Irrigation Scheduling for Small-Scale Crops Based on Crop Water Content Patterns Derived from UAV Multispectral Imagery
Abstract
:1. Introduction
2. Materials and Methods
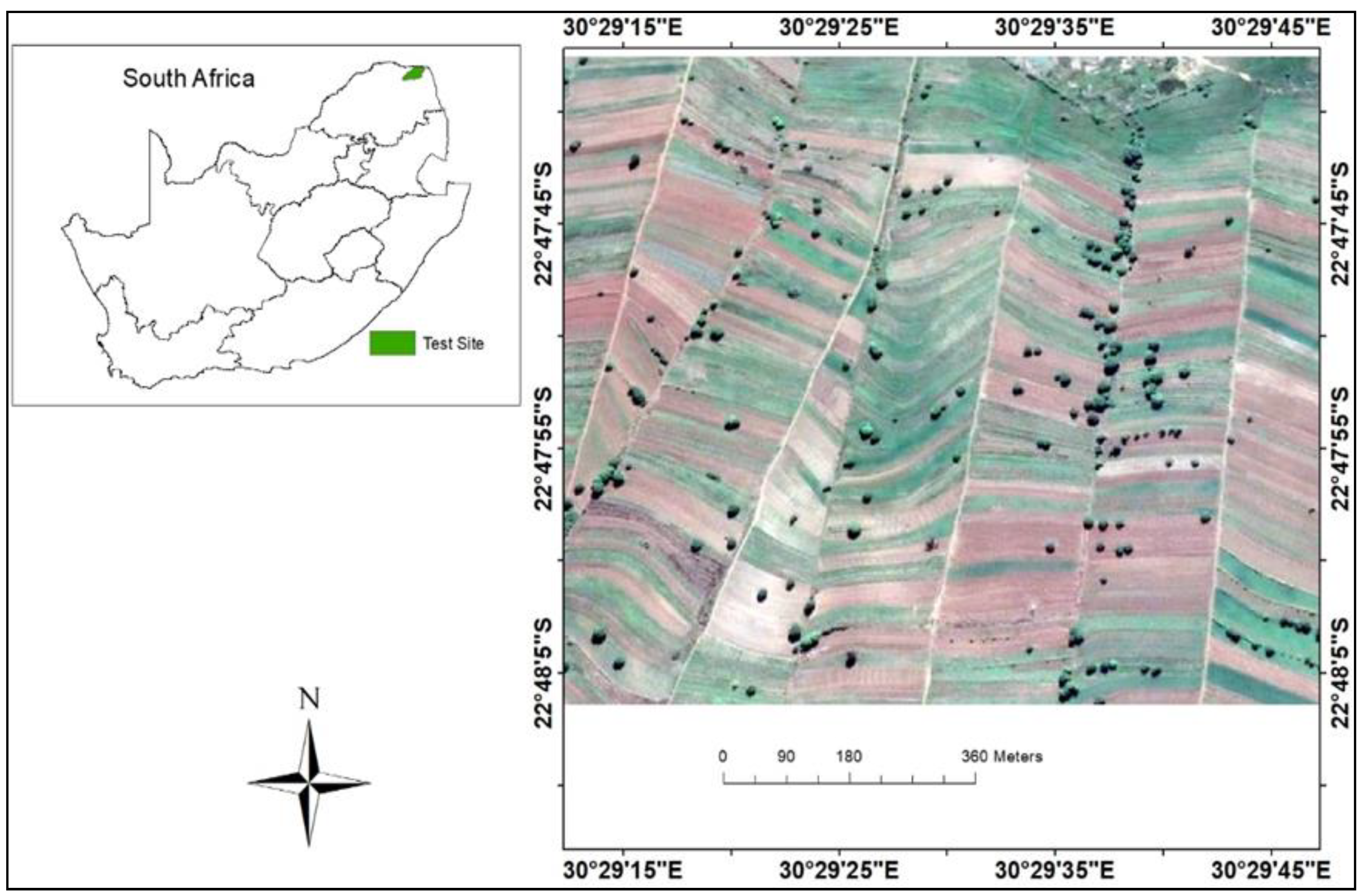
2.1. Study Area
2.2. Data Acquisition
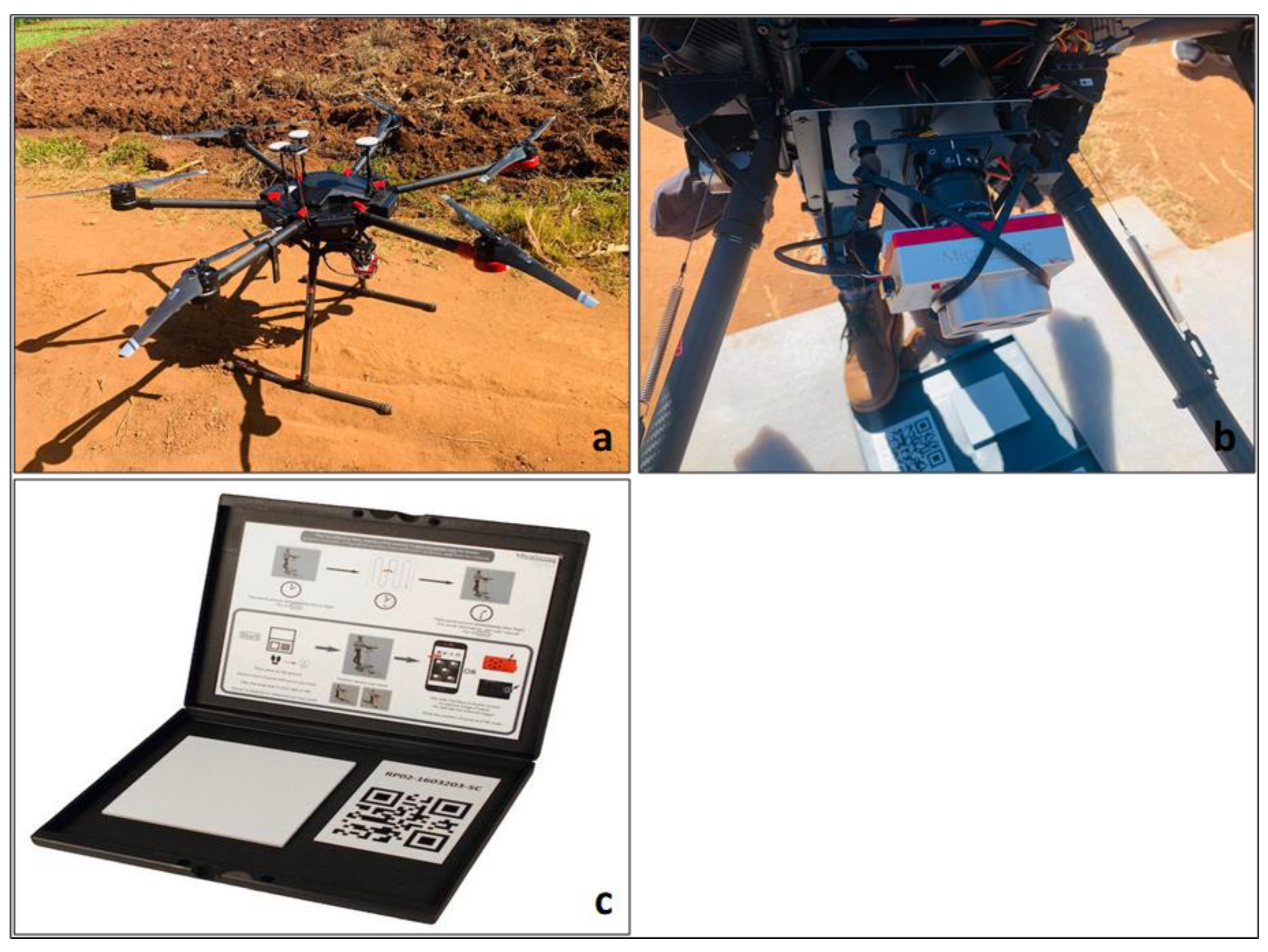
2.2.1. The UAV Data
2.2.2. Reference Data
2.3. UAV Camera Calibration
2.4. UAV Data Processing
2.5. Spectral Characterization of Crops
2.6. Derivation of Spectral Vegetation Indices Related to Crop Water Content
2.7. Analysing Spatial Patterns in Water Content across Various Crops
2.8. Modelling Crop Water Content Based on Spectral Vegetation Indices
2.9. Empirical Model Validation
2.10. Simulation of Changes in Crop Water Content
3. Results
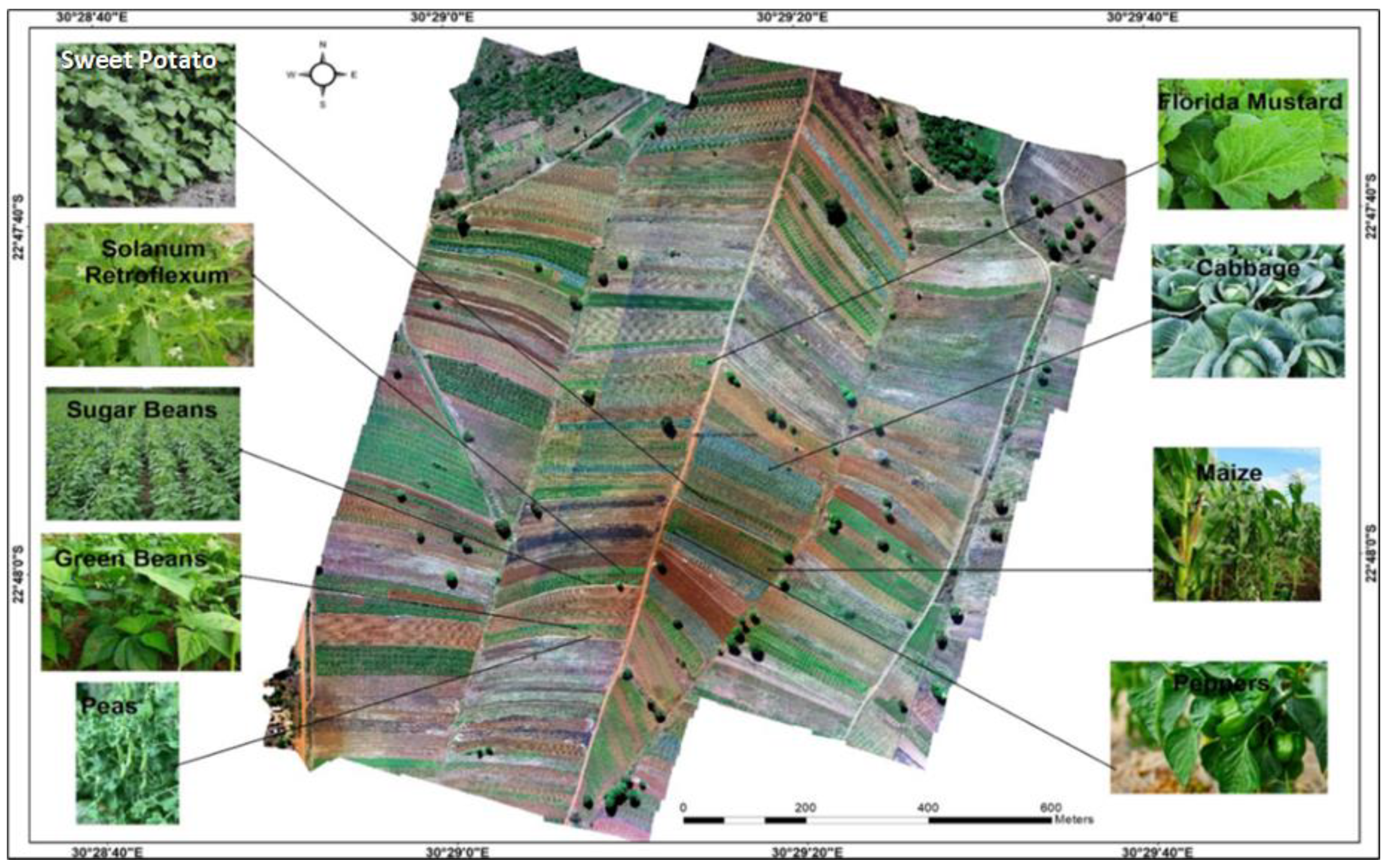
3.1. Crop Types Cultivated in the Study Area
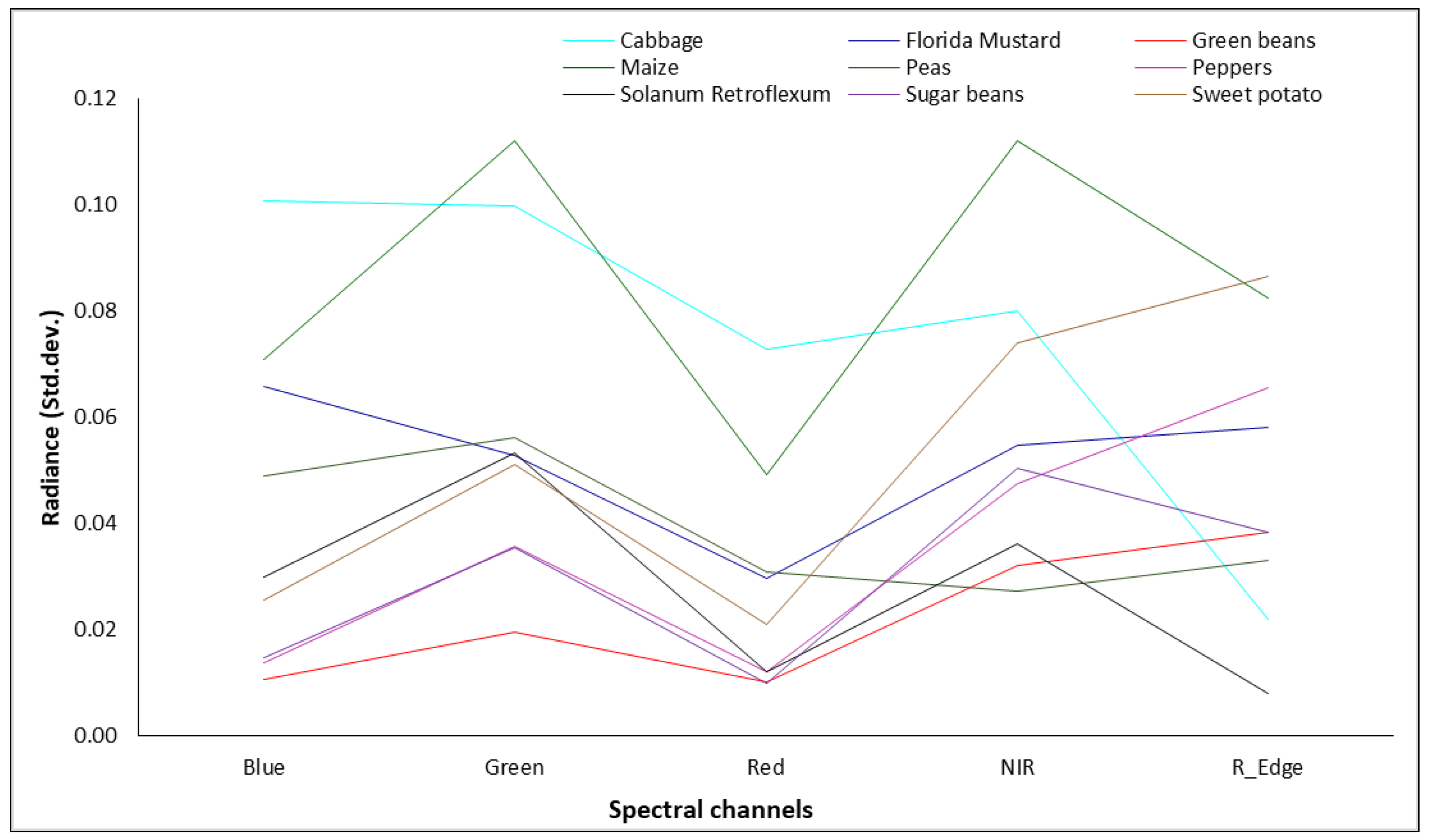
3.2. Spectral Characterization of Crops
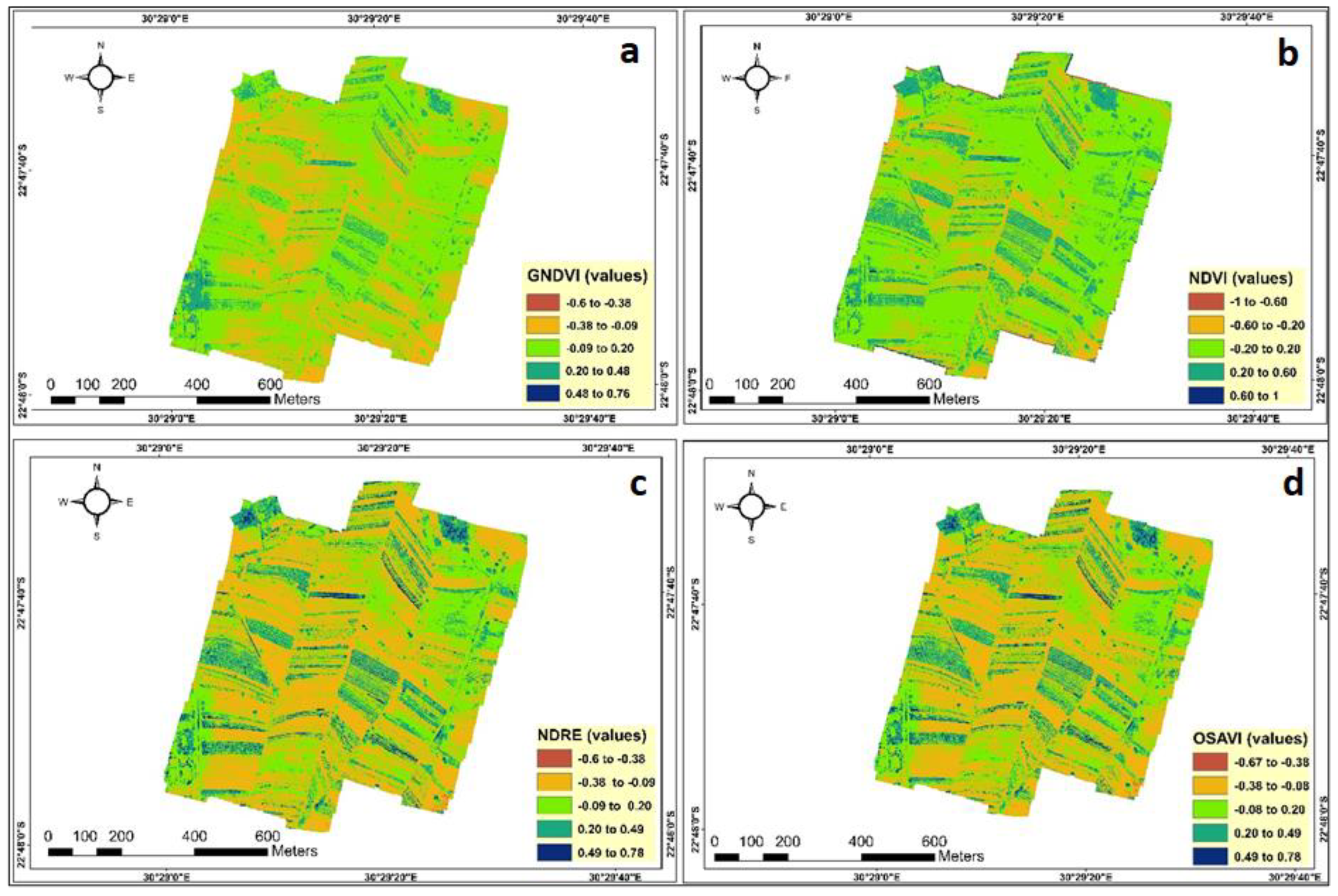
3.3. Spectral Indices for Characterizing Crop Water Content
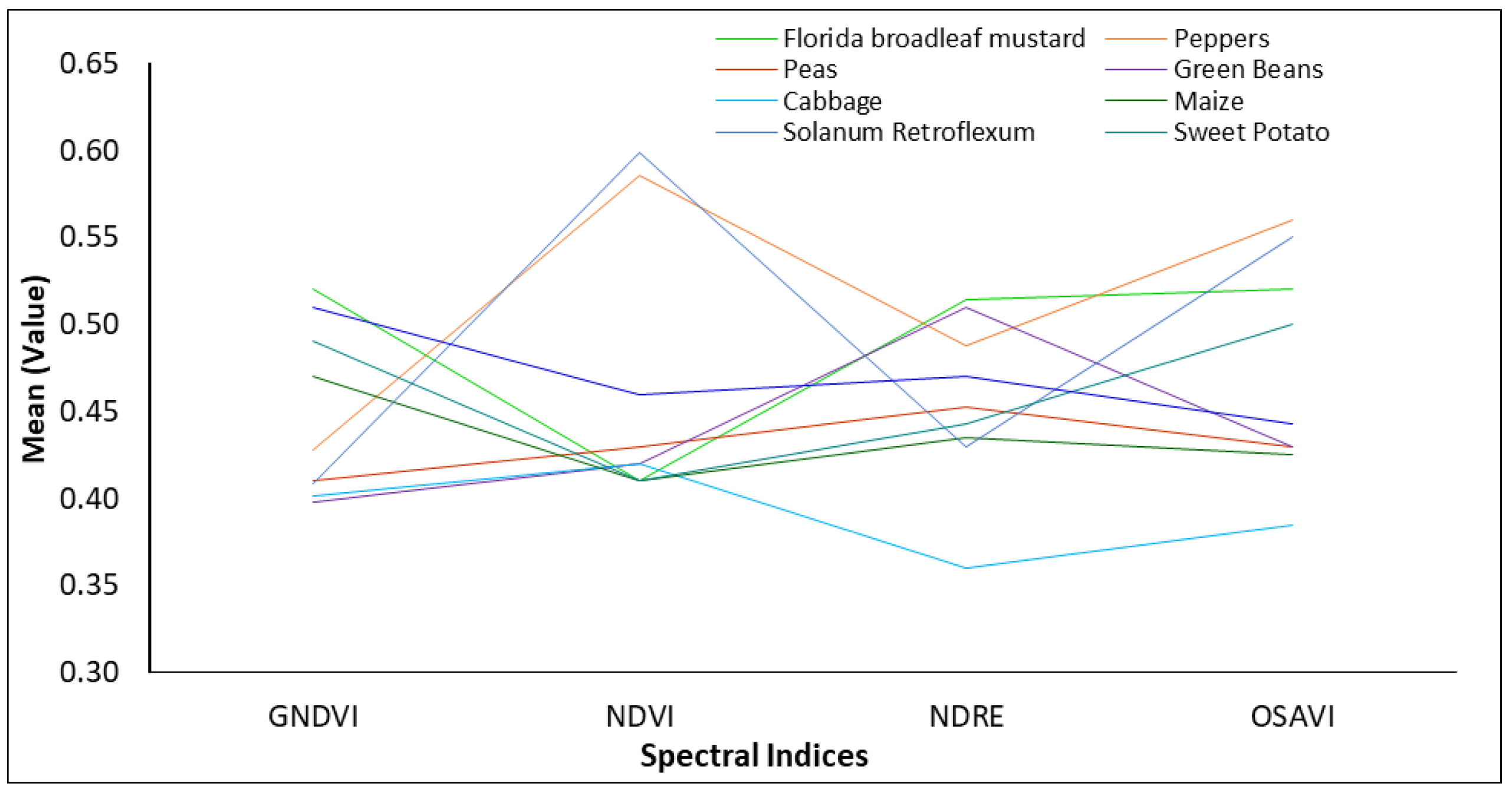
3.4. Mean Spectral Index Profile of Crops
3.5. Pattern Analysis of Water Content across Surveyed Crops
3.6. Sensitivity of Spectral Vegetation Indices to Crop Water Content
3.6.1. Greenness Normalized Difference Vegetation Index
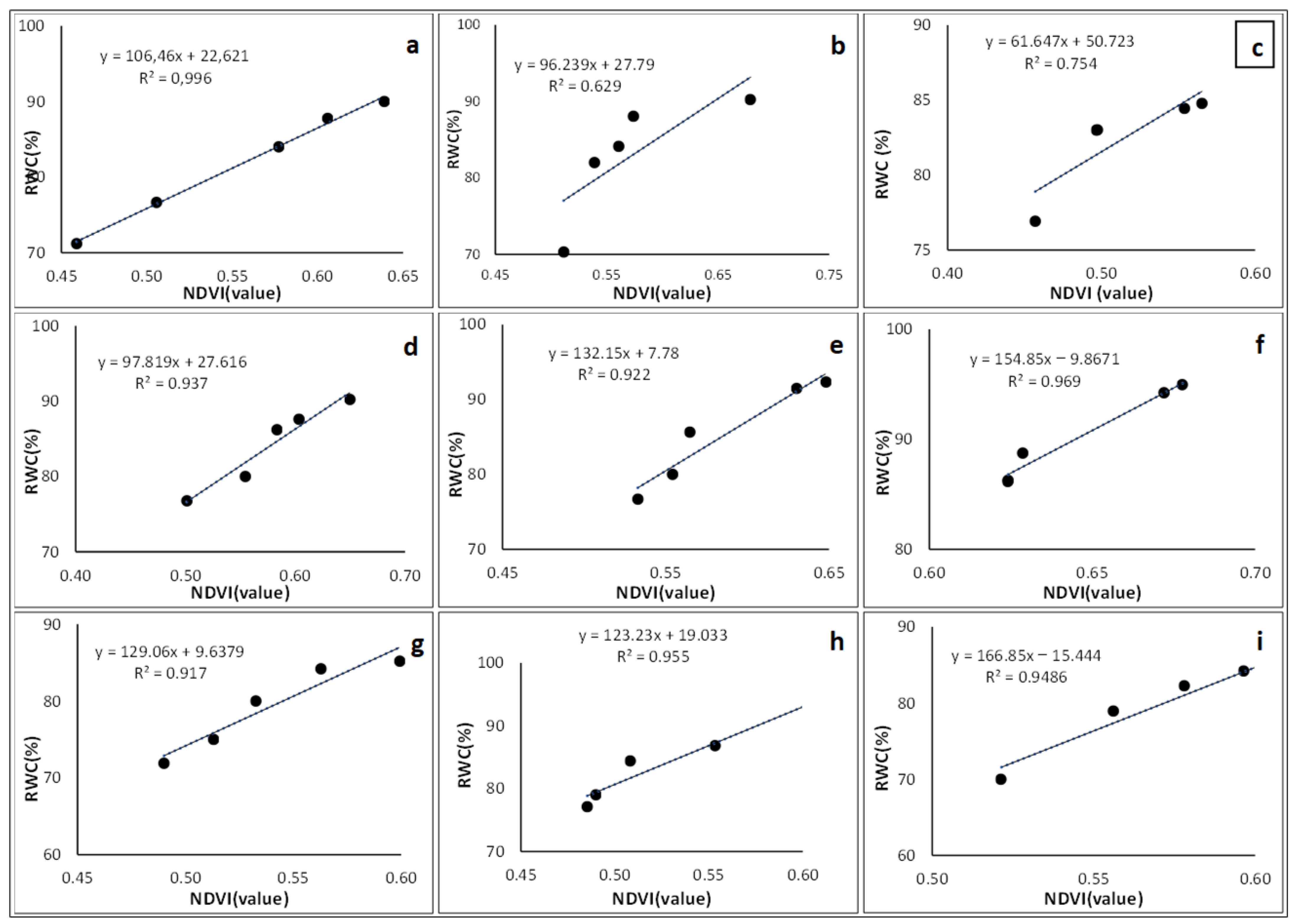
3.6.2. Normalized Difference Vegetation Index
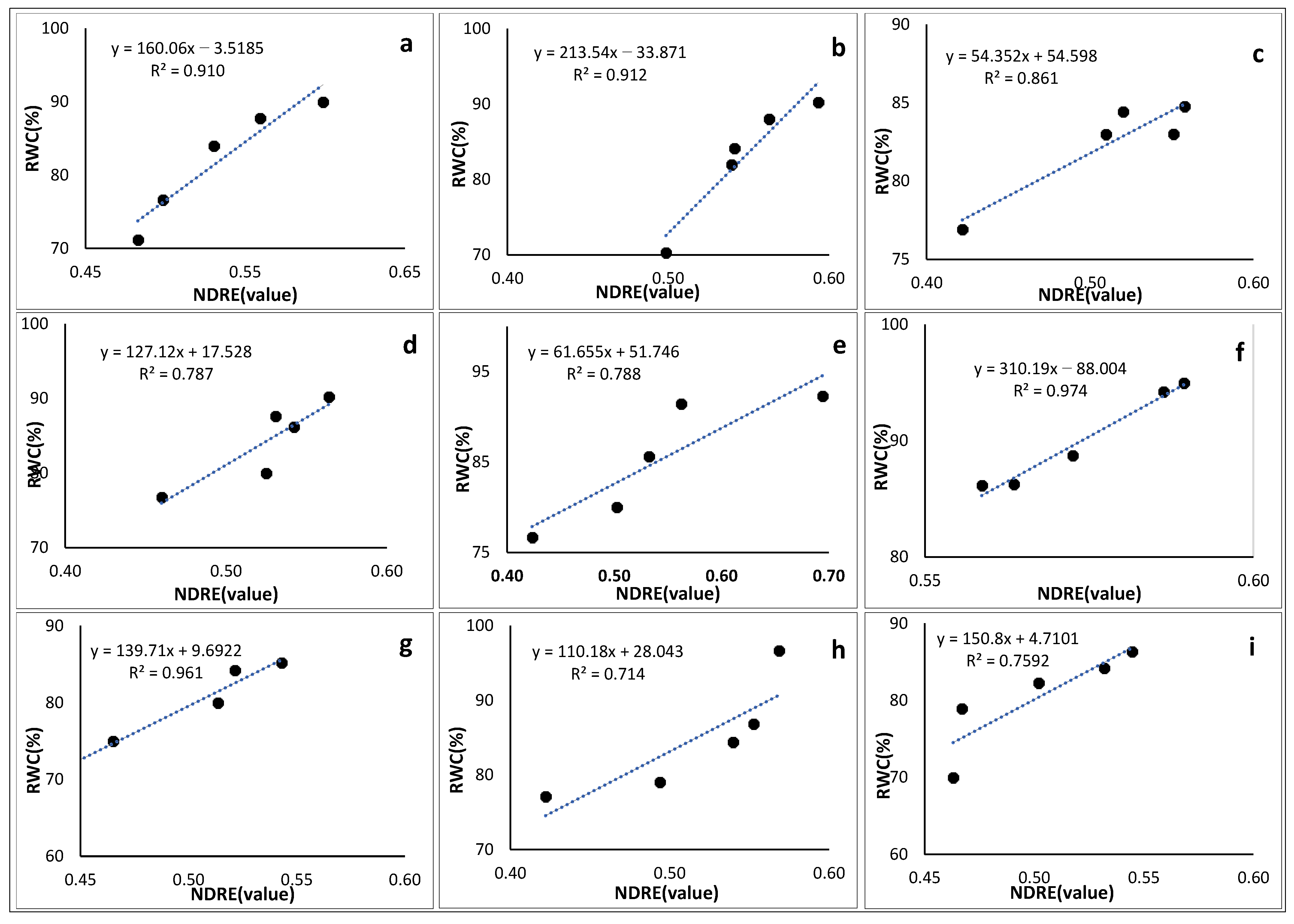
3.6.3. Normalized Difference Red-Edge Index
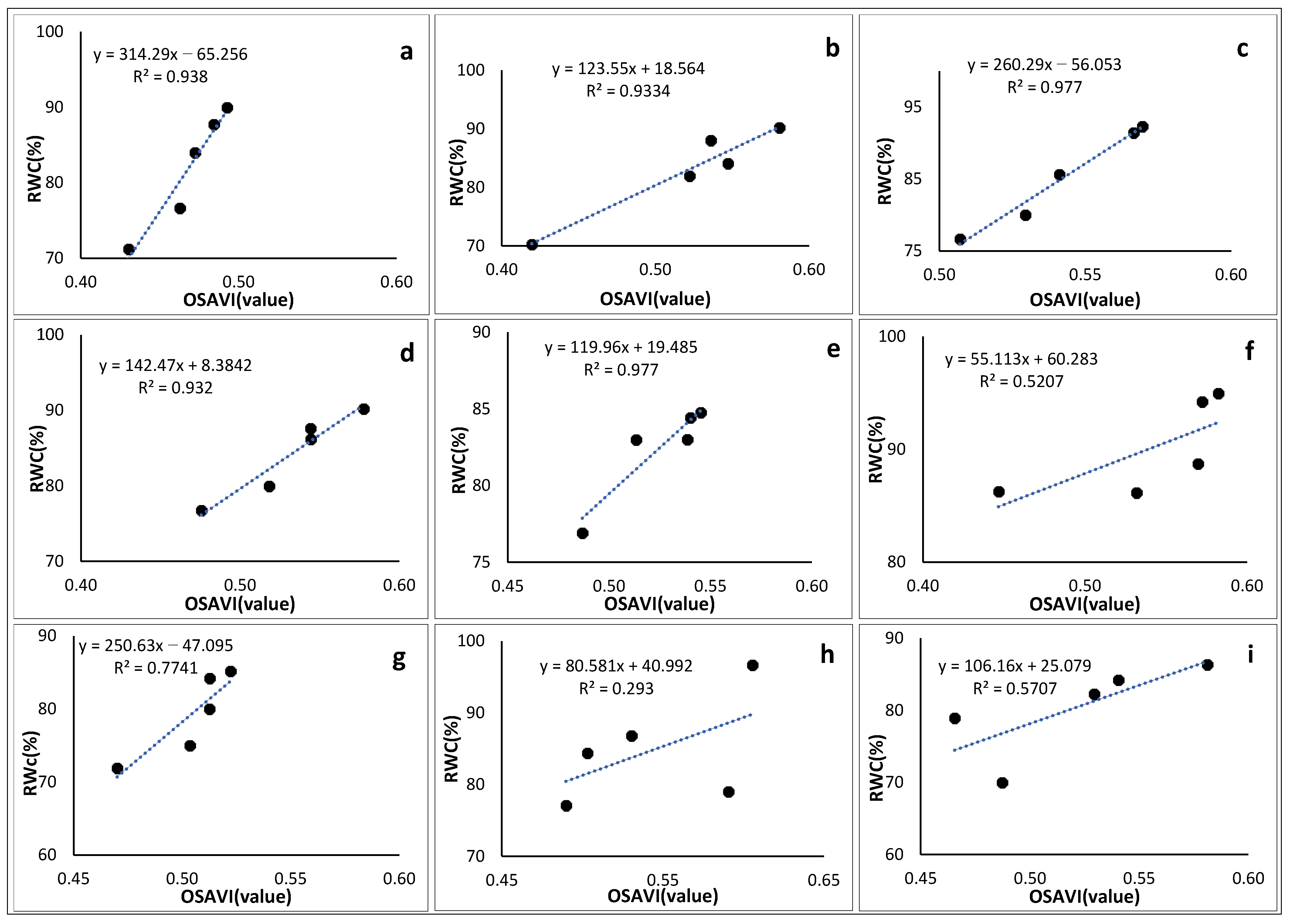
3.6.4. Optimized Soil-Adjusted Vegetation Index
3.7. Empirical Models for Modelling Crop Water Content
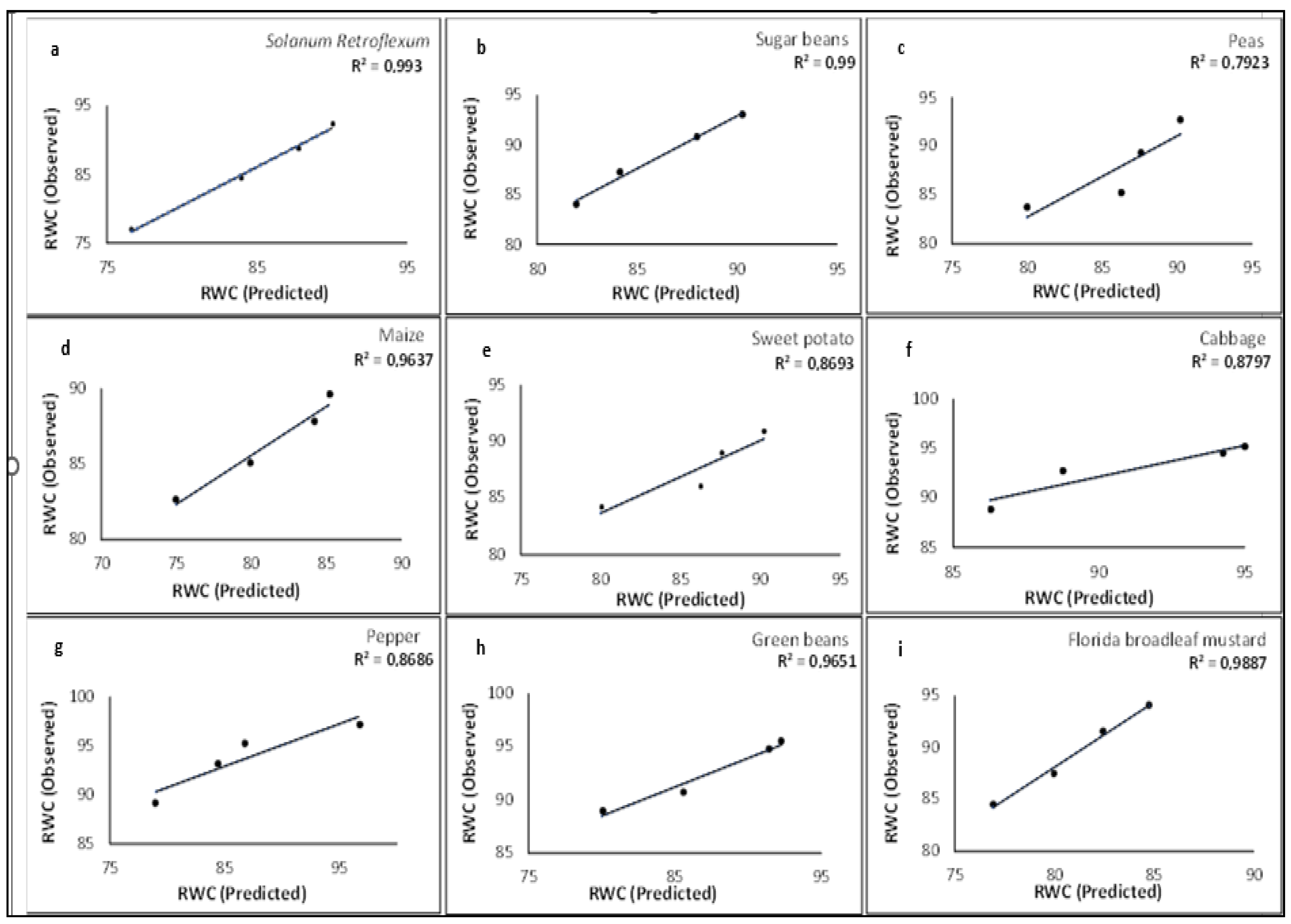
3.8. Crop Water Content Model Validation
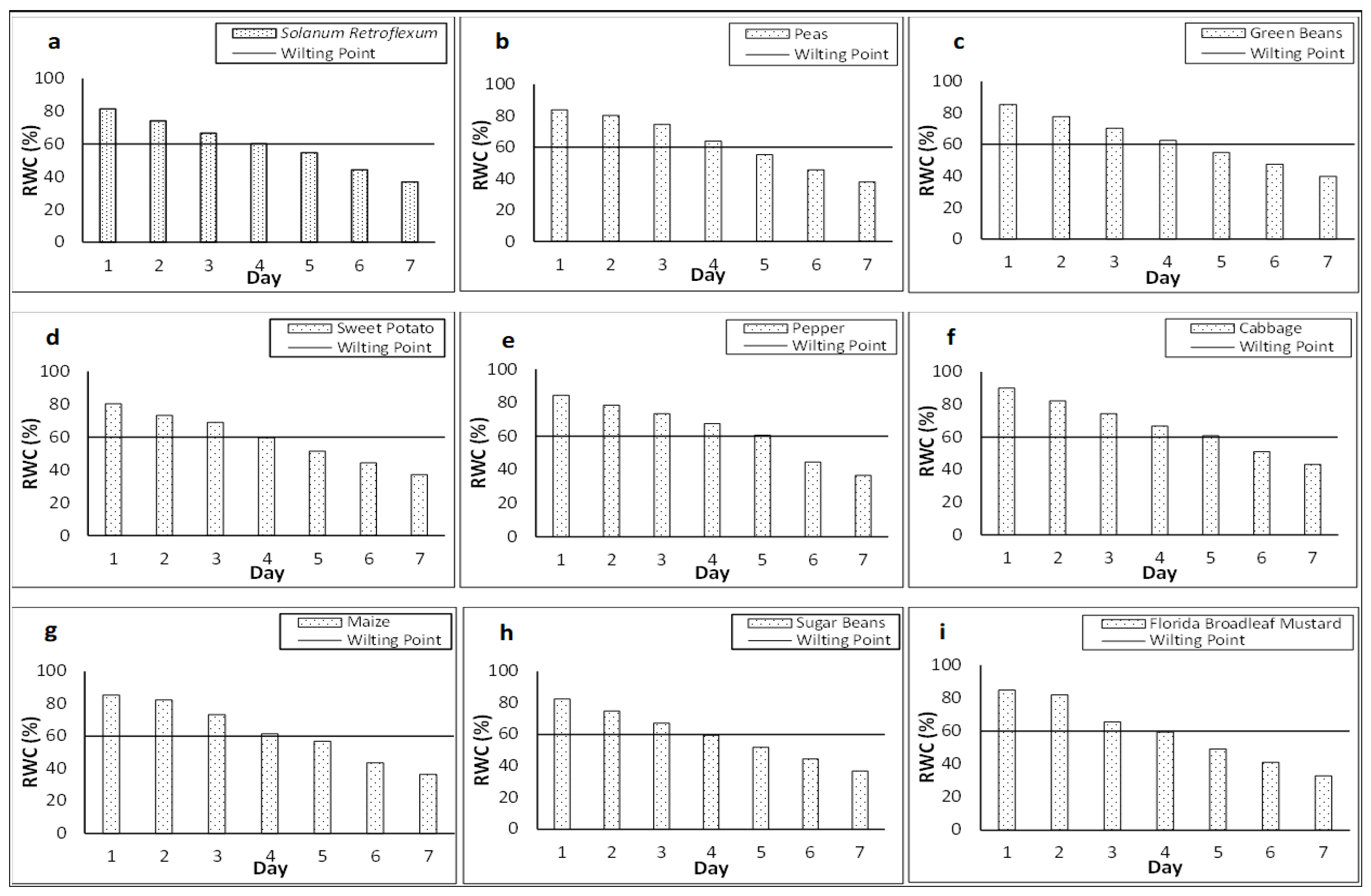
3.9. Temporal Patterns in Crop Water Content for Irrigation Scheduling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lowder, S.K.; Skoet, J.; Singh, S. What do We Really Know about the Number and Distribution of Farms and Family Farms in the World? Background Paper for the State of Food and Agriculture 2014; FAO: Rome, Italy, 2014. [Google Scholar]
- Fan, S.; Rue, C. The role of smallholder farms in a changing world. In The Role of Smallholder Farms in Food and Nutrition Security; Springer: Cham, Switzerland, 2020; pp. 13–28. [Google Scholar]
- Rapsomanikis, G. The Economic Lives of Smallholder Farmers: An Analysis Based on Household Data from Nine Countries; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; p. 48. [Google Scholar]
- Dixon, J.; Taniguchi, K.; Wattenbach, H.; TanyeriArbur, A. Smallholders, Globalization and Policy Analysis; Food & Agriculture Organization: Rome, Italy, 2004; Volume 5. [Google Scholar]
- Baiphethi, M.N.; Jacobs, P.T. The contribution of subsistence farming to food security in South Africa. Agrekon 2009, 48, 459–482. [Google Scholar] [CrossRef]
- Hlophe-Ginindza, S.N.; Mpandeli, N.S. The Role of Small-Scale Farmers in Ensuring Food Security in Africa. In Food Security in Africa; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Hanjra, M.A.; Qureshi, M.E. Global water crisis and future food security in an era of climate change. Food Policy 2010, 35, 365–377. [Google Scholar] [CrossRef]
- Booker, J.F.; Trees, W.S. Implications of Water Scarcity for Water Productivity and Farm Labor. Water 2020, 12, 308. [Google Scholar] [CrossRef] [Green Version]
- Muzerengi, T.; Mapuranga, D. Impact of small-scale irrigation schemes in addressing food shortages in semi-arid areas: A case of Ingwizi Irrigation scheme in Mangwe District, Zimbabwe. Int. J. Humanit. Soc. Stud. 2017, 5, 298–312. [Google Scholar]
- Lee, M.; Gambiza, J. The adoption of conservation agriculture by smallholder farmers in southern Africa: A scoping review of barriers and enablers. J. Rural. Stud. 2022, 92, 214–225. [Google Scholar] [CrossRef]
- Beringer, T.I.M.; Lucht, W.; Schaphoff, S. Bioenergy production potential of global biomass plantations under environmental and agricultural constraints. Gcb Bioenergy 2011, 3, 299–312. [Google Scholar] [CrossRef]
- Hussain, I.; Turral, H.; Molden, D.; Ahmad, M.U.D. Measuring and enhancing the value of agricultural water in irrigated river basins. Irrig. Sci. 2007, 25, 263–282. [Google Scholar] [CrossRef]
- Asayehegn, K. Irrigation versus rain-fed agriculture: Driving for households’ income disparity, a study from Central Tigray, Ethiopia. Agric. Sci. Res. J. 2012, 2, 20–29. [Google Scholar]
- Romero, R.; Muriel, J.L.; García, I.; de la Peña, D.M. Research on automatic irrigation control: State of the art and recent results. Agric. Water Manag. 2012, 114, 59–66. [Google Scholar] [CrossRef]
- Osroosh, Y.; Peters, R.T.; Campbell, C.S.; Zhang, Q. Comparison of irrigation automation algorithms for drip-irrigated apple trees. Comput. Electron. Agric. 2016, 128, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Andrew, R.; Guan, H.; Batelaan, O. Estimation of GRACE water storage components by temporal decomposition. J. Hydrol. 2017, 552, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Babaeian, E.; Sadeghi, M.; Jones, S.B.; Montzka, C.; Vereecken, H.; Tuller, M. Ground, proximal, and satellite remote sensing of soil moisture. Rev. Geophys. 2019, 57, 530–616. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, X.; Long, H.; Xu, X.; Bao, Y. Monitoring the effects of land use and cover type changes on soil moisture using remote-sensing data: A case study in China′s Yongding River basin. Catena 2010, 82, 135–145. [Google Scholar] [CrossRef]
- Shafian, S.; Maas, S.J. Index of Soil Moisture Using Raw Landsat Image Digital Count Data in Texas High Plains. Remote Sens. 2015, 7, 2352–2372. [Google Scholar] [CrossRef] [Green Version]
- Przeździecki, K.; Zawadzki, J. Modification of the Land Surface Temperature–Vegetation Index Triangle Method for soil moisture condition estimation by using SYNOP reports. Ecol. Indic. 2020, 119, 106823. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Q.G.; Horn, R.; Baumgartl, T. Shear strength of surface soil as affected by soil bulk density and soil water content. Soil Tillage Res. 2001, 59, 97–106. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, J.; Liu, J.; Wen, Y.; Wang, Y. Soil Moisture Retrieval Using Microwave Remote Sensing Data and a Deep Belief Network in the Naqu Region of the Tibetan Plateau. Sustainability 2021, 13, 12635. [Google Scholar] [CrossRef]
- Deng, X.; Yang, L.; Fu, Z.; Du, C.; Lyu, H.; Cui, L.; Zhang, L.; Zhang, J.; Jia, B. A calibration-free capacitive moisture detection method for multiple soil environments. Measurement 2020, 173, 108599. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Li, H.; Guo, J. Soil Moisture Retrieval in Farmland Areas with Sentinel Multi-Source Data Based on Regression Convolutional Neural Networks. Sensors 2021, 21, 877. [Google Scholar] [CrossRef]
- Sobrino, J.A.; Franch, B.; Mattar, C.; Jiménez-Muñoz, J.C.; Corbari, C. A method to estimate soil moisture from Airborne Hyperspectral Scanner (AHS) and ASTER data: Application to SEN2FLEX and SEN3EXP campaigns. Remote Sens. Environ. 2011, 10, 18. [Google Scholar] [CrossRef]
- Sun, J.; Yang, L.; Yang, X.; Wei, J.; Li, L.; Guo, E.; Kong, Y. Using spectral reflectance to estimate the leaf chlorophyll content of maize inoculated with arbuscular mycorrhizal fungi under water stress. Front. Plant Sci. 2021, 12, 646173. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Tian, J.; Tian, Q.; Xu, K.; Xu, N. Development of soil moisture indices from differences in water absorption between shortwave-infrared bands. ISPRS J. Photogramm. Remote Sens. 2019, 154, 216–230. [Google Scholar] [CrossRef]
- Pramudita, A.A.; Wahyu, Y.; Rizal, S.; Prasetio, M.D.; Jati, A.N.; Wulansari, R.; Ryanu, H.H. Soil Water Content Estimation With the Presence of Vegetation Using Ultra Wideband Radar-Drone. IEEE Access 2022, 10, 85213–85227. [Google Scholar] [CrossRef]
- Homayoun, H. Remobilization of stem reserves in wheat genotypes under normal and drought stress conditions. Adv. Environ. Biol. 2011, 5, 1721–1725. [Google Scholar]
- Bryla, D.R.; Gartung, J.L.; Strik, B.C. Evaluation of irrigation methods for highbush blueberry—I. Growth and water requirements of young plants. HortScience 2011, 46, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Lal, R.; Stewart, B.A. (Eds.) Soil-Specific Farming: Precision Agriculture; CRC Press: Boca Raton, FL, USA, 2021; p. 22. [Google Scholar]
- Neupane, J.; Guo, W. Agronomic basis and strategies for precision water management: A review. Agronomy 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Vereecken, H.; Huisman, J.A.; Bogena, H.; Vanderborght, J.; Vrugt, J.A.; Hopmans, J.W. On the value of soil moisture measurements in vadose zone hydrology: A review. Water Resour. Res. 2008, 44, 4. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Her, Y.; Yu, X.; Li, Y.; Smyth, A.; Martens-Habbena, W. Effect of information-driven irrigation scheduling on water use efficiency, nutrient leaching, greenhouse gas emission, and plant growth in South Florida. Agric. Ecosyst. Environ. 2022, 333, 107954. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Huh, M.K.; Lee, B. The Change of Chlorophyll Content and Chlorophyll Efficiency in Epipremnum aureum by Water and pH. Eur. J. Bot. 2022, 1, 898. [Google Scholar] [CrossRef]
- Kahil, M.T.; Connor, J.D.; Albiac, J. Efficient water management policies for irrigation adaptation to climate change in Southern Europe. Ecol. Econ. 2015, 120, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Farias, S.; Espinoza-Meza, S.; López-Olivari, R.; Araya-Alman, M.; Carrasco-Benavides, M. Effects of different irrigation levels on plant water status, yield, fruit quality, and water productivity in a drip-irrigated blueberry orchard under Mediterranean conditions. Agric. Water Manag. 2021, 249, 106805. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Kingfield, D.M.; de Beurs, K.M. Landsat identification of tornado damage by land cover and an evaluation of damage recovery in forests. J. Appl. Meteorol. Climatol. 2017, 56, 965–987. [Google Scholar] [CrossRef]
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Grégoire, J.M. Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Lin, C.; Tsogt, K.; Chang, C.I. An empirical model-based method for signal restoration of SWIR in ASD field spectroradiometry. Photogramm. Eng. Remote Sens. 2012, 78, 119–127. [Google Scholar] [CrossRef]
- Yao, J.; Wu, J.; Xiao, C.; Zhang, Z.; Li, J. The classification method study of crops remote sensing with deep learning, machine learning, and Google Earth engine. Remote Sens. 2022, 14, 2758. [Google Scholar] [CrossRef]
- Zygielbaum, A.I.; Gitelson, A.A.; Arkebauer, T.J.; Rundquist, D.C. Non-destructive detection of water stress and estimation of relative water content in maize. Geophys. Res. Lett. 2009, 36, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Crusiol, L.G.T.; Sun, L.; Sun, Z.; Chen, R.; Wu, Y.; Ma, J.; Song, X. In-Season Monitoring of Maize Leaf Water Content Using Ground-Based and UAV-Based Hyperspectral Data. Sustainability 2022, 14, 9039. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Niu, Y.; Han, W. Mapping Maize Water Stress Based on UAV Multispectral Remote Sensing. Remote Sens. 2019, 11, 605. [Google Scholar] [CrossRef] [Green Version]
- Lacerdaa, L.N.; Snider, J.L.; Cohen, Y.; Liakos, V.; Gobbo, S.; Vellidis, G. Using UAV-based thermal imagery to detect crop water status variability in cotton. Smart Agric. Technol. 2022, 2, 100029. [Google Scholar] [CrossRef]
- Ndlovu, H.S.; Odindi, J.; Sibanda, M.; Mutanga, O.; Clulow, A.; Chimonyo, V.G.P.; Mabhaudhi, T. A Comparative Estimation of Maize Leaf Water Content Using Machine Learning Techniques and Unmanned Aerial Vehicle (UAV)-Based Proximal and Remotely Sensed Data. Remote Sens. 2021, 13, 4091. [Google Scholar] [CrossRef]
- Cai, X.; Magidi, J.; Nhamo, L.; van Koppen, B. Mapping Irrigated Areas in the Limpopo Province, South Africa; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2017; Volume 172. [Google Scholar]
- Mudzielwana, R.V.A. Analysing food security status among farmworkers in the Tshiombo Irrigation Scheme, Vhembe district, Limpopo Province (Doctoral dissertation). Agriculture 2022, 12, 999. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Mugandani, R.; Mafongoya, P. Localized Institutional Actors and Smallholder Irrigation Scheme Performance in Limpopo Province of South Africa. Agriculture 2020, 10, 418. [Google Scholar] [CrossRef]
- Lahiff, E.P. Agriculture and Rural Livelihoods in a South African ‘Homeland’: A Case Study from Venda; University of London, School of Oriental and African Studies: London, UK, 1997. [Google Scholar]
- Bernal, A.; Hernández, A.; Mesa, M.; Rodríguez, O.; González, P.J.; Reyes, R. Characteristics of soil and its limiting factors of regional Murgas, Havana Province. Cultiv. Trop. 2015, 36, 30–40. [Google Scholar]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Mullan, D.; Pietragalla, J. Leaf relative water content. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: Texcoco, Mexico, 2012; pp. 25–27. [Google Scholar]
- Dang, L.M.; Wang, H.; Li, Y.; Min, K.; Kwak, J.T.; Lee, O.N.; Park, H.; Moon, H. Fusarium wilt of radish detection using RGB and near infrared images from Unmanned Aerial Vehicles. Remote Sens. 2020, 12, 2863. [Google Scholar] [CrossRef]
- Filgueiras, R.; Mantovani, E.C.; Althoff, D.; Fernandes Filho, E.I.; Cunha, F.F.D. Crop NDVI monitoring based on sentinel 1. Remote Sens. 2019, 11, 1441. [Google Scholar] [CrossRef] [Green Version]
- Mangewa, L.J.; Ndakidemi, P.A.; Alward, R.D.; Kija, H.K.; Bukombe, J.K.; Nasolwa, E.R.; Munishi, L.K. Comparative assessment of UAV and sentinel-2 NDVI and GNDVI for preliminary diagnosis of habitat conditions in Burunge wildlife management area, Tanzania. Earth 2022, 3, 769–787. [Google Scholar] [CrossRef]
- Bastiaanssen, W.G.; Molden, D.J.; Makin, I.W. Remote sensing for irrigated agriculture: Examples from research and possible applications. Agric. Water Manag. 2000, 46, 137–155. [Google Scholar] [CrossRef]
- Crema, A.; Boschetti, M.; Nutini, F.; Cillis, D.; Casa, R. Influence of soil properties on maize and wheat nitrogen content assessment from Sentinel-2 data. Remote Sens. 2020, 12, 2175. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests of Homogeneity of Variance. In Contributions to Probability and Statistics; Stanford University Press: Stanford, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Pierce, M.W.; Thornton, C.I.; Abt, S.R. Predicting peak outflow from breached embankment dams. J. Hydrol. Eng. 2010, 15, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice; OTexts: Melbourne, Australia, 2018. [Google Scholar]
- Kalariya, K.A.; Singh, A.L.; Goswami, N.; Mehta, D.; Mahatma, M.K.; Ajay, B.C.; Chakraborty, K.; Zala, P.V.; Chaudhary, V.; Patel, C.B. Photosynthetic characteristics of peanut genotypes under excess and deficit irrigation during summer. Physiol. Mol. Biol. Plants 2015, 21, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Li, B.; Zhang, W.; Porter, J.R.; Zhang, F. Evaluation of Sustainability of Irrigated Crops in Arid Regions, China. Sustainability 2021, 13, 342. [Google Scholar] [CrossRef]
- Quimbita, W.; Toapaxi, E.; Llanos, J. Smart Irrigation System Considering Optimal Energy Management Based on Model Predictive Control (MPC). Appl. Sci. 2022, 12, 4235. [Google Scholar] [CrossRef]
- Svedin, J.D.; Kerry, R.; Hansen, N.C.; Hopkins, B.J. Identifying Within-Field Spatial and Temporal Crop Water Stress to Conserve Irrigation Resources with Variable-Rate Irrigation. Agronomy 2021, 11, 1377. [Google Scholar] [CrossRef]
- Yetbarek, E.; Ojha, R. Spatio-temporal variability of soil moisture in a cropped agricultural plot within the Ganga Basin, India. Agric. Water Manag. 2020, 234, 10610. [Google Scholar] [CrossRef]
- Attarzadeh, R.; Amini, J.; Notarnicola, C.; Greifeneder, F. Synergetic Use of Sentinel-1 and Sentinel-2 Data for Soil Moisture Mapping at Plot Scale. Remote Sens. 2018, 10, 1285. [Google Scholar] [CrossRef] [Green Version]
- Acharya, U.; Daigh, A.L.M.; Oduor, P.G. Soil Moisture Mapping with Moisture-Related Indices, OPTRAM, and an Integrated Random Forest-OPTRAM Algorithm from Landsat 8 Images. Remote Sens. 2022, 14, 3801. [Google Scholar] [CrossRef]
- Zheng, X.; Feng, Z.; Li, L.; Li, B.; Jiang, T.; Li, X.; Li, X.; Chen, S. Simultaneously Estimating Surface Soil Moisture and Roughness of Bare Soils by Combining Optical and Radar Data. Int. J. Appl. Earth Obs. Geoinf. 2021, 100, 102345. [Google Scholar] [CrossRef]
- Li, B.; Ti, C.; Zhao, Y.; Yan, X. Supplementary Materials: Estimating Soil Moisture with Landsat Data and its Application in Extracting the Spatial Distribution of Winter Flooded Paddies. Remote Sens. 2016, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Gangat, R.; van Deventer, H.; NaidooI, L.; Adam, E. Estimating soil moisture using Sentinel-1 and Sentinel-2 sensors for dryland and palustrine wetland areas. S. Afr. J. Sci. 2020, 116, 7–8. [Google Scholar] [CrossRef]
- Rabiei, S.; Jalilvand, E.; Tajrishy, M. A Method to Estimate Surface Soil Moisture and Map the Irrigated Cropland Area Using Sentinel-1 and Sentinel-2 Data. Sustainability 2021, 13, 11355. [Google Scholar] [CrossRef]
- Ambrosone, M.; Matese, A.; Di Gennaro, S.F.; Gioli, B.; Tudoroiu, M.; Genesio, L.; Toscano, P. Retrieving soil moisture in rainfed and irrigated fields using Sentinel-2 observations and a modified OPTRAM approach. Int. J. Appl. Earth Obs. Geoinf. 2020, 89, 102113. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, G. Estimation of canopy water content by means of hyperspectral indices based on drought stress gradient experiments of maize in the north plain China. Remote Sens. 2015, 7, 15203–15223. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Parkash, V.; Singh, S. A Review on Potential Plant-Based Water Stress Indicators for Vegetable Crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Hillel, D.; Hatfield, J.L. Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3. [Google Scholar]
- De Swaef, T.; Steppe, K.; Lemeur, R. Determining reference values for stem water potential and maximum daily trunk shrinkage in young apple trees based on plant responses to water deficit. Agric. Water Manag. 2009, 96, 541–550. [Google Scholar] [CrossRef]
- Junttila, S.; Campos, M.; Hölttä, T.; Lindfors, L.; Issaoui, A.E.; Vastaranta, M.; Hyyppä, H.; Puttonen, E. Tree Water Status Affects Tree Branch Position. Forests 2022, 13, 728. [Google Scholar] [CrossRef]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Easterday, K.; Kislik, C.; Dawson, T.E.; Hogan, S.; Kelly, M. Remotely sensed water limitation in vegetation: Insights from an experiment with unmanned aerial vehicles (UAVs). Remote Sens. 2019, 11, 1853. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, U.; Bonel, A.G.; David-Schwartz, R.; Degu, A.; Fait, A.; Cochard, H.; Peterlunger, E.; Herrera, J.C. Grapevine acclimation to water deficit: The adjustment of stomatal and hydraulic conductance differs from petiole embolism vulnerability. Plants 2017, 245, 1091–1104. [Google Scholar] [CrossRef]
- Orynbaikyzy, A.; Gessner, U.; Mack, B.; Conrad, C. Crop Type Classification Using Fusion of Sentinel-1 and Sentinel-2 Data: Assessing the Impact of Feature Selection, Optical Data Availability, and Parcel Sizes on the Accuracies. Remote Sens. 2020, 12, 2779. [Google Scholar] [CrossRef]
- Seeley, M.M.; Martin, R.E.; Vaughn, N.R.; Thompson, D.R.; Dai, J.; Asner, G.P. Quantifying the variation in refectance spectra of Metrosideros polymorpha canopies across environmental gradients. Remote Sens. 2023, 15, 1614. [Google Scholar] [CrossRef]
- Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.; Henry, W.B.; Gao, W.; Reddy, K.R. Physiological assessment of water deficit in soybean using midday leaf water potential and spectral features. J. Plant Interact. 2019, 1, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Wang, J.; Ding, J.; Cao, X.; Zhang, Z.; Liu, J.; Li, X. Combining UAV-based hyperspectral imagery and machine learning algorithms for soil moisture content monitoring. PeerJ. 2019, 7, 6926. [Google Scholar] [CrossRef]
- Franco, A.C.; Haag-Kerwer, A.; Herzog, B.; Grams, T.E.E.; Ball, E.; de Mattos, E.A.; Scarano, F.R.; Barreto, S.; Garcia, M.A.; Mantovani, A.; et al. The effect of light levels on daily patterns of chlorophyll fluorescence and organic acid accumulation in the tropical CAM Treeclusia Hilariana. Trees 1996, 10, 359–365. [Google Scholar] [CrossRef]
- Bahadur, A.; Lama, T.D.; Chaurasia, S.N.S. Gas exchange, chlorophyll fluorescence, biomass production, water use and yield response of tomato (Solanum lycopersicum) grown under deficit irrigation and varying nitrogen levels. Indian J. Agric. Sci. 2015, 85, 224–228. [Google Scholar]
- Liu, F.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Effects of deficit irrigation (DI) and partial root drying (PRD) on gas exchange, biomass partitioning, and water use efficiency in potato. Sci. Hortic. 2006, 109, 113–117. [Google Scholar] [CrossRef]
- Ismail, M.R.; Davies, W.J.; Awad, M.H. Leaf growth and stomatal sensitivity to ABA in droughted pepper plants. Sci. Hortic. 2002, 96, 313–327. [Google Scholar] [CrossRef]

| Spectral Vegetation Index | Formula | Author(s) | |
|---|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (3) | Filgueiras et al. [57] | |
| Greenness Normalized Difference Vegetation Index (GNDVI) | (4) | Mangewa et al. [58] | |
| Optimized Soil Adjusted Vegetation Index (OSAVI) | (5) | Bastiaanssen et al. [59] | |
| Normalized Difference Red-Edge Index (NDRE) | (6) | Crema et al. [60] | |
| Crop Type | GNDVI | NDVI | NDRE | OSAVI |
|---|---|---|---|---|
| Cabbage | 0.402 | 0.422 | 0.371 | 0.369 |
| Maize | 0.461 | 0.418 | 0.448 | 0.452 |
| Florida Broadleaf Mustard | 0.514 | 0.418 | 0.512 | 0.504 |
| Sweet Potato | 0.494 | 0.418 | 0.463 | 0.489 |
| Sugar Beans | 0.505 | 0.489 | 0.475 | 0.434 |
| Green Beans | 0.398 | 0.437 | 0.511 | 0.434 |
| Peas | 0.409 | 0.433 | 0.458 | 0.428 |
| Pepper | 0.429 | 0.574 | 0.488 | 0.572 |
| Solanum retroflexum | 0.411 | 0.594 | 0.439 | 0.590 |
| Spectral Vegetation Index | Crop Type(s) |
|---|---|
| GNDVI | Sweet potato; maize; sugar beans; Florida broadleaf mustard |
| NDVI | Solanum retroflexum; pepper; cabbage |
| NDRE | Peas; green beans |
| OSAVI | - |
| Statistics | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| N | 81 | 81 | 81 |
| Levene’s Test Statistic | 0.512 | 0.296 | 0.321 |
| DF (categories) | 8 | 8 | 8 |
| DF Den | 36 | 36 | 36 |
| p-Value | 0.839 | 0.963 | 0.953 |
| Crop Type | Empirical Equation | r2 |
|---|---|---|
| Solanum retroflexum | 166.85 × (NDVI) − 15.444 | 0.949 |
| Peas | 139.71 × (NDRE) + 9.6922 | 0.961 |
| Green Beans | 310.19 × (NDRE) − 88.004 | 0.974 |
| Sweet Potato | 9.616 × (GNDVI) + 45.259 | 0.948 |
| Pepper | 123.23 × (NDVI) + 19.033 | 0.955 |
| Cabbage | 106.46 × (NDVI) + 22.621 | 0.996 |
| Maize | 244.89 × (GNDVI) − 51.1 | 0.995 |
| Sugar Beans | 205.01 × (GNDVI) − 25.4 | 0.978 |
| Florida Broadleaf Mustard | 88.588 × (GNDVI) + 35.805 | 0.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mndela, Y.; Ndou, N.; Nyamugama, A. Irrigation Scheduling for Small-Scale Crops Based on Crop Water Content Patterns Derived from UAV Multispectral Imagery. Sustainability 2023, 15, 12034. https://doi.org/10.3390/su151512034
Mndela Y, Ndou N, Nyamugama A. Irrigation Scheduling for Small-Scale Crops Based on Crop Water Content Patterns Derived from UAV Multispectral Imagery. Sustainability. 2023; 15(15):12034. https://doi.org/10.3390/su151512034
Chicago/Turabian StyleMndela, Yonela, Naledzani Ndou, and Adolph Nyamugama. 2023. "Irrigation Scheduling for Small-Scale Crops Based on Crop Water Content Patterns Derived from UAV Multispectral Imagery" Sustainability 15, no. 15: 12034. https://doi.org/10.3390/su151512034
APA StyleMndela, Y., Ndou, N., & Nyamugama, A. (2023). Irrigation Scheduling for Small-Scale Crops Based on Crop Water Content Patterns Derived from UAV Multispectral Imagery. Sustainability, 15(15), 12034. https://doi.org/10.3390/su151512034





