Mushroom Biomass Waste Is a Source of the Antioxidants Ergothioneine and Glutathione
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom and Chemical Materials
2.2. Glutathione Metabolite Analysis
2.3. Ergothioneine Analysis
2.4. Ergothioneine Capture from Mushroom Biomass Waste
2.5. Statistical Analysis
3. Results and Discussion
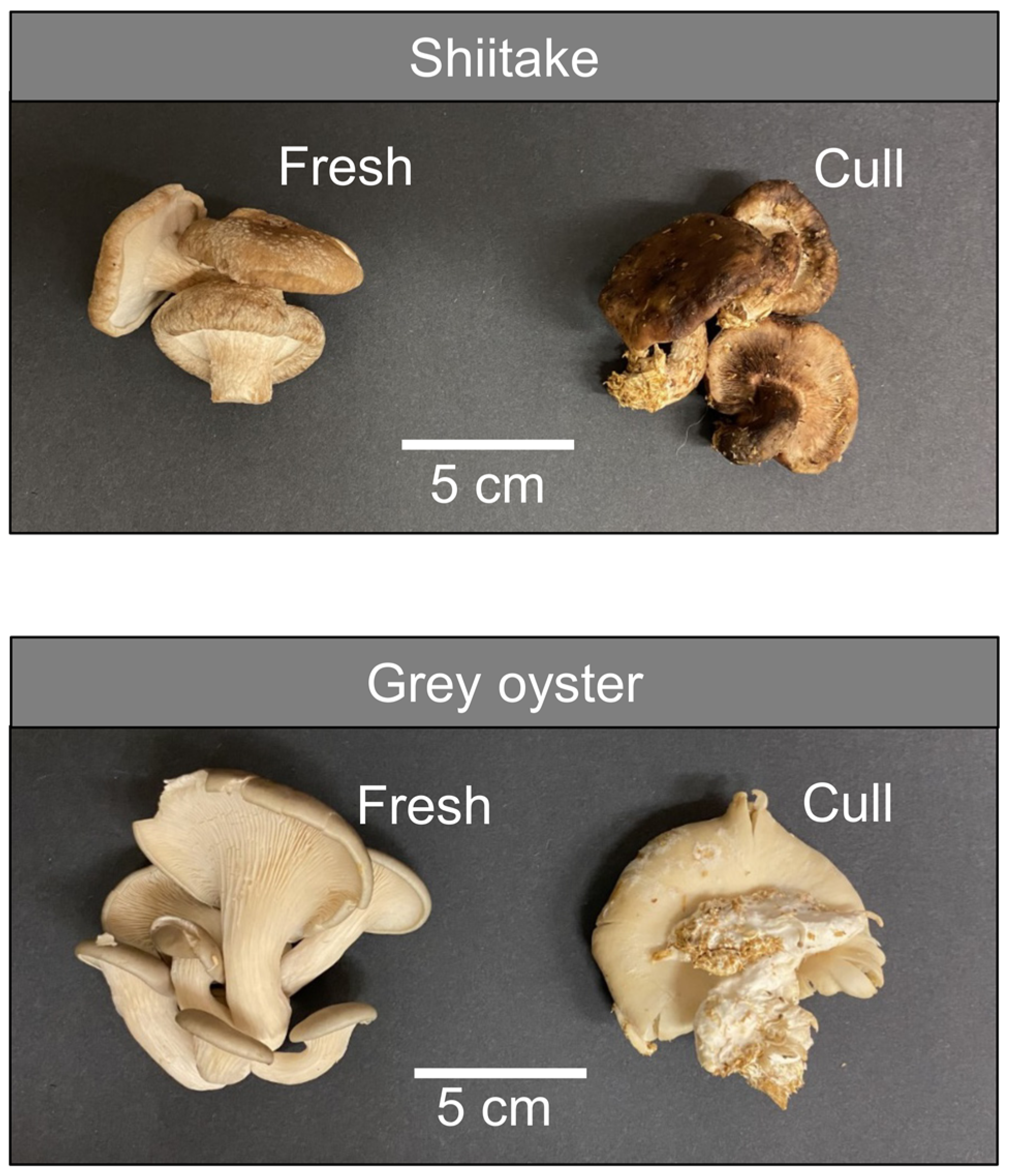
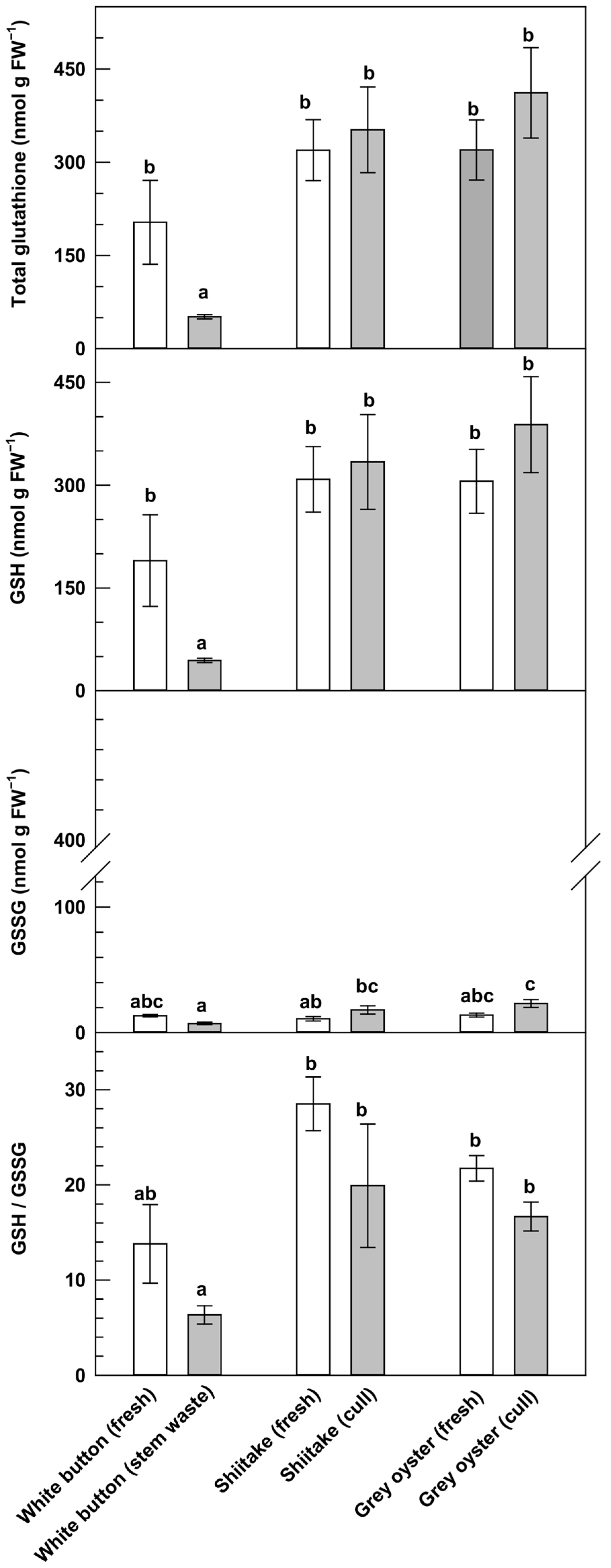
3.1. Analysis of Glutathione Metabolites in Fresh and Waste Mushrooms
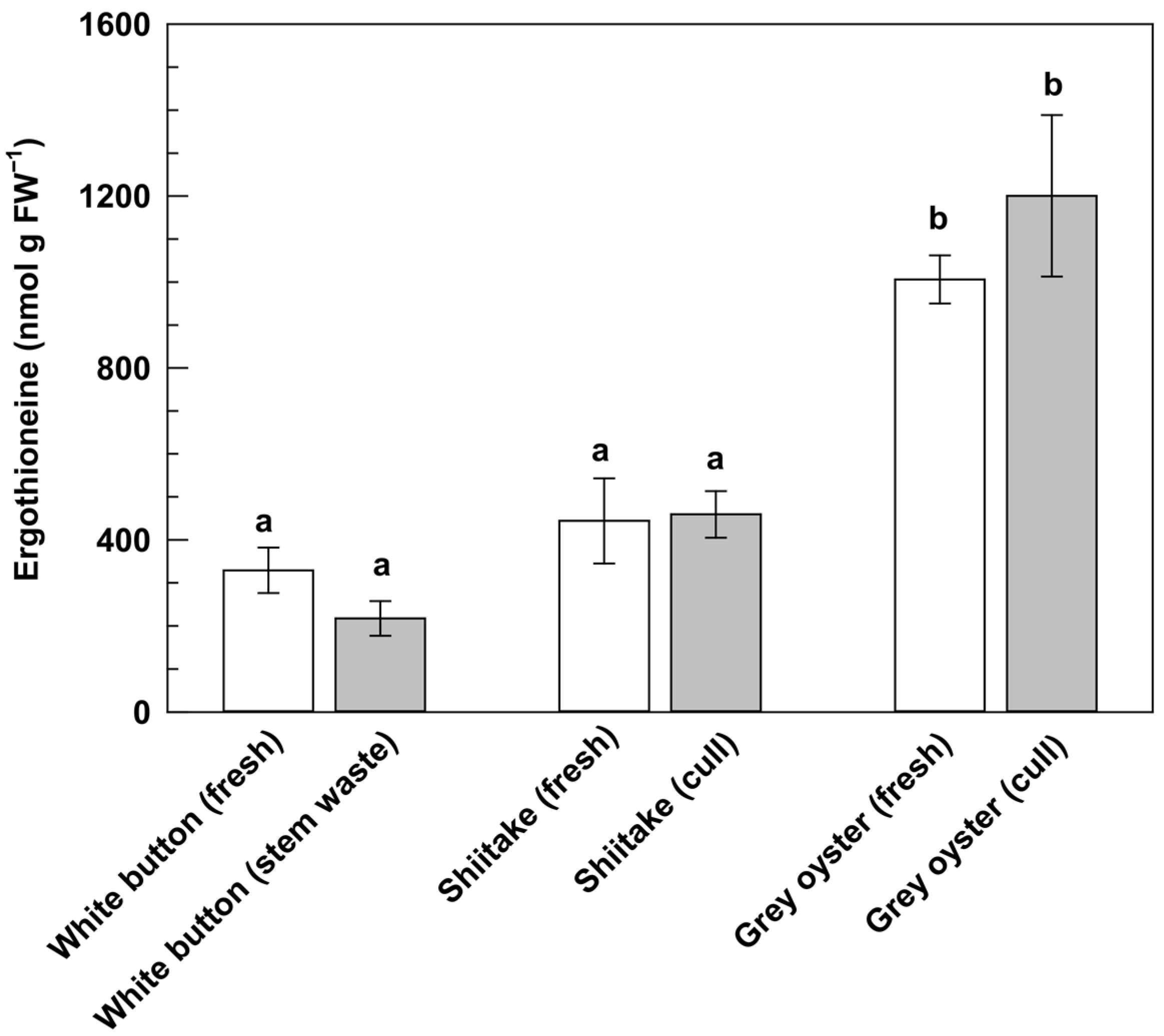
3.2. Analysis of Ergothioneine in Fresh and Waste Mushrooms
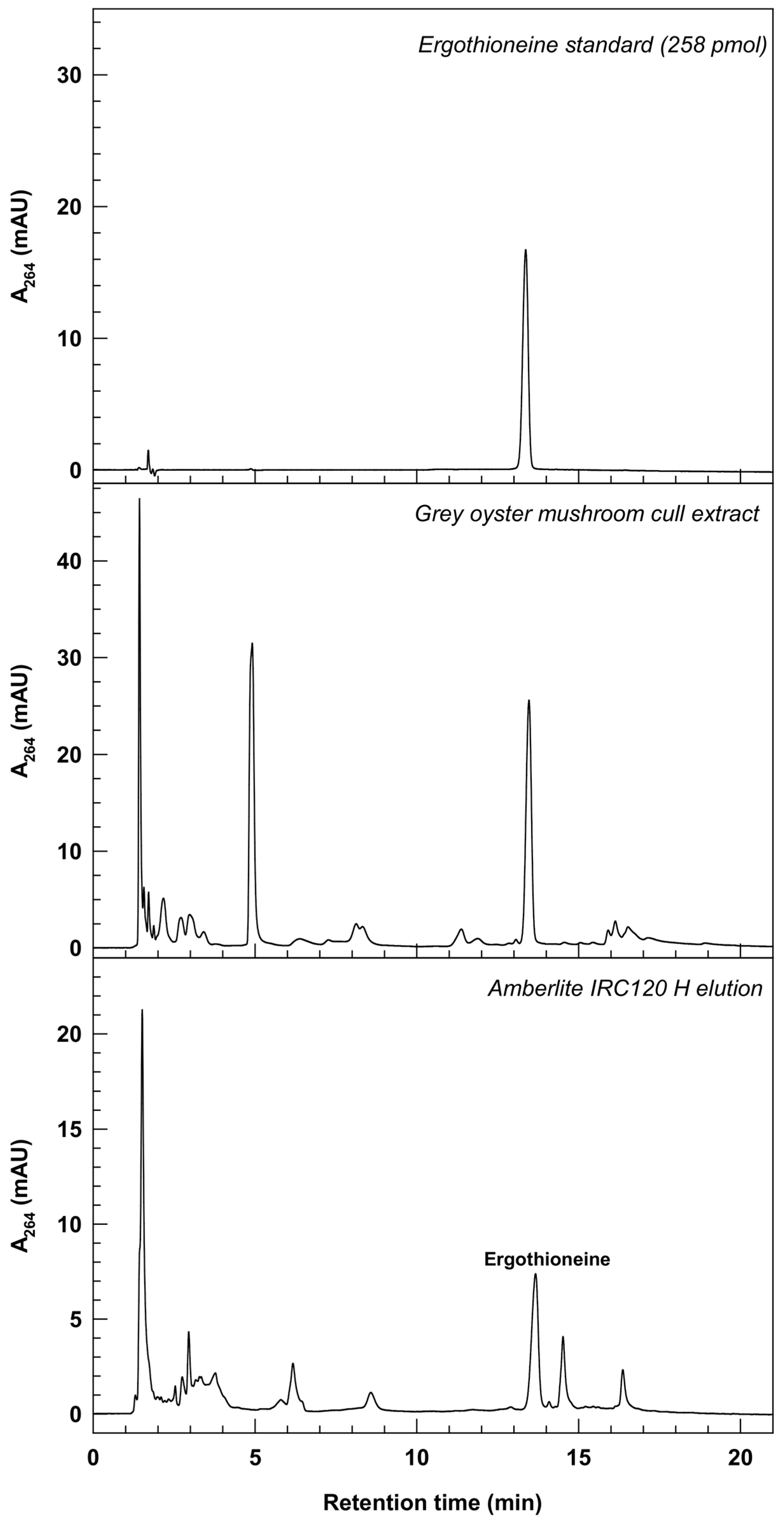
3.3. Capture of Ergothioneine from Oyster Mushroom Culls
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fulgoni, V.L., III; Agarwal, S. Nutritional impact of adding a serving of mushrooms on usual intakes and nutrient adequacy using National Health and Nutrition Examination Survey 2011–2016 data. Food Sci. Nutr. 2021, 9, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Fenny, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef] [PubMed]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. Arab. Book 2011, 9, e0142. [Google Scholar] [CrossRef]
- Lum, G.B.; Shelp, B.J.; DeEll, J.R.; Bozzo, G.G. Oxidative metabolism is associated with physiological disorders in fruits stored under multiple environmental stresses. Plant Sci. 2016, 245, 143–152. [Google Scholar] [CrossRef]
- Yamauchi, N. Postharvest chlorophyll degradation and oxidative stress. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 101–113. [Google Scholar]
- Jones, D.P.; Coates, R.J.; Flagg, E.W.; Eley, J.W.; Block, G.; Greenberg, R.S.; Gunter, E.W.; Jackson, B. Glutathione in foods listed in the National Cancer Institute’s Health habits and history food frequency questionnaire. Nutr. Cancer 1992, 17, 57–75. [Google Scholar] [CrossRef]
- Tsiasioti, A.; Zotou, A.S.; Tzanavaras, P.D. Single run analysis of glutathione and its disulfide in food samples by liquid chromatography coupled to on-line post-column derivatization. Food Chem. 2021, 361, 130173. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Ey, J.; Schömig, E.; Taubert, D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef]
- Stampfli, A.R.; Blankenfeldt, W.; Seebeck, F.P. Structural basis of ergothioneine biosynthesis. Curr. Opin. Struct. Biol. 2020, 65, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Lin, S.; Lin, J.; Wang, Y.; Lin, J.F.; Guo, L. The biosynthetic pathway of ergothioneine in culinary–medicinal winter mushroom, Flammulina velutipes (Agaricomycetes). Int. J. Med. Mushrooms 2020, 22, 171–181. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QI (accessed on 20 June 2023).
- Statistics Canada. Mushroom Growers’ Survey. 2021. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/220620/dq220620d-eng.htm (accessed on 20 June 2023).
- Statistics Canada. Economic Profile of the Mushroom Industry in Canada. 2019. Available online: https://www150.statcan.gc.ca/n1/en/catalogue/21-004-X201900100001 (accessed on 4 April 2023).
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of mushroom by-products as a source of value-added compounds and potential applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, M.; Fang, Z. Valorization of mushroom by-products: A review. J. Sci. Food Agric. 2022, 102, 5593–5605. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Halim-Lim, S.A.; Kamarudin, N.Z.; Rukayadi, Y.; Abd Rahim, M.H.; Jamaludin, A.A.; Ilham, Z. Fruiting-body-base flour from an oyster mushroom waste in the development of antioxidative chicken patty. J Food Sci. 2020, 85, 3124–3133. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.K.; Das, A.K.; Banerjee, R.; Pateiro, M.; Nanda, P.K.; Gadekar, Y.P.; Biswas, S.; McClements, D.J.; Lorenzo, J.M. Application of enoki mushroom (Flammulina velutipes) stem wastes as functional ingredients in goat meat nuggets. Foods 2020, 9, 432. [Google Scholar] [CrossRef]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.C.; Bhuyan, D.J. Recovery of ergosterol and vitamin D2 from mushroom waste—Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Cebin, A.V.; Petravić-Tominac, V.; Djakovic, S.; Srecec, S.; Zechner-Krpan, V.; Piljac-Zegarac, J.; Isikhuemhen, O.S. Polysaccharides and antioxidants from culinary-medicinal white button mushroom, Agaricus bisporus (Agaricomycetes), waste biomass. Int. J. Med. Mushrooms 2018, 20, 797–808. [Google Scholar] [CrossRef]
- Lum, G.B.; Brikis, C.J.; Deyman, K.L.; Subedi, S.; DeEll, J.R.; Shelp, B.J.; Bozzo, G.G. Pre-storage conditioning ameliorates the negative impact of 1-methylcyclopropene on physiological injury and modifies the response of antioxidants and γ-aminobutyrate in ‘Honeycrisp’ apples exposed to controlled-atmosphere conditions. Postharvest Biol. Technol. 2016, 116, 115–128. [Google Scholar] [CrossRef]
- Dubost, N.J.; Beelman, R.B.; Peterson, D.; Royse, D.J. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int. J. Med. Mushrooms 2006, 8, 215–222. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Wang, H.; Li, Y.; Liu, W.; Wang, Q.; Liu, D.; Chen, N.; Jiang, W. Validation of a HILIC method for the analysis of ergothioneine in fermentation broth. J. Chromatogr. Sci. 2016, 54, 934–938. [Google Scholar] [CrossRef]
- Park, S.H.; Lovejoy, K.; Grosse, S.; De Pra, M.; Meding, S.; Steiner, F. Underivatized Amino Acid Analysis in Wine by HILIC Separation and Mass Detection. Thermo Scientific Application Notes 73151. 2019. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-73151-hilic-ms-amino-acids-wine-an73151-en.pdf (accessed on 18 April 2022).
- Christianson, D.D.; Wall, J.S.; Dimler, R.J.; Senti, F.R. Separation and determination of quaternary nitrogen compounds and other nitrogenous substances by ion exchange chromatography. Application to analysis of corn extracts. Anal. Chem. 1960, 32, 874–878. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 29 May 2023).
- Dogan, H.; Coteli, E.; Karatas, F. Determination of glutathione, selenium, and malondialdehyde in different edible mushroom species. Biol. Trace Elem. Res. 2016, 174, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Oumari, M.; Goldfuss, B.; Stoffels, C.; Schmalz, H.G.; Gründemann, D. Regeneration of ergothioneine after reaction with singlet oxygen. Free Radic. Biol. Med. 2019, 134, 498–504. [Google Scholar] [CrossRef]
- Jenny, K.A.; Mose, G.; Haupt, D.J.; Hondal, R.J. Oxidized forms of ergothioneine are substrates for mammalian thioredoxin reductase. Antioxidants 2022, 11, 185. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Giri, A.; Ohshima, T. A rapid HPLC post-column reaction analysis for the quantification of ergothioneine in edible mushrooms and in animals fed a diet supplemented with extracts from the processing waste of cultivated mushrooms. Food Chem. 2012, 133, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Ho, K.J.; Hsieh, Y.J.; Wang, L.T.; Mau, J.L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT—Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Fan, T.W.M. Considerations of sample preparation for metabolomics investigation. In The Handbook of Metabolomics; Methods in Pharmacology and Toxicology; Fan, T.M., Lane, A., Higashi, R., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 7–27. [Google Scholar]
- Rodriguez Estrada, A.E.; Lee, H.J.; Beelman, R.B.; Jimenez-Gasco, M.D.M.; Royse, D.J. Enhancement of the antioxidants ergothioneine and selenium in Pleurotus eryngii var. eryngii basidiomata through cultural practices. World J. Microbiol. Biotechnol. 2009, 25, 1597–1607. [Google Scholar] [CrossRef]
- Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the identification and quantification of ergothioneine and lovastatin in various mushroom species: Assets and challenges of different analytical approaches. Molecules 2021, 26, 1832. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Chen, Z.Y. Influence of cold storage and processing of edible mushroom on ergothioneine concentration. Int. J. Food Eng. 2019, 5, 159–163. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nagasaka, R.; Ohshima, T. Effects of extraction solvents, cooking procedures and storage conditions on the contents of ergothioneine and phenolic compounds and antioxidative capacity of the cultivated mushroom Flammulina velutipes. Int. J. Food Sci. Technol. 2012, 47, 1193–1205. [Google Scholar] [CrossRef]
- Takusagawa, S.; Satoh, Y.; Ohtsu, I.; Dairi, T. Ergothioneine production with Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2019, 83, 181–184. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Z.; Su, E.; Wang, L.; Sun, L.; Lei, P.; Xu, H.; Li, S. Recent strategies for the biosynthesis of ergothioneine. J. Agric. Food Chem. 2021, 69, 13682–13690. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, C.; Delaunay, N.; Costanza, C.; Eudes, V.; Mallet, J.M.; Gareil, P. New avenue for mid-UV-range detection of underivatized carbohydrates and amino acids in capillary electrophoresis. Anal. Chem. 2011, 83, 7381–7387. [Google Scholar] [CrossRef] [PubMed]
- Panthong, S.; Boonsathorn, N.; Chuchawankul, S. Antioxidant activity, anti-proliferative activity, and amino acid profiles of ethanolic extracts of edible mushrooms. Genet. Mol. Res. 2016, 15, gmr15048886. [Google Scholar] [CrossRef] [PubMed]
- Yadan, J.C. Matching chemical properties to molecular biological activities opens a new perspective on L-ergothioneine. FEBS Lett. 2022, 596, 1299–1312. [Google Scholar] [CrossRef]
- Bessieres, M.A.; Gibon, Y.; Lefeuvre, J.C.; Larher, F. A single-step purification for glycine betaine determination in plant extracts by isocratic HPLC. J. Agric. Food Chem. 1999, 47, 3718–3722. [Google Scholar] [CrossRef]
- Apparoo, Y.; Phan, C.W.; Kuppusamy, U.R.; Sabaratnam, V. Ergothioneine and its prospects as an anti-ageing compound. Exp. Gerentol. 2022, 170, 111982. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Fan, S.; Ma, J.; Wang, Y.; Li, Q.; Song, Z.; Sun, Y.; Zhang, Y. Determination of L-ergothioneine in cosmetics based on ultraperformance liquid chromatography-tandem mass spectrometry. Int. J. Anal. Chem. 2022, 2022, 4372295. [Google Scholar] [CrossRef]
- Kitsanayanyong, L.; Ohshima, T. Ergothioneine: A potential antioxidative and antimelanosis agent for food quality preservation. FEBS Lett. 2022, 596, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumar, D.; Bozzo, G. Mushroom Biomass Waste Is a Source of the Antioxidants Ergothioneine and Glutathione. Sustainability 2023, 15, 11961. https://doi.org/10.3390/su151511961
Sivakumar D, Bozzo G. Mushroom Biomass Waste Is a Source of the Antioxidants Ergothioneine and Glutathione. Sustainability. 2023; 15(15):11961. https://doi.org/10.3390/su151511961
Chicago/Turabian StyleSivakumar, Dhanya, and Gale Bozzo. 2023. "Mushroom Biomass Waste Is a Source of the Antioxidants Ergothioneine and Glutathione" Sustainability 15, no. 15: 11961. https://doi.org/10.3390/su151511961
APA StyleSivakumar, D., & Bozzo, G. (2023). Mushroom Biomass Waste Is a Source of the Antioxidants Ergothioneine and Glutathione. Sustainability, 15(15), 11961. https://doi.org/10.3390/su151511961







