Application of Multi-Plant Symbiotic Systems in Phytoremediation: A Bibliometric Review
Abstract
1. Introduction
2. Data and Methodology
2.1. Data Sources
2.2. Analysis Methodology
3. Research Results
3.1. Annual Changes in the Number of Articles Published and the Main Countries Studied
3.2. Publication Characteristics Analysis
3.3. Keyword Co-Occurrence Network and Clustering Analysis
3.4. Literature Co-Citation Analysis
4. Discussion
4.1. Factors Influencing the Restoration of Multi-Plant Symbiotic Systems
- (1)
- Plant diversity. Generally, plant diversity increases plant community productivity, community stability, and biomass and decreases the toxicity of pollutants to plants via multiple plant–soil feedback mechanisms, thereby enhancing phytoremediation [21,93]. Furthermore, plant diversity is comprised primarily of species diversity and functional diversity. Studies have demonstrated that plant diversity is influenced by soil heterogeneity [94], habitat heterogeneity [95], and spatial heterogeneity [96]. Species diversity has a significant positive effect on plant biomass above the ground and microbial communities below the ground [97,98]. Nevertheless, some scholars contend that functional diversity is more important than species diversity [99,100]. That functional trait diversity can be enhanced by promoting ecological niche complementarity to modify mutual competitive effects and reduce interspecific competition among plants [101]. It has also been discovered that increasing the number of genotypes in plant populations can enhance the functional diversity of communities and indirectly promote resource complementarity and ecological niche differentiation among different genotypes in order to increase resource use efficiency [102].
- (2)
- Interspecific relationships. Appropriate species combinations, planting ratios, planting densities, and planting patterns can enhance the facilitative relationships between plant assemblages by increasing the resource use efficiency of plants. Studies have shown that nutrients may be a major limiting factor for species coexistence and plant productivity in polluted environments [86,103]. However, plant assemblages can increase the ability of each species to obtain key elements from the soil nutrient pool [104]. Thus, when ecological niche overlap among plants is high (e.g., Zea mays and Suaeda saltbush I [16], Solanum melongena and Sedum alfredii [52]), it will increase nutrient competition among species and weaken the facilitative effect of plant combination cropping systems. This can be enhanced through fertilization, microorganism inoculation, and the addition of exogenous substances [52].
- (3)
- Sex of plant species. According to studies, dioecious plants exhibit sex differences in response to environmental stress, with female plants tending to allocate more resources to reproductive growth and male plants tending to increase their own tolerance [105]. Mixed plantings of plants of different sexes can increase the abundance of microbial communities such as actinomycetes and β-amastigotes to improve the rhizosphere environment, thereby affecting the remediation capacity of plant assemblages and tolerance to polluted environments [106]. Plants of different sexes can also enhance plant community promotion through the complementary effect of ecological niches, such as a study by Bu Chunlan et al. [107], who discovered that when Morus alba of different sexes was mixed in cultivation, the transfer of nutrients elements between males and females was achieved through hyphal links, which improved the photosynthetic capacity of the plants, thereby promoting the biomass of mulberry in the mixed female–male planting.
4.2. Mechanisms for the Restoration of Multi-Plant Symbiotic Systems
- (1)
- Plant–soil feedback (PSF) mechanisms, which primarily consist of plant–pathogenic fungi, plant–mycorrhizal fungi, and plant–soil enzymes, in which different plant species drive different changes in soil properties, and these changes, in turn, affect aspects of plant remediation efficiency, resilience, and competitiveness [108,109]. Studies have shown that plant species composition is the dominant factor influencing microbial community composition at the soil surface (0–10 cm), but microbial community changes are more sensitive to plant height responses in deeper soil layers (11–20 cm) [110]. Plant–soil feedbacks primarily promote the restoration of multi-plant systems by promoting the secretion of root exudates, the diversity and abundance of rhizosphere microorganisms, and the formation of symbiotic networks of clumping mycorrhizal fungi, which alter soil physicochemical properties and provide a favorable environment for plant growth. Mycorrhizal fungi that form clumps are the primary mechanism for the plant–soil feedback effect that drives phytoremediation [111]. Multi-plant co-cropping can increase microbial population diversity and activity of phosphatases, dehydrogenases, and especially urease [97] while reducing soil fungal pathogen abundance, thereby increasing plant productivity, because soil bacteria can produce disease-resistance-related defense enzymes such as chitinases to degrade the cell walls of fungal pathogens [21]. However, although plant–soil feedback mechanisms are a hot topic of research in this field [63,112,113], studies on their interactions with plants must still be conducted due to their small size and complex habitat heterogeneity.
- (2)
- The majority of plants have their own resistance mechanisms, and multi-plant symbiotic systems can enhance the resistance mechanisms of plants in the system or increase detoxification pathways, thereby decreasing the bioavailability and toxicity of pollutants. For instance, the tolerance and detoxification mechanisms of plants to heavy metals are related to their subcellular distribution; plants primarily use cell wall fixation or vesicle storage of heavy metal elements to reduce the degree of stress; and different forms of heavy metal elements have different migration abilities and produce different levels of toxicity in plants. In addition, Yue et al. [114] discovered that the Syngonium podophyllum-Peperomia tetraphylla co-planting system inhibited the reduction of metallic uranium (U) by the root system of Peperomia tetraphylla, facilitated the transport of U from roots to the plant parts above the ground, and enhanced the barrier effect of cell walls and vesicles on U, thereby reducing its toxic effects on plants. It effectively increased the biomass of both plants, as well as significantly increasing the bioaccumulation (BA), transport factor (TF), and bioaccumulation factor (BCF) of U in plants. The multi-plant combination system can also reduce oxidative stress while accelerating pollutant metabolism by increasing antioxidant substances in plants and antioxidant enzyme activity and detoxification enzyme activities, such as cytochrome P450 reductase (CPR), glutathione sulfhydryl transferase (GST), and glycosyl transferase (GT) [115].
- (3)
- Through plant–plant interactions, multi-plant symbiotic systems can enhance the overall remediation capacity of plant communities. According to the stress gradient hypothesis (SGH), competition dominates plant–plant interactions in benign or low-stress environments, whereas competition typically decreases and facilitation increases as environmental stress increases [19]. In addition, additional studies have demonstrated that facilitation is more likely to benefit plant species with low tolerance but high competitive ability [116]. It has also been demonstrated that litter decomposition and chemosensory effects in plant communities can increase the competitive advantage of hyperaccumulating plants, thereby decreasing the toxic effects of pollutants on neighboring plants [117]. Koelbener et al. [118] discovered, for instance, that competitive interactions between Salix caprea and Carex flava promoted the uptake of Zn by Salix caprea, thereby mitigating the negative effects of heavy metals on Carex flava. Consequently, a suitable phytocommunity composition can enhance plant–plant interactions to maximize a phytocommunity’s ability to degrade pollutants, thereby enhancing phytoremediation efficiency.
5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaney, R.L.; Malik, M.; Li, Y.M.; Brown, S.L.; Brewer, E.P.; Angle, J.S.; Baker, A.J. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997, 8, 279–284. [Google Scholar] [CrossRef]
- Huang, K.; Lin, L.; Chen, F.; Liao, M.A.; Wang, J.; Tang, Y.; Lai, Y.; Liang, D.; Xia, H.; Wang, X.; et al. Effects of live Myriophyllum aquaticum and its straw on cadmium accumulation in Nasturtium officinale. Environ. Sci. Pollut. Res. 2017, 24, 22503–22509. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, A.S.; Memarian, R. Phytoremediation of mixed soil contaminants. Water Air Soil Pollut. 2012, 223, 511–518. [Google Scholar] [CrossRef]
- Dai, J.; Chen, X.; Wang, J.; Cheng, Q.; Li, R.; Lin, L.; Wang, L. Solanum spp. straw improves phytoremediation ability of hyperaccumulator Galinsoga parviflora on cadmium-contaminated soil. Environ. Prog. Sustain. Energy 2022, 42, e13969. [Google Scholar] [CrossRef]
- Favas, P.J.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of soils contaminated with metals and metalloids at mining areas: Potential of native flora. Environ. Risk Assess. Soil Contam. 2014, 3, 485–516. [Google Scholar]
- Deng, L.; Li, Z.; Wang, J.; Liu, H.; Li, N.; Wu, L.; Hu, P.; Luo, Y.; Christie, P. Long-term field phytoextraction of zinc/cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int. J. Phytoremediation 2016, 18, 134–140. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, D.; Brereton, N.J.; Marchand, L.; Brisson, J.; Pitre, F.E.; Labrecque, M. Complementarity of three distinctive phytoremediation crops for multiple-trace element contaminated soil. Sci. Total Environ. 2018, 610, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Zheng, H.S.; Zhang, S.M.; Han, Z.; Shao, L.; He, W.H.; Gao, Y.; Wang, L.G.; He, P.M. Effects of different plant combinations on purification effect of simulated wastewater treatment plant tail water and root microbial community. Chin. J. Appl. Environ. 2022, 28, 387–393. [Google Scholar]
- Choudhury, M.I.; McKie, B.G.; Hallin, S.; Ecke, F. Mixtures of macrophyte growth forms promote nitrogen cycling in wetlands. Sci. Total Environ. 2018, 635, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.; Isbell, F.; Manning, P.; Connolly, J.; Bruelheide, H.; Ebeling, A.; Roscher, C.; Van Ruijven, J.; Weigelt, A.; Wilsey, B.; et al. Plant diversity effects on grassland productivity are robust to both nutrient enrichment and drought. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150277. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Qi, S.; Gu, X.S.; Wang, J.; Xie, X. An evaluation of EDTA additions for improving the phytoremediation efficiency of different plants under various cultivation systems. Ecotoxicology 2016, 25, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Brisson, J.; Rodriguez, M.; Martin, C.A.; Proulx, R. Plant diversity effect on water quality in wetlands: A meta-analysis based on experimental systems. Ecol. Appl. 2020, 30, e02074. [Google Scholar] [CrossRef]
- Fagundes, M.V.; Oliveira, R.S.; Fonseca, C.R.; Ganade, G. Nurse-target functional match explains plant facilitation strength. Flora 2022, 292, 152061. [Google Scholar] [CrossRef]
- Wang, S.; Ge, S.; Mai, W.; Tian, C. Nitrogen Promotes the Salt-Gathering Capacity of Suaeda salsa and Alleviates Nutrient Competition in the Intercropping of Suaeda salsa/Zea mays L. Int. J. Mol. Sci. 2022, 23, 15495. [Google Scholar] [CrossRef]
- Cuevas, J.G.; Silva, S.I.; León Lobos, P.; Ginocchio Cea, R. Nurse effect and herbivory exclusion facilitate plant colonization in abandoned mine tailings storage facilities in north-central Chile. Rev. Chil. Hist. Nat. 2013, 86, 63–74. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Nie, X.G.; Wang, L. Response of Three Aquatic Plant Combinations to Bisphenol A Stress. J. Nucl. Agric. Sci. 2021, 35, 1221–1230. [Google Scholar]
- Jia, P.; Liang, J.L.; Yang, S.X.; Zhang, S.C.; Liu, J.; Liang, Z.W.; Li, F.M.; Zeng, Q.W.; Fang, Z.; Liao, B.; et al. Plant diversity enhances the reclamation of degraded lands by stimulating plant–soil feedbacks. J. Appl. Ecol. 2020, 57, 1258–1270. [Google Scholar] [CrossRef]
- Klaus, V.H.; Whittingham, M.J.; Báldi, A.; Eggers, S.; Francksen, R.M.; Hiron, M.; Lellei-Kovács, E.; Rhymer, C.M.; Buchmann, N. Do biodiversity-ecosystem functioning experiments inform stakeholders how to simultaneously conserve biodiversity and increase ecosystem service provisioning in grasslands? Biol. Conserv. 2020, 245, 108552. [Google Scholar] [CrossRef]
- Liu, K.; Guan, X.; Li, C.; Zhao, K.; Yang, X.; Fu, R.; Li, Y.; Yu, F. Global perspectives and future research directions for the phytoremediation of heavy metal-contaminated soil: A knowledge mapping analysis from 2001 to 2020. Front. Environ. Sci. Eng. 2022, 16, 73. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemospheres 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. System and Method for Automatically Generating Systematic Reviews of a Scientific Field. U.S. Patent 8,566,360, 22 October 2013. [Google Scholar]
- Cui, X.; Guo, X.; Wang, Y.; Wang, X.; Zhu, W.; Shi, J.; Lin, C.; Gao, X. Application of remote sensing to water environmental processes under a changing climate. J. Hydrol. 2019, 574, 892–902. [Google Scholar] [CrossRef]
- Qin, X.N.; Lu, X.L.; Wu, C.Y. The knowledge mapping of domestic ecological security research: Bibliometric analysis based on citespace. Acta Ecol. Sin. 2014, 34, 3693–3703. [Google Scholar]
- Chen, Y.; Chen, C.M.; Hu, Z.G. Principles and Applications of Citation Space Analysis: A Practical Guide to CiteSpace; Science Press: Beijing, China, 2014. [Google Scholar]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S1), 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.Y.; Xie, Z.L.; Ba, Z.C. Research on the Influence of Mixed Keyword Selection Strategy on Co-word Analysis Results. Inf. Stud. Theory Appl. 2017, 40, 110–116. [Google Scholar]
- Langeveld, H.; Quist-Wessel, F.; Dimitriou, I.; Aronsson, P.; Baum, C.; Schulz, U.; Bolte, A.; Baum, S.; Köhn, J.; Weih, M.; et al. Assessing environmental impacts of short rotation coppice (SRC) expansion: Model definition and preliminary results. Bioenergy Res. 2012, 5, 621–635. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.W.; Chen, S.; Gao, X.J. Actualities, damage and management of soil cadmium pollution in China. Anhui Agric. Sci. Bull. 2015, 21, 104–107. [Google Scholar]
- Bian, F.Y.; Zhong, Z.K.; Li, C.Z.; Zhang, X.P.; Gu, L.J.; Huang, Z.C.; Gai, X.; Huang, Z.Y. Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J. Hazard. Mater. 2021, 416, 125898. [Google Scholar] [CrossRef] [PubMed]
- Epelde, L.; Becerril, J.M.; Barrutia, O.; González-Oreja, J.A.; Garbisu, C. Interactions between plant and rhizosphere microbial communities in a metalliferous soil. Environ. Pollut. 2010, 158, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Pan, G.; Wei, Y.; Zhao, N.N.; Gu, M.H.; He, B.; Wang, X.L. Effects of Claroideoglomus etunicatum Fungi Inoculation on Arsenic Uptake by Maize and Pteris vittata L. Toxics 2022, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Cao, X.R.; Yao, Z.Y.; Lin, Q.; Yan, B.B.; Cui, X.Q.; He, Z.L.; Yang, X.E.; Wang, C.W.; Chen, G.Y. Phytoremediation of Cd-contaminated farmland soil via various Sedum alfredii-oilseed rape cropping systems: Efficiency comparison and cost-benefit analysis. J. Hazard. Mater. 2021, 419, 126489. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Wan, X.; Shi, H.; Yang, J.; Ma, C.; Lei, M.; Chen, T. Temporal and spatial differentiation characteristics of soil arsenic during the remediation process of Pteris vittata L. and Citrus reticulata Blanco intercropping. Sci. Total Environ. 2022, 812, 152475. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C. Dynamic response of enzymatic activity and microbial community structure in metal (loid)-contaminated soil with tree-herb intercropping. Geoderma 2019, 345, 5–16. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C. Effects of tree-herb co-planting on the bacterial community composition and the relationship between specific microorganisms and enzymatic activities in metal (loid)-contaminated soil. Chemosphere 2019, 220, 237–248. [Google Scholar] [CrossRef]
- Wei, Z.B.; Guo, X.F.; Wu, Q.T.; Long, X.X.; Penn, C.J. Phytoextraction of heavy metals from contaminated soil by co-cropping with chelator application and assessment of associated leaching risk. Int. J. Phytoremediation 2011, 13, 717–729. [Google Scholar] [CrossRef]
- Verret, V.; Gardarin, A.; Pelzer, E.; Mediene, S.; Makowski, D.; Valantin-Morison, M. Can legume companion plants control weeds without decreasing crop yield? A meta-analysis. Field Crops Res. 2017, 204, 158–168. [Google Scholar] [CrossRef]
- Powlson, D.S.; Prookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Yang, W.; Pan, Y.; Yu, X.; Xiao, S.; Wang, W.; Lu, M. Biochar and Cropping Systems Changed Soil Copper Speciation and Accumulation in Sweet Corn and Soybean. Plants 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Chen, H. The effects of biochar and intercropping on the Cd, Cr and Zn speciation in soils and plant uptake by Machilus pauhoi. Bull. Environ. Contam. Toxicol. 2017, 98, 574–581. [Google Scholar] [CrossRef]
- Wolfe, A.K.; Bjornstad, D.J. Why would anyone object? An exploration of social aspects of phytoremediation acceptability. Crit. Rev. Plant Sci. 2002, 21, 429–438. [Google Scholar] [CrossRef]
- Milić, D.; Luković, J.; Ninkov, J.; Zeremski-Skoric, T.; Zoric, L.; Vasin, J.; Milic, S. Heavy metal content in halophytic plants from inland and maritime saline areas. Cent. Eur. J. Biol. 2012, 7, 307–317. [Google Scholar] [CrossRef]
- Wieshammer, G.; Unterbrunner, R.; Garcia, T.B.; Zivkovic, M.F.; Puschenreiter, M.; Wenzel, W.W. Phytoextraction of Cd and Zn from agricultural soils by Salix ssp. and intercropping of Salix caprea and Arabidopsis halleri. Plant Soil 2007, 298, 255–264. [Google Scholar] [CrossRef]
- Sun, M.; Fu, D.; Teng, Y.; Shen, Y.; Luo, Y.; Li, Z.; Christie, P. In situ phytoremediation of PAH-contaminated soil by intercropping alfalfa (Medicago sativa L.) with tall fescue (Festuca arundinacea Schreb.) and associated soil microbial activity. J. Soils Sediments 2011, 11, 980–989. [Google Scholar] [CrossRef]
- Li, Y.F.; Zheng, G.D.; Yang, J.X.; Guo, J.M.; Yang, J.; Chen, T.B. Effects of water-soluble chitosan on Hylotelephium spectabile and soybean growth, as well as Cd uptake and phytoextraction efficiency in a co-planting cultivation system. Int. J. Phytoremediation 2023, 25, 339–349. [Google Scholar] [CrossRef]
- Ma, L.; Huang, L.; Liu, Q.; Xu, S.; Wen, Z.; Qin, S.; Li, T.; Feng, Y. Positive effects of applying endophytic bacteria in eggplant-Sedum intercropping system on Cd phytoremediation and vegetable production in cadmium polluted greenhouse. J. Environ. Sci. 2022, 115, 383–391. [Google Scholar] [CrossRef]
- Zou, J.; Song, F.; Lu, Y.; Zhuge, Y.; Niu, Y.; Lou, Y.; Pan, H.; Zhang, P.; Pang, L. Phytoremediation potential of wheat intercropped with different densities of Sedum plumbizincicola in soil contaminated with cadmium and zinc. Chemosphere 2021, 276, 130223. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Michael, P.I.; Krishnaswamy, M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ. Exp. Bot. 2011, 74, 171–177. [Google Scholar] [CrossRef]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amezaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Wang, S.; Cao, Y.; Geng, B.; Yang, K.; Bai, Z. Succession law and model of reconstructed soil quality in an open-pit coal mine dump in the loess area. J. Environ. Manag. 2022, 312, 114923. [Google Scholar] [CrossRef]
- Caravaca, F.; Aiguacil, M.M.; Torres, P.; Roldan, A. Plant type mediates rhizospheric microbial activities and soil aggregation in a semiarid Mediterranean salt marsh. Geoderma 2005, 124, 375–382. [Google Scholar] [CrossRef]
- Garcia, C.; Roldan, A.; Hernandez, T. Ability of different plant species to promote microbiological processes in semiarid soil. Geoderma 2005, 124, 193–202. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, F.L.; Lu, J.L.; Feng, S.W.; Wu, Z.H.; Liang, J.L.; Jia, P.; Li, J.T. Soil enzyme activities and influencing factors in farmlands around metalliferous mine wastelands in China. J. Agro-Environ. Sci. 2022, 41, 2797–2804. [Google Scholar]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total Environ. 2017, 607, 1419–1427. [Google Scholar] [CrossRef]
- Gómez-Sagasti, M.T.; Garbisu, C.; Urra, J.; Miguez, F.; Artetxe, U.; Hernandez, A.; Vilela, J.; Alkorta, I.; Becerril, J.M. Mycorrhizal-assisted phytoremediation and intercropping strategies improved the health of contaminated soil in a peri-urban area. Front. Plant Sci. 2021, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Fang, L.; Wang, M.; Jiang, M.; Shen, G. Intercropping of gramineous pasture ryegrass (Lolium perenne L.) and leguminous forage alfalfa (Medicago sativa L.) increases the resistance of plants to heavy metals. J. Chem. 2018, 7803408. [Google Scholar] [CrossRef]
- Schneider, J.; Bundschuh, J.; do Nascimento CW, A. Arbuscular mycorrhizal fungi-assisted phytoremediation of a lead-contaminated site. Sci. Total Environ. 2016, 572, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Li, T.K.; Chen, L.; Pang, D.B.; Gao, F. Research Progress on Plant-Soil Feedback Based on Bibliometrics. Chin. J. Grassl. 2022, 44, 73–86. [Google Scholar]
- Burges, A.; Epelde, L.; Blanco, F.; Becerril, J.M.; Garbisu, C. Ecosystem services and plant physiological status during endophyte-assisted phytoremediation of metal contaminated soil. Sci. Total Environ. 2017, 584, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Requena, N. Measuring quality of service: Phosphate’a la carte’by arbuscular mycorrhizal fungi. New Phytol. 2005, 168, 268–271. [Google Scholar] [CrossRef]
- Wang, F.Y.; Lin, X.G.; Yin, R. Role of microbial inoculation and chitosan in phytoextraction of Cu, Zn, Pb and Cd by Elsholtzia splendens–a field case. Environ. Pollut. 2007, 147, 248–255. [Google Scholar] [CrossRef]
- Hu, J.; Chan, P.T.; Wu, F.; Wu, S.; Zhang, J.; Lin, X.; Wong, M.H. Arbuscular mycorrhizal fungi induce differential Cd and P acquisition by Alfred stonecrop (Sedum alfredii Hance) and upland kangkong (Ipomoea aquatica Forsk.) in an intercropping system. Appl. Soil Ecol. 2013, 63, 29–35. [Google Scholar] [CrossRef]
- Giasson, P.; Karam, A.; Jaouich, A. Arbuscular mycorrhizae and alleviation of soil stresses on plant growth. In Mycorrhizae: Sustainable Agriculture and Forestry; Springer: Dordrecht, The Nertherlands, 2008; pp. 99–134. [Google Scholar]
- Humel, S.; Schmidt, S.N.; Sumetzberger-Hasinger, M.; Mayer, P.; Loibner, A.P. Enhanced accessibility of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic PAHs in industrially contaminated soil after passive dosing of a competitive sorbate. Environ. Sci. Technol. 2017, 51, 8017–8026. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.F.; Zhang, X.; Zhu, F.J.; Li, Y.F.; Cai, M.; Kallenborn, R. Polycyclic aromatic hydrocarbons in the marine atmosphere from the Western Pacific to the Southern Ocean: Spatial variability, Gas/particle partitioning, and source apportionment. Environ. Sci. Technol. 2022, 56, 6253–6261. [Google Scholar] [PubMed]
- Zhang, X.T.; Peng, S.C.; Wang, J.Z.; Zhang, X.X.; Huang, G.F.; Chen, G.Z. Pollution characteristics, source apportionment and risk assessmenl of polyeyeliearomatic hydrocarbons in Lake Chaohu. Acta Sci. Circumstantiae 2023, 43, 47–57. [Google Scholar]
- US EPA. Toxic and Priority Pollutants under the Clean Water Act. US EPA. Available online: https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 14 July 2023).
- Umeh, A.C.; Vázquez-Cuevas, G.M.; Semple, K.T. Mineralisation of 14C-phenanthrene in PAH-diesel contaminated soil: Impact of Sorghum bicolor and Medicago sativa mono-or mixed culture. Appl. Soil Ecol. 2018, 125, 46–55. [Google Scholar] [CrossRef]
- Olson, P.E.; Castro, A.; Joern, M.; DuTeau, N.M.; Pilon-Smits, E.; Reardon, K.F. Effects of agronomic practices on phytoremediation of an aged PAH-contaminated soil. J. Environ. Qual. 2008, 37, 1439–1446. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Leimer, S.; Meusel, H.; Velescu, A.; Dassen, S.; Eisenhauer, N.; Hoffmann, T.; Oelmann, Y.; Wilcke, W. Plant diversity enhances the natural attenuation of polycyclic aromatic compounds (PAHs and oxygenated PAHs) in grassland soils. Soil Biol. Biochem. 2019, 129, 60–70. [Google Scholar] [CrossRef]
- Meng, L.; Qiao, M.; Arp, H.P.H. Phytoremediation efficiency of a PAH-contaminated industrial soil using ryegrass, white clover, and celery as mono-and mixed cultures. J. Soils Sediments 2011, 11, 482–490. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.L.; Beri, W.T.; Khan, M.B.; Yang, X.E. Fava bean intercropping with Sedum alfredii inoculated with endophytes enhances phytoremediation of cadmium and lead co-contaminated field. Environ. Pollut. 2020, 265, 114861. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, J.; Yu, X.N.; Xie, Y.D.; Lin, L.J.; Sun, G.C.; Li, H.X.; Liao, M.A.; Liang, D.; Xia, H.; et al. Intercropping with Solanum nigrum and Solanum photeinocarpum from Two Ecoclimatic Regions Promotes Growth and Reduces Cadmium Uptake of Eggplant Seedlings. Pedosphere 2017, 27, 638–644. [Google Scholar] [CrossRef]
- Wan, X.M.; Lei, M. Intercropping efficiency of four arsenic hyperaccumulator Pteris vittata populations as intercrops with Morus alba. Environ. Sci. Pollut. Res. 2018, 25, 12600–12611. [Google Scholar] [CrossRef]
- Zu, Y.Q.; Qin, L.; Zhan, F.D.; Wu, J.; Li, Y.; Chen, J.J.; Wang, J.X.; Hu, W.Y. Intercropping of Sonchus asper and Vicia faba affects plant cadmium accumulation and root responses. Pedosphere 2020, 30, 457–465. [Google Scholar] [CrossRef]
- Xia, H.; Liang, D.; Chen, F.B.; Liao, M.A.; Lin, L.J.; Tang, Y.; Lv, X.L.; Li, H.X.; Wang, Z.H.; Wang, X.; et al. Effects of mutual intercropping on cadmium accumulation by the accumulator plants Conyza canadensis, Cardamine hirsuta, and Cerstium glomeratum. Int. J. Phytoremediation 2018, 20, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Echevarria, G.; Sulce, S.; Morel, J.L. Improving the agronomy of Alyssum murale for extensive phytomining: A five-year field study. Int. J. Phytoremediation 2015, 17, 117–127. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Pan, Y.; Wang, Z.; Zhu, C. Heavy metal absorption status of five plant species in monoculture and intercropping. Plant Soil 2011, 345, 237–245. [Google Scholar] [CrossRef]
- Wei, S.; Pan, S. Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species. J. Soils Sediments 2010, 10, 886–894. [Google Scholar] [CrossRef]
- Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011.
- Geng, K.; Sun, S.; Huang, Z.; Huang, C.; Wu, C.; Deng, T.; Tang, Y.; Ruan, J.; He, C.; Morel, J.L.; et al. Key processes and progress in phytomining of nickel contaminated soils: A review. Chin. J. Biotechnol. 2020, 36, 436–449. [Google Scholar]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, H.T.; Chang, X.; Wu, G.L. Higher species diversity improves soil water infiltration capacity by increasing soil organic matter content in semiarid grasslands. Land Degrad. Dev. 2019, 13, 1599–1606. [Google Scholar] [CrossRef]
- Baer, S.G.; Adams, T.; Scott, D.A.; Blair, J.M.; Collins, S.L. Soil heterogeneity increases plant diversity after 20 years of manipulation during grassland restoration. Ecol. Appl. 2020, 30, e02014. [Google Scholar] [CrossRef]
- Gornish, E.S.; Shaw, J.; Gillespie, B.M. Using strip seeding to test how restoration design affects randomness of community assembly. Restor. Ecol. 2019, 27, 1199–1205. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wang, J.; Zhao, T.; Cheng, S.; Shao, D.; Xu, J. Effects of species diversity on plant growth and remediation of Cd contamination in soil. Acta Sci. Circumst. 2016, 36, 2103–2113. [Google Scholar]
- Gao, Y.; Miao, C.; Xia, J.; Mao, L.; Wang, Y.; Zhou, P. Plant diversity reduces the effect of multiple heavy metal pollution on soil enzyme activities and microbial community structure. Front. Environ. Sci. Eng. 2012, 6, 213–223. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, S.; Zhang, R.; Qi, W.; Zhang, R.; Knops, J.M.H.; Lu, J. Magnitude of species diversity effect on aboveground plant biomass increases through successional time of abandoned farmlands on the eastern Tibetan Plateau of China. Land Degrad. Dev. 2017, 28, 370–378. [Google Scholar] [CrossRef]
- Abu Hanif, M.; Yu, Q.; Rao, X.; Shen, W. Disentangling the contributions of plant taxonomic and functional diversities in shaping aboveground biomass of a restored forest landscape in Southern China. Plants 2019, 8, 612. [Google Scholar] [CrossRef]
- Fujii, S.; Mori, A.S.; Koide, D.; Makoto, K.; Matsuoka, S.; Osono, T.; Isbell, F. Disentangling relationships between plant diversity and decomposition processes under forest restoration. J. Appl. Ecol. 2017, 54, 80–90. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S.; Roumet, C. Complementarity as a mechanism of coexistence between functional groups of grasses. J. Ecol. 2007, 95, 1296–1305. [Google Scholar] [CrossRef]
- Hou, S.S.; Wang, L.; Xu, H.S.; Wang, H.; Wang, X.X. Ecological mechanisms and guiding principles of mixed cropping of crop varieties. Chin. J. Eco-Agric. 2023, 31, 1–10. [Google Scholar]
- Zhang, L.; Liu, W.; Liu, S.; Zhang, P.; Ye, C.; Liang, H. Revegetation of a barren rare earth mine using native plant species in reciprocal plantation: Effect of phytoremediation on soil microbiological communities. Environ. Sci. Pollut. Res. 2020, 27, 2107–2119. [Google Scholar] [CrossRef]
- Wei, Z.; Maxwell, T.; Robinson, B.; Dickinson, N. Plant Species Complementarity in Low-Fertility Degraded Soil. Plants 2022, 11, 1370. [Google Scholar] [CrossRef]
- Lin, T.; Tang, J.; He, F.; Chen, G.; Shi, Y.; Wang, X.; Han, S.; Li, S.; Zhu, T.; Chen, L. Sexual differences in above-and belowground herbivore resistance between male and female poplars as affected by soil cadmium stress. Sci. Total Environ. 2022, 803, 150081. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Liu, X.; Korpelainen, H.; Li, C. Intra-and intersexual interactions shape microbial community dynamics in the rhizosphere of Populus cathayana females and males exposed to excess Zn. J. Hazard. Mater. 2021, 402, 123783. [Google Scholar] [CrossRef]
- Bu, C.L.; Yan, M.J.; Dong, T.F.; Liu, G.; Huang, G.Q.; Xu, X. Effects of arbuscular mycorrhizal fungi (AMF) on biomass, photosynthetic characteristics and infection rate of mulberry (Morusalba) in different combination groups. Plant Physiol. J. 2022, 58, 2181–2190. [Google Scholar]
- Kinnebrew, E.; Champlin, L.K.; Galford, G.L.; Neill, C. Woody plant encroachment into coastal grasslands: Consequences for soil properties and plant diversity. Reg. Environ. Chang. 2020, 20, 1–13. [Google Scholar]
- Wei, W.; Zhu, P.; Chen, P.; Huang, Q.; Bai, X.; Ni, G.; Hou, Y. Mixed evidence for plant–soil feedbacks in forest invasions. Oecologia 2020, 193, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Norton, B.A.; Bending, G.D.; Clark, R.; Corstanje, R.; Dunnett, N.; Evans, K.L.; Grafius, D.R.; Gravestock, E.; Grice, S.M.; Harris, J.A.; et al. Urban meadows as an alternative to short mown grassland: Effects of composition and height on biodiversity. Ecol. Appl. 2019, 29, e01946. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.; Bever, J.D. Mycorrhizal feedbacks generate positive frequency dependence accelerating grassland succession. J. Ecol. 2019, 107, 622–632. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Li, Q.; Huang, Z. Bamboo-based agroforestry changes phytoremediation efficiency by affecting soil properties in rhizosphere and non-rhizosphere in heavy metal-polluted soil (Cd/Zn/Cu). J. Soils Sediments 2022, 23, 368–378. [Google Scholar] [CrossRef]
- Wan, T.; Dong, X.; Yu, L.; Huang, H.; Li, D.; Han, H.; Jia, Y.; Zhang, Y.; Liu, Z.; Zhang, Q.; et al. Comparative study of three Pteris vittata-crop intercropping modes in arsenic accumulation and phytoremediation efficiency. Environ. Technol. Innov. 2021, 24, 101923. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, D.; Li, G.; Yi, H.; Zhai, K.; Hu, N.; Zhang, H.; Dai, Z.; Ma, J.; Li, F.; et al. Enhanced effects and mechanisms of Syngonium podophyllum-Peperomia tetraphylla co-planting on phytoremediation of low concentration uranium-bearing wastewater. Chemosphere 2021, 279, 130810. [Google Scholar]
- Nie, X.; Wang, L. Plant species compositions alleviate toxicological effects of bisphenol A by enhancing growth, antioxidant defense system, and detoxification. Environ. Sci. Pollut. Res. 2022, 29, 65755–65770. [Google Scholar] [CrossRef]
- Gross, N.; Liancourt, P.; Choler, P.; Suding, K.N.; Lavorel, S. Strain and vegetation effects on local limiting resources explain the outcomes of biotic interactions. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 9–19. [Google Scholar] [CrossRef]
- Morris, C.; Grossl, P.R.; Call, C.A. Elemental allelopathy: Processes, progress, and pitfalls. Plant Ecol. 2009, 202, 1–11. [Google Scholar] [CrossRef]
- Koelbener, A.; Ramseier, D.; Suter, M. Competition alters plant species response to nickel and zinc. Plant Soil 2008, 303, 241–251. [Google Scholar] [CrossRef]
- Ullah, H.; Treesubsuntorn, C.; Thiravetyan, P. Enhancing mixed toluene and formaldehyde pollutant removal by Zamioculcas zamiifolia combined with Sansevieria trifasciata and its CO2 emission. Environ. Sci. Pollut. Res. 2021, 28, 538–546. [Google Scholar] [CrossRef]
- Siswanto, D.; Permana, B.H.; Treesubsuntorn, C.; Thiravetyan, P. Sansevieria trifasciata and Chlorophytum comosum botanical biofilter for cigarette smoke phytoremediation in a pilot-scale experiment—Evaluation of multi-pollutant removal efficiency and CO2 emission. Air Qual. Atmos. Health 2020, 13, 109–117. [Google Scholar] [CrossRef]
- Perreault, R.; Laforest-Lapointe, I. Plant-microbe interactions in the phyllosphere: Facing challenges of the anthropocene. ISME J. 2022, 16, 339–345. [Google Scholar]
- Shafiq, M.; Jamil, S. Role of plant growth regulators and a saprobic fungus in enhancement of metal phytoextraction potential and stress alleviation in pearl millet. J. Hazard. Mater. 2012, 237, 186–193. [Google Scholar]
- Martinez-Oro, D.; Parraga-Aguado, I.; Querejeta, J.I.; Alvarez-Rogel, J.; Conesa, H.M. Nutrient limitation determines the suitability of a municipal organic waste for phytomanaging metal (loid) enriched mine tailings with a pine-grass co-culture. Chemosphere 2019, 214, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Clemente, R.; Arco-Lázaro, E.; Pardo, T.; Martín, I.; Sánchez-Guerrero, A.; Sevilla, F.; Bernal, M.P. Combination of soil organic and inorganic amendments helps plants overcome trace element induced oxidative stress and allows phytostabilisation. Chemosphere 2019, 223, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cicatelli, A.; Guarino, F.; Baldan, E.; Castiglione, S. Genetic and biochemical characterization of rhizobacterial strains and their potential use in combination with chelants for assisted phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 8866–8878. [Google Scholar] [CrossRef]
- Yung, L.; Sirguey, C.; Azou-Barre, A.; Blaudez, D. Natural fungal endophytes from Noccaea caerulescens mediate neutral to positive effects on plant biomass, mineral nutrition and Zn phytoextraction. Front. Microbiol. 2021, 12, 1726. [Google Scholar]
- Liu, A.; Wang, W.; Zheng, X.; Chen, X.; Fu, W.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 2022, 302, 134900. [Google Scholar] [CrossRef] [PubMed]
- Mahohi, A.; Raiesi, F. Functionally dissimilar soil organisms improve growth and Pb/Zn uptake by Stachys inflata grown in a calcareous soil highly polluted with mining activities. J. Environ. Manag. 2019, 247, 780–789. [Google Scholar]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar]
- Tsyganov, V.E.; Tsyganova, A.V.; Gorshkov, A.P.; Seliverstova, E.V.; Kim, V.E.; Chizhevskaya, E.P.; Belimov, A.A.; Serova, T.A.; Ivanova, K.A.; Kulaeva, O.A.; et al. Efficacy of a plant-microbe system: Pisum sativum (L.) cadmium-tolerant mutant and Rhizobium leguminosarum strains, expressing pea metallothionein genes PsMT1 and PsMT2, for cadmium phytoremediation. Front. Microbiol. 2020, 11, 15. [Google Scholar]
- Fernandes, J.P.; Guiomar, N. Simulating the stabilization effect of soil bioengineering interventions in Mediterranean environments using limit equilibrium stability models and combinations of plant species. Ecol. Eng. 2016, 88, 122–142. [Google Scholar]
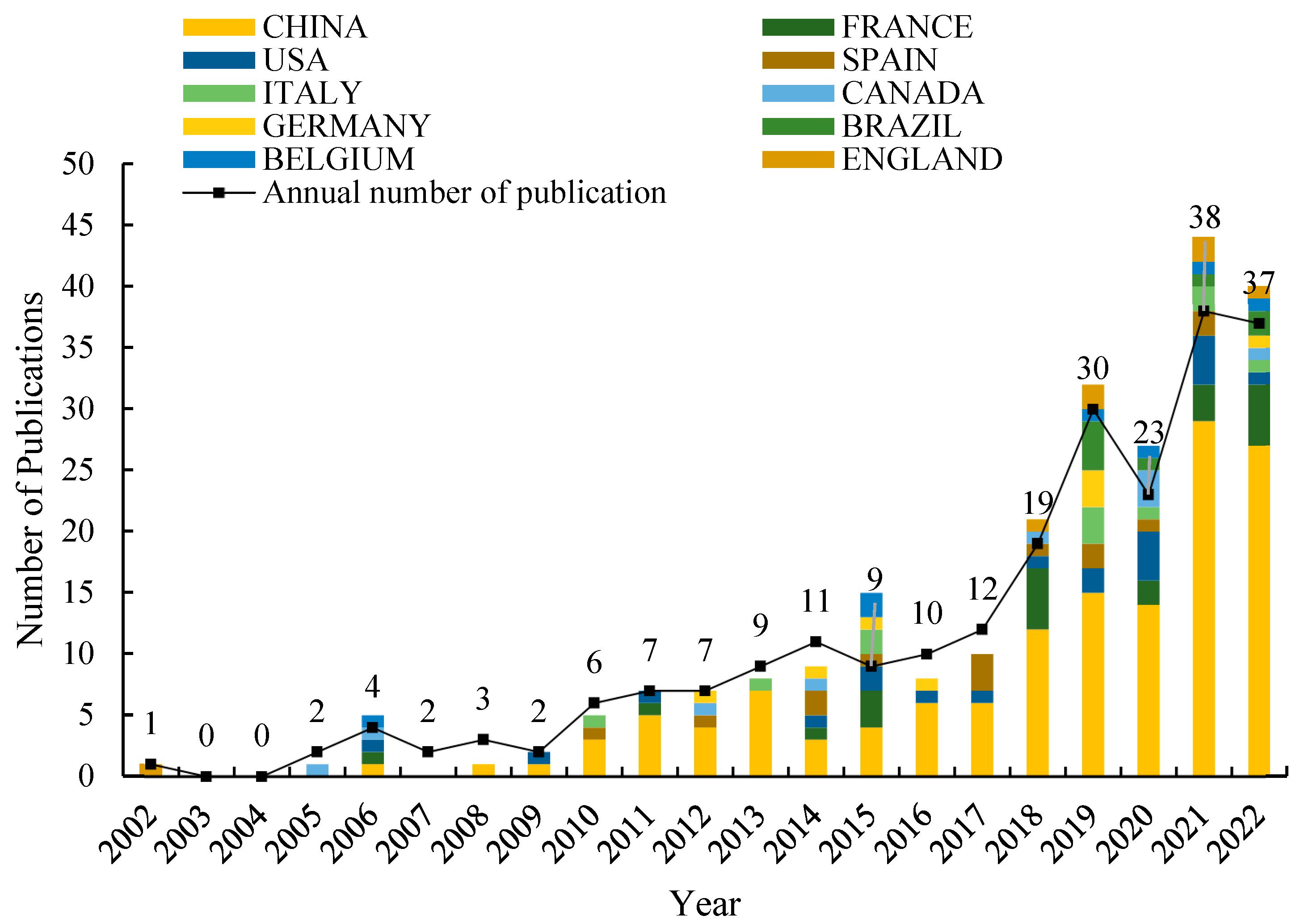
| Rank | Country | Institution | Category | Journal Source | Author |
|---|---|---|---|---|---|
| 1 | China (138, 59.48%) | Chinese Academy of Sciences (33, 14.22%) | Environmental Sciences (176, 75.86%) | International Journal of Phytoremediation (26, 11.21%) | Lin LJ (12, 5.17%) |
| 2 | France (21, 9.05%) | Inrae (17, 7.33%) | Soil Science (32, 13.79%) | Environmental Science and Pollution Research (22, 9.48%) | Li HS (8, 3.45%) |
| 3 | USA (20, 8.62%) | University of Chinese Academy of Sciences Cas (16, 6.90%) | Engineering Environmental (24, 10.35%) | Science of the Total Environment (22, 9.48%) | Tang Y (8, 3.45%) |
| 4 | Spain (14, 6.03%) | Sichuan Agricultural University (15, 6.47%) | Plant Sciences (19, 8.19%) | Chemosphere (18, 7.76%) | Wang J (8, 3.45%) |
| 5 | Italy (11, 4.74%) | South China Agricultural University (14, 6.03%) | Ecology (18, 7.76%) | Environmental Pollution (9, 3.88%) | Wang X (8, 3.45%) |
| 6 | Canada (9, 3.88%) | Institute of Soil Science Cas (13, 5.60%) | Agronomy (13, 5.60%) | Journal of Soils and Sediments (9, 3.88%) | Christie P (7, 3.02%) |
| 7 | Germany (8, 3.45%) | Zhejiang University (13, 5.60%) | Toxicology (12, 5.17%) | Ecotoxicology and Environmental Safety (8, 3.45%) | Liang D (7, 3.02%) |
| 8 | Brazil (8, 3.45%) | Institute of Geographic Sciences Natural Resources Research Cas (12, 5.17%) | Water Resources (8, 3.45%) | Journal of Hazardous Materials (7, 3.02%) | Liao MA (7, 3.02%) |
| 9 | Belgium (7, 3.02%) | Ministry of Agriculture Rural Affairs (11, 4.74%) | Meteorology Atmospheric Sciences (6, 2.59%) | Plant and Soil (7, 3.02%) | Luo J (7, 3.02%) |
| 10 | England (7, 3.02%) | Universite de Lorraine (10, 4.31%) | Green Sustainable Science Technology (5, 2.16%) | Ecological Engineering (6, 2.59%) | Luo YM (7, 3.02%) |
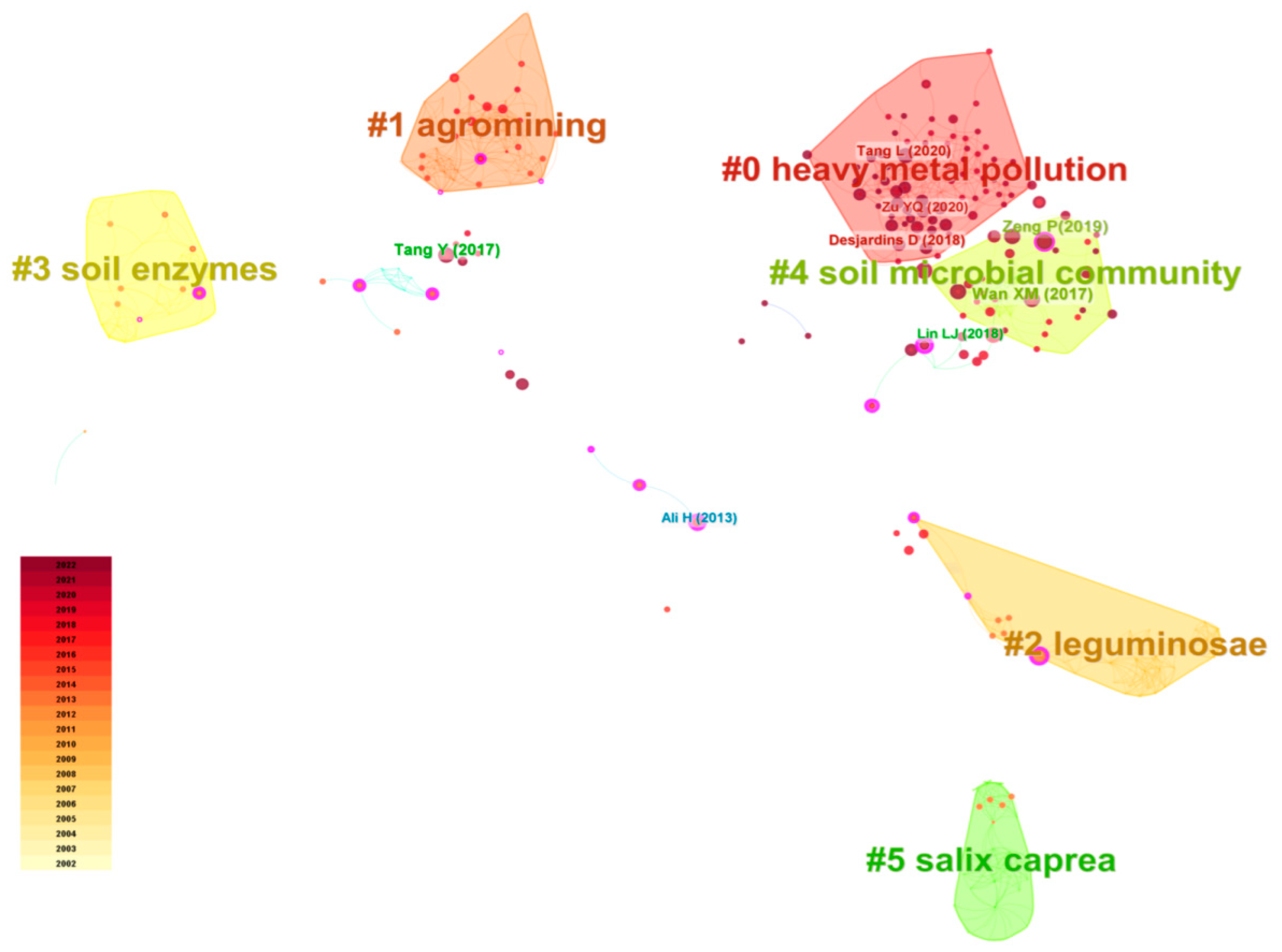
| Cluster | Size | Silhouette | Top Team (LLR) |
|---|---|---|---|
| #0 short rotation coppice | 63 | 0.67 | Remediation (0.1), Pteris vittata L., cadmium accumulation, microbial community, organic acid |
| #1 straw | 50 | 0.73 | Phytoremediation (0.22), Cd (0.13), Zn, maize, heavy metal pollution |
| #2 heavy metal | 44 | 0.81 | Phytoextraction (0.13), soil, cadmium (0.18), zinc, lead |
| #3 soil enzymes | 41 | 0.83 | heavy metal (0.26), biodiversity, enzyme activity, water |
| #4 glomus caledonium | 38 | 0.83 | Accumulation (0.29), plant, Sedum alfredii, Pb, Cu |
| #5 phenanthrene | 37 | 0.93 | Rhizosphere (0.16), degradation, bioremediation, polycyclic aromatic hydrocarbon, biodegradation |
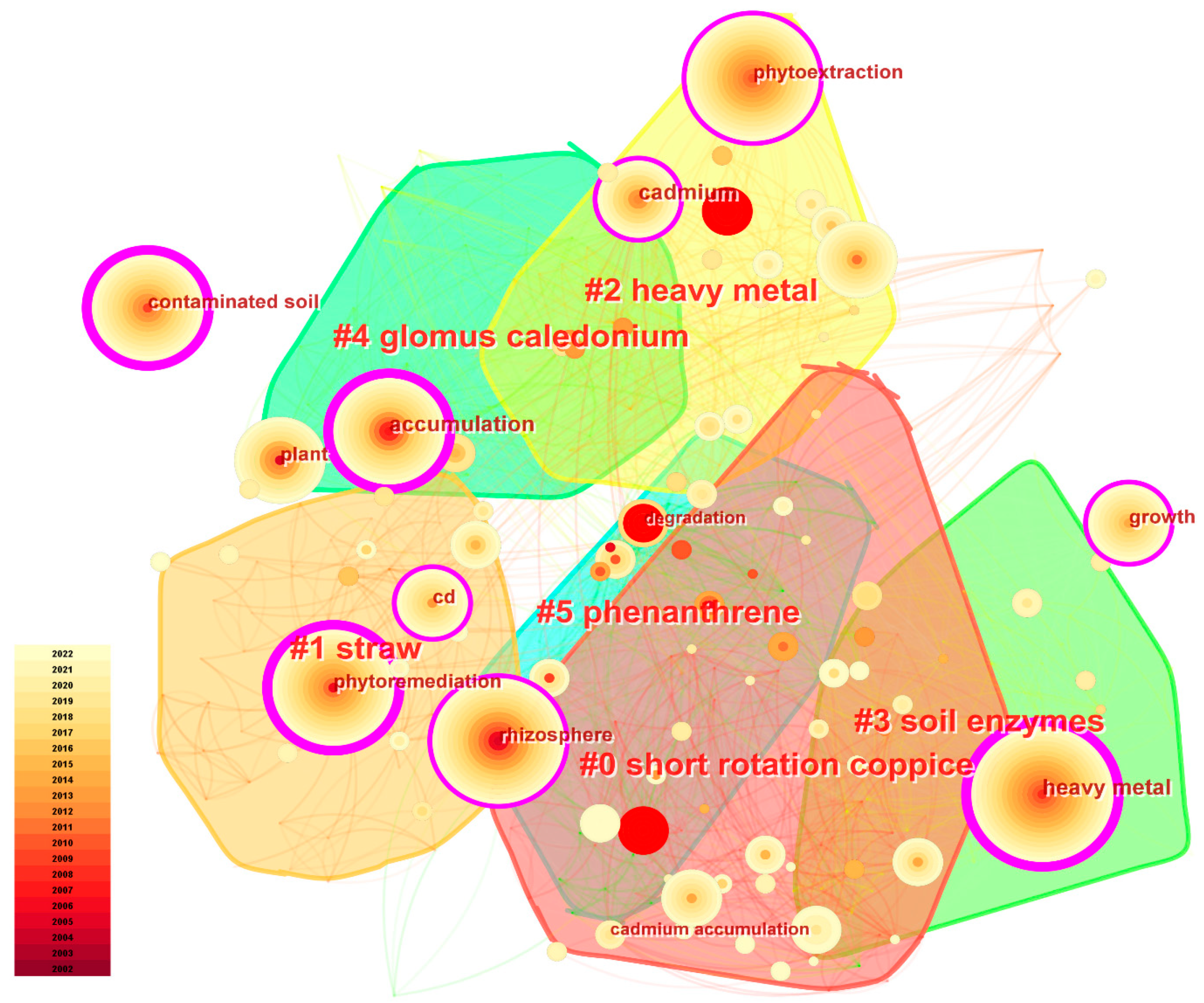
| Cluster | Author | Journal | Title | CF × BC | Time Horizon |
|---|---|---|---|---|---|
| #0 heavy metal pollution | Desjardins D [9] | Science of the Total Environment | Complementarity of three distinctive phytoremediation crops for multiple-trace element contaminated soil | 0.39 | 2014–2018 |
| #1 agromining | Bani A [86] | International Journal of Phytoremediation | Improving the Agronomy of Alyssum murale for Extensive Phytomining: A Five-Year Field Study | 0.72 | 2010–2018 |
| #2 Leguminosae | An LY [87] | Plant Soil | Heavy metal absorption status of five plant species in monoculture and intercropping | 0.99 | 2004–2012 |
| #3 soil enzymes | Wei SQ [88] | J Soils Sediments | Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species | 0.80 | 2005–2011 |
| #4 soil microbial community | Zeng P [80] | Science of the Total Environment | Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb, and Zn in contaminated soil | 3.42 | 2014–2021 |
| #5 Salix caprea | Marschner H [89] | Academic press | Marschner’s Mineral Nutrition of Higher Plants | 0.27 | 2003–2012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Sheng, Q.; Zhu, Z.; Liu, Y. Application of Multi-Plant Symbiotic Systems in Phytoremediation: A Bibliometric Review. Sustainability 2023, 15, 12252. https://doi.org/10.3390/su151612252
Song S, Sheng Q, Zhu Z, Liu Y. Application of Multi-Plant Symbiotic Systems in Phytoremediation: A Bibliometric Review. Sustainability. 2023; 15(16):12252. https://doi.org/10.3390/su151612252
Chicago/Turabian StyleSong, Shuang, Qianqian Sheng, Zunling Zhu, and Yanli Liu. 2023. "Application of Multi-Plant Symbiotic Systems in Phytoremediation: A Bibliometric Review" Sustainability 15, no. 16: 12252. https://doi.org/10.3390/su151612252
APA StyleSong, S., Sheng, Q., Zhu, Z., & Liu, Y. (2023). Application of Multi-Plant Symbiotic Systems in Phytoremediation: A Bibliometric Review. Sustainability, 15(16), 12252. https://doi.org/10.3390/su151612252