From the Sea to Mosquito Control: The Potential of Halymenia dilatata Marine Alga as an Eco-Friendly Mosquitocidal Agent
Abstract
:1. Introduction
2. Methodology
2.1. Red Alga Extraction and Harvesting
2.2. Mosquito Rearing
2.3. Chemicals
2.4. Larvicidal Assay
2.5. Enzyme Assay
2.6. Repellent Assay
2.7. Non-Target Toxicity
2.8. Photomicrography Assay
2.9. Data Analysis
3. Results
3.1. Larval Toxicity of Mx-Hd
3.2. Enzyme Inhibition of Mx-Hd
3.3. Repellent Activity of Mx-Hd
3.4. Toxicity of Mx-Hd against Aquatic Predators
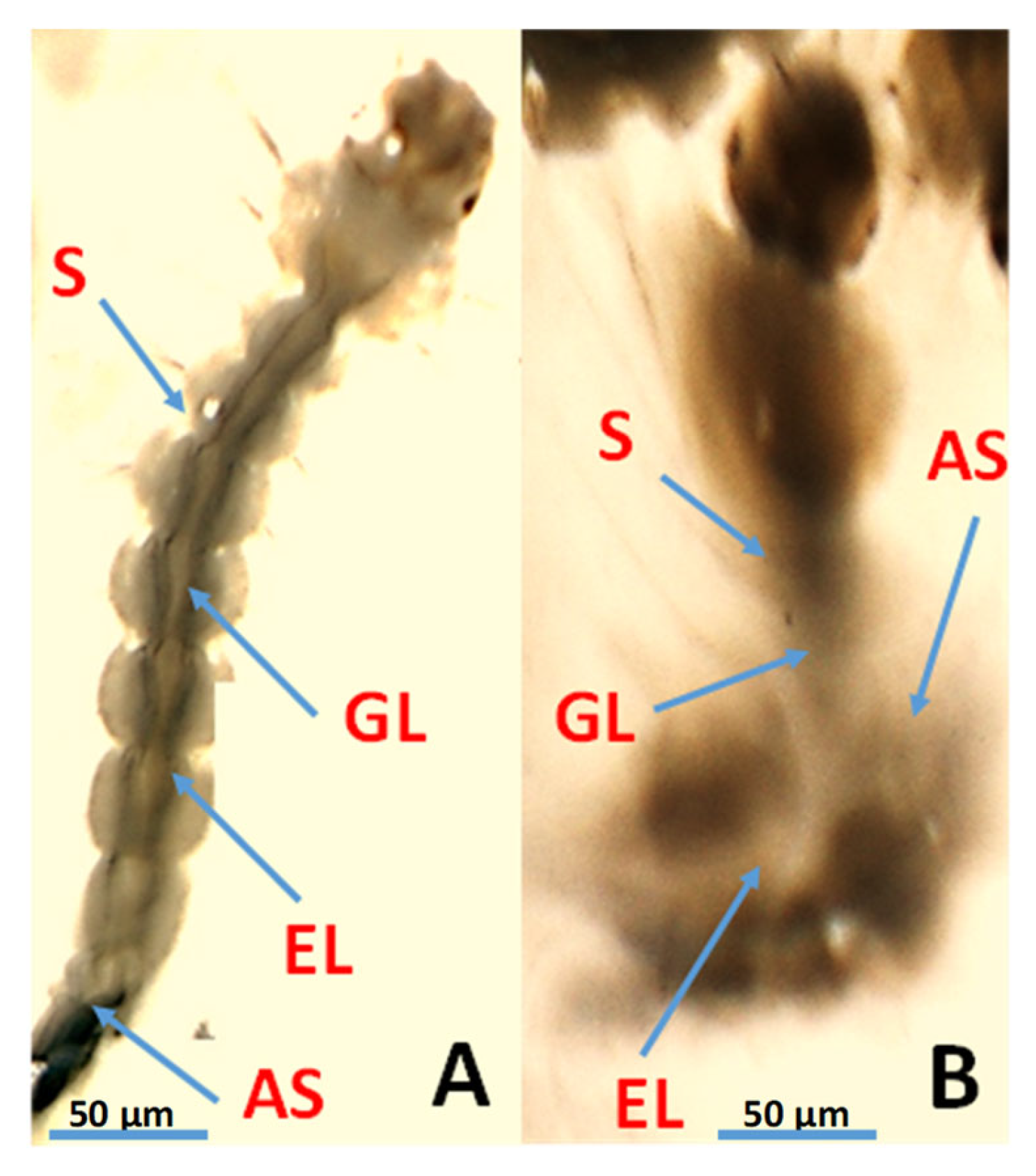
3.5. Photomicrography of Ae. aegypti Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chellappandian, M.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Karthi, S.; Thanigaivel, A.; Ponsankar, A.; Kalaivani, K.; Hunter, W.B. Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ. Int. 2018, 113, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Edwin, E.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Ponsankar, A.; Pradeepa, V.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Abdel-Megeed, A.; et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae). Acta Tropica 2016, 163, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.S.; Vasantha-Srinivasan, P.; Altemimi, A.B.; Keerthana, R.; Radhakrishnan, N.; Senthil-Nathan, S.; Kalaivani, K.; Chandrasekar, N.; Karthi, S.; Ganesan, R.; et al. Bioactive molecules derived from plants in managing dengue vector Aedes aegypti (Linn.). Molecules 2023, 28, 2386. [Google Scholar] [CrossRef] [PubMed]
- Vasantha-Srinivasan, P.; Thanigaivel, A.; Edwin, E.S.; Ponsankar, A.; Senthil-Nathan, S.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Duraipandiyan, V.; Al-Dhabi, N.A. Toxicological effects of chemical constituents from Piper against the environmental burden Aedes aegypti Liston and their impact on non-target toxicity evaluation against biomonitoring aquatic insects. Environ. Sci. Pollut. Res. 2018, 25, 10434–10446. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Chellappandian, M.; Senthil-Nathan, S.; Ponsankar, A.; Thanigaivel, A.; Karthi, S.; Edwin, E.S.; Selin-Rani, S.; Kalaivani, K.; Maggi, F.; et al. A novel herbal product based on Piper betle and Sphaeranthus indicus essential oils: Toxicity, repellent activity and impact on detoxifying enzymes GST and CYP450 of Aedes aegypti Liston (Diptera: Culicidae). J. Asia-Pacific Entomol. 2018, 21, 1466–1472. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Panikar, S.; Sethuraman, V.; Usha-Raja-Nanthini, A.; Shivakumar, M.S. Toxic and synergetic effect of plant essential oils along with nano-emulsion for control of three mosquito species. J. Nat. Pestic. Res. 2023, 5, 100045. [Google Scholar] [CrossRef]
- Kamaraj, C.; Gandhi, P.R.; Kumar, R.C.S.; Balasubramani, G.; Malafaia, G. Biosynthesis and extrinsic toxicity of copper oxide nanoparticles against cattle parasites: An eco-friendly approach. Environ. Res. 2022, 214, 114009. [Google Scholar] [CrossRef]
- Sanga, A.G.; Mazigo, H.D.; Manjurano, A.; Morona, D.; Thomas, A. Measuring repellence and mortality effects of clove and cinnamon essential oils impregnated nets against Anopheles gambiae senso stricto using tunnel test. J. Nat. Pestic. Res. 2023, 5, 100046. [Google Scholar] [CrossRef]
- Amala, K.; Karthi, S.; Ganesan, R.; Radhakrishnan, N.; Srinivasan, K.; Mostafa, A.E.Z.M.; Al-Ghamdi, A.A.; Alkahtani, J.; Elshikh, M.S.; Senthil-Nathan, S.; et al. Bioefficacy of Epaltes divaricata (L.) n-Hexane extracts and their major metabolites against the Lepidopteran pests Spodoptera litura (fab.) and dengue mosquito Aedes aegypti (Linn.). Molecules 2021, 26, 3695. [Google Scholar] [CrossRef]
- Karthi, S.; Vasantha-Srinivasan, P.; Ganesan, R.; Ramasamy, V.; Senthil-Nathan, S.; Khater, H.F.; Radhakrishnan, N.; Amala, K.; Kim, T.J.; El-Sheikh, M.A.; et al. Target activity of Isaria tenuipes (Hypocreales: Clavicipitaceae) fungal strains against dengue vector Aedes aegypti (Linn.) and its non-target activity against aquatic predators. J. Fungi 2020, 6, 196. [Google Scholar] [CrossRef]
- Moola, A.K.; Ayyadurai, T.; Balasubramani, S.; Vignesh, R.; Mohan, P.K.; Sathish, S. Chemical composition and larvicidal activity against Aedes aegypti larvae from Hyptis suaveolens (L.) Poit essential oil. J. Nat. Pestic. Res. 2023, 3, 100018. [Google Scholar] [CrossRef]
- Bibi, R.; Tariq, R.M.; Rasheed, M. Toxic assessment, growth disrupting and neurotoxic effects of red seaweeds’ botanicals against the dengue vector mosquito Aedes aegypti L. Ecotoxicol. Environ. Saf. 2020, 195, 110451. [Google Scholar] [CrossRef]
- Aziz, M.; Hashan Arif, E.I.; Muhammad Dimyati, N.I.; Ishak, I.H.; Hamdan, R.H.; Syazwan, S.A.; Peng, T.L. Larvicidal effect of Vitex ovata Thunb. (lamiales: Lamiaceae) leaf extract towards Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera: Culicidae). Parasitologia 2021, 1, 210–217. [Google Scholar] [CrossRef]
- Yogarajalakshmi, P.; Poonguzhali, T.V.; Ganesan, R.; Karthi, S.; Senthil-Nathan, S.; Krutmuang, P.; Radhakrishnan, N.; Mohammad, F.; Kim, T.J.; Vasantha-Srinivasan, P. Toxicological screening of marine red algae Champia parvula (C. Agardh) against the dengue mosquito vector Aedes aegypti (Linn.) and its non-toxicity against three beneficial aquatic predators. Aquat. Toxicol. 2020, 222, 105474. [Google Scholar] [CrossRef]
- Jainab, S.B.; Azeez, A.; Fathima, A.; Kumar, R.R. GC-MS analysis of the marine algae Halymenia dilatata Zanardini a potential source of fish feed in future. Indian. Hydrobiol. 2019, 18, 164–169. [Google Scholar]
- Sathiyaraj, G.; Vinosha, M.; Sangeetha, D.; Manikandakrishnan, M.; Palanisamy, S.; Sonaimuthu, M.; Manikandan, R.; You, S.; Prabhu, N.M. Bio-directed synthesis of Pt-nanoparticles from aqueous extract of red algae Halymenia dilatata and their biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126434. [Google Scholar]
- Rondevaldova, J.; Quiao, M.A.; Drabek, O.; Dajcl, J.; Dela Pena-Galanida, G.D.; Leopardas, V.E.; Kokoska, L. Mineral composition of seaweeds and seagrasses of the Philippines. Phycologia 2023, 62, 217–224. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kamada, T.; Lee, W.W.; Jeon, Y.J. Anti-inflammatory activity of halogenated secondary metabolites of Laurencia snackeyi (Weber-van Bosse) Masuda in LPS-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1805–1813. [Google Scholar] [CrossRef]
- Manilal, A.; Sujith, S.; Sabarathnam, B.; Kiran, G.S.; Selvin, J.; Shakir, C.; Lipton, A.P. Biological activity of red alga Laurencia brandenii. Acta Bot. Croatica. 2011, 70, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Vinosha, M.; Palanisamy, S.; Muthukrishnan, R.; Selvam, S.; Kannapiran, E.; You, S.; Prabhu, N.M. Biogenic synthesis of gold nanoparticles from Halymenia dilatata for pharmaceutical applications: Antioxidant, anti-cancer and antibacterial activities. Process. Biochem. 2019, 85, 219–229. [Google Scholar] [CrossRef]
- Hassan, S.W.M.; Shobier, A.H. GC/MS identification and applications of bioactive seaweed extracts from Mediterranean coast of Egypt. Egypt. J. Aquat. Biol. Fish. 2018, 22, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Vinosha, M.; Palanisamy, S.; Anjali, R.; Li, C.; Yelithao, K.; Marudhupandi, T.; Tabarsa, M.; You, S.; Prabhu, N.M. Sulfated galactan from Halymenia dilatata enhance the antioxidant properties and prevents Aeromonas hydrophila infection in tilapia fish: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 158, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Shameel, M.; Aisha, K.; Khan, S.H. A preliminary survey of seaweeds from the coast of Makran, Pakistan. Bot. Mar. 1996, 39, 223–230. [Google Scholar] [CrossRef]
- Li, Y.; Cui, J.; Zhang, G.; Liu, Z.; Guan, H.; Hwang, H.; Aker, W.G.; Wang, P. Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour. Technol. 2016, 214, 144–149. [Google Scholar] [CrossRef]
- Deepak, P.; Sowmiya, R.; Balasubramani, G.; Aiswarya, D.; Arul, D.; Josebin, M.P.D.; Perumal, P. Mosquito-larvicidal efficacy of gold nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Part. Sci. Technol. 2018, 36, 974–980. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report: 2005: Make Every Mother and Child Count; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Finney, D.J. Statistical logic in the monitoring of reactions to therapeutic drugs. Methods Inf. Med. 1971, 10, 237–245. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Edwin, E.S.; Ponsankar, A.; Chellappandian, M.; Selin-Rani, S.; Lija-Escaline, J.; Kalaivani, K. Impact of Terminalia chebula Retz. against Aedes aegypti L. and non-target aquatic predatory insects. Ecotoxicol. Environ. Saf. 2017, 137, 210–217. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Special Programme for Research and Training in Tropical Diseases (TDR); Department of Control of Neglected Tropical Diseases. Epidemic and Pandemic Alert, 2009. In Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Thanigaivel, A.; Chanthini, K.M.P.; Karthi, S.; Vasantha-Srinivasan, P.; Ponsankar, A.; Sivanesh, H.; Stanley-Raja, V.; Shyam-Sundar, N.; Narayanan, K.R.; Senthil-Nathan, S. Toxic effect of essential oil and its compounds isolated from Sphaeranthus amaranthoides Burm. f. against dengue mosquito vector Aedes aegypti Linn. Pestic. Biochem. Physiol. 2019, 160, 163–170. [Google Scholar] [CrossRef]
- Biber, P.A.; Dueñas, J.R.; Almeida, F.L.; Gardenal, C.N.; Almirón, W.R. Laboratory evaluation of susceptibility of natural subpopulations of Aedes aegypti larvae to temephos. J. Am. Mosq. Cont. Assoc. 2006, 22, 408–411. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lija-Escaline, J.; Senthil-Nathan, S.; Thanigaivel, A.; Pradeepa, V.; Vasantha-Srinivasan, P.; Ponsankar, A.; Edwin, E.S.; Selin-Rani, S.; Abdel-Megeed, A. Physiological and biochemical effects of chemical constituents from Piper nigrum Linn (Piperaceae) against the dengue vector Aedes aegypti Liston (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4239–4249. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian. J. Med. Res. 2012, 135, 581. [Google Scholar] [PubMed]
- Yee, D.A.; Kaufman, M.G.; Juliano, S.A. The significance of ratios of detritus types and micro-organism productivity to competitive interactions between aquatic insect detritivores. J. Animal Ecol. 2007, 76, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepak, P.; Balamuralikrishnan, B.; Park, S.; Sowmiya, R.; Balasubramani, G.; Aiswarya, D.; Amutha, V.; Perumal, P. Phytochemical profiling of marine red alga, Halymenia palmata and its bio-control effects against Dengue Vector, Aedes aegypti. S. Afr. J. Bot. 2019, 121, 257–266. [Google Scholar] [CrossRef]
- Yu, K.X.; Wong, C.L.; Ahmad, R.; Jantan, I. Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2015, 8, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Bianco, E.M.; Pires, L.; Santos, G.K.; Dutra, K.A.; Reis, T.N.; Vasconcelos, E.R.; Cocentino, A.L.; Navarro, D.M. Larvicidal activity of seaweeds from northeastern Brazil and of a halogenated sesquiterpene against the dengue mosquito (Aedes aegypti). Ind. Crops Prod. 2013, 43, 270–275. [Google Scholar] [CrossRef]
- Haleem, D.R.A.; El Tablawy, N.H.; Alkeridis, L.A.; Sayed, S.; Saad, A.M.; El-Saadony, M.T.; Farag, S.M. Screening and evaluation of different algal extracts and prospects for controlling the disease vector mosquito Culex pipiens L. Saudi J. Biol. Sci. 2022, 29, 933–940. [Google Scholar] [CrossRef]
- Kiran, S.K.; Prakash, S.S.; Chamegowda, T.C.; Krishnamurthy, R.; Yogananda, S.B.; Asha, N.N. Effect of different biostimulants on growth parameters of maize in red soils of Karnataka. J. Pharmaco. Phytochem. 2020, 9, 541–545. [Google Scholar]
- Yu, X.Q.; He, W.F.; Liu, D.Q.; Feng, M.T.; Fang, Y.; Wang, B.; Feng, L.H.; Guo, Y.W.; Mao, S.C. A seco-laurane sesquiterpene and related laurane derivatives from the red alga Laurencia okamurai Yamada. Phytochemistry 2014, 103, 162–170. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Kumar, M.; Bijo, A.J.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation. Plant Physiol. Biochem. 2012, 51, 129–138. [Google Scholar] [CrossRef]
- Xu, P.; Choo, Y.M.; De La Rosa, A.; Leal, W.S. Mosquito odorant receptor for DEET and methyl jasmonate. Proc. Natl. Acad. Sci. USA 2014, 111, 16592–16597. [Google Scholar] [CrossRef]
- Chellappandian, M.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Thanigaivel, A.; Kalaivani, K.; Sivanesh, H.; Stanley-Raja, V.; Chanthini, K.M.P.; Shyam-Sundar, N. Target and non-target botanical pesticides effect of Trichodesma indicum (Linn) R. Br. and their chemical derivatives against the dengue vector, Aedes aegypti L. Environ. Sci. Pollut. Res. 2019, 26, 16303–16315. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Karthi, S.; Chellappandian, M.; Ponsankar, A.; Thanigaivel, A.; Senthil-Nathan, S.; Chandramohan, D.; Ganesan, R. Aspergillus flavus (Link) toxins reduces the fitness of dengue vector Aedes aegypti (Linn.) and their non-target toxicity against aquatic predator. Microbial Pathog. 2019, 128, 281–287. [Google Scholar] [CrossRef]
- Jayakodi, S.; Shanmugam, V.K. Green synthesis of CuO nanoparticles and its application on toxicology evaluation. Biointerface Res. Appl. Chem. 2020, 10, 6343–6353. [Google Scholar]
- Rajapandian, R.; Kadarkarai, M. Encapsulation of silver nano crystals using Salvinia molesta against the Anopheles stephensi and oxidative stress enzyme activity of larvivorous fish. J. Nat. Pesti. Res. 2023, 3, 100022. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Karthi, S.; Ganesan, R.; Senthil-Nathan, S.; Krutmuang, P.; Chellappandian, M.; Radhakrishnan, N.; Ponsankar, A.; Karthick, K.; Nelofer, A.R. The efficacy of methanolic extract of Swietenia mahagoni Jacq.(Meliaceae) and a commercial insecticide against laboratory and field strains of Aedes aegypti (Linn.) and their impact on its predator Toxorhynchites splendens. Biocatal. Agric. Biotechnol. 2021, 31, 101915. [Google Scholar] [CrossRef]
- D’Adamo, I.; Gastaldi, M.; Morone, P.; Rosa, P.; Sassanelli, C.; Settembre-Blundo, D.; Shen, Y. Bioeconomy of sustainability: Drivers, opportunities and policy implications. Sustainability 2021, 14, 200. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussni Hasan, N.R.; Yogarajalakshmi, P.; Vasantha-Srinivasan, P.; Shehata, W.F.; Radhakrishnan, N.; Jayakodi, S.; Karthi, S.; Senthil-Nathan, S.; Hamed Mansour, H.E.; Ghazzawy, H.S.; et al. From the Sea to Mosquito Control: The Potential of Halymenia dilatata Marine Alga as an Eco-Friendly Mosquitocidal Agent. Sustainability 2023, 15, 11900. https://doi.org/10.3390/su151511900
Hussni Hasan NR, Yogarajalakshmi P, Vasantha-Srinivasan P, Shehata WF, Radhakrishnan N, Jayakodi S, Karthi S, Senthil-Nathan S, Hamed Mansour HE, Ghazzawy HS, et al. From the Sea to Mosquito Control: The Potential of Halymenia dilatata Marine Alga as an Eco-Friendly Mosquitocidal Agent. Sustainability. 2023; 15(15):11900. https://doi.org/10.3390/su151511900
Chicago/Turabian StyleHussni Hasan, Nadia Rebhi, Parthiban Yogarajalakshmi, Prabhakaran Vasantha-Srinivasan, Wael F. Shehata, Narayanaswamy Radhakrishnan, Santhoshkumar Jayakodi, Sengodan Karthi, Sengottayan Senthil-Nathan, Hossam Eldin Hamed Mansour, Hesham S. Ghazzawy, and et al. 2023. "From the Sea to Mosquito Control: The Potential of Halymenia dilatata Marine Alga as an Eco-Friendly Mosquitocidal Agent" Sustainability 15, no. 15: 11900. https://doi.org/10.3390/su151511900
APA StyleHussni Hasan, N. R., Yogarajalakshmi, P., Vasantha-Srinivasan, P., Shehata, W. F., Radhakrishnan, N., Jayakodi, S., Karthi, S., Senthil-Nathan, S., Hamed Mansour, H. E., Ghazzawy, H. S., Bushara, M. A., & Abdou, A. H. (2023). From the Sea to Mosquito Control: The Potential of Halymenia dilatata Marine Alga as an Eco-Friendly Mosquitocidal Agent. Sustainability, 15(15), 11900. https://doi.org/10.3390/su151511900











