Preparation of Coal Gangue-Based Porous Ceramics and Its Application on Pb2+ Cycling Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Main Instruments
2.3. Synthesis of Coal Gangue-Based Porous Ceramic Adsorbent
3. Results and Discussion
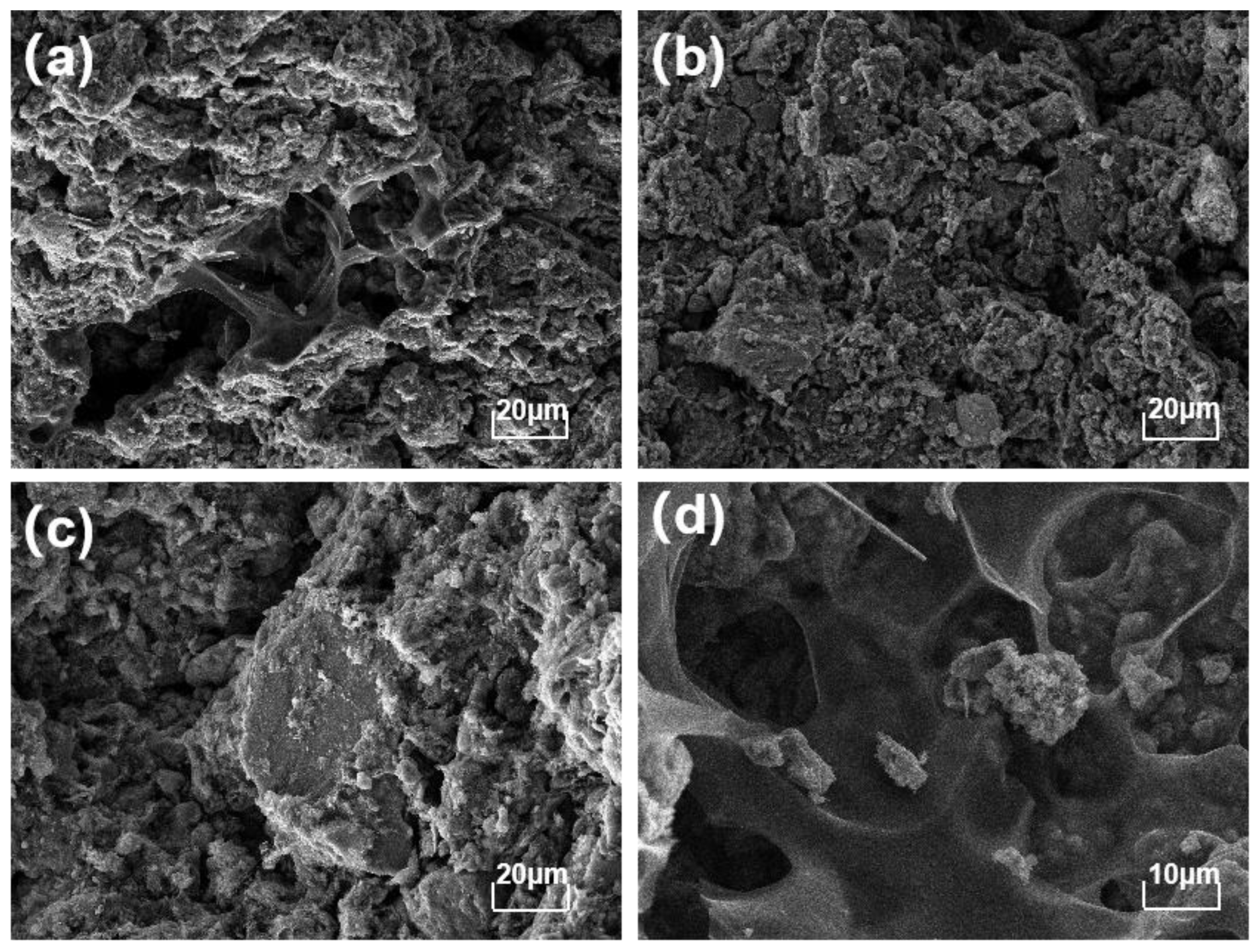
3.1. Composition and Morphology of Raw Materials
3.2. Factors Affecting the Preparation of CGPC
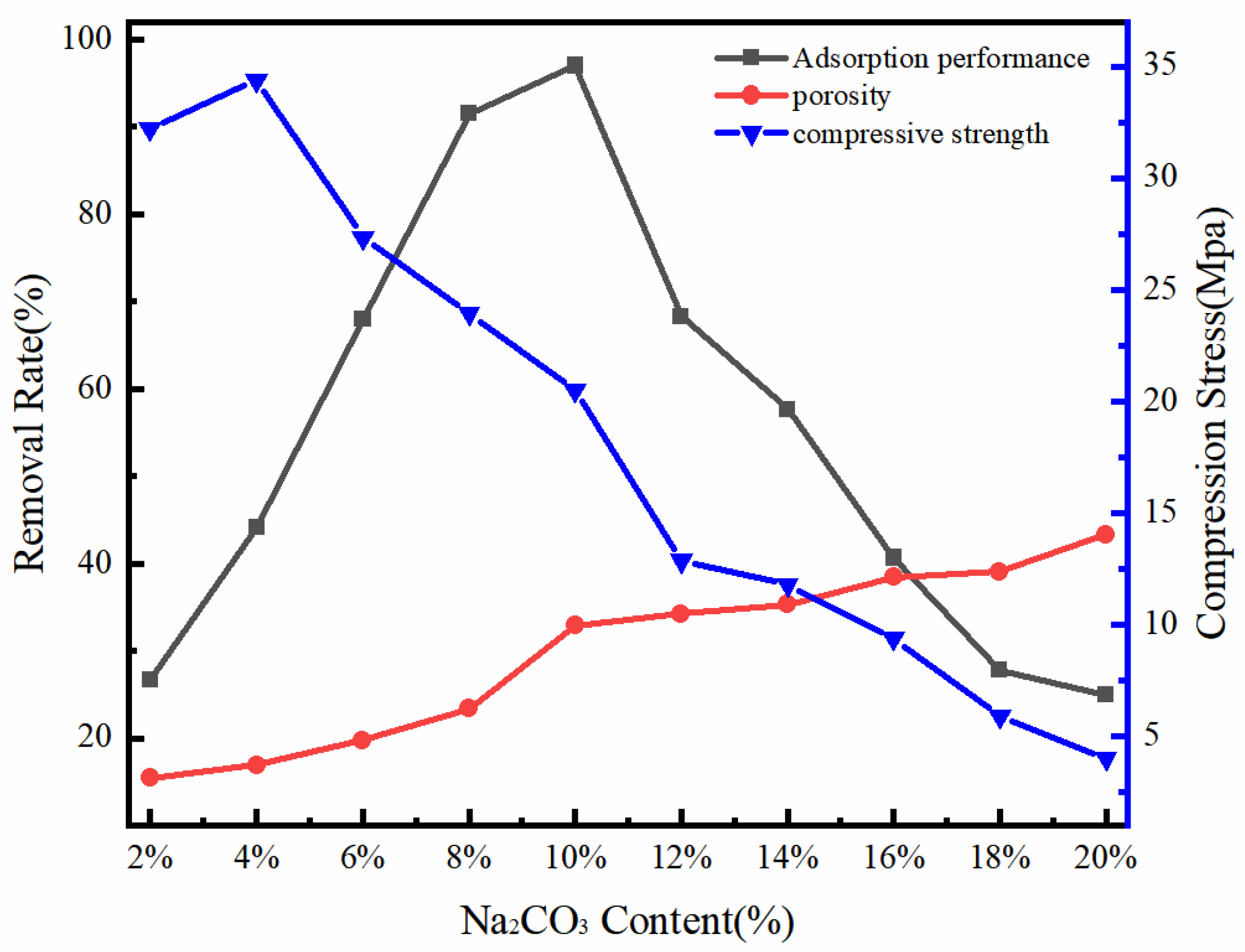
3.2.1. The Effect of the Proportion of Pore Forming Agent on the Performance of CGPC
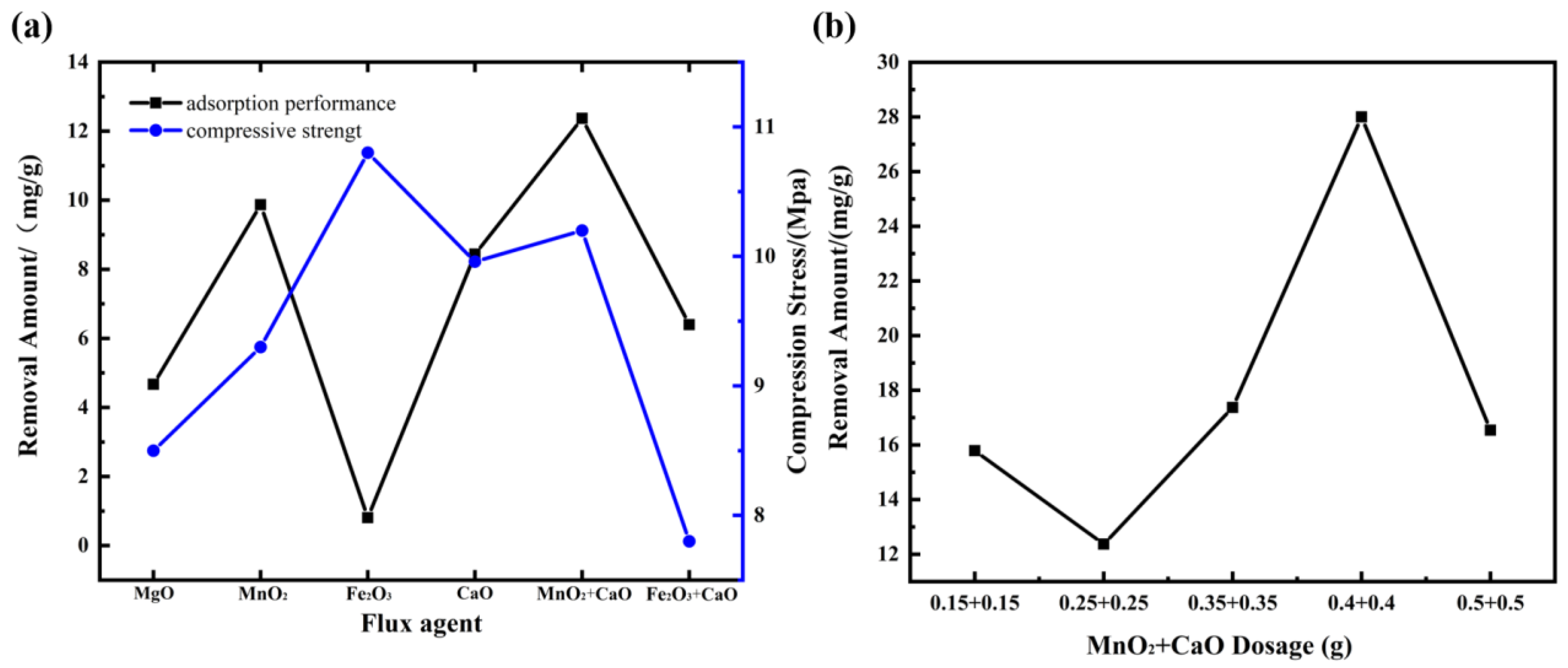
3.2.2. Effect of Flux on the Performance of CGPC
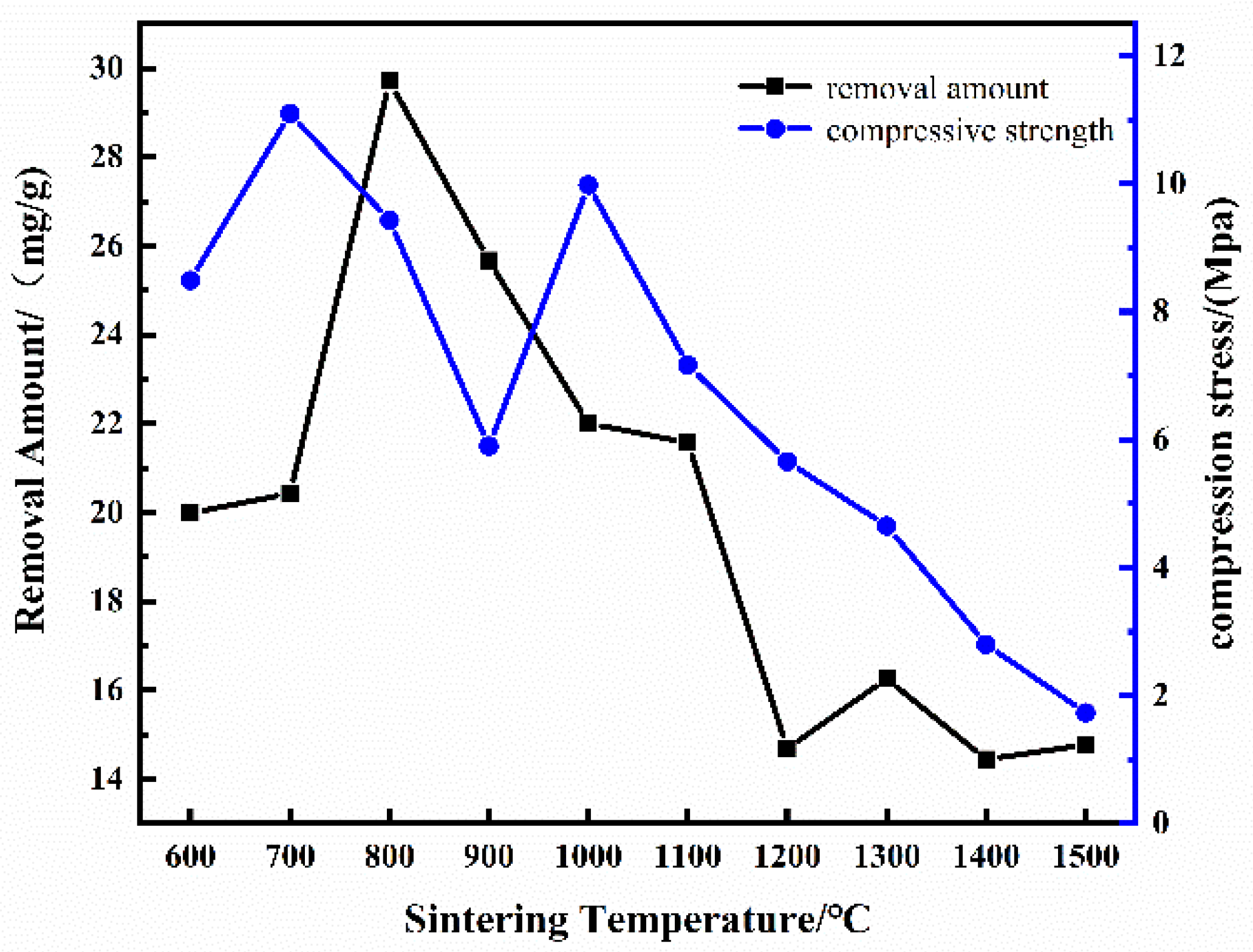
3.2.3. Effect of Sintering Temperature on the Performance of CGPC
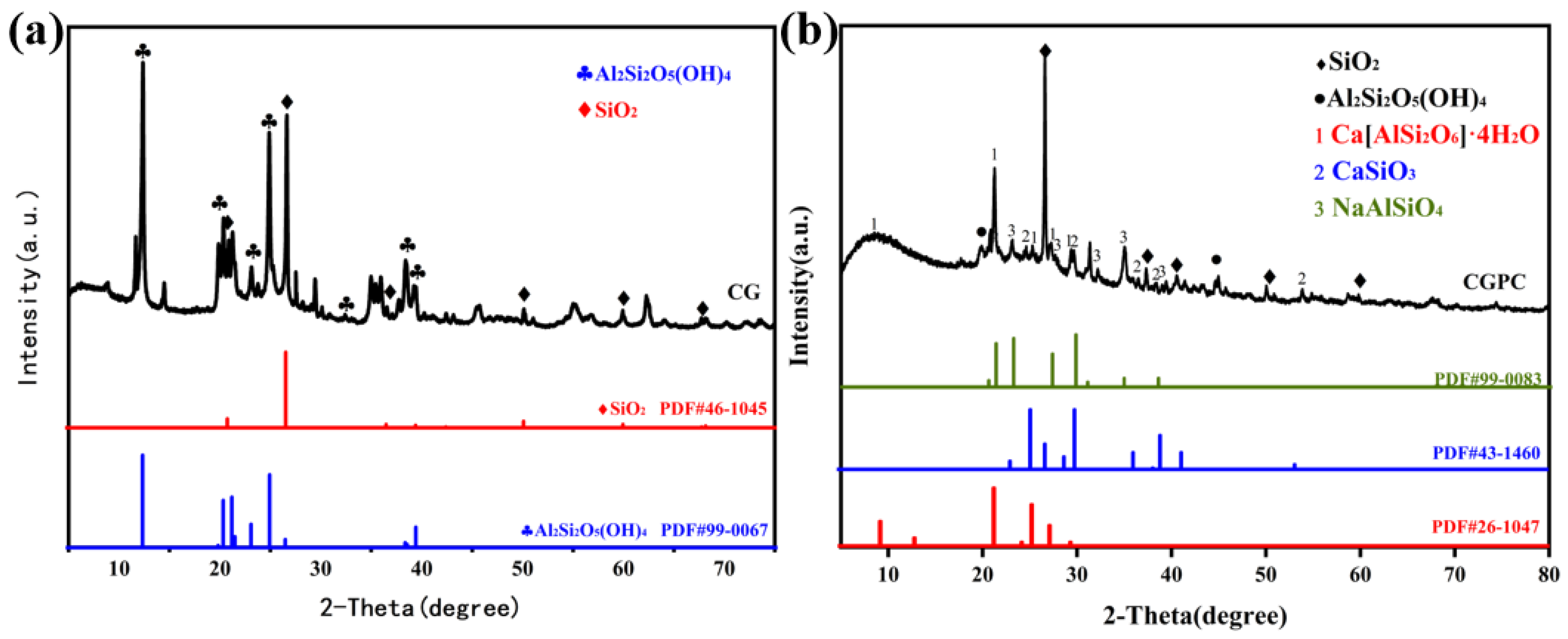
3.3. XRD Analysis of CGPC
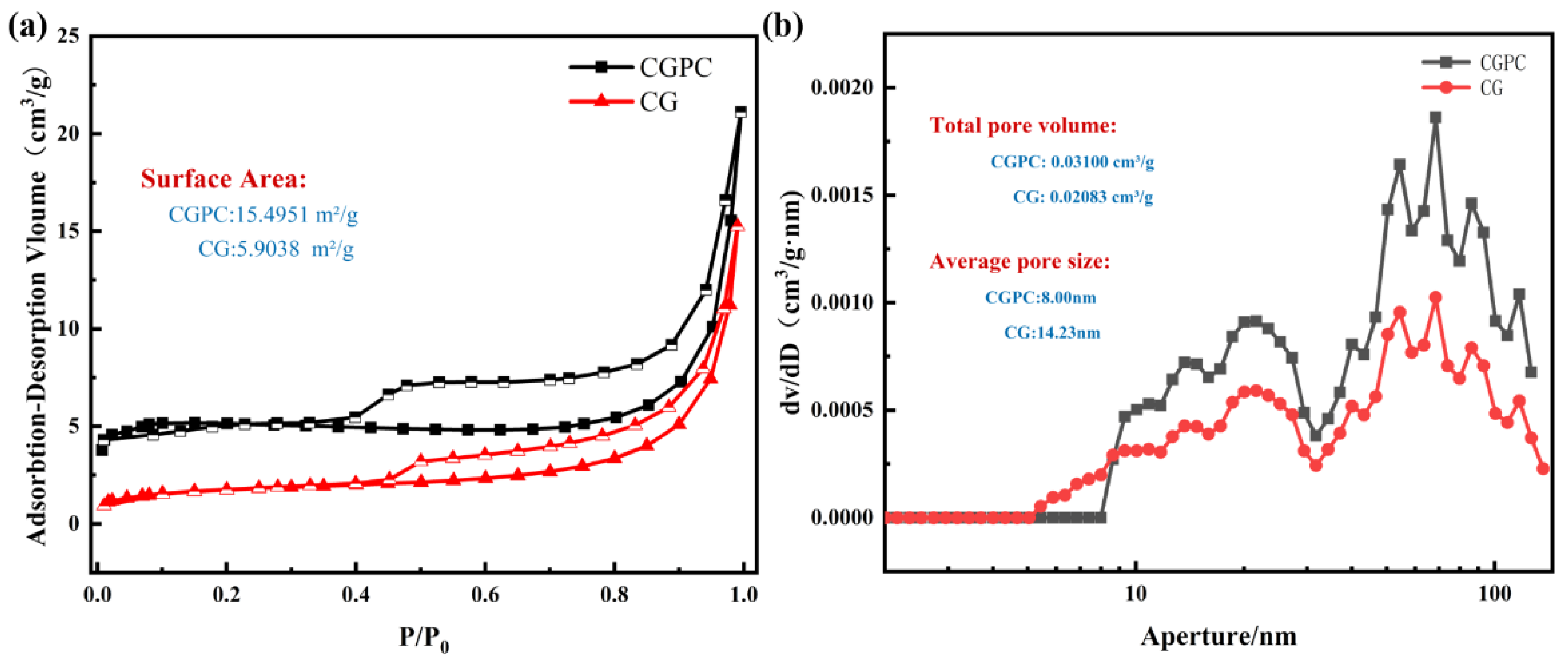
3.4. Pore Structure of CGPC
3.5. Mechanism of Pb2+ Removal by CGPC
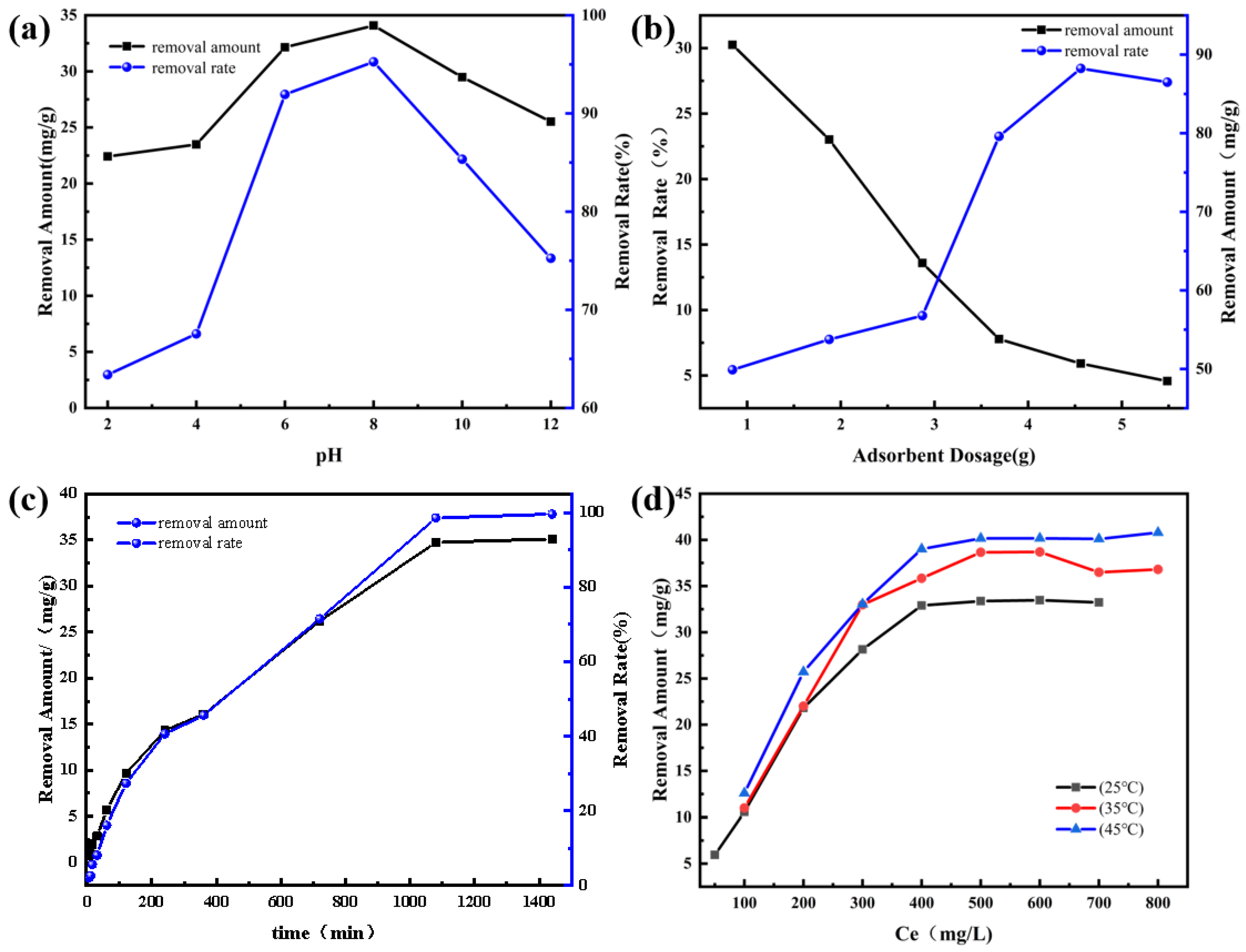
3.6. Factors Affecting the Removal of Pb2+ by CGPC
3.7. Study on Desorption Performance of CGPC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awual, M.R. An efficient composite material for selective lead(II) monitoring and removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 103087. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M. Novel conjugate adsorbent for visual detection and removal of toxic lead(II) ions from water. Microporous Mesoporous Mater. Off. J. Int. Zeolite Assoc. 2014, 196, 261–269. [Google Scholar] [CrossRef]
- Awual, M.R. Assessing of lead(III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem. Eng. J. 2016, 289, 65–73. [Google Scholar] [CrossRef]
- Awual, R.; Hasan, M.; Shahat, A. Functionalized novel mesoporous adsorbent for selective lead(II) ions monitoring and removal from wastewater. Sens. Actuators B Chem. 2014, 203, 854–863. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011.
- US Environmental Protection Agency. 2012 Guidelines for Water Reuse; US Environmental Protection Agency: Washington, DC, USA, 2012.
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. In-Depth Study of Heavy Metal Removal by an Etidronic Acid-Functionalized Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2022, 14, 7450–7463. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xia, M.; Wang, F.; Dong, L.; Zhu, S. Efficient absorption properties of surface grafted HEDP-HAP composites for Pb(2+) and Cu(2+): Experimental study and visualization study of interaction based on Becke surface analysis and independent gradient model. J. Hazard. Mater. 2021, 401, 123748. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, N.; Ola, O.; Xia, Y.; Zhu, Y. Porous ceramics: Light in weight but heavy in energy and environment technologies. Mater. Sci. Eng. R. Rep. Rev. J. 2021, 143, 100589. [Google Scholar]
- Aprianti, T.; Miskah, S.; Moeksin, R.; Sisnayati, S.; Nasir, S. Pb removal in pulp and paper industry leachate wastewater using activated carbon-ceramic composite adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2019, 298, 012011. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Lin, X.; Chen, Z. Porous Fe(0)/C ceramsites for removal of aqueous Pb(ii) ions: Equilibrium, long-term performance and mechanism studies. RSC Adv. 2018, 8, 25445–25455. [Google Scholar] [CrossRef]
- Ribeiro, J.; Suárez-Ruiz, I.; Flores, D. Geochemistry of self-burning coal mining residues from El Bierzo Coalfield (NW Spain): Environmental implications. Int. J. Coal Geol. 2016, 159, 155–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T.-C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar]
- Xu, H.; Du, H.; Kang, L.; Cheng, Q.; Feng, D.; Xia, S. Constructing Straight Pores and Improving Mechanical Properties of GangueBased Porous Ceramics. J. Renew. Mater. 2021, 9, 2129. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hu, Y.; Yan, S.; Yang, J. Adsorption removal of cationic dyes from aqueous solutions using ceramic adsorbents prepared from industrial waste coal gangue. J. Environ. Manag. 2019, 234, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, Y. Removal of Pb(II) and Zn(II) from aqueous solution by ceramisite prepared by sintering bentonite, iron powder and activated carbon. Chem. Eng. J. 2013, 215, 432–439. [Google Scholar] [CrossRef]
- Lu, M.; Wang, R.; Xue, Y.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Wang, T.; Li, J. Eco-friendly ceramsite from dredged sediment/biomass for Pb(II) removal: Process optimization and adsorption mechanistic insights. J. Environ. Chem. Eng. 2022, 10, 108939. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Seabra, M.P.; Labrincha, J.A. Novel porous fly-ash containing geopolymer monoliths for lead adsorption from wastewaters. J. Hazard. Mater. 2016, 318, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Panda, L.; Rath, S.S.; Rao, D.S.; Nayak, B.B.; Das, B.; Misra, P.K. Thorough understanding of the kinetics and mechanism of heavy metal adsorption onto a pyrophyllite mine waste based geopolymer. J. Mol. Liq. 2018, 263, 428–441. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Y.; Qiu, X.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. Novel porous phosphoric acid-based geopolymer foams for adsorption of Pb(II), Cd(II) and Ni(II) mixtures: Behavior and mechanism. Ceram. Int. 2023, 49, 7030–7039. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, F.; Wang, L.; Rong, Y.; He, P.; Jia, D.; Yang, J. A green and low-cost hollow gangue microsphere/geopolymer adsorbent for the effective removal of heavy metals from wastewaters. J. Environ. Manag. 2019, 246, 174–183. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Wang, S.; Xu, C.; Yang, K.; Xu, M. Removal of Pb(II) from wastewater using Al2O3-NaA zeolite composite hollow fiber membranes synthesized from solid waste coal fly ash. Chemosphere 2018, 206, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Rong, Y.; Yan, S.; Yu, M.; Li, X.; Wang, Z.; Zhang, T.; Yang, J. Novel design of microsphere adsorbent for efficient heavy metals adsorption. Int. J. Appl. Ceram. Technol. 2020, 17, 2228–2239. [Google Scholar] [CrossRef]
- Kim, H.S.; Moon, D.J.; Yoo, H.Y.; Park, J.S.; Park, M.; Lim, W.T. A crystallographic study of Sr2+ and K+ ion-exchanged zeolite Y (FAU, Si/Al = 1.56) from binary solution with different mole ratio of Sr2+ and K+. J. Porous Mater. 2019, 27, 63–71. [Google Scholar] [CrossRef]
- Cheng, Y.; Xing, J.; Bu, C.; Zhang, J.; Piao, G.; Huang, Y.; Xie, H.; Wang, X. Dehydroxylation and Structural Distortion of Kaolinite as a High-Temperature Sorbent in the Furnace. Minerals 2019, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R. Preparation of low-cost ceramic membranes for microfiltration using sugarcane bagasse ash as a pore-forming agent. Ceramic 2019, 65, 620–625. [Google Scholar]
- Kushwaha, R.; Kaleeswaran, D.; Haldar, S.; Chakraborty, D.; Mullangi, D.; Borah, A.; Vinod, C.P.; Murugavel, R.; Vaidhyanathan, R. Nanoporous Covalent Organic Framework Embedded with Fe/Fe3O4 Nanoparticles as Air-Stable Low-Density Nanomagnets. ACS Appl. Nano Mater. 2020, 3, 9088–9096. [Google Scholar] [CrossRef]
- Mocciaro, A.; Lombardi, M.B.; Scian, A.N. Ceramic Material Porous Structure Prepared Using Pore-Forming Additives. Refract. Ind. Ceram. 2017, 58, 65–68. [Google Scholar] [CrossRef]
- Heraiz, M.; Merrouche, A.; Saheb, N. Effect of MgO addition and sintering parameters on mullite formation through reaction sintering kaolin and alumina. Adv. Appl. Ceram. 2013, 105, 285–290. [Google Scholar] [CrossRef]
- Miljkovic, M.; Ilic, S.; Zec, S. Sol-gel synthesis and characterization of iron doped mullite. J. Alloys Compd. 2014, 612, 259–264. [Google Scholar]
- Liu, Z.; Zhao, J.; Li, Y.; Zeng, Z.; Mao, J.; Peng, Y.; He, Y. Low-temperature sintering of bauxite-based fracturing proppants containing CaO and MnO2 additives. Mater. Lett. 2016, 171, 300–303. [Google Scholar] [CrossRef]
- Mma, B.; Ab, D.; Aab, C.; Yek, E.; Ab, A.; Yeh, A.; Yt, A.; Mo, B.; Ya, B.; As, D. Effect of sintering temperature on the microstructure and mechanical behavior of porous ceramics made from clay and banana peel powder. Results Mater. 2019, 4, 100028. [Google Scholar]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar]
- Sun, G.; Zhang, J.; Hao, B.; Li, X.; Yan, M.; Liu, K. Feasible synthesis of coal fly ash based porous composites with multiscale pore structure and its application in Congo red adsorption. Chemosphere 2022, 298, 134136. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.C.; Yan, X.B.; Lou, D.L.; Li, D.X. Influence on Activation Property of Coal Gangue by Calcining Temperature. Appl. Mech. Mater. 2012, 174–177, 1137–1140. [Google Scholar] [CrossRef]
- Sugiarti, S. Synthesis of NaP1 and faujasite zeolite from natural zeolite of ende-ntt as lead (Pb(II))adsorbent. Rasayan J. Chem. 2019, 12, 650–658. [Google Scholar]
- Qiu, G.; Luo, Z.; Shi, Z.; Ni, M. Utilization of Coal Gangue and Copper Tailings as Clay for Cement Clinker Calcinations. J. Wuhan Univ. Technol. Mater. Sci. 2011, 26, 1205–1210. [Google Scholar] [CrossRef]
- Armesto, L.; Merino, J.L. Characterization of some coal combustion solid residues. Fuel 1999, 78, 613–618. [Google Scholar] [CrossRef]
- Jonas, B.; Martin, S.; Tobias, F. Porous Alumina Ceramics with Multimodal Pore Size Distributions. Materials 2021, 14, 3294. [Google Scholar]
- Yan, S.; Pan, Y.; Wang, L.; Liu, J.; Zhang, Z.; Huo, W.; Yang, J.; Huang, Y. Synthesis of low-cost porous ceramic microspheres from waste gangue for dye adsorption. J. Adv. Ceram. 2018, 7, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Liu, J.L. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Bai, Y.; Hong, J. Preparation of a Novel Millet Straw Biochar-Bentonite Composite and Its Adsorption Property of Hg2+ in Aqueous Solution. Materials 2021, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Lu, J.; Niu, Q.; Chen, X.; Wang, Z.; Zhang, J. Preparation and characterization of benzoic acid-modified activated carbon for removal of gaseous mercury chloride—ScienceDirect. Fuel 2015, 160, 440–445. [Google Scholar] [CrossRef]
- Mmka, B.; Hja, B. Synthesis of nano-crystalline zeolite-A and zeolite-X from Indian coal fly ash, its characterization and performance evaluation for the removal of Cs+ and Sr2+ from simulated nuclear waste. J. Hazard. Mater. 2022, 423, 127085. [Google Scholar]
- Zhu, Q.; Wu, J.; Wang, L.; Yang, G.; Zhang, X. Adsorption Characteristics of Pb2+ onto Wine Lees-Derived Biochar. Bull. Environ. Contam. Toxicol. 2016, 97, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Physicochemical property and chromium leaching behavior in different environments of glass ceramics prepared from AOD stainless steel slag. J. Alloys Compd. 2019, 805, 1106–1116. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhu, M.; Cheng, F.; Zhang, D. Decomposition of key minerals in coal gangues during combustion in O2/N2 and O2/CO2 atmospheres. Appl. Therm. Eng. 2018, 148, 977–983. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Adsorption of Cr(VI) from aqueous solution by prepared high surface area activated carbon from Fox nutshell by chemical activation with H3PO4. J. Environ. Chem. Eng. 2017, 5, 2032–2041. [Google Scholar] [CrossRef]
- Li, Q.; Lv, L.; Zhao, X.; Wang, Y.; Wang, Y. Cost-effective microwave-assisted hydrothermal rapid synthesis of analcime-activated carbon composite from coal gangue used for Pb. Environ. Sci. Pollut. Res. Int. 2022, 29, 77788–77799. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Shao, N.; Hu, T.; Han, L.; Wang, D. Geopolymerization enhanced hydrothermal synthesis of analcime from steel slag and CFBC fly ash and heavy metal adsorption on analcime. Environ. Technol. 2018, 41, 1753–1765. [Google Scholar] [CrossRef]
- Maity, R.; Chakraborty, D.; Nandi, S.; Yadav, A.K.; Mullangi, D.; Vinod, C.P.; Vaidhyanathan, R. Aqueous-Phase Differentiation and Speciation of Fe3+ and Fe2+ Using Water-Stable Photoluminescent Lanthanide-Based Metal–Organic Framework. ACS Appl. Nano Mater. 2019, 2, 5169–5178. [Google Scholar] [CrossRef]
- Huang, T.; Yan, M.; He, K.; Huang, Z.; Zeng, G.; Chen, A.; Peng, M.; Li, H.; Yuan, L.; Chen, G. Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci. 2019, 543, 43–51. [Google Scholar] [CrossRef] [PubMed]



| Sorbent Type | Ion Species | Concentration | Condition | Adsorption Capacity | Reference | |
|---|---|---|---|---|---|---|
| pH | T | |||||
| Geopolymer foam | Pb | 50 mg/L | 5 | 24 h | 6.3 mg/g | [19] |
| Pyrophyllite mine waste-based geopolymer | Pb | 50 mg/L | 7.8 | 1.5 h | 7.54 mg/g | [20] |
| Porous phosphoric acid activated eopolymer | Pb | 50 mg/L | 7 | 24 h | 11.99 mg/g | [21] |
| Hollow gangue microsphere | Pb | 50 mg/L | 6 | 4 h | 138.89 mg/g | [22] |
| Porous chromium carbon biomorphic ceramics | Pb | 200 mg/L | 6 | 2.5 h | 8.97 mg/g | [23] |
| Metallic iron/carbon ceramsites | Pb | 50 mg/L | 6 | 3 h | 112.36 mg/g | [11] |
| Coal gangue microsphere | Pb | 50 mg/L | 6 | 2 h | 18.90 mg/g | [24] |
| Coal gangue-based porous ceramics | Pb | 200 mg/L | 6 | 18 h | 32.15 mg/g | This work |
| Composition | Al2O3 | SiO2 | Fe3O4 | SO3 | CaO | TiO2 | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| Wt./% | 34.54 | 48.36 | 5.65 | 4.057 | 2.73 | 2.29 | 1.36 | 0.1092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Liu, H.; Han, S.; Liu, J.; Wang, Y. Preparation of Coal Gangue-Based Porous Ceramics and Its Application on Pb2+ Cycling Adsorption. Sustainability 2023, 15, 11879. https://doi.org/10.3390/su151511879
Jia Y, Liu H, Han S, Liu J, Wang Y. Preparation of Coal Gangue-Based Porous Ceramics and Its Application on Pb2+ Cycling Adsorption. Sustainability. 2023; 15(15):11879. https://doi.org/10.3390/su151511879
Chicago/Turabian StyleJia, Yansen, Hongwei Liu, Shaoxiong Han, Jun Liu, and Yongzhen Wang. 2023. "Preparation of Coal Gangue-Based Porous Ceramics and Its Application on Pb2+ Cycling Adsorption" Sustainability 15, no. 15: 11879. https://doi.org/10.3390/su151511879
APA StyleJia, Y., Liu, H., Han, S., Liu, J., & Wang, Y. (2023). Preparation of Coal Gangue-Based Porous Ceramics and Its Application on Pb2+ Cycling Adsorption. Sustainability, 15(15), 11879. https://doi.org/10.3390/su151511879






