Enrichment of Anammox Bacteria Using Anammox Sludge as a Primer Combined with Ordinary Activated Sludge
Abstract
1. Introduction
| Cultivation Strategy | Inf. NH4+-N | Inf. NO2−-N | TN Removal Efficiency | NRR | Start-Up Time | References |
|---|---|---|---|---|---|---|
| mg/L | mg/L | % | kg N/(m3·d) | d | ||
| Addition of extracellular polymeric substances. | 60–200 | 80–250 | - | 0.05–0.75 | 19 | [13] |
| Activated sludge as inoculum, fed with a low concentration of anammox sludge. | 30–160 | 40–180 | >70 | - | 5 | [19] |
| Biochar accelerates start-up | 140 | 140 | 80.0 ± 9.6 | 1.48 | 85 | [27] |
| low frequency and intensity ultrasound. | 70 | 70 | 86 | 0.68 | 53 | [28] |
| Inoculating denitrifying granular sludge mixed AnAOB | 50–220 | 50–300 | - | 0.72 | 28 | [29] |
| inoculated with flocculent nitritation sludge | 75–210 | 75–230 | - | 0.5 | 73 | [30] |
| feed in ferrous iron. | 50 | 50 | - | 0.16 | 50 | [31] |
| Inoculating perchlorate reduction sludge and a small amount of anammox sludge. | 200 | 200 | 73.20 ± 6.79% | - | 41 | [32] |
| A novel electrolysis-integrated anammox system | 420 | 420 | 90.12–90.80 | - | - | [33] |
| cultivation strategy | 90–95 | 105–110 | 75.3 | 0.67 | - | [34] |
2. Materials and Methods
2.1. Experimental Setup and Operation Strategy
2.2. Seed Sludge and Feed Wastewater
2.3. Batch Test
2.4. Extraction and Determination of Extracellular Polymeric Substances
2.5. Scanning Electron Microscopy Observations
2.6. Microbial Analysis Methods
3. Results and Discussion
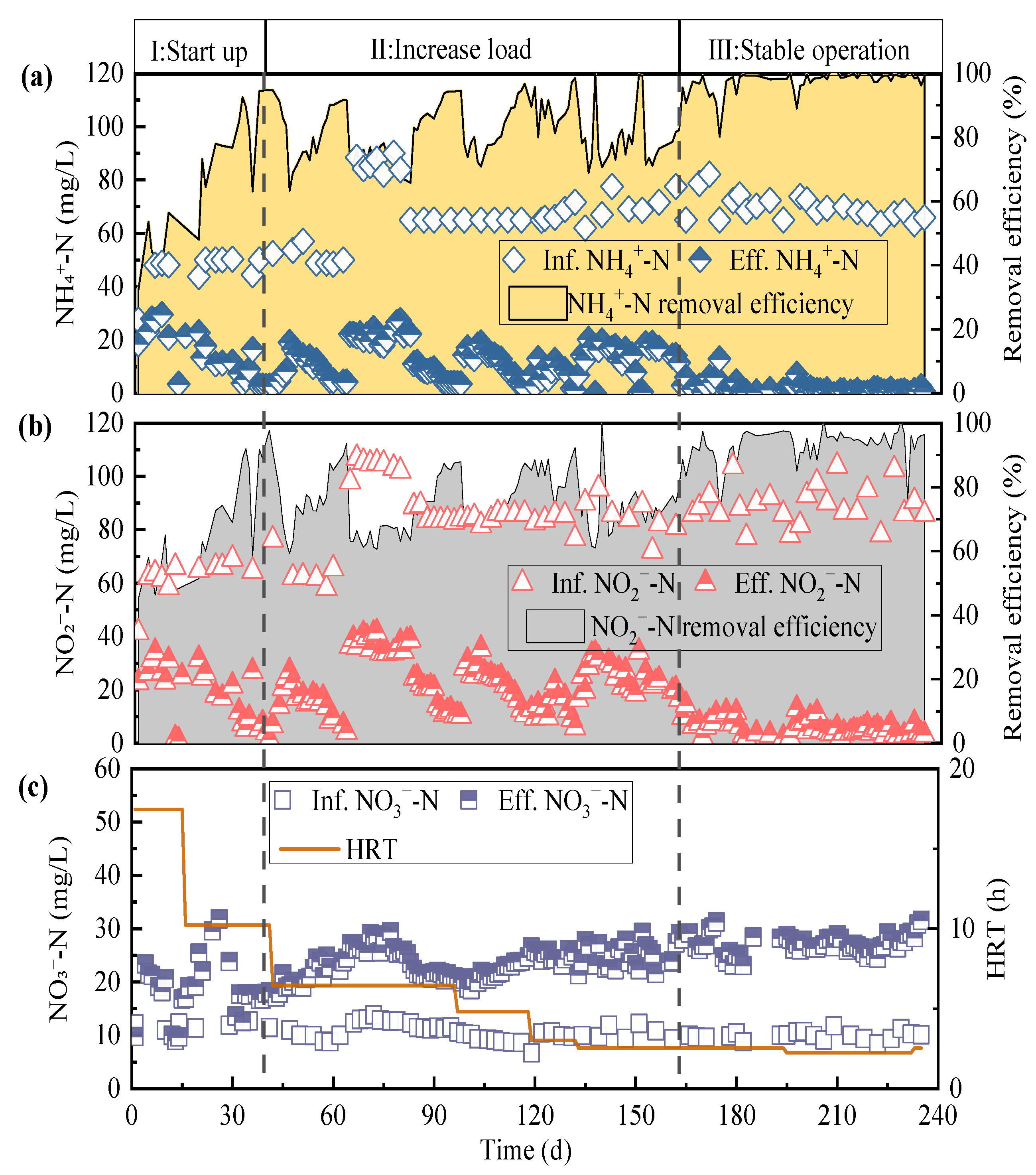
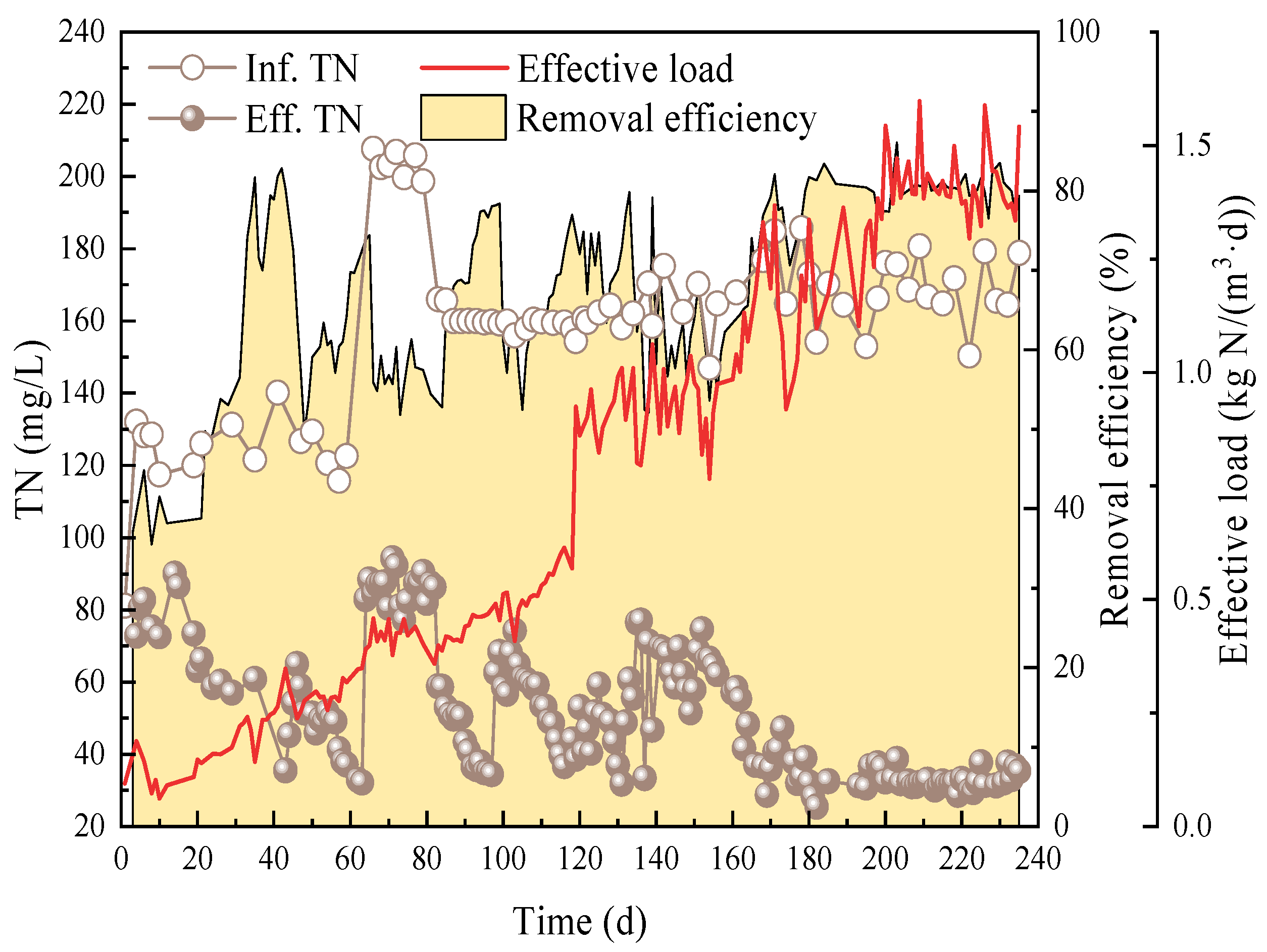
3.1. Nitrogen Removal Performance
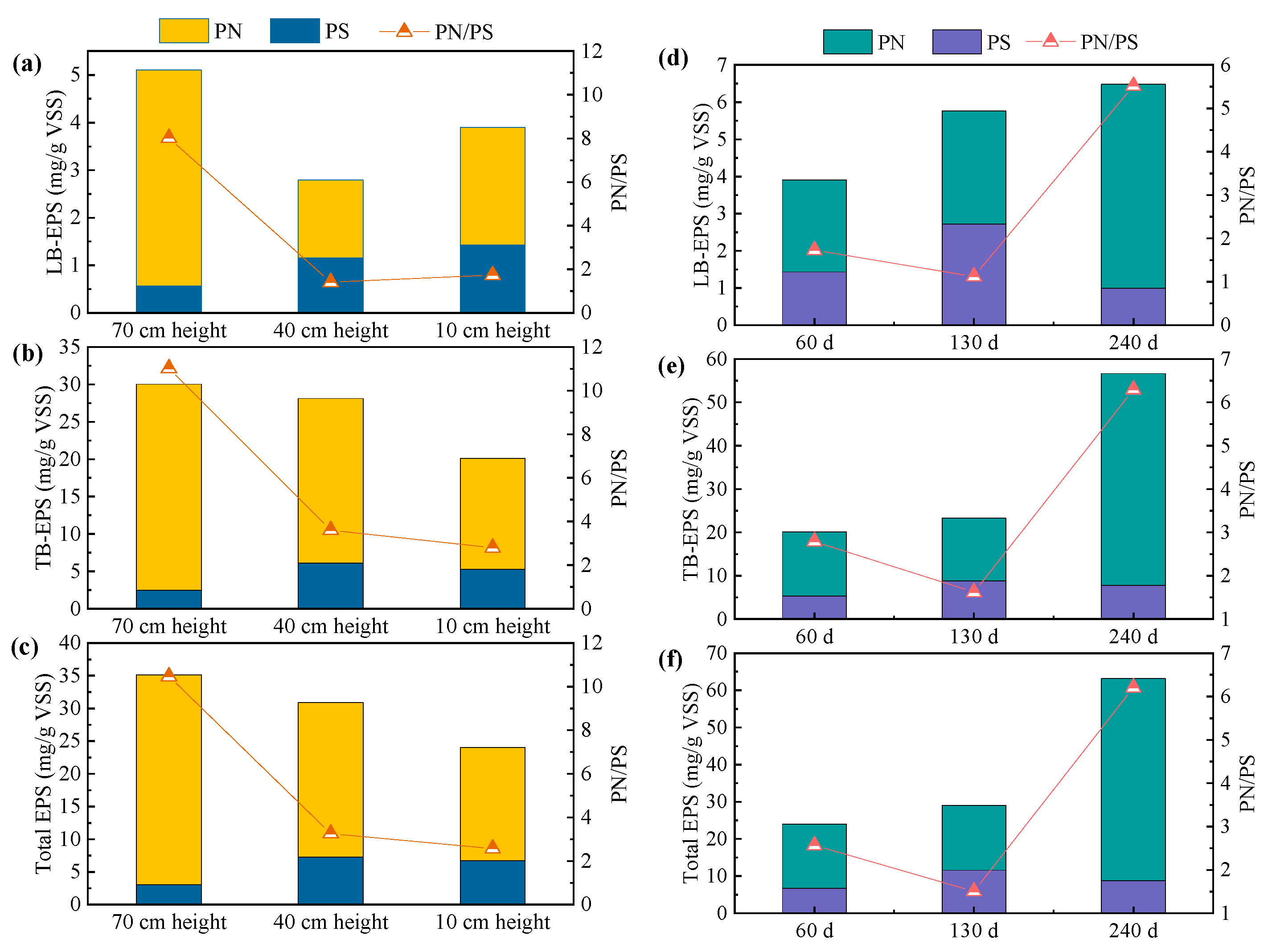
3.2. Changes in Sludge Extracellular Polymeric Substances
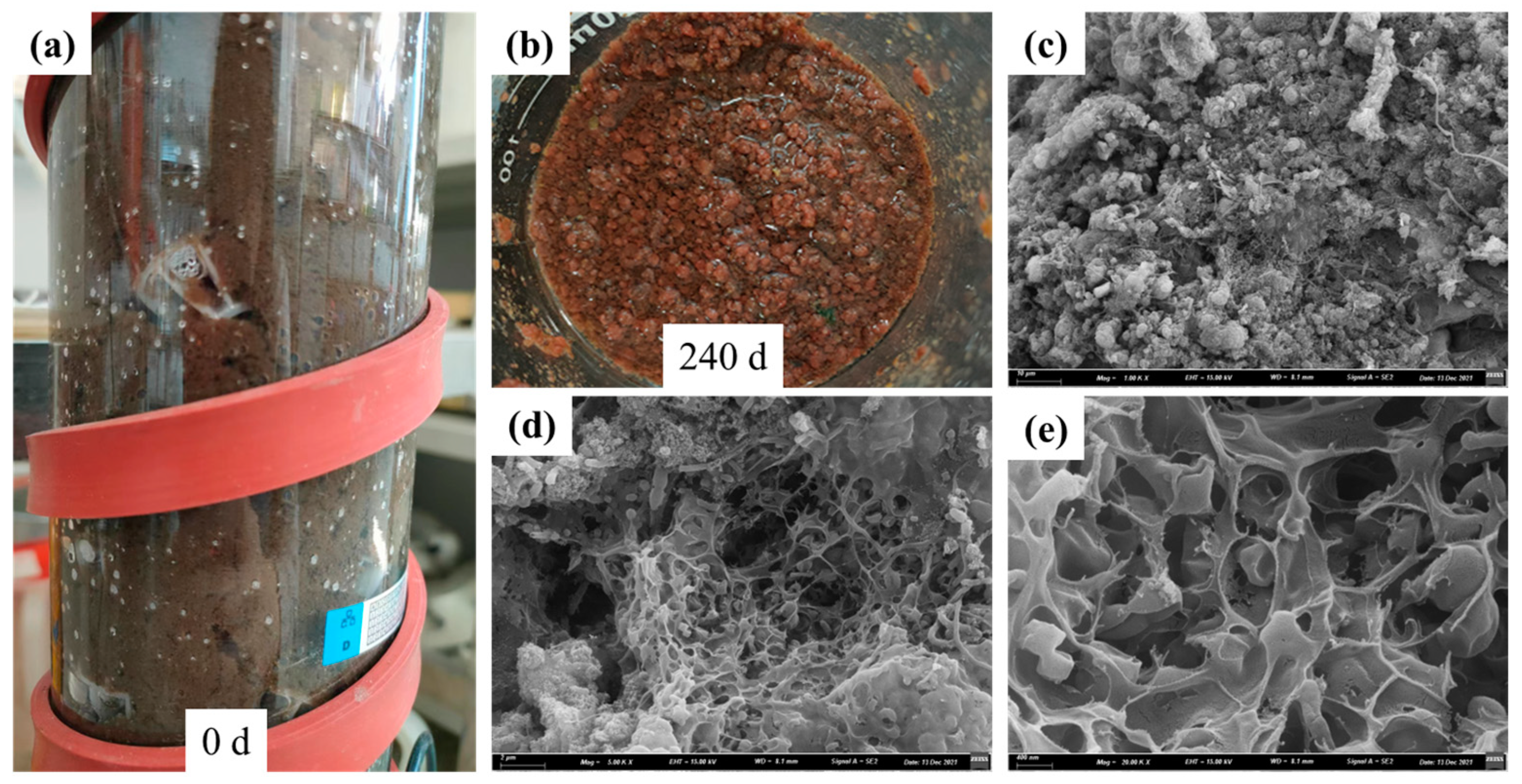
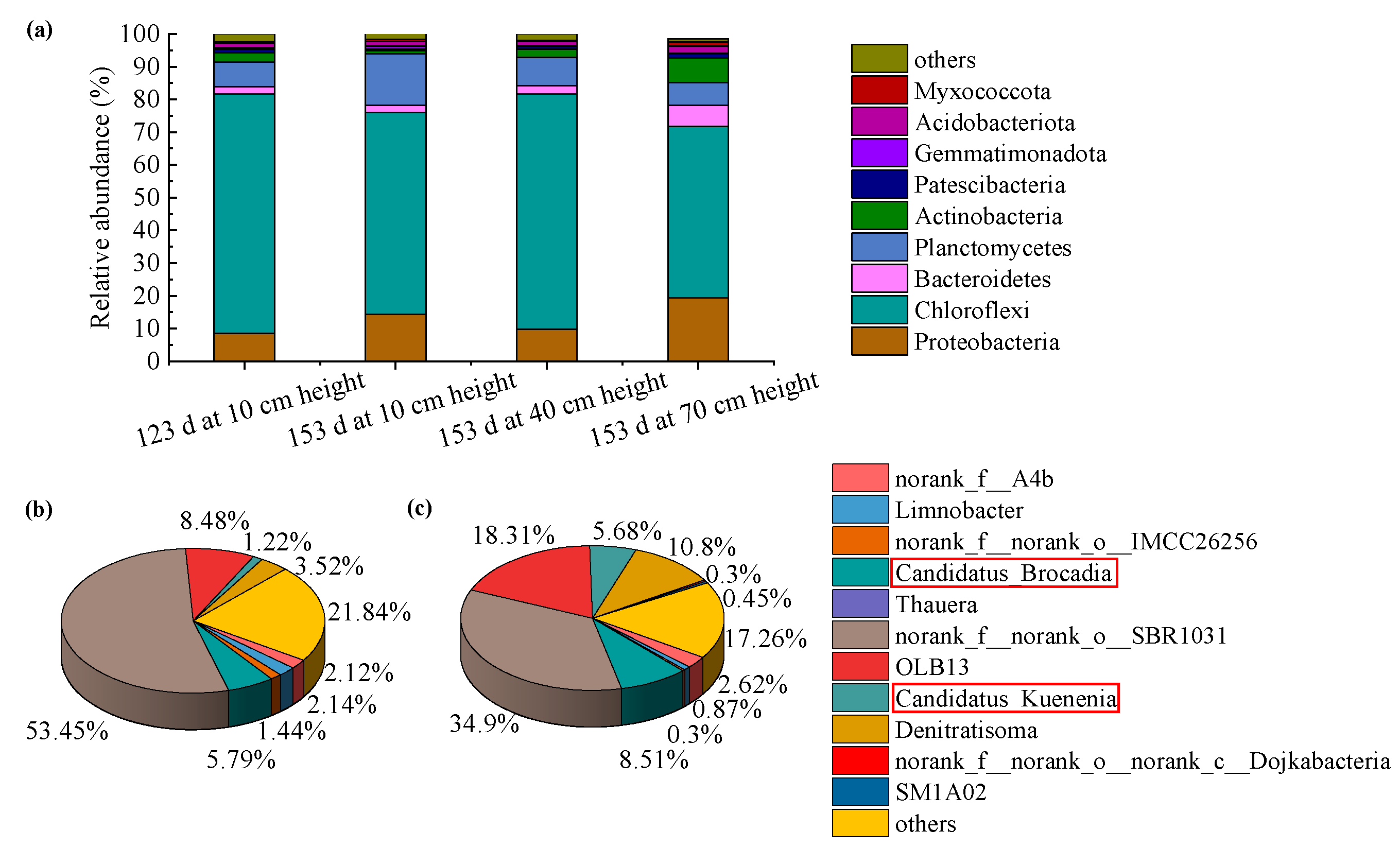
3.3. Sludge Surface Characteristics and Microbial Community Structure
3.4. Effect of COD, NH4+-N and NO2−-N on the Anammox Reaction in the Batch Test
3.5. Discussion of Factors Influencing Anammox Enrichment
4. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Peng, Y.; Zhang, L.; Gao, R.; Yang, L.; Liu, Q.; Zhang, Q.; Li, X.; Wang, S. Enhanced nitrogen removal assisted by mainstream partial-anammox from real sewage in a continuous flow A2/O reactor. Chem. Eng. J. 2020, 400, 125893. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Peng, Y.; Wu, C.; Du, R.; Gong, L.; Ma, B. Achieving partial denitrification with sludge fermentation liquid as carbon source: The effect of seeding sludge. Bioresour. Technol. 2013, 149, 570–574. [Google Scholar] [CrossRef]
- Dai, B.; Yang, Y.; Wang, Z.; Wang, J.; Yang, L.; Cai, X.; Wang, Z.; Xia, S. Enhancement and mechanisms of iron-assisted anammox process. Sci. Total Environ. 2023, 858, 159931. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, L.; Zhang, Q.; Li, X.; Peng, Y.; Wang, C. Enhancing nitrogen removal of carbon-limited municipal wastewater in step-feed biofilm batch reactor through integration of anammox. Bioresour. Technol. 2023, 381, 129091. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M.; Logemann, S.; Muyzer, G.; Robertson, L.A.; De Vries, S.; Van Loosdrecht, M.C.M.; Kuenen, J.G. Novel principles in the microbial conversion of nitrogen compounds. Int. J. Gen. Mol. Microbiol. 1997, 71, 75–93. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Li, J.; Yang, H. Municipal wastewater treatment via the two-stage partial nitrification-anammox (PN/A) process with gel immobilization. J. Water Process Eng. 2022, 50, 103267. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Niu, M.; Li, B.; Wang, S.; Peng, Y. Performance of partial-denitrification process providing nitrite for anammox in sequencing batch reactor (SBR) and upflow sludge blanket (USB) reactor. Int. Biodeter. Biodegr. 2017, 122, 38–46. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, Y.; Wang, Q.; Zhang, Z.; Wu, C.; Gao, M.; Liu, F. The Summary of Nitritation Process in Mainstream Wastewater Treatment. Sustainability 2022, 14, 16453. [Google Scholar] [CrossRef]
- Yin, X.; Qiao, S.; Zhou, J.; Tang, X. Fast start-up of the anammox process with addition of reduced graphene oxides. Chem. Eng. J. 2016, 283, 160–166. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Zhang, L.; Liu, J.; Wang, X.; Gao, R.; Pang, L.; Zhou, Y. Quantify the contribution of anammox for enhanced nitrogen removal through metagenomic analysis and mass balance in an anoxic moving bed biofilm reactor. Water Res. 2019, 160, 178–187. [Google Scholar] [CrossRef]
- Huang, S.; Wu, D. Start-up Strategies for Anaerobic Ammonia Oxidation (Anammox) in In-Situ Nitrogen Removal from Polluted Groundwater in Rare Earth Mining Areas. Sustainability 2021, 13, 4591. [Google Scholar] [CrossRef]
- Ma, M.; Cao, Q.; Mabruk, A.; Xie, J.; Wu, P.; Liu, W.; Chen, C. Promotion of nitrogen removal and microbial enrichment on anammox by exogenous substance addition: A critical review. J. Water Process Eng. 2022, 49, 103096. [Google Scholar] [CrossRef]
- Yang, D.; Zuo, J.; Jiang, C.; Wang, D.; Gu, L.; Zhang, S.; Lu, H.; Wang, D.; Xu, S.; Bai, Z.; et al. Fast start-up of anammox process: Effects of extracellular polymeric substances addition on performance, granule properties, and bacterial community structure. J. Environ. Manag. 2023, 338, 117836. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xu, Z.; Liu, Y.; Yang, G.; Mu, J.; Jin, R.; Yang, Q.; Zhang, X. Performance, kinetics characteristics and enhancement mechanisms in anammox process under Fe(II) enhanced conditions. Biodegradation 2020, 31, 223–234. [Google Scholar] [CrossRef]
- Li, D.; Dang, Z.; Zhang, J. Study on two anammox start-up and operation strategies: Low-intensity direct current electric field and negative pressure. Chem. Eng. J. 2022, 435, 134791. [Google Scholar] [CrossRef]
- Zekker, I.; Artemchuk, O.; Rikmann, E.; Ohimai, K.; Dhar Bhowmick, G.; Madhao Ghangrekar, M.; Burlakovs, J.; Tenno, T. Start-Up of Anammox SBR from Non-Specific Inoculum and Process Acceleration Methods by Hydrazine. Water 2021, 13, 350. [Google Scholar] [CrossRef]
- Yuan, Q.; Jia, Z.; Roots, P.; Wells, G. A strategy for fast anammox biofilm formation under mainstream conditions. Chemosphere 2023, 318, 137955. [Google Scholar] [CrossRef]
- Christensson, M.; Ekstrom, S.; Chan, A.A.; Le Vaillant, E.; Lemaire, R. Experience from start-ups of the first ANITA Mox Plants. Water Sci. Technol. 2013, 67, 2677–2684. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Lin, J.; Tang, R.; Wang, W.; Zhan, X.; Hu, Z. Selection of seeding strategy for fast start-up of Anammox process with low concentration of Anammox sludge inoculum. Bioresour. Technol. 2018, 268, 638–647. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhao, Y.; Li, X.; Zhang, Q.; Sui, J.; Wang, C.; Peng, Y. Mechanism of suspended sludge impact on anammox enrichment in anoxic biofilm through long term operation and microbial analysis. Water Res. 2023, 229, 119412. [Google Scholar] [CrossRef]
- Lotti, T.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C.M. Physiological and kinetic characterization of a suspended cell anammox culture. Water Res. 2014, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, J.; Deng, L.; Jia, T.; Zhao, Y.; Li, X.; Peng, Y. From hybrid process to pure biofilm anammox process: Suspended sludge biomass management contributing to high-level anammox enrichment in biofilms. Water Res. 2023, 236, 119959. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Gao, D.; Wang, H.; de Kreuk, M.; Ren, N. Ecological characteristics of seeding sludge triggering a prompt start-up of anammox. Bioresour. Technol. 2013, 133, 475–481. [Google Scholar] [CrossRef]
- Egli, K.; Fanger, U.; Alvarez, P.J.J.; Siegrist, H.; van der Meer, J.R.; Zehnder, A.J.B. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 2001, 175, 198–207. [Google Scholar] [CrossRef]
- Geng, L.; Yang, H.; Wang, X.; Liu, X.; Wang, J.; Su, Y. Inhibition and recovery of ANAMMOX with Na2SO3: From performance to microbial community analysis. J. Environ. Chem. Eng. 2023, 11, 109051. [Google Scholar] [CrossRef]
- Hu, M.; Liu, X.; Ya, T.; Liu, J.; Zhang, M.; Wang, X. Dissolved oxygen alters the microbial interactions of anammox consortia in PN/A process. J. Water Process Eng. 2023, 53, 103697. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Chen, L.; Zhang, Z.; Tian, J.; Jin, R.; Yao, J. Biochar accelerates the start-up of the anammox process: Phenomenon and potential mechanisms. J. Water Process Eng. 2023, 53, 103662. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Sun, Y.; Zhou, S.; Li, L.; Shao, J. Using low frequency and intensity ultrasound to enhance start-up and operation performance of Anammox process inoculated with the conventional sludge. Ultrason. Sonochem. 2018, 42, 283–292. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Lian, J.; Ngo, H.H.; Guo, W.; Liu, Y.; Song, Y. Rapid start-up of the anammox process by denitrifying granular sludge and the mechanism of the anammox electron transport chain. Biochem. Eng. J. 2016, 115, 101–107. [Google Scholar] [CrossRef]
- Tang, C.; Zheng, P.; Chai, L.; Min, X. Characterization and quantification of anammox start-up in UASB reactors seeded with conventional activated sludge. Int. Biodeter. Biodegr. 2013, 82, 141–148. [Google Scholar] [CrossRef]
- Bi, Z.; Qiao, S.; Zhou, J.; Tang, X.; Zhang, J. Fast start-up of Anammox process with appropriate ferrous iron concentration. Bioresour. Technol. 2014, 170, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Guo, J.; Song, Y.; Gu, J.; Lian, J.; Lu, C.; Han, Y.; Li, H.; Hou, Y. Rapid start up anammox process through a new strategy with inoculating perchlorate reduction sludge and a small amount of anammox sludge. Biochem. Eng. J. 2020, 164, 107784. [Google Scholar] [CrossRef]
- He, X.; Shi, S.; Huang, W.; Fan, X.; Zhou, J.; Chen, Y.; Wang, Y. A novel Electrolysis-integrated anammox system for intensified nitrogen removal and simultaneous phosphorus recovery as vivianite. Chem. Eng. J. 2023, 466, 143299. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.; Su, R.; An, N.; Zhang, Y.; Wei, Y.; Ma, B. A novel method for immobilizing anammox bacteria in polyurethane foam carriers through dewatering. J. Water Process Eng. 2023, 53, 103738. [Google Scholar] [CrossRef]
- Qi, W.; Liu, L.; Shi, Q.; Su, X.; Lv, Q.; Peng, Y.; Wang, C. Process research of one-stage partial nitrification-anammox (S-PN/A) technology in toilet water treatment and related kinetic simulation. J. Chem. Technol. Biotechnol. 2021, 96, 3414–3425. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Zhang, H.; Peng, Y. Formation of partial-denitrification (PD) granular sludge from low-strength nitrate wastewater: The influence of loading rates. J. Hazard. Mater. 2020, 384, 121273. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Yu, Y.; Hu, J.; Liu, X.; Deng, X.; Yu, J.; Chi, R.; Xiao, C. Recovery of anammox process performance after substrate inhibition: Reactor performance, sludge morphology, and microbial community. Bioresour. Technol. 2022, 357, 127351. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Yang, Y.; Yang, Y.; Yang, J.; Li, S.; Mu, X.; Luo, J.; Li, X. Alleviation of substrate inhibition in an anaerobic ammonia oxidation reactor: Recovery process performance and microbial community. J. Water Process Eng. 2023, 51, 103439. [Google Scholar] [CrossRef]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Li, X.; Wang, J.; Peng, Y. Efficient partial-denitrification/anammox (PD/A) process through gas-mixing strategy: System evaluation and microbial analysis. Bioresour. Technol. 2020, 300, 122675. [Google Scholar] [CrossRef]
- Qiao, X.; Fu, C.; Chen, Y.; Fang, F.; Zhang, Y.; Ding, L.; Yang, K.; Pan, B.; Xu, N.; Yu, K.; et al. Molecular insights into enhanced nitrogen removal induced by trace fluoroquinolone antibiotics in an anammox system. Bioresour. Technol. 2023, 374, 128784. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, J.; Zhao, L.; Liu, W.; Chen, L.; Cai, T.; Ji, X. Succession of microbial communities reveals the inevitability of anammox core in the development of anammox processes. Bioresour. Technol. 2023, 371, 128645. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X.; Dang, Y.; Shi, T.; Sun, D. Enhancement of nitrogen removal in coupling Anammox and DAMO via Fe-modified granular activated carbon. J. Environ. Manag. 2023, 340, 118001. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Chen, H.; Liu, G.; Zhu, J.; Ma, X.; Piao, C.; Li, X.; Wang, K. Investigation of the mechanism of anammox granules alleviating the inhibition of organic matter in pharmaceutical wastewater. J. Clean. Prod. 2023, 398, 136129. [Google Scholar] [CrossRef]
- Fan, W.; Chen, Y.; Yuan, B.; Chen, X.; Wang, Q.; Zhao, S.; Jia, W. Enhancing anammox nitrogen removal by static magnetic field exposure: Performance, microbial community and symbiotic relationship analysis. J. Water Process Eng. 2023, 53, 103709. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Jiang, B.; Feng, Y.; Zhu, T.; Tao, H.; Tang, X.; Liu, S. Genome-Centered Metagenomics Analysis Reveals the Symbiotic Organisms Possessing Ability to Cross-Feed with Anammox Bacteria in Anammox Consortia. Environ. Sci. Technol. 2018, 52, 11285–11296. [Google Scholar] [CrossRef]
- Gao, M.; Zou, X.; Dang, H.; Mohammed, A.N.; Yang, S.; Zhou, Y.; Yao, Y.; Guo, H.; Liu, Y. Exploring interactions between quorum sensing communication and microbial development in anammox membrane bioreactor. J. Environ. Chem. Eng. 2023, 11, 109339. [Google Scholar] [CrossRef]
- Kindaichi, T.; Yuri, S.; Ozaki, N.; Ohashi, A. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci. Technol. 2012, 66, 2556–2561. [Google Scholar] [CrossRef]
- Ya, T.; Du, S.; Li, Z.; Liu, S.; Zhu, M.; Liu, X.; Jing, Z.; Hai, R.; Wang, X. Successional Dynamics of Molecular Ecological Network of Anammox Microbial Communities under Elevated Salinity. Water Res. 2021, 188, 116540. [Google Scholar] [CrossRef] [PubMed]
- Daigger, G.T. Oxygen and Carbon Requirements for Biological Nitrogen Removal Processes Accomplishing Nitrification, Nitritation, and Anammox. Water Environ. Res. 2014, 86, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, J.; Park, J.; Lee, C. Response of granular anammox process under mainstream conditions to continuous and transient organic loads. Chem. Eng. J. 2023, 464, 142681. [Google Scholar] [CrossRef]
- Li, L.; Bian, D.; Wang, Q.; Xue, C.; Zhang, Q.; Zhang, S.M. Performance of anammox enchanced by pulsed electric fields under added organic carbon sources using integrated network and metagenomics analyses. Bioresour. Technol. 2023, 380, 129116. [Google Scholar] [CrossRef]
- Fu, W.; Zhu, R.; Lin, H.; Zheng, Y.; Hu, Z. Effect of organic concentration on biological activity and nitrogen removal performance in an anammox biofilm system. Water Sci. Technol. 2021, 84, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Key Physiology of Anaerobic Ammonium Oxidation. Appl. Environ. Microb. 1999, 65, 3248–3250. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Z.; Zhou, J.; Ma, X.; Sun, Y.; Liu, J.; Zhao, J.; Jin, R. Roles of Fe(II), Fe(III) and Fe0 in denitrification and anammox process: Mechanisms, advances and perspectives. J. Water Process Eng. 2023, 53, 103746. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ni, S.; Zhuang, X.; Lee, T. Nano zero-valent iron improves anammox activity by promoting the activity of quorum sensing system. Water Res. 2021, 202, 117491. [Google Scholar] [CrossRef]
- Hao, X.; Wang, X.; Liu, R.; Li, S.; van Loosdrecht, M.C.M.; Jiang, H. Environmental impacts of resource recovery from wastewater treatment plants. Water Res. 2019, 160, 268–277. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Bi, Y.; Meng, F.; Wang, D.; Qiu, C.; Yu, J.; Wang, S. A review of anammox metabolic response to environmental factors: Characteristics and mechanisms. Environ. Res. 2023, 223, 115464. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Li, Z.; Huang, B.; Yang, M.; Fan, N.; Jin, R. Metabolomics and molecular simulation reveal the responding mechanism of anammox consortia to perfluorooctanoic acid by regulating metabolic network. Chem. Eng. J. 2023, 460, 141712. [Google Scholar] [CrossRef]
| Number | Initial COD Concentration | Initial NH4+-N Concentration | Initial NO2−-N Concentration | Average Conversion Rate of NH4+-N | Average Conversion Rate of NO2−-N | ΔNO2−-N/ΔNH4+-N |
|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/(L·h) | mg/(L·h) | ||
| 1 | 0 | 37.49 ± 3.2 | 71.16 ± 5.6 | 4.17 ± 0.5 | 8.18 ± 0.7 | 1.96 ± 0.2 |
| 2 | 50 ± 5 | 34.47 ± 3.2 | 76.26 ± 5.6 | 3.32 ± 0.5 | 8.58 ± 0.7 | 2.59 ± 0.2 |
| 3 | 100 ± 10 | 38.66 ± 3.2 | 75.26 ± 5.6 | 3.80 ± 0.5 | 8.61 ± 0.7 | 2.26 ± 0.2 |
| 4 | 500 ± 50 | 38.19 ± 3.2 | 74.72 ± 5.6 | 3.33 ± 0.5 | 8.30 ± 0.7 | 2.49 ± 0.2 |
| 5 | 800 ± 80 | 38.60 ± 3.2 | 75.97 ± 5.6 | 3.18 ± 0.5 | 8.65 ± 0.7 | 2.72 ± 0.2 |
| Number | Initial NH4+-N Concentration | Initial NO2−-N Concentration | Initial TN Concentration | Average Conversion Rate of NH4+-N | Average Conversion Rate of NO2−-N | ΔNO2−-N/ΔNH4+-N |
|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/(L·h) | mg/(L·h) | ||
| 1 | 39.90 ± 3.5 | 65.54 ± 5.2 | 105.4 ± 8.4 | 3.64 ± 0.3 | 7.28 ± 0.5 | 2.00 ± 0.1 |
| 2 | 87.14 ± 6.7 | 64.27 ± 5.2 | 151.4 ± 12.1 | 5.12 ± 0.4 | 7.34 ± 0.5 | 1.43 ± 0.1 |
| 3 | 127.7 ± 9.6 | 65.43 ± 5.2 | 193.1 ± 15.4 | 5.60 ± 0.4 | 7.45 ± 0.5 | 1.33 ± 0.1 |
| 4 | 193.8 ± 15.3 | 64.19 ± 5.2 | 258.0 ± 20.6 | 9.26 ± 0.5 | 6.99 ± 0.5 | 0.75 ± 0.1 |
| 5 | 210.7 ± 17.5 | 62.09 ± 5.2 | 272.8 ± 21.8 | 6.36 ± 0.4 | 5.91 ± 0.5 | 0.93 ± 0.1 |
| Number | Initial NH4+-N Concentration | Initial NO2−-N Concentration | Initial TN Concentration | Average Conversion Rate of NH4+-N | Average Conversion Rate of NO2−-N | ΔNO2−-N/ΔNH4+-N |
|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | mg/(L·h) | mg/(L·h) | ||
| 1 | 33.75 ± 2.6 | 64.97 ± 5.2 | 98.72 ± 8.0 | 4.22 ± 0.3 | 7.14 ± 0.6 | 1.69 ± 0.2 |
| 2 | 34.96 ± 2.6 | 121.8 ± 9.7 | 156.7 ± 12.5 | 4.23 ± 0.3 | 10.79 ± 0.8 | 2.55 ± 0.2 |
| 3 | 33.62 ± 2.6 | 170.5 ± 13.6 | 204.2 ± 16.3 | 4.05 ± 0.3 | 11.48 ± 0.9 | 2.83 ± 0.2 |
| 4 | 33.81 ± 2.6 | 244.9 ± 19.6 | 277.7 ± 22.2 | 3.81 ± 0.2 | 12.07 ± 0.9 | 3.16 ± 0.2 |
| 5 | 33.12 ± 2.6 | 288.1 ± 23.0 | 319.2 ± 25.5 | 3.33 ± 0.2 | 10.85 ± 0.9 | 3.26 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Hu, M.; Wang, C.; Qi, W.; Peng, Y. Enrichment of Anammox Bacteria Using Anammox Sludge as a Primer Combined with Ordinary Activated Sludge. Sustainability 2023, 15, 12123. https://doi.org/10.3390/su151612123
Liu L, Hu M, Wang C, Qi W, Peng Y. Enrichment of Anammox Bacteria Using Anammox Sludge as a Primer Combined with Ordinary Activated Sludge. Sustainability. 2023; 15(16):12123. https://doi.org/10.3390/su151612123
Chicago/Turabian StyleLiu, Lifang, Meiling Hu, Cong Wang, Weikang Qi, and Yongzhen Peng. 2023. "Enrichment of Anammox Bacteria Using Anammox Sludge as a Primer Combined with Ordinary Activated Sludge" Sustainability 15, no. 16: 12123. https://doi.org/10.3390/su151612123
APA StyleLiu, L., Hu, M., Wang, C., Qi, W., & Peng, Y. (2023). Enrichment of Anammox Bacteria Using Anammox Sludge as a Primer Combined with Ordinary Activated Sludge. Sustainability, 15(16), 12123. https://doi.org/10.3390/su151612123





