1. Introduction
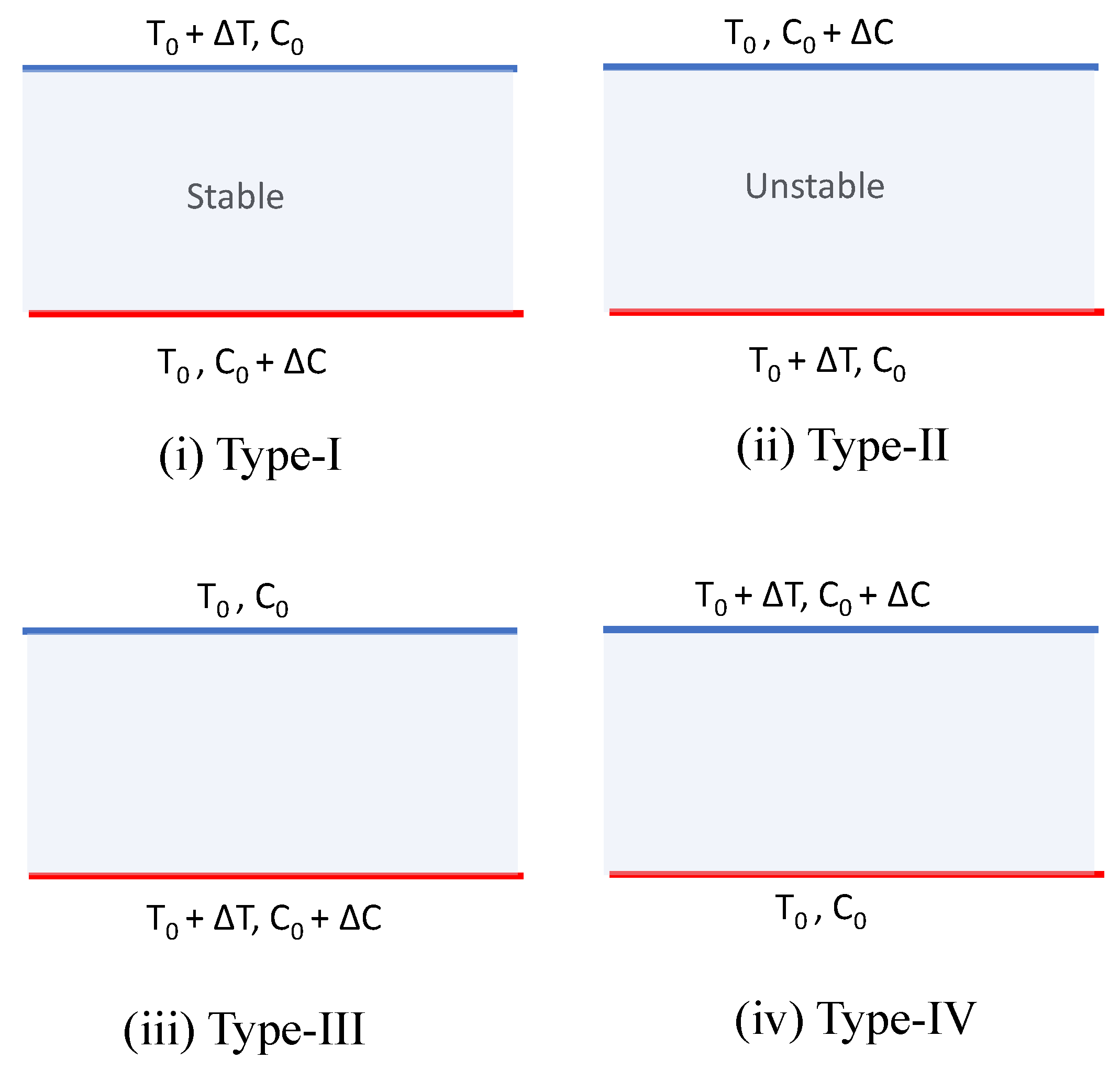
In nature, generally stability/instability is seen in a two-component system (temperature and concentration), in four different ways, as shown in
Figure 1. To analyze the stability of any system, it is necessary to check the type of configuration in a two-component system. In a Type-I system, both components are in a stable configuration, whereas in a Type-II system, both components are destabilizing. Hence, the study of the stability of Type-I and Type-II systems is not required. However, in Type-III or Type-IV system configurations, one of the components is stabilizing and the other one is destabilizing. In these cases, stability can be achieved at certain conditions. In Type-III instability, temperature shows the destabilizing effect, whereas the solute concentration shows the stabilizing effect. This type of instability is termed the diffusive or Bénard instability (both in reactive and non-reactive fluids). In Type-IV instability, temperature is stabilizing the system, wherein the solute concentration is destabilizing the system. This type of instability in non-reactive, and chemically reactive fluid systems are, respectively, called the finger and the chemical instabilities. In the Bénard type of instability, the first bifurcation point is stationary in nature. However, in a finger or chemical type of instability, the first bifurcation point may be oscillatory or stationary. In the present analysis, we intended to predict the effect of the chemical parameters and the aspect ratio on the formation of Bénard cells, at the onset of convection. Hence, the main attention was focused on the Bénard type of instability.
After the discussion on the types of instabilities, now we shall proceed with the introduction to chemically driven convection (
) and its applications. The problem of double-diffusive convection (
) in a chemically active fluid-saturated porous medium is a well-explored phenomenon due to its wide range of applications in many fields including chemical engineering, meteorology, condensed matter physics, etc. Chemical engineering involves the designing of equipment, systems, etc. However, most of the chemical equipment includes curvature shapes such as cylinders. In the present study, we made an attempt to investigate a chemically driven convection in cylindrical enclosures analytically. The chemical reaction can be either exothermic or endothermic or polymerization reactions. In all these processes, some amount of thermal energy either releases or absorbs, which allows the system to generate an adverse density gradient. Hence, the contribution of the chemical reaction to the stability of the system is more significant compared to the contribution by the thermal effect. This type of convection is called the chemically driven convection. The process of the chemical reaction can either occur (i) throughout the volume of the enclosure(homogeneous kinetics) or (ii) exclusively at the surfaces of the enclosures (heterogeneous kinetics). In the former case, the fundamental equations need to be modified to include the transformation process. In the latter case, the boundary conditions need to be modified to include the changes. Furthermore, in modeling the chemical reaction problem, it is important to consider the rate of the chemical reaction, viz. whether the chemical reaction is fast, moderate, or slow, by which the effects of the diffusion terms can be predicted. In a fast chemical reaction problem, the solute diffusion term can be omitted because it occurs at a larger time scale compared to the relaxation time of the chemical reaction. The authors of [
1,
2] presented a model coupling the chemical reaction parameters with the hydrodynamic equations to analyze how convection occurs in a system due to a chemical reaction.
Moving on to the literature survey, in the first part of this section, we shall discuss the works on
in rectangular enclosures (
s). Later, the literature survey is carried out for
in cylindrical enclosures (
s). Many authors have investigated chemically driven convection in a rectangular porous enclosure [
3,
4] or in an infinite horizontal porous medium saturated by a chemically active binary fluid [
5,
6,
7,
8,
9]. Gatica et al. [
3] analytically performed a stability analysis of a natural convection in a rectangular porous cavity with non-isothermal and first-order isothermal chemical reactions. The results of the classical
were obtained as a particular case. They also predicted the stable mode of convection for a given aspect ratio. Hernocourt et al. [
4] classified various possible instabilities, viz. Rayleigh–Bénard, Rayleigh–Taylor, and double-diffusive mechanisms depending on the parameter space in a rectangular porous cavity saturated by an exothermic autocatalytic reacting fluid. Nandakumar and Weintscheke [
5] presented a bifurcation study of a chemically reactive fluid-saturated porous medium in a tilted rectangular box. They employed the perturbation method to study the bifurcation structure in a buoyancy-driven convection (weak convection). Later, they developed a numerical method to study the complex bifurcation structure of the chemically driven convection. Steinberg and Brand investigated the convective instability of a binary mixture with a fast chemically active fluid in an infinite horizontal porous medium. Two types of instabilities (Types III and IV) were considered, and in both instabilities, they observed that the first bifurcation could be stationary or oscillatory depending on the sign and magnitude of the heat of reaction. Mckay [
6] determined the onset of convection in a reactive fluid layer overlying the porous medium saturated by the same reactive fluid using the collocation method. The effect of various parameters, viz. the Frank–Kamenetskii (F-K) number, the fluid/porous medium depth ratio, and the boundary conditions, on the onset was determined. Linear and nonlinear stability analyses were carried out by Malashetty and Biradar [
7] in a double-diffusive chemically reactive binary mixture saturated in an anisotropic porous medium. They showed that the anisotropic porous parameters have a significant influence on the stability of the system. Jotkar et al. [
8] showed that the convective instability in a porous medium arises due to a second-order chemical reaction. They performed linear stability analysis to find the onset of chemically driven convection. Later, they performed a nonlinear simulation and showed that the chemical reaction gives rise to large dissociation fluxes. Later, Jotkar et al. [
9] numerically analyzed the differential diffusive convective dissolution dynamics (DDDCD) in a chemically driven convection.
In cylindrical porous enclosures, not many authors have studied chemically driven convection [
10,
11,
12]. Farr et al. [
10] investigated the chemically driven convection in a cylindrical enclosure in terms of Bessel functions, the solution of which were extracted through the numerical method. Pop et al. [
11] analyzed the natural convection of a boundary layer flow in a cylindrical porous body saturated by a chemically active fluid, which undergoes an exothermic chemical reaction. Roy et al. [
12] numerically investigated the flow characteristics of a free convection driven by an exothermic chemical reaction in an annular region confined between two wavy wall cylinders using the finite difference method. Many authors have investigated the chemically driven convection in a fluid- [
13,
14,
15,
16,
17] and in a single-fluid-saturated porous medium [
18,
19,
20,
21,
22,
23].
To the best of the authors knowledge, there is no analytical work reported in the literature studying the chemically driven convection in cylindrical porous enclosures saturated by a chemically active binary mixture. In the literature, all the works concerning an infinite horizontal extent (in both chemically reactive or non-reactive fluids) neglect the vertical boundary effects. Furthermore, no work has reported the value of the aspect ratio at which the vertical boundary effect becomes negligible. Furthermore, the effect of the chemical reaction on the formation of the Bénard cells at the onset of convection has not been studied. In the present paper, we analyzed all the above-mentioned unconsidered aspects in a homogeneous type of fast chemically reactive binary fluid embedded in densely packed cylindrical/rectangular porous enclosures with the Bénard type of instability. We also present a unified model that combines the problems of natural convection in s and s of infinite horizontal extent saturated by a chemically reactive/non-reactive binary fluid mixture.
2. Mathematical Formulation
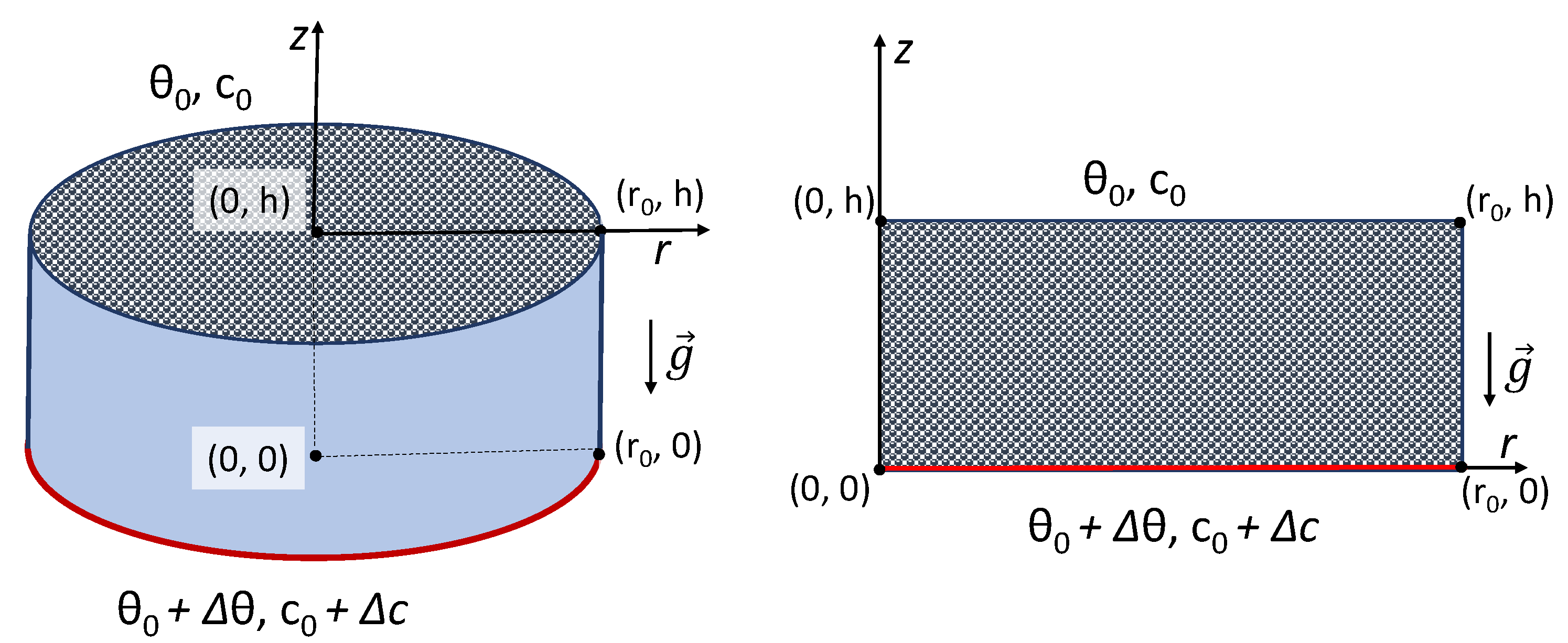
We considered for the investigation a low-porosity cylindrical/rectangular enclosure of depth
h and radius/breadth
saturated by a chemically reactive fluid. The radial/horizontal and vertical directions were, respectively, assumed to be
r and
z, as shown in
Figure 2. In the cylindrical enclosure, due to the centro-symmetric flow pattern, we assumed an axisymmetric condition, which makes the analysis two-dimensional. The vertical temperature and concentration gradients were applied in the presence of a gravitational field,
. As mentioned in the Introduction Section, our main focus was on the Bénard type of instability. Hence, we assumed a warm, high-concentration fluid and a cold, low-concentration fluid at the lower and upper boundaries, respectively. To keep the study simple, the porous medium was assumed to be isotropic, homogeneous, and made up of spherically shaped porous materials. Furthermore, we made the local thermal equilibrium assumption between the fluid and the solid phases.
Under the Boussinesq approximation and the assumption of small-scale convective motion, the fundamental hydrodynamic equations [
1,
24] for the two-dimensional velocity,
, temperature,
, and solute concentration,
c, in the binary liquid are given by
where
is the gradient operator,
is the Laplacian operator, and
is the horizontal Laplacian operator. The parameter
is an artificial curvature parameter, which is deliberately introduced in the continuity equation and in the Laplacian operator. It takes two discrete values, 1 and 0, representing the conservation equations in cylindrical and Cartesian coordinates, respectively. The quantities
, and
are the effective density, porosity, time, pressure, dynamic coefficient of viscosity, permeability, ratio of the product of the density and specific heat at constant pressure, effective thermal diffusivity, thermal expansion coefficient, solutal analog of
, and equilibrium value of the solute concentration. The subscript
l represents the liquid, and the quantities without subscripts represent the effective quantities. The quantity
is the relaxation time, and
is the scaled form of the relaxation time; the two quantities are related by:
where
is the specific heat at constant pressure,
is the chemical parameter, and
is the chemical potential.
In the analysis, we assumed a fast chemical reaction, and hence, the relaxation time,
, is very much less compared to the diffusion time,
, where
is the solute diffusion rate. Thus, the assumption of a fast chemical reaction results in the following condition:
In Newtonian liquids, . This condition also justifies the neglecting of the terms concerning cross-diffusion effects (Soret and Dufour) in Equations (3) and (4).
From the right-hand side of Equations (3) and (4), it is clear that the reaction rate is proportional to the local deviation of the solute concentration from its equilibrium value. This equilibrium value is dependent on the temperature. For small temperature perturbations and neglecting all pressure variations, the term
can be expanded around the constant values
as
This expression also yields
where
represents the difference in quantities. For sufficiently low densities, the chemical potential
is given by
where
is the heat of reaction [
25],
Energy is the energy release during the chemical reaction, and
is the Boltzmann constant. Now, substituting
H in Equation (
9) yields
The sign of
is dependent on the type of reaction under consideration. The parameter
is negative for reactions that produce the lighter component (exothermic) and also for reactions that produce the denser component (endothermic). Even for polymerization reactions with
,
is negative. However, for a dissociating reaction, the sign of
is positive.
Now, we eliminate the pressure term in Equation (3) by operating the curl twice and simplifying it. The
z-component of the resulting equation is given by
Initially, at the quiescent basic state, the fluid is motionless due to the fact that the chemical reaction effect is compensated by the stable solute concentration effect. In this motionless state, vertical gradients are only in the form of diffusion. Hence, the basic state assumes the values
, and
, where the subscript
b represents the basic state. In the basic state, Equation (3) and the second-order derivative of Equation (4) with respect to
z yield
Mutually independent temperature and concentration boundary conditions that can be defined at the boundaries are:
where
and
are the difference in the temperature and concentration, respectively, prescribed at the walls. Using the boundary condition (
13) in Equation (
12), we obtain the following solution in the basic state:
Next, we superimpose small perturbations as follows:
Substituting Equation (
15) in Equations (
1), (
11), (3), and (4) and using the basic state solution (
14), we obtain
Further on, we neglect primes for simplicity. The dimensional quantities are now converted into their non-dimensional form by applying the following scaling of variables:
Using Equation (
20) in Equations (
16)–(19), we obtain:
where
and
are the dimensionless form of the Laplacian and horizontal Laplacian operators, respectively. The non-dimensional parameters
, and
are the Vadasz number, the Darcy–Rayleigh number, the scaled form of the chemical potential, and the ratio of thermal diffusion time to the relaxation time, respectively, and are defined as:
The parameter
in Equation (22) represents the contribution of the chemical reaction to the density distribution.
The non-dimensional form of the governing Equations (
21)–(24) is solved subject to the following boundary condition:
where
is the aspect ratio. In the case of the cylindrical
, there is no motion in a slender cylindrical region around the axis of the cylindrical enclosure,
, and it is termed a pseudo-boundary.
Equation (
26) signifies the use of solvent-impermeable, isothermal, and iso-solute conditions at the horizontal boundaries. In other words, the temperature and the solute concentration are fed to the system through horizontal boundaries, whereas the solvent is confined in an enclosure. The vertical boundaries are solvent-impermeable, adiabatic, solute-impermeable conditions.
To determine the critical Rayleigh number at which convection occurs, we perform linear stability analysis in the next section.
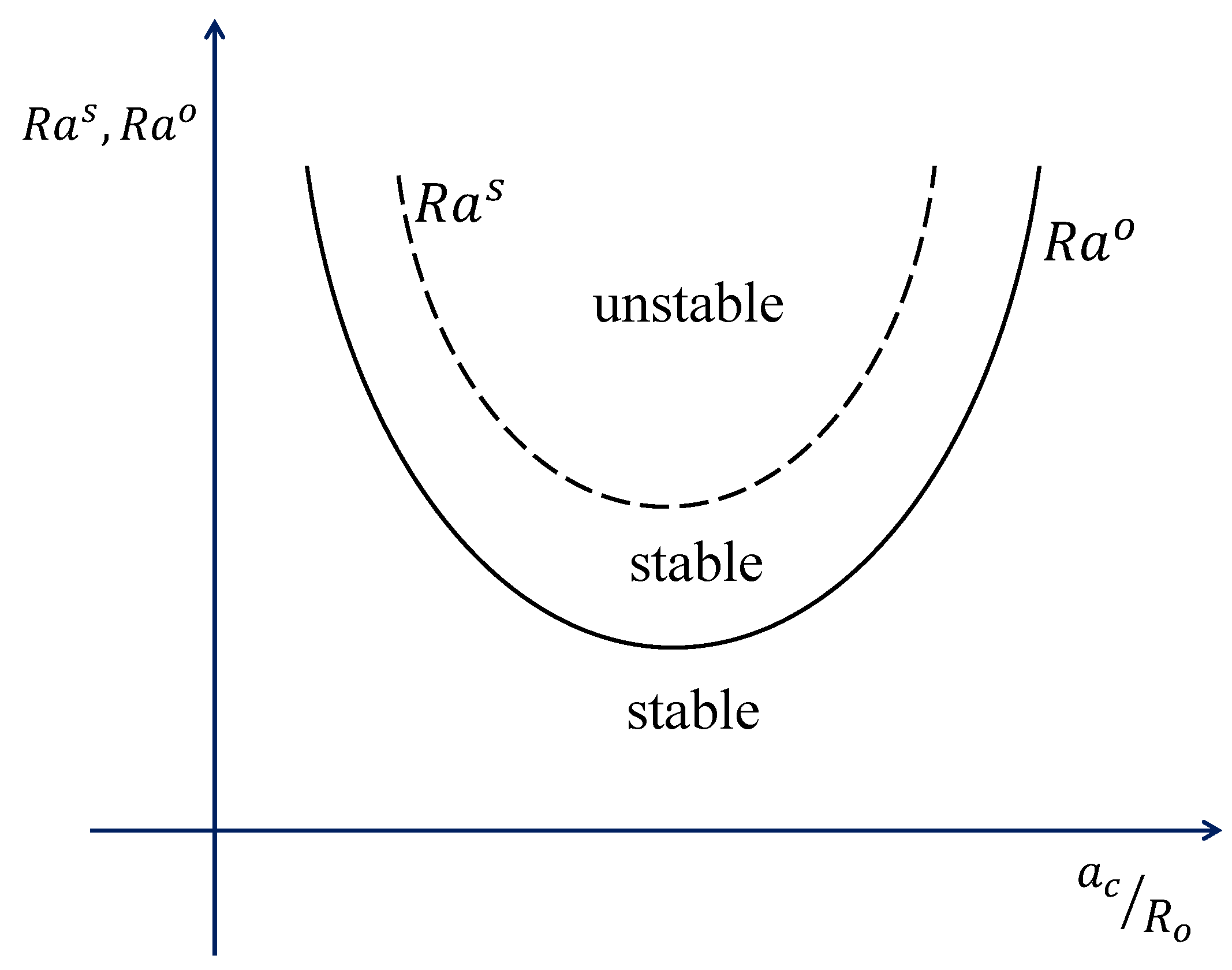
The principle of the exchange of stabilities is schematically shown below in
Figure 3.
The proof of the validity of the principle is extremely tedious. For the sake of being sure about the validity, we shall for the present only refer to earlier works that preferred stationary convection over oscillatory ([
1,
14]). A separate work on the subject of the principle of the exchange of stabilities in the chemically reactive fluid problem in a Bénard-type situation shall be taken in the future.
3. Linear Stability Analysis under the Assumption of the Principle of Exchange of Stabilities
The linearized forms of Equations (
21)–(24) are given by:
The variable separable eigenfunctions corresponding to the periodically appearing roll planform (velocity), isothermal, and iso-solute conditions, are of the form
. Hence, we may take
where
and
. The solution (
30) satisfies the
Z-boundary conditions of Equation (
26). In what follows, we write Equations (
27)–(29) in terms of
, and hence, we write
for
in these equations. Decoupling these transformed equations, we can write
where
To solve Equation (
31), we need four boundary conditions with respect to
R on each of
, and
. Hence, we convert the
U boundary conditions present in Equation (
26) into
W boundary conditions using Equation (
21). This procedure gives us:
At this point, we are short of two boundary conditions with respect to
R on
. The additional boundary conditions required are obtained using Equations (
21)–(24) and the boundary conditions in Equation (
26). The additional boundary conditions on
can be obtained in the form:
Having settled matters pertaining to the boundary conditions, we now factorize Equation (
31) as follows:
where
and
are to be determined. Upon multiplying together the two factors in Equation (
35), we obtain
Upon comparing Equations (
31) and (
36), we obtain the equations for
and
in the form:
Equation (
37) may be used to solve for
, but it involves the eigenvalue
. In view of this, we rearrange Equation (
37) to obtain the expression for
in the form:
The obtaining of
required for evaluating
shall be achieved by using the constraint condition
at
.
To that end, we shift back our attention to the solution of
in Equation (
35) and, hence, choose one factor in Equation (
35), which is the Helmholtz equation:
The solution of Equation (
40) is:
where
and
is the Bessel function of the first kind and of order
. The solution in Equation (
41) is used in Equation (
30) to obtain the complete solution of
in the form:
From the solution (
42), it is clear that the parameter
represents the wave number. Equation (
42) satisfies the boundary condition in Equation (
34), provided:
From the above, it becomes evident that the critical
, viz.
, is not just the result of the minimization of
with respect to
. It also involves the constraint condition (
43). Thus, we have in hand a constrained minimization problem involving Equations (
39) and (
43). To simplify the constrained minimization problem further, we make the substitution
in Equations (
39) and (
43). With this substitution, Equations (
39) and (
43) now take the form:
There are infinitely many solutions for
that satisfy the condition (
45). Among these values of
, a particular value that minimizes
is the required critical value of
, namely
.
After having evolved the procedure for obtaining the critical values of , and thereby and , in the case of the unified problem, we next discuss the results obtained in the study.
4. Results and Discussion
The two-dimensional Darcy–Bénard convection of a fast chemically reacting fluid occupying cylindrical/rectangular enclosures (s/s) was analyzed analytically using the degeneracy technique. A single-term Galerkin solution was employed for solving the problem in cylindrical/rectangular coordinate systems. The eigenfunction in the problem of a rectangular enclosure involves trigonometric functions only, while that in a cylindrical enclosure involves a product of the Bessel and trigonometric functions. The intention of the present study was to determine the onset of convection and predict the number of Bénard cells that manifest in the two enclosures at the onset of convection. In view of this, we first obtained an expression for the critical Rayleigh number, , which determines the onset of convection. Later, using the expression of , we developed an explicit expression for the number of cells as a function of various system parameters.
Generally, a chemical reaction is studied as Bénard instability or chemical instability. In Bénard instability (Type-III), a higher temperature and a higher concentration are present near the lower boundary. In this case, instability arises in two different ways: High-energy components near the bottom plate allow the system to have more chemical reaction near the lower boundary. This produces a lighter component near the bottom of the enclosure, which sets up an adverse density gradient in the system. Furthermore, the chemical reaction releases some amount of thermal energy near the bottom plate, which accelerates the instability in the system. In chemical instability (Type-IV), a higher temperature and a higher solute concentration are present at the top wall. This allows the system to have high-energy, lighter components at the top. Even though this configuration is a stable configuration, a higher concentration of more-energetic lighter components diffuses towards the region of its lower concentration (bottom plate). This raises the temperature near the lower boundary, which accelerates the chemical reaction locally. This process produces more-energetic lighter components near the lower boundary, and it starts moving upwards, causing buoyancy-driven convection. With this phenomenon, when the concentration of the lighter components near the upper boundary reduces, the diffusion process slows down, and the system leads to oscillatory convection.
The main concern of the present study was to predict the number of Bénard cells, and this manifests in Type-III instability. Hence, we chose a fast chemically reacting, fluid-saturated, densely packed porous medium with the Bénard type of instability. This allows the system to have only stationary convection.
Before moving on to the discussion of the results, we first estimated the permissible range of values of the parameters appearing in the study. Gitterman and Steinberg [
26] provided typical values for the parameters governing the fast chemically reacting Rayleigh–Bénard convection problem. They chose the values:
The negative value of
represents the Type-IV instability which makes
due to a negative temperature gradient value in
. In our analysis, we mainly focused on the Type-III instability. In this case, we need to choose a positive value for the parameter
. The parameters’ values mentioned in Equation (
46) are for the case of a non-porous medium. In the work of Steinberg and Brand [
1], the parameters’ values given for a chemically active fluid mixture in a densely packed porous medium were as follows:
With these values, the system exhibits both an oscillatory and a stationary nature of convection. However, in the present analysis, our interest was to study the stationary convection, and hence, we modified the parameters’ values using Equations (
46) and (
47).
The parameter
can be rewritten as
[
25]. In the presence of a porous medium, some amount of thermal energy gets absorbed by the porous material. Hence, the value of
gets reduced in its presence compared to the case of a non-porous medium. We hence chose
in the calculation of the present investigation.
Recalling the definition of
,
and using the condition
, we can write
. In the presence of a porous medium, the diffusion rate,
, reduces further. Hence, we have
. As mentioned in
Section 2, the parameter
takes values in the range of
for Newtonian liquids. Hence,
for Newtonian liquids. The thermal diffusivity,
, of a Newtonian-liquid-saturated porous medium [
27] is found to be of the order of
. With these values of
and
,
is constrained by the condition:
.
The value of the other parameter
is dependent on the type of instability under consideration. For Type-III and Type-IV instabilities,
takes positive and negative values, respectively. From Equation (
10),
is given by
Since
is dependent on
D, a similar argument as the case of
can be made to find the value of
in the presence of a porous medium. Hence, in our computations, we chose
.
Having arrived at permissible ranges of the parameters’ values, in the next subsection, we first determine the critical Rayleigh number and then proceed to find an expression for the number of cells in .
4.1. Expression for the Critical Thermal Rayleigh Number, , with
From Equations (
44) and (
45), it is clear that the problem involves a constrained minimization problem. There are infinite positive roots satisfying Equation (
45). Let us arrange them as
. The first four positive roots of Equation (
45) are tabulated in the first four columns of
Table 1. For various sets of parameter values, different
’s,
, yield the critical Rayleigh number,
. The dependency of
on different parameters is recorded in
Table 1. The particular
that yields
is represented in bold, and we call it
. At larger values of the aspect ratio, the wave number is given by
. Although, from the constraint (
45),
seems independent of the parameters, the critical value,
, varies with the parameters’ values due to the dependence of
on them. Hence, the purpose of
Table 1 is to show that the wave number is dependent on the chemical parameters.
In
Table 1, the discrete roots,
, of Equations (
45) obtained from the numerical methods are recorded. To represent the roots of Equation (
45) analytically, we arrived at a general solution for it. We tabulated the first 100 numerical solutions,
, of
and made some observations. Surprisingly, we found that
for
and for
,
. Hence, we adopted
as the line of best fit for
. Hence, we may write
The successive integral values of
n in Equation (
49), respectively, yield successive numerical roots of Equation (
45) with a maximum percentage error of 0.01103. Now, by the inspection method, we found that, for different parameters’ values, a different
n yields
and is called the critical
n,
. Using Equation (
49),
may be written as
. Now, using
in Equation (
44), the expression
is obtained in the form:
Having obtained an expression for , in the next section, we were interested in finding the physical significance of the parameter, n. Intuition tells us that n may represent the number of cells.
4.2. Expression for the Number of Cells in
To analyze the number of cells that manifest in the given enclosure, we made use of the expression of the stream function,
:
Using Equation (
42) in Equation (
51), we obtain
The integral constant in Equation (
52) becomes zero as the boundary of the enclosure is also a streamline. In
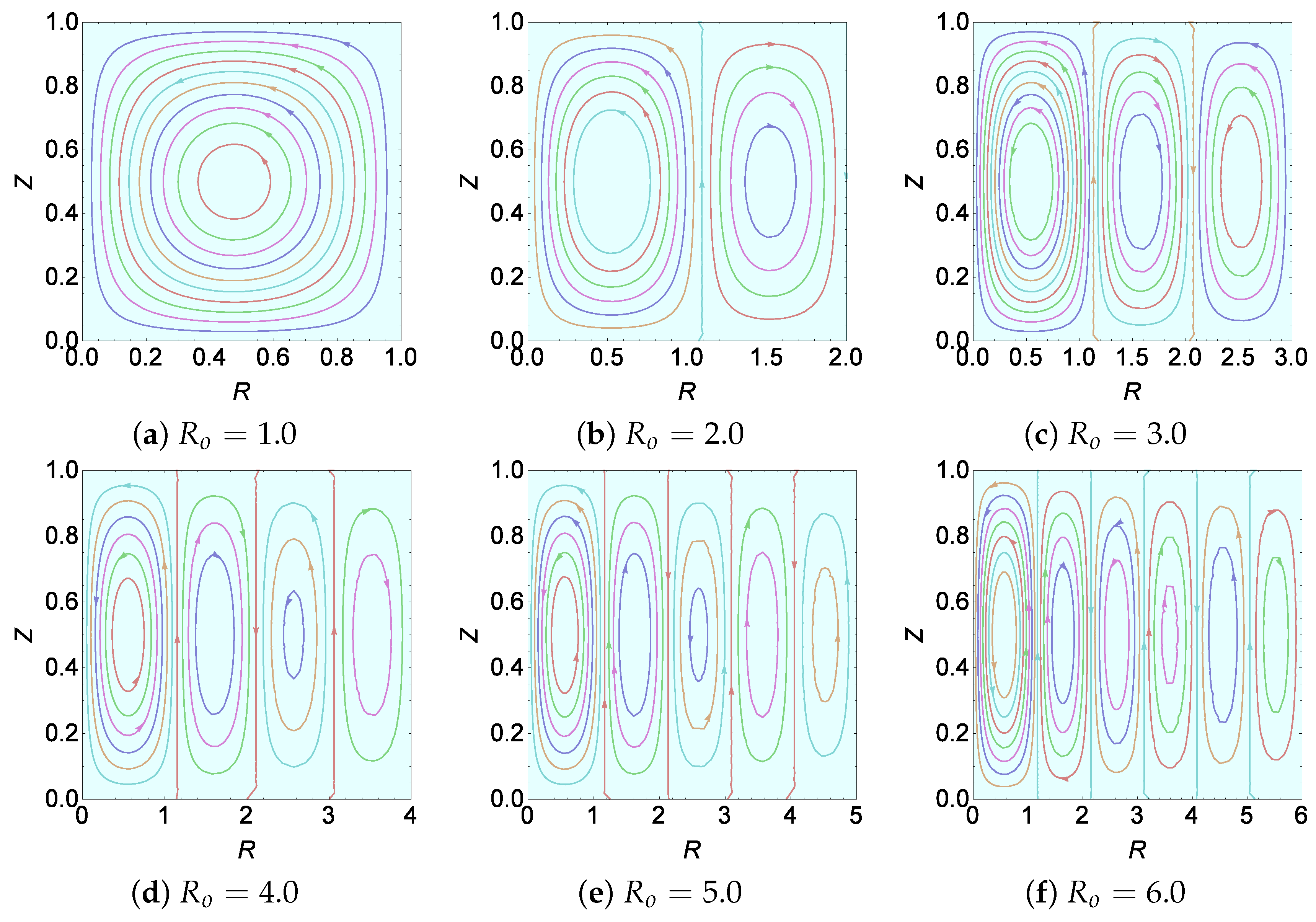
Figure 4, we plot the streamlines for different values of
(by fixing all other parameters’ values). The value of
was chosen in such a way that each value of
falls in the range of different marginal stability curves of the upper-most graph of
Figure 5c. Now, by observing
Figure 5c (upper-most graph) and
Figure 4 together, we arrive at the information that the value
represents the number of Bénard cells that manifest in the system for a given
.
Thus, we note that the intersection point between any two successive marginal stability curves in
Figure 5 represents the point of increase of the number of cells. Now, we used this information in obtaining an explicit expression for the number of cells. This intersection point between any two marginal stability curves in
Figure 5 can be obtained from the condition:
Using Equation (
50) in Equation (
53) and simplifying for
, we obtain a fifth-degree polynomial of
. The ceil value of the real and positive root (only one in fact) of the polynomial equation yields the value of the number of cells for the considered set of parameters’ values and is given by
where
and the operator
on the right-hand side of Equation (
54) represents the ceil function, which returns the maximum positive integer of the corresponding function.
After having obtained the expression for the number of cells, we shall discuss in the next section the effect of various parameters on and .
4.3. Effect of the Chemical Parameters, , and , and the Aspect Ratio, , on and
In the last two columns of
Table 1, we record the influence of the chemical parameters and the aspect ratio on
and
. We observed from the tabulated values that the parameters
and
showed a destabilizing effect in the system. The reason behind this observation is that, from the definitions of
and
, it is clear that an increase in the values of
and
increases the energy release during the chemical reaction,
D, in the system. This forces the onset of convection to happen earlier.
The parameter shows a stabilizing effect in the system. For any considered chemically reactive fluid, increasing the value of increases the thermal diffusion rate and decreases the chemical reaction rate. The thermal diffusion rate of any fluid-saturated porous medium depends inversely on the thermal conductivity of the porous material. Thus, an increase in the value of implies the consideration of a low thermally conducting porous medium and a slower rate of chemical reaction, hence the delay in the onset of convection.
The effect of increasing
is to increase the horizontal dimension of the enclosure. This allows more space in the system for the manifestation of more cells. The fact that
increases with the increase in
is shown in the fourth row of
Table 1.
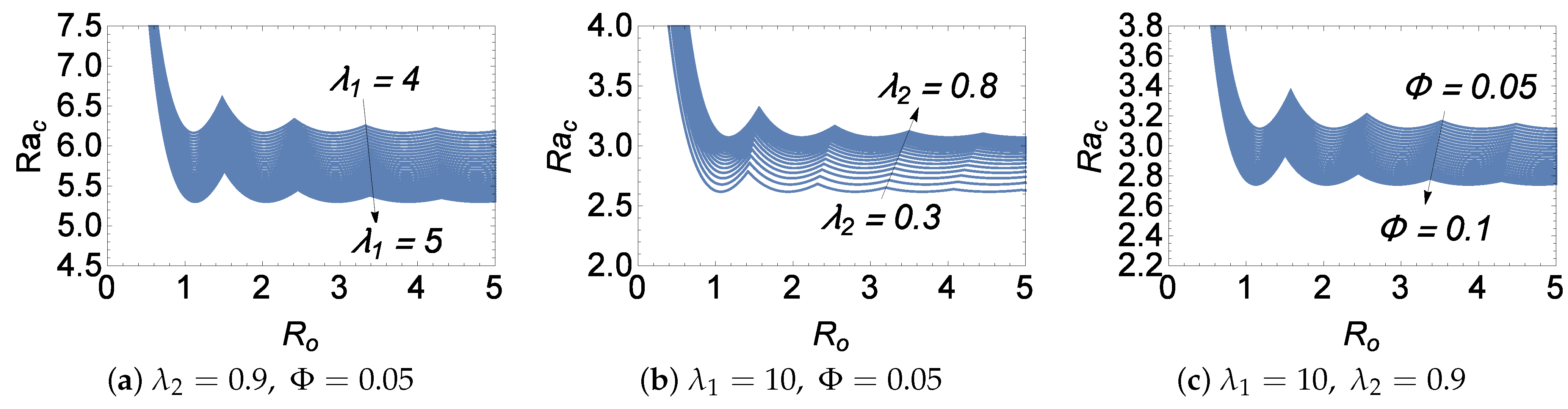
Figure 5 is the plot of
versus
for different chemical parameters’ values, and it reiterates the earlier discussion concerning the effects of
,
, and
on the stability of the system. It is observed from the figure that the increase in the values of
and
decreases the value of
, confirming its destabilizing nature, and the opposite effect is observed for the parameter
. The locus of intersection points represents the increasing and decreasing effects of
, and
on the number of cells depending on whether the loci lean to the left or the right, respectively.
We now discuss the limiting cases of the present model in the next subsection.
4.4. Limiting Cases
4.4.1. Results on Rectangular Enclosures
The Rayleigh number expression (
44) remains the same in the case of
. The constraint function (
45) in
reduces to
The solution of Equation (
55) is
. With this value of
and from the definition of the critical Rayleigh number, we obtain
where
is the value of
at which
attains its minimum value. Following a procedure similar to that considered earlier for
, we obtain an expression for the number of cells as
We note at this juncture that, from the results, the effects of various parameters, viz. , , and , on and follow . Thus, to avoid duplication, we skipped the corresponding plots here of the s.
4.4.2. Results of in a Non-Reactive Fluid-Saturated Porous Medium
Mathematically, the chemical reaction in the system is governed by the non-dimensional parameters , , and .
By choosing the values:
, and
, in Equations (
21)–(24), the solute concentration equation gets decoupled. The resultant set of equations now represents the problem of
s in a non-reactive fluid-saturated porous medium and are given by:
Now, upon simplifying Equations (
58)–(60) and following the standard procedure, the expressions of
and
yield:
Equations (
61) and (62) are unified equations representing the
.
4.4.3. Results of Classical ()
As we considered very shallow enclosures (
), the concept of the wave number can be applied in both the
and
. The expression of the critical wave number is given by
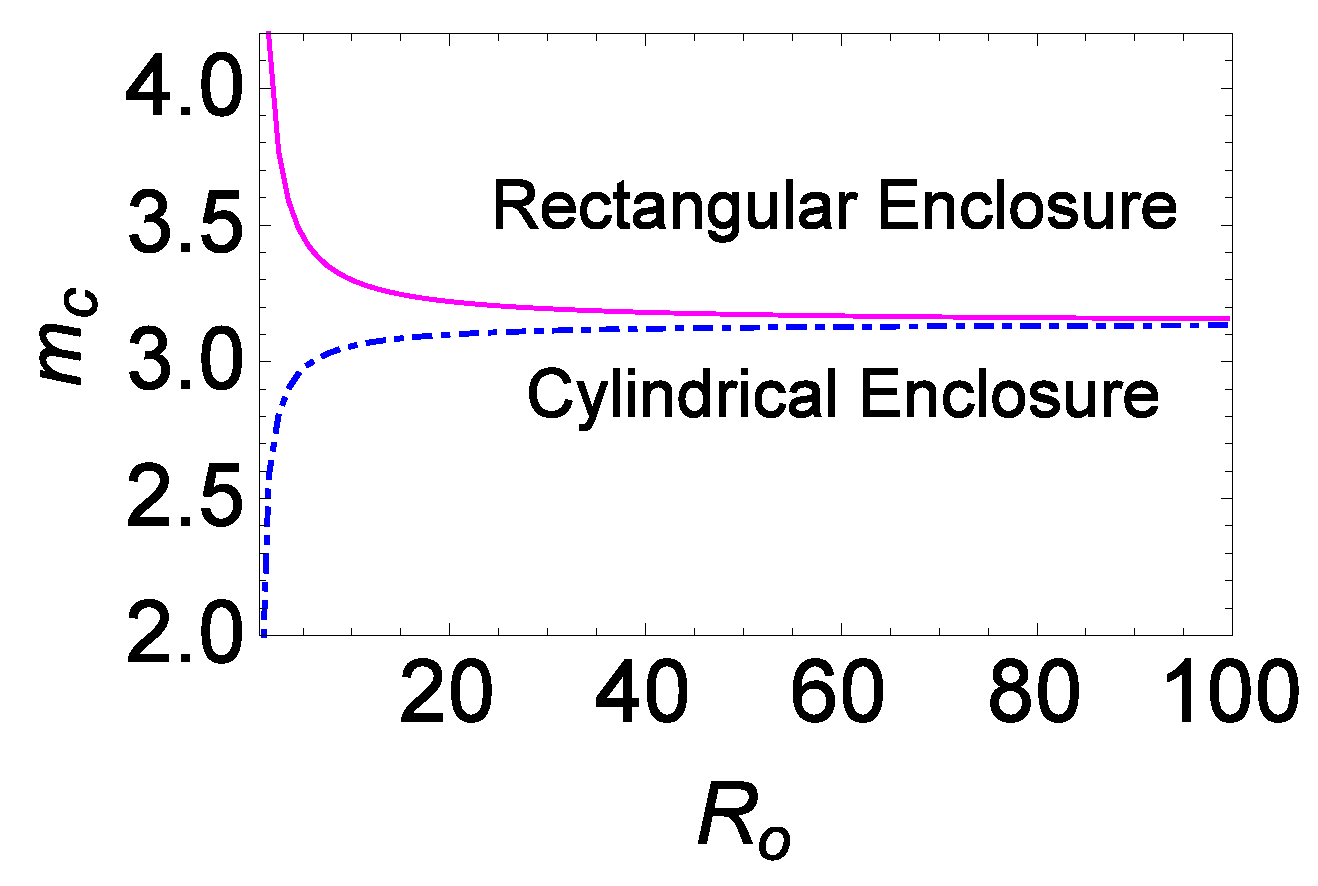
We discussed in
Section 4.1 that the wave number is intrinsically dependent on the chemical parameters, aspect ratio, and type of enclosures under consideration (nature of vertical boundaries). For fixed chemical parameters’ values, the wave number is dependent only on the aspect ratio and the type of enclosure. Upon increasing
, the value of
in both the
and
starts converging to a particular value,
, after which the wave number becomes independent of both the aspect ratio and the type of enclosure. The value of
at which the boundary effects become negligible is taken as
. This nature of
and
is recorded in
Table 2 for different sets of parameters’ values, and it is also represented graphically in
Figure 6 for one set of chemical parameter values. Similar plots were observed for all other combinations of chemical parameters. Hence, the corresponding plots were omitted here.
In
Table 2, it is observed that the value of
decreases with the increase in the values of
and
(first and second rows of
Table 2). The opposite effect is observed for the variation of the parameter
. This pattern of the variation of
follows the pattern of the number of cells formed in the system (see the last column of
Table 1).
In this case, also the effects of various chemical parameters on and remained the same as in the case of the . Hence, the corresponding plots were omitted here.
Having discussed the results and the limiting cases, in the next section, we shall validate the present model results by comparing it with the existing works.
4.5. Validation of the Present Model
The results of the present model were validated by comparing them with those of Steinberg and Brand [
1] for the problem of chemically driven convection in a densely packed porous layer of infinite horizontal extent. At large values of
, their results can be recovered from those of the present model.
From Equation (
25), the expression for the thermal gradient,
, is given by
The expression for the critical temperature gradient shall be written as
The values of the critical thermal gradient for two sets of parameter values are explicitly available in Steinberg and Brand [
1] and are tabulated in
Table 3 along with the values obtained from the present model (Equation (
65)). It is clear from the tabulated values that the present model’s results are in good agreement with those of Steinberg and Brand [
1].
In
Table 3, the permeability,
, is found from the semiempirical Blake–Kozeny formula [
28]:
where
is the diameter of the spherical porous material.
At this point, we made the observation that we shall endeavor to consider a similar problem that involves nanofluids, especially hybrid ones, as considered by Yaseen et al. [
29,
30].
After the validation of the results of the present model, in the next section, we draw some major conclusions of the study.