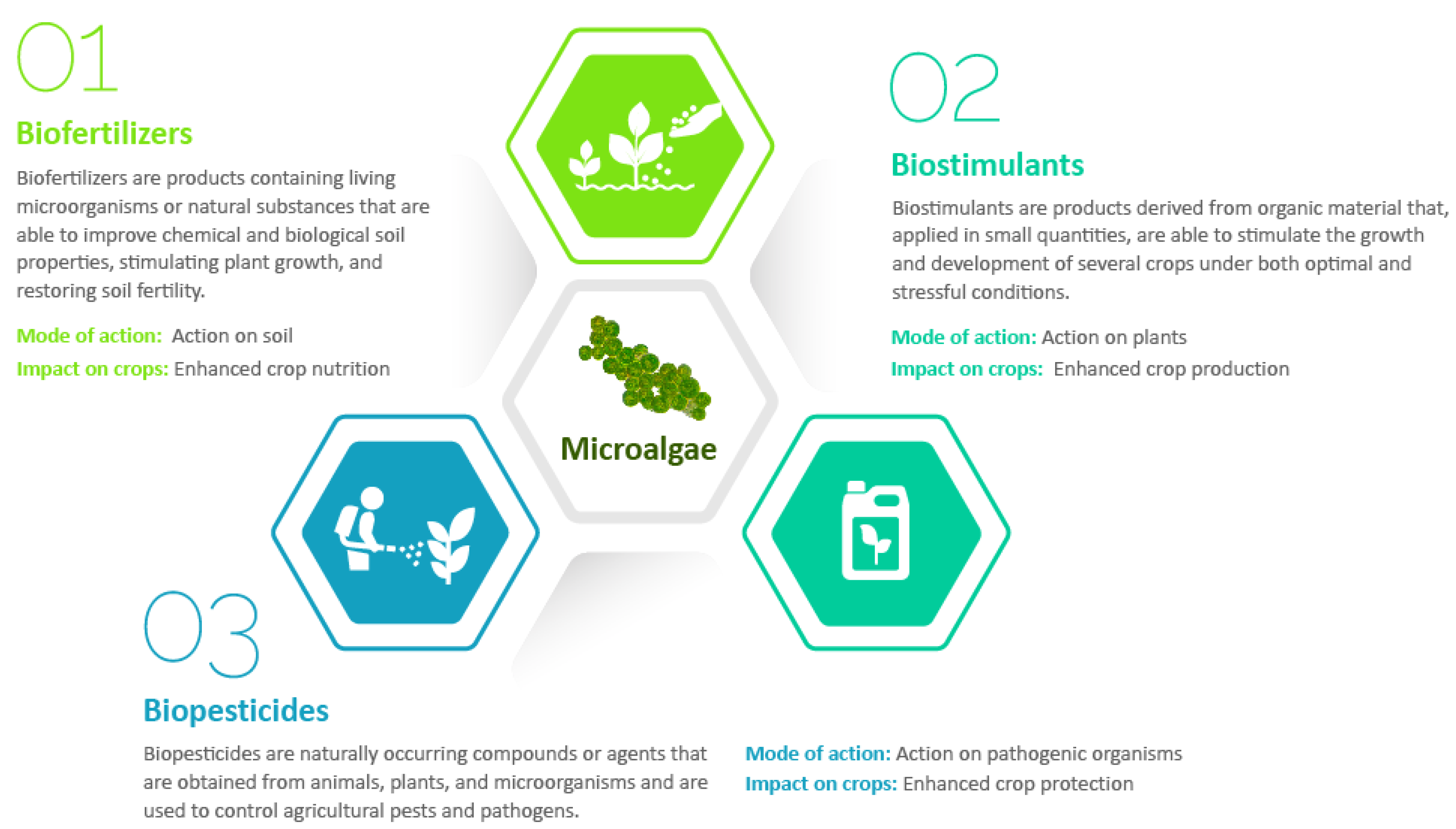
3. Microalgae as Biofertilizers
4. Production and Application Techniques of Microalgae-Based Fertilizers
5. Limitations of Microalgae Based-Biofertilizers
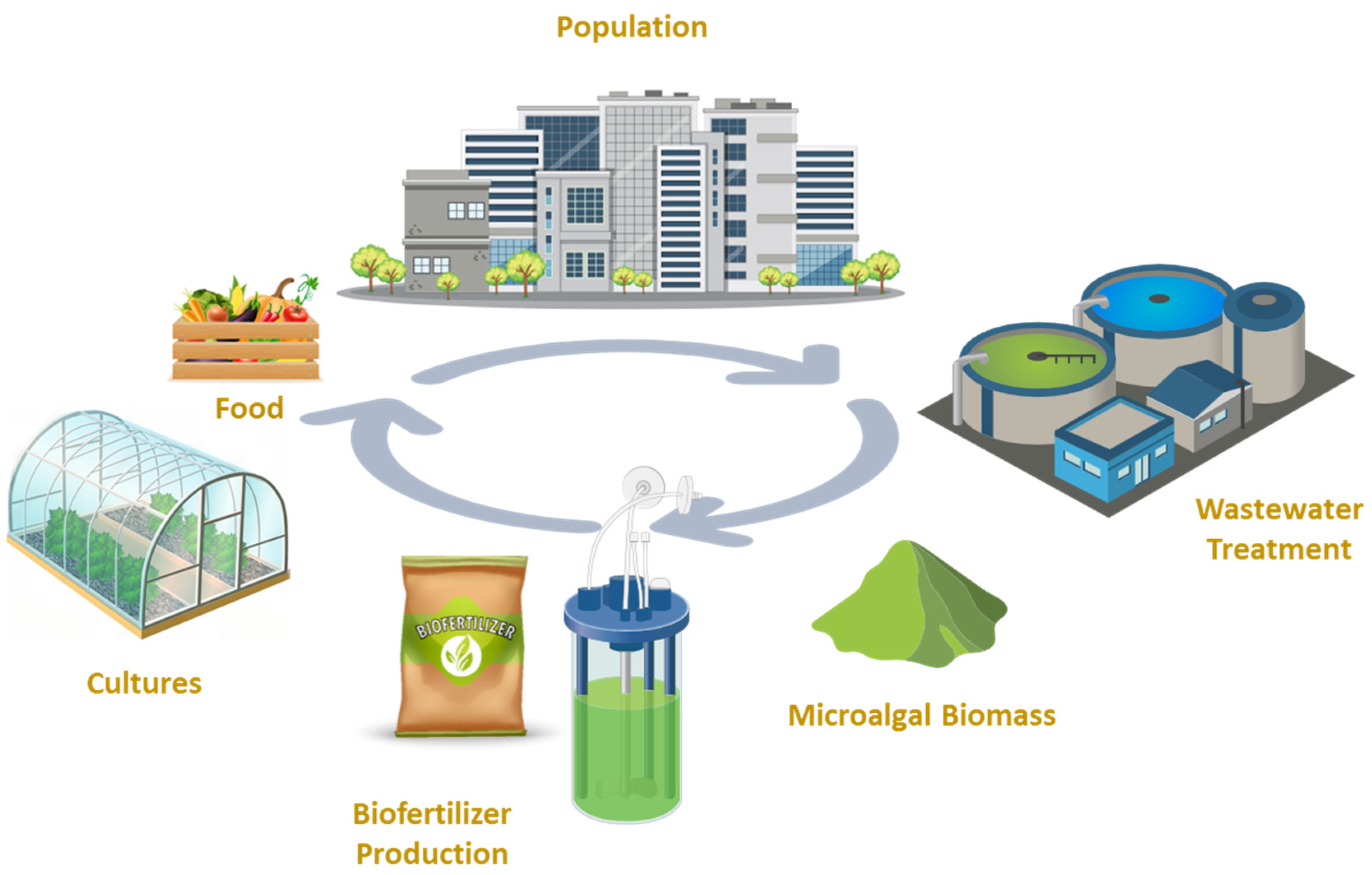
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murata, M.M.; Ito Morioka, L.R.; Da Silva Marques, J.B.; Bosso, A.; Suguimoto, H.H. What do patents tell us about microalgae in agriculture? AMB Express 2021, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef]
- Pan, D.; Kong, F.; Zhang, N.; Ying, R. Knowledge training and the change of fertilizer use intensity: Evidence from wheat farmers in China. J. Environ. Manag. 2017, 197, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Řezbová, H.; Slaboch, J.; Mach, J. Emissions from Managed Agricultural Soils in Context of Consumption of Inorganic Nitrogen Fertilisers in Selected EU Countries. Agronomy 2023, 13, 159. [Google Scholar] [CrossRef]
- Ward, M.H. Too much of a good thing? Nitrate from nitrogen fertilizers and cancer. Rev. Environ. Health 2009, 24, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.; Chauhan, P.S. Excessive and Disproportionate Use of Chemicals Cause Soil Contamination and Nutritional Stress. In Soil Contamination; Marcelo, L.L., Sonia, S., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Quan, Z.; Huang, B.; Lu, C.; Shi, Y.; Chen, X.; Zhang, H.; Fang, Y. The fate of fertilizer nitrogen in a high nitrate accumulated agricultural soil. Sci. Rep. 2016, 6, 21539. [Google Scholar] [CrossRef]
- Fakhri, Y.; Bjørklund, G.; Bandpei, A.M.; Chirumbolo, S.; Keramati, H.; Hosseini Pouya, R.; Asadi, A.; Amanidaz, N.; Sarafraz, M.; Sheikhmohammad, A.; et al. Concentrations of arsenic and lead in rice (Oryza sativa L.) in Iran: A systematic review and carcinogenic risk assessment. Food Chem. Toxicol. 2018, 113, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Douna, B.K. Risk of Nitrate Residues in Food Products and Drinking Water. Asian Pac. J. Environ. Cancer 2023, 6, 69–79. [Google Scholar]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [PubMed]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as Biofertilizer in Modern Agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 397–411. [Google Scholar]
- Çakirsoy, I.; Miyamoto, T.; Ohtake, N. Physiology of microalgae and their application to sustainable agriculture: A mini-review. Front. Plant Sci. 2022, 13, 1005991. [Google Scholar] [CrossRef]
- Fernandes, I.; Pinto, R.; Aguiar, R.; Correia, R. Perspective Application of the Circular Economy in the Blue Biotechnology: Microalgae as Sources of Health Promoting Compounds. Glob. J. Nutr. Food Sci. 2020, 3. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Sun, L.; Wang, Q. Converting carbon dioxide to high value-added products: Microalgae-based green biomanufacturing. GCB Bioenergy 2023, 15, 386–398. [Google Scholar] [CrossRef]
- Khaligh, S.F.; Asoodeh, A. Recent advances in the bio-application of microalgae-derived biochemical metabolites and development trends of photobioreactor-based culture systems. 3 Biotech 2022, 12, 260. [Google Scholar] [CrossRef]
- De Souza, M.H.B.; Calijuri, M.L.; Assemany, P.P.; Castro, J.d.S.; de Oliveira, A.C.M. Soil application of microalgae for nitrogen recovery: A life-cycle approach. J. Clean. Prod. 2019, 211, 342–349. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Chandini; Kumar, R.; Kumar, R.; Prakash, O. The Impact of Chemical Fertilizers on our Environment and Ecosystem. In Research Trends in Environmental Sciences, 2nd ed.; AkiNik Publications: Delhi, India, 2019; pp. 71–86. [Google Scholar]
- Tayoh, L.N. Destruction of Soil Health and Risk of Food Contamination by Application of Chemical Fertilizer. In Ecological and Practical Applications for Sustainable Agriculture; Bauddh, K., Kumar, S., Singh, R.P., Korstad, J., Eds.; Springer: Singapore, 2020; pp. 53–64. [Google Scholar] [CrossRef]
- Krasilnikov, P.; Taboada, M.A.; Amanullah. Fertilizer Use, Soil Health and Agricultural Sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of Soil Organic Carbon, pH, Electrical Conductivity, and Water Stable Aggregates to Long-Term Annual Manure and Inorganic Fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Park, J.-R.; Jang, Y.-H.; Kim, E.-G.; Lee, G.-S.; Kim, K.-M. Nitrogen Fertilization Causes Changes in Agricultural Characteristics and Gas Emissions in Rice Field. Sustainability 2023, 15, 3336. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Lu, Z.; Meng, F.; Cong, R.; Li, X.; Ren, T.; Lu, J. Imbalance between nitrogen and potassium fertilization influences potassium deficiency symptoms in winter oilseed rape (Brassica napus L.) leaves. Crop J. 2022, 10, 565–576. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of Different Rates of Nitrogen Fertilization on Crop Yield, Soil Properties and Leaf Physiological Attributes in Banana Under Subtropical Regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, C.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Effect of Nitrogen Fertilization on Tree Growth and Nutrient Content in Soil and Cherry Leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Liu, C.-W.; Sung, Y.; Chen, B.-C.; Lai, H.-Y. Effects of Nitrogen Fertilizers on the Growth and Nitrate Content of Lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Akhtar, M.; Mukhtar, Z.; Saeed, N.A. Hazards of nitrogen fertilizers and ways to reduce nitrate accumulation in crop plants. Environ. Sci. Pollut. Res. 2020, 27, 17661–17670. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. Int. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Wei, B.; Yu, J.; Cao, Z.; Meng, M.; Yang, L.; Chen, Q. The Availability and Accumulation of Heavy Metals in Greenhouse Soils Associated with Intensive Fertilizer Application. Int. J. Environ. Res. Public Health 2020, 17, 5359. [Google Scholar] [CrossRef]
- Gambús, F.; Wieczorek, J. Pollution of fertilizers with heavy metals. Ecol. Chem. Eng. A 2012, 19, 353–360. [Google Scholar]
- Thomas, E.Y.; Omueti, J.A.I.; Ogundayomi, O. The effect of phosphate fertilizer on heavy metal in soils and Amaranthus caudatus. Agric. Biol. J. N. Am. 2012, 3, 145–149. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Xu, Z.-M.; Li, Q.-S.; Yang, P.; Ye, H.-J.; Chen, Z.-S.; Guo, S.-H.; Wang, L.-L.; He, B.-Y.; Zeng, E.Y. Impact of osmoregulation on the differences in Cd accumulation between two contrasting edible amaranth cultivars grown on Cd-polluted saline soils. Environ. Pollut. 2017, 224, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium Toxicity in Plants and Role of Mineral Nutrients in Its Alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Yan, L.-J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Lampe, B.J.; Park, S.K.; Robins, T.; Mukherjee, B.; Litonjua, A.A.; Amarasiriwardena, C.; Weisskopf, M.; Sparrow, D.; Hu, H. Association between 24-hour urinary cadmium and pulmonary function among community-exposed men: The VA Normative Aging Study. Environ. Health Perspect. 2008, 116, 1226–1230. [Google Scholar] [CrossRef]
- Lamas, G.A.; Bhatnagar, A.; Jones, M.R.; Mann, K.K.; Nasir, K.; Tellez-Plaza, M.; Ujueta, F.; Navas-Acien, A. Contaminant Metals as Cardiovascular Risk Factors: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2023, 12, e029852. [Google Scholar] [CrossRef]
- Reyes-Hinojosa, D.; Lozada-Pérez, C.A.; Zamudio Cuevas, Y.; López-Reyes, A.; Martínez-Nava, G.; Fernández-Torres, J.; Olivos-Meza, A.; Landa-Solis, C.; Gutiérrez-Ruiz, M.C.; Rojas del Castillo, E.; et al. Toxicity of cadmium in musculoskeletal diseases. Environ. Toxicol. Pharmacol. 2019, 72, 103219. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P. Cadmium carcinogenesis. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2003, 533, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Thompson, R.B.; Xu, J.; Liao, S.; Suo, L.; Peng, Y.; Chen, Q.; Yang, J.; Li, Y.; Zou, G.; et al. Arsenic and Cadmium Accumulation in Soil as Affected by Continuous Organic Fertilizer Application: Implications for Clean Production. Agronomy 2021, 11, 2272. [Google Scholar] [CrossRef]
- Ai-Qasi, B.A.; Sharqi, M.M.; Faiath, S.E. Effect of Nitrogen Fertilizer Sources, Lead and Cadmium Pollution on Some Properties of Barley (Hordeum vulgare). IOP Conf. Ser. Earth Environ. Sci. 2021, 904, 012057. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Mise, N.; Ichihara, S. Arsenic contamination in food chain in Bangladesh: A review on health hazards, socioeconomic impacts and implications. Hyg. Environ. Health Adv. 2022, 2, 100004. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Kumar, S.; Islam, R.; Akash, P.B.; Khan, M.H.R.; Proshad, R.; Karmoker, J.; MacFarlane, G.R. Lead (Pb) Contamination in Agricultural Products and Human Health Risk Assessment in Bangladesh. Water Air Soil Pollut. 2022, 233, 257. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, M.; Yang, Y.; Wu, Y.; Ni, H.; Yu, X.; Shi, J.; Chen, H.; Bian, X.; Pan, D.; et al. Enhanced growth of ginger plants by an eco-friendly nitrogen-fixing Pseudomonas protegens inoculant in glasshouse fields. J. Sci. Food Agric. 2022, 102, 3038–3046. [Google Scholar] [CrossRef]
- Stavi, I.; Lal, R. Achieving Zero Net Land Degradation: Challenges and opportunities. J. Arid Environ. 2015, 112, 44–51. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.; Esteban Lucas-Borja, M.; Pereira, P.; Muñoz-Rojas, M. Cyanobacteria as a Nature-Based Biotechnological Tool for Restoring Salt-Affected Soils. Agronomy 2020, 10, 1321. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef]
- Rocha, M.; Pereira, P.; Melo, P. Cianobactérias e Microalgas: Organismos Promissores para a Agricultura e para a Reabilitação dos Solos. Captar 2021, X, 1–8. [Google Scholar]
- Swarnalakshmi, K.; Prasanna, R.; Kumar, A.; Pattnaik, S.; Chakravarty, K.; Shivay, Y.S.; Singh, R.; Saxena, A.K. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 2013, 55, 107–116. [Google Scholar] [CrossRef]
- Osman, M.E.H.; El-Sheekh, M.M.; El-Naggar, A.H.; Gheda, S.F. Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol. Fertil. Soils 2010, 46, 861–875. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular economy fertilization: Testing micro and macro algal species as soil improvers and nutrient sources for crop production in greenhouse and field conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Siangdung, W.; Paithoonrangsari, K.; Bunnag, B. Cultivation of Spirulina platensis using pig wastewater in a semi-continuous process. J. Microbiol. Biotechnol. 2010, 20, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Pooja, K.; Priyanka, V.; Rao, B.C.S.; Raghavender, V. Cost-effective treatment of sewage wastewater using microalgae Chlorella vulgaris and its application as bio-fertilizer. Energy Nexus 2022, 7, 100122. [Google Scholar] [CrossRef]
- Roychowdhury, D.; Paul, M.; Banerjee, S.K. A review on the effects of biofertilizers and biopesticides on rice and tea cultivation and productivity. Int. J. Sci. Eng. Technol. 2014, 2, 96–106. [Google Scholar]
- Afkairin, A.; Ippolito, J.A.; Stromberger, M.; Davis, J.G. Solubilization of organic phosphorus sources by cyanobacteria and a commercially available bacterial consortium. Appl. Soil Ecol. 2021, 162, 103900. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as Bio-fertilizers for Rice Growth and Seed Yield Productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Özer Uyar, G.E.; Mısmıl, N. Symbiotic association of microalgae and plants in a deep water culture system. PeerJ 2022, 10, e14536. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Lo Piero, A.R.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Elarroussi, H.; Elmernissi, N.; Benhima, R.; Kadmiri, I.M.E.; Bendaou, N.; Smouni, A.; Wahbya, I. Microalgae polysaccharides a promising plant growth biostimulant. J. Algal Biomass Utln. 2016, 7, 55–63. [Google Scholar]
- Jimenez, R.; Markou, G.; Tayibi, S.; Barakat, A.; Chapsal, C.; Monlau, F. Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes. Appl. Sci. 2020, 10, 3890. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef]
- Sharma, G.K.; Khan, S.A.; Shrivastava, M.; Bhattacharyya, R.; Sharma, A.; Gupta, D.K.; Kishore, P.; Gupta, N. Circular economy fertilization: Phycoremediated algal biomass as biofertilizers for sustainable crop production. J. Environ. Manag. 2021, 287, 112295. [Google Scholar] [CrossRef]
- Kusvuran, S. Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Hortic. Plant J. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- Suchithra, M.R.; Muniswami, D.M.; Sri, M.S.; Usha, R.; Rasheeq, A.A.; Preethi, B.A.; Dineshkumar, R. Effectiveness of green microalgae as biostimulants and biofertilizer through foliar spray and soil drench method for tomato cultivation. S. Afr. J. Bot. 2022, 146, 740–750. [Google Scholar] [CrossRef]
- Chu, Q.; Lyu, T.; Xue, L.; Yang, L.; Feng, Y.; Sha, Z.; Yue, B.; Mortimer, R.J.G.; Cooper, M.; Pan, G. Hydrothermal carbonization of microalgae for phosphorus recycling from wastewater to crop-soil systems as slow-release fertilizers. J. Clean. Prod. 2021, 283, 124627. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Gopalsamy, J.; Jayasingam, P.; Arumugam, A.; Kannadasan, S.; Sampathkumar, P. The Impact of Using Microalgae as Biofertilizer in Maize (Zea mays L.). Waste Biomass Valorization 2019, 10, 1101–1110. [Google Scholar] [CrossRef]
- El Arroussi, H.; Elbaouchi, A.; Benhima, R.; Bendaou, N.; Smouni, A.; Wahby, I. Halophilic microalgae Dunaliella salina extracts improve seed germination and seedling growth of Triticum aestivum L. under salt stress. Acta Hortic. 2016, 1148, 13–26. [Google Scholar] [CrossRef]
- Elhafiz, A.; Gaur, A.E.S.S.; Hamdany, N.; Maryam, O.; Lakshmi, T.V.R. Chlorella vulgaris and Chlorella pyrenoidosa live cells appear to be promising sustainable biofertilizer to grow rice, lettuce, cucumber and eggplant in the UAE soils. Recent Res. Sci. Technol. 2015, 7, 14–21. [Google Scholar]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Renuka, N.; Prasanna, R.; Sood, A.; Ahluwalia, A.S.; Bansal, R.; Babu, S.; Singh, R.; Shivay, Y.S.; Nain, L. Exploring the efficacy of wastewater-grown microalgal biomass as a biofertilizer for wheat. Environ. Sci. Pollut. Res. Int. 2016, 23, 6608–6620. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Kabi, S.R.; Verma, S.; Adak, A.; Pal, M.; Shivay, Y.S.; Prasanna, R.; Nain, L. Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro- and micronutrients in grains in rice–wheat cropping sequence. Cogent Food Agric. 2015, 1, 1037379. [Google Scholar] [CrossRef]
- Sharma, V.; Prasanna, R.; Hossain, F.; Muthusamy, V.; Nain, L.; Das, S.; Shivay, Y.S.; Kumar, A. Priming maize seeds with cyanobacteria enhances seed vigour and plant growth in elite maize inbreds. 3 Biotech 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Consortia of cyanobacteria/microalgae and bacteria in desert soils: An underexplored microbiota. Appl. Microbiol. Biotechnol. 2018, 102, 7351–7363. [Google Scholar] [CrossRef]
- Cañedo, J.C.G.; Lizárraga, G.L.L. Considerations for Photobioreactor Design and Operation for Mass Cultivation of Microalgae. In Algae; Nooruddin, T., Dharumadurai, D., Eds.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P.L. A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal Reactors: A Review of Enclosed System Designs and Performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef]
- Kumar, N.; Banerjee, C.; Negi, S.; Shukla, P. Microalgae harvesting techniques: Updates and recent technological interventions. Crit. Rev. Biotechnol. 2023, 43, 342–368. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sust. Energ. Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Lourenço, K.S.; Clark, C.; Luz, R.L.; da Silva, G.H.R.; Vet, L.E.M.; Cantarella, H.; Fernandes, T.V.; Kuramae, E.E. From toilet to agriculture: Fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resour. Conserv. Recycl. 2020, 161, 104924. [Google Scholar] [CrossRef]
- Lorentz, J.F.; Calijuri, M.L.; Assemany, P.P.; Alves, W.S.; Pereira, O.G. Microalgal biomass as a biofertilizer for pasture cultivation: Plant productivity and chemical composition. J. Clean. Prod. 2020, 276, 124130. [Google Scholar] [CrossRef]
- Youssef, S.M.; El-Serafy, R.S.; Ghanem, K.Z.; Elhakem, A.; Abdel Aal, A.A. Foliar Spray or Soil Drench: Microalgae Application Impacts on Soil Microbiology, Morpho-Physiological and Biochemical Responses, Oil and Fatty Acid Profiles of Chia Plants under Alkaline Stress. Biology 2022, 11, 1844. [Google Scholar] [CrossRef]
- Bumandalai, O.; Tserennadmid, R. Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquat. Biol. 2019, 7, 95–99. [Google Scholar]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef]
- Ruiz, J.; Wijffels, R.H.; Dominguez, M.; Barbosa, M.J. Heterotrophic vs autotrophic production of microalgae: Bringing some light into the everlasting cost controversy. Algal Res. 2022, 64, 102698. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pahl, S.L.; Lee, A.K.; Kalaitzidis, T.; Ashman, P.J.; Sathe, S.; Lewis, D.M. Harvesting, Thickening and Dewatering Microalgae Biomass. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 165–185. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients From Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Ferrer, I.; Díez-Montero, R. Can microalgae grown in wastewater reduce the use of inorganic fertilizers? J. Environ. Manage 2022, 323, 116224. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, J.M.; González-López, C.V.; Brindley, C.; Fernández-Sevilla, J.M.; Acién-Fernández, F.G. Simulation and Techno-Economical Evaluation of a Microalgal Biofertilizer Production Process. Biology 2022, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Chemical Fertilizers | Biofertilizer | ||
|---|---|---|---|---|
| Fungi | Bacteria | Microalgae/ Cyanobacteria | ||
| Formation of symbiotic bonds between plant roots and soil microorganisms. |  |  |  |  |
| An important role in the nitrogen cycle is making nitrogen available in a form that plants can use. |  |  |  |  |
| Ability to promote phosphorus solubilization. |  |  |  |  |
| The ability for nitrogen fixation by individual strains, phosphorous solubilization, and hormone production promote plant growth. |  |  |  |  |
| Ability to capture CO2 and reduce greenhouse emissions during the addition of organic carbon to the soil. |  |  |  |  |
| Gradual release of nutrients for plant uptake. |  |  |  |  |
| Negative environmental impacts through degradation of soil, water contamination, and induction of eutrophication. |  |  |  |  |
| Industrial-scale production and widespread use in the agricultural sector. |  |  |  |  |
 Not applicable.
Not applicable.  Applicable.
Applicable.| Bioactive Compounds | Biological Activity | Role in Agriculture | Microalgae/Cyanobacteria Sources |
|---|---|---|---|
| Phenolic compounds | Antioxidant, antibacterial and antifungal | Crop protection against pathogens and various biotic and abiotic stress conditions | Chlorella vulgaris; Isochrysis sp.; Botryococcus braunii; Odontella sinensis; Chaetoceros calcitrans; Phaeodactylum tricornutum; Isochrysis galbana; Tetraselmis suecica; Saccharina japonica; Neochloris oleoabundans; Skeletonema costatum |
| Carotenoids | Antioxidant, anti-inflammatory and anticancer | Crop fortification; Soil bioremediation and fertilization; Crop protection against biotic and abiotic stress conditions | Spirulina sp.; Dunaliella salina; Chlorella pyrenoidosa; Haematococcus pluvialis; Chlorella protothecoides; Muriellopsis sp.; Chlorella zofingiensis; Phaeodactylum tricornutum |
| Terpenoids | Antioxidant, antibacterial and anticarcinogenic | Crop protection against insects, bacteria, and other organisms; Attraction of pollinators; Stimulation of plant growth and development | Pseudanabaena articulate; Sphaerococcus coronopifolius; Chondrococcus hornemanni; Hypnea pannosa; Plocamium cornutum; Oscillatoria perornata; Planktothricoids raciborskii; Thermosynechococcus elongate; Portieria hornemann; Pseudanabaena sp.; Synechocystis sp.; Plocamium leptophyllum |
| Polysaccharides | Antioxidant, anti-inflammatory, antibacterial; anticoagulant and anticancer | Crop protection against biotic and abiotic stress conditions; Improvement of soil quality; Stimulation of plant growth | Dunaliella; Chlorella; Navicula; Aphanothece; Cylindrotheca; Scytonema; Arthrospira; Rhodella; Phaeodactylum; Chlamydomonas; Porphyridium; Nostoc |
| Free fatty acids | Antioxidant, antifungal, antiviral, antibiotic, and anticarcinogenic | Crop protection against various biotic and abiotic stress conditions | Dunaliella; Spirulina; Chlorella; Porphyridium; Nannochloropsis; Anabaena; Scenedesmus |
| Phytohormones | Chemical messengers | Crop response to stress conditions; Regulation of cellular activities in crops; Stimulation of plant growth | Chlamydomonas; Chlorella; Protococcus; Scenedesmus; Arthrospira; Phormidium |
| Microalgae/Cyanobacteria | Application Form | Crops | Effects | Ref. * |
|---|---|---|---|---|
| Spirulina platensis | Biomass | Eruca sativa, Ameranthus gangeticus, Brassica rapa ssp. chinensis, and Brassica oleracea alboglabra | Enhancing plant growth; Improves the germination process. | [69] |
| Spirulina platensis | Hydrolyzed biomass | Lactuca sativa L. | Promote seedling growth; Increases the spermine content in leaves. | [74] |
| Spirulina platensis | Total polysaccharides extract | Solanum lycopersium and Capsicum annum | Improve plant growth; Increase in root weight; Increase in the number and size of nodes. | [75] |
| Monoraphidium sp. | Dried biomass | Solanum lycopersicum | Reduction of nitrate leaching from the soil; Plant growth is comparable to that of synthetic fertilizer. | [76] |
| Arthrospira platensis, Dunaliella salina, and Porphyridium sp. | Crude polysaccharides extract | Solanum lycopersicum | Promote plant growth; Improved the nodes number; Increase carotenoid, chlorophyll, proteins content, and Nitrate Reductase, NAD-Glutamate Dehydrogenase activities. | [77] |
| Chlorella minutissima | Dried biomass | Zea mays and Spinacia oleracea | Increase soil nutrient content; Promote plant growth. | [78] |
| Chlorella vulgaris | Extract | Brassica oleracea var. italica | Promote plant growth; Increase plant nutrient content; Increase plant phenolic and flavonoid contents; Increase plant antioxidant activity. | [79] |
| Chlorella vulgaris | Extract | Solanum lycopersicum L. | Improves plant growth and yield; Increases the availability of macronutrients and micronutrients. | [80] |
| Chlorella vulgaris and Microcystis sp. | Hydrochar | Triticum aestivum | Increase the content of available phosphorus in the soil; Increase the efficiency of phosphorus used by the plant. | [81] |
| Chlorella vulgaris and Spirulina platensis | Dried Biomass | Oryza sp. | Increases the availability of macronutrients; Improves the chemical properties of the soil and biological activity. | [70] |
| Chlorella vulgaris and Spirulina platensis | Dried Biomass | Zea mays L. | Improves germination rate and increases plant yields; Increase in shoot length and number of leaves; Increase in both fresh and dry weights of root, shoot, and whole plant. | [82] |
| Dunaliella salina | Exopolysaccharides extract | Triticum aestivum | Increase in tolerance to salt stress; Enhance germination rate and seedling growth; Increase in root length and coleoptile height. | [83] |
| Chlorella vulgaris and Chlorella pyrenoidosa | Solution | Lactuca sativa, Oryza sp., Solanum Melongena and Cucumis sativus | Improves the germination process and salinity tolerance; Enhance chlorophyll content. | [84] |
| Chlorella vulgaris and Scenedesmus quadricauda | Extract | Beta vulgaris L. | Biostimulant effects on the expression of root traits and genes related to nutrient acquisition; Improves plant growth. | [85] |
| Microalgae consortia | ||||
| Chlorella, Anabaena, Scenedesmus, Chroococcus, Chlorococcum, Fischerella, Phormidium, Westiellopsis, and Spirogyra | Microalgal consortia | Triticum aestivum L. HD2967 | Increase grain yield and weight; Increases the availability of macronutrients; Enhanced microbial activity; Enhance crop productivity and yield. | [86] |
| Anabaena oscillarioides CR3, Brevundimonas diminuta PR7, and Ochrobactrum anthropi PR10 | Cyanobacteria and microbe consortia | Triticum aestivum L. | Increases the availability of macronutrients and improves plant yield; Increase in plant dry weight. | [87] |
| Anabaena torulosa and Trichoderma viride | Biofilm constituted by cyanobacteria and a fungus | Inbred genotypes of maize viz. V6 (HKI323PV) and V7 (HKI161PV) | Increase seedling attributes and seed enzymatic activities; Improve mobilization of nutrients at the seed stage. | [88] |
| Harvesting Technique | Advantages | Limitations |
|---|---|---|
| Sedimentation | Simple and inexpensive method; Do not require complex equipment. | Slow process, may take a long time for complete separation; May result in lower biomass recovery; Possibility of biomass degradation. |
| Flotation | Suitable for large-scale operations; Low-cost method; Short operation time; Low space requirement. | Requires the use of chemical surfactants. |
| Filtration | Efficient separation of microalgae from the medium; Cost-effective; Can achieve high biomass recovery rates; Suitable for both large and small-scale operations; Low energy consumption (natural and pressure filter); It can be used for the separation of shear-sensitive species. | Requires regular maintenance of membranes; Membrane fouling/clogging and replacement increase operational costs. |
| Centrifugation | Rapid and efficient separation of microalgae; High biomass recovery rates; Applicable for almost all microalgae species. | Expensive equipment and maintenance costs; High energy consumption; Possibility of cell damage. Suitable for the recovery of high-value products. |
| Coagulation/flocculation | Aggregates microalgae, making harvesting easier; It can be used in combination with other methods for improved efficiency; Cost-effective for large-scale operations; Applicable to a wide range of microalgae species. | Requires the use of chemicals for flocculant formation; Chemicals may be expensive; Recycling of culture medium is limited. |
| Electrical based processes | Do not require the use of chemicals; Applicable to a wide range of microalgae species. | High energy consumption and equipment costs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. https://doi.org/10.3390/su151612413
Gonçalves J, Freitas J, Fernandes I, Silva P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability. 2023; 15(16):12413. https://doi.org/10.3390/su151612413
Chicago/Turabian StyleGonçalves, João, Jorge Freitas, Igor Fernandes, and Pedro Silva. 2023. "Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth" Sustainability 15, no. 16: 12413. https://doi.org/10.3390/su151612413
APA StyleGonçalves, J., Freitas, J., Fernandes, I., & Silva, P. (2023). Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability, 15(16), 12413. https://doi.org/10.3390/su151612413







