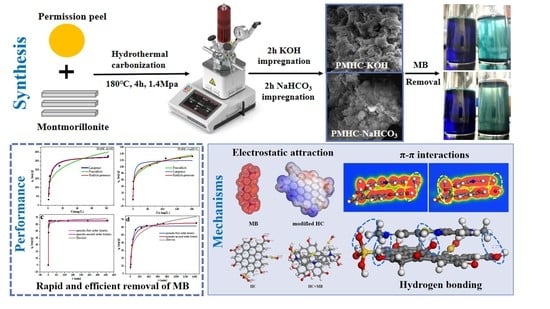
Simple Alkali-Modified Persimmon Peel–Montmorillonite Composite Hydrochar for Rapid and Efficient Removal of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrochar
2.3. Batch Adsorption Experiments
2.4. Characterization Methods
2.5. DFT Calculation
3. Results and Discussion
3.1. Hydrochar Characterization
3.1.1. SEM Analysis
3.1.2. XRD Analysis
3.1.3. FTIR and XPS Analysis
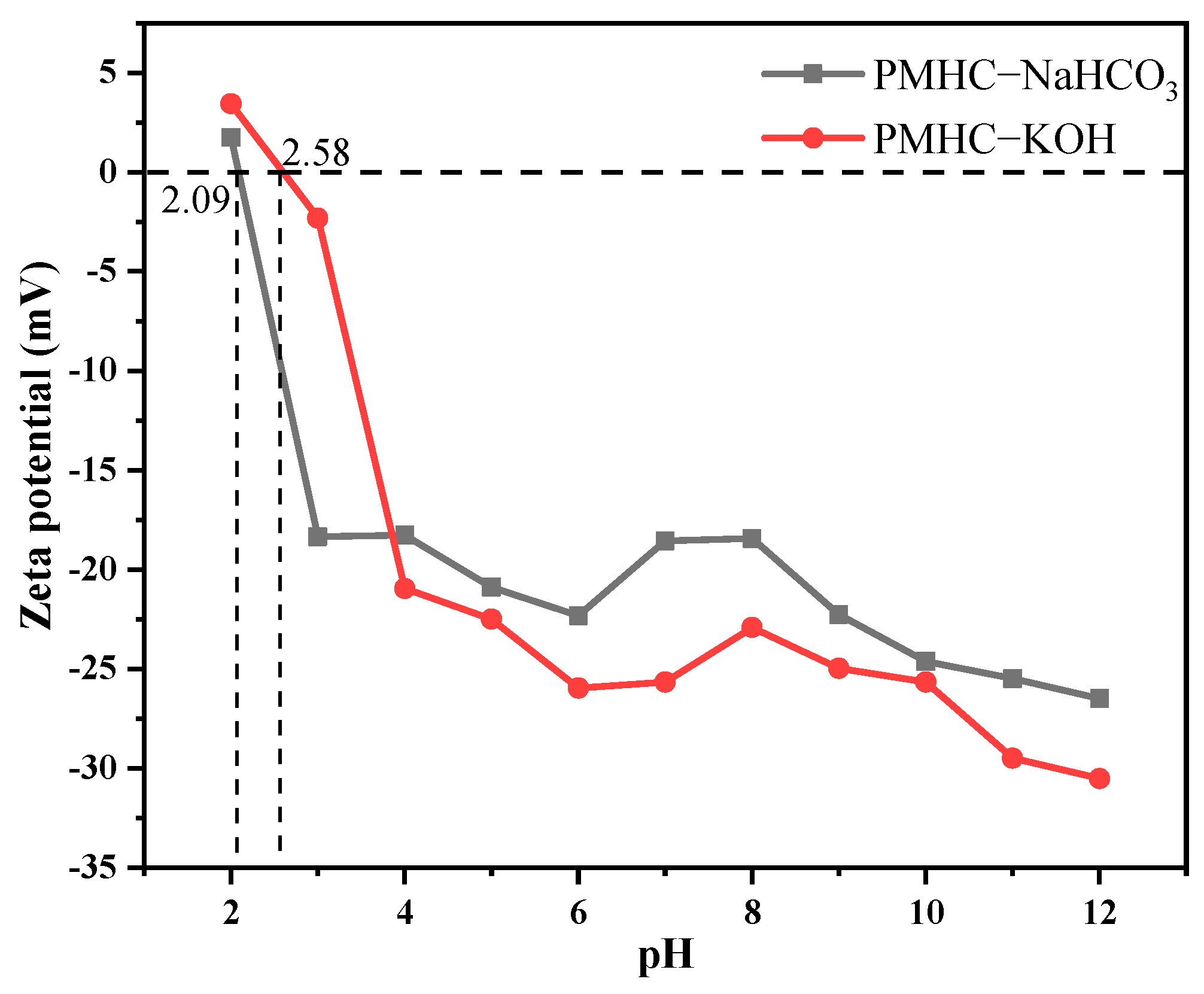
3.1.4. Zeta Potential Analysis
3.2. Adsorption Study
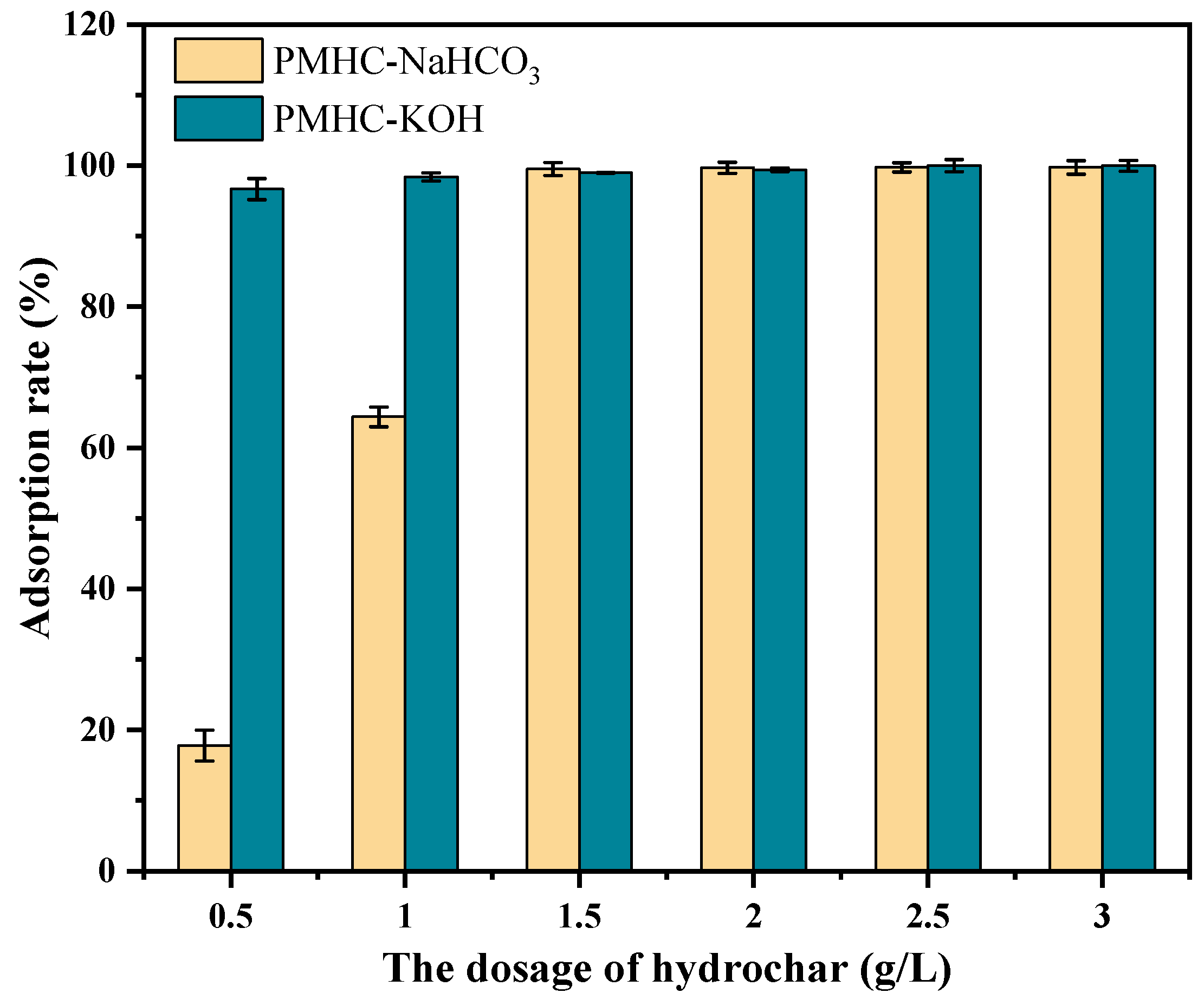
3.2.1. Effect of Additional Amounts of Modified Hydrochars on MB Adsorption
3.2.2. Adsorption Isotherms of MB onto Modified Hydrochars
3.2.3. Adsorption Kinetics of MB onto Modified Hydrochars
3.3. Adsorption Mechanisms
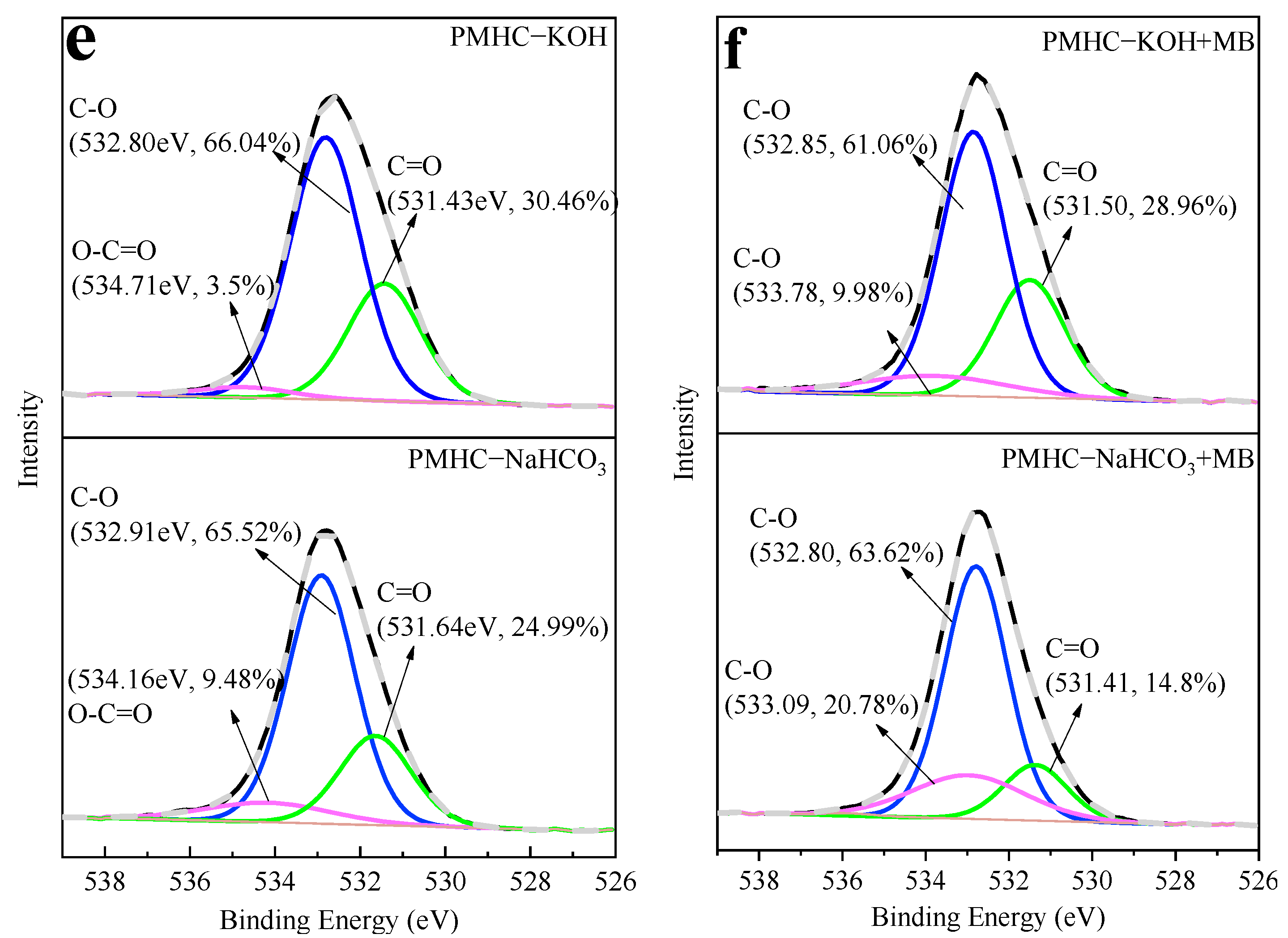
3.3.1. FTIR and XPS Analysis
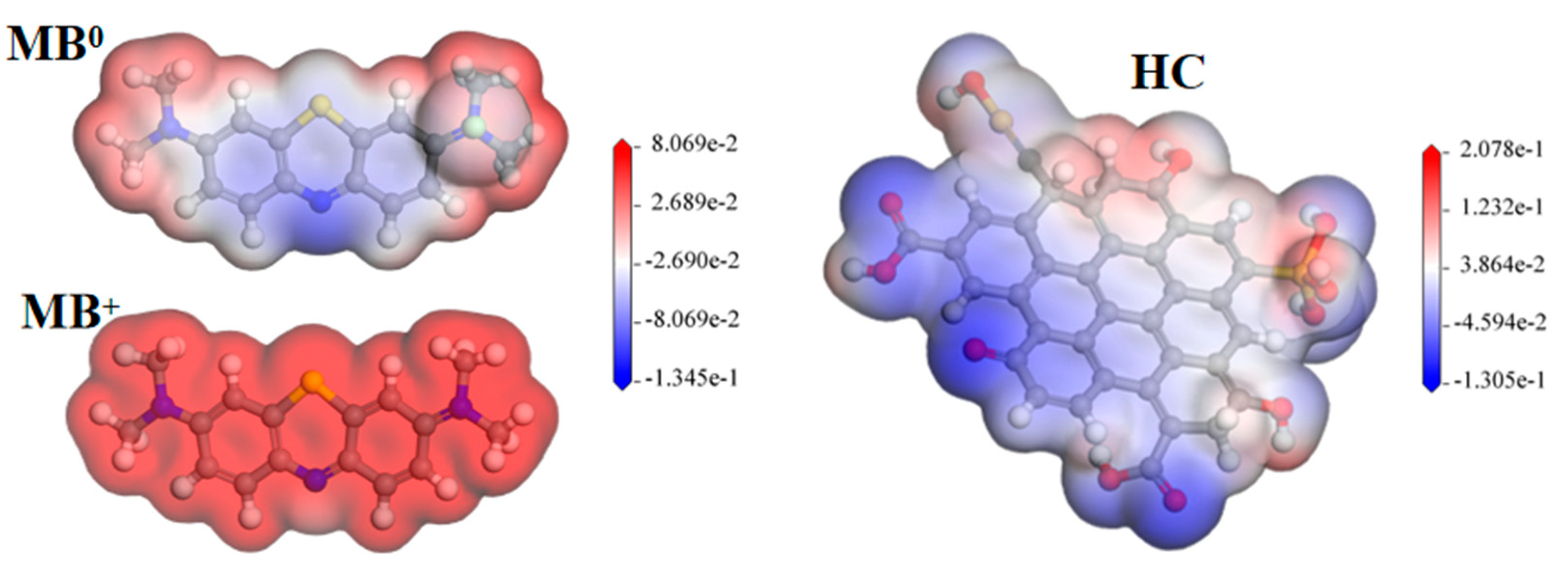
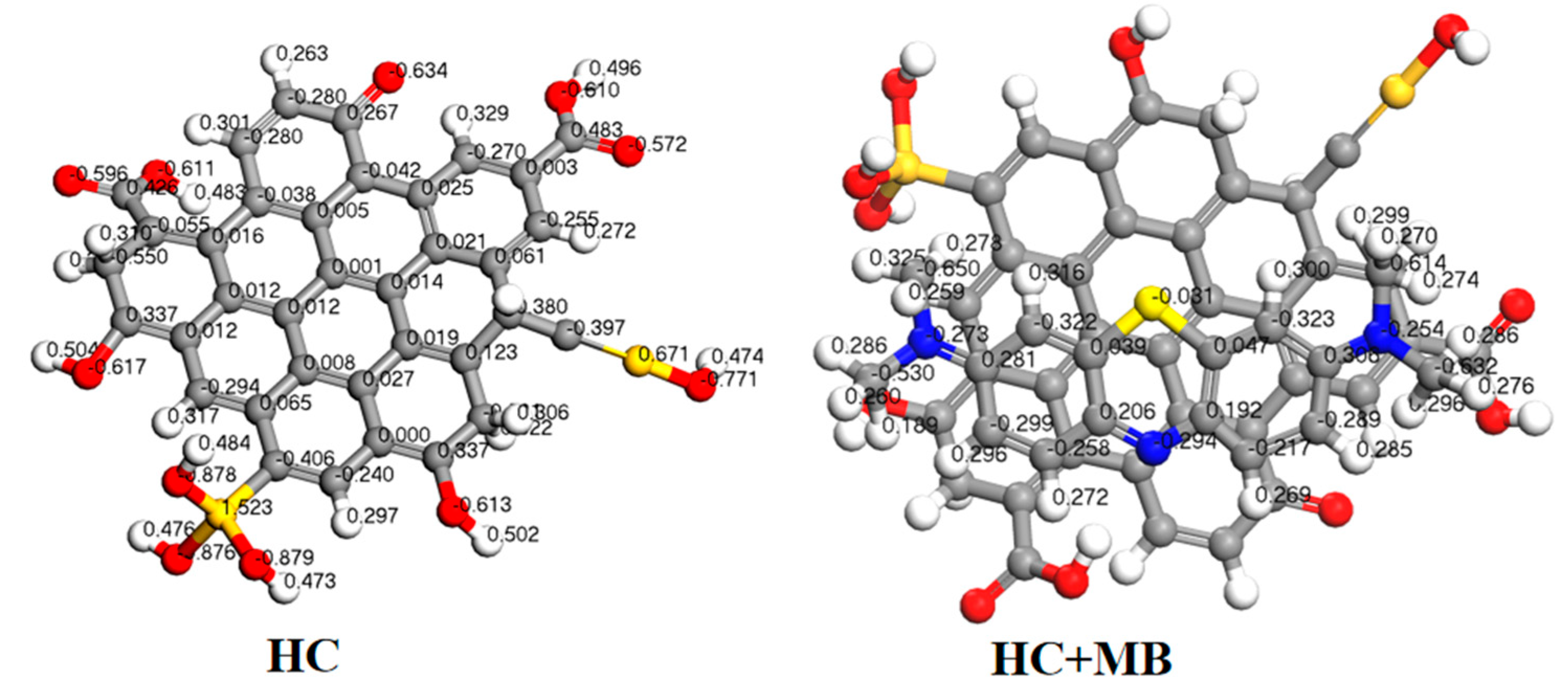
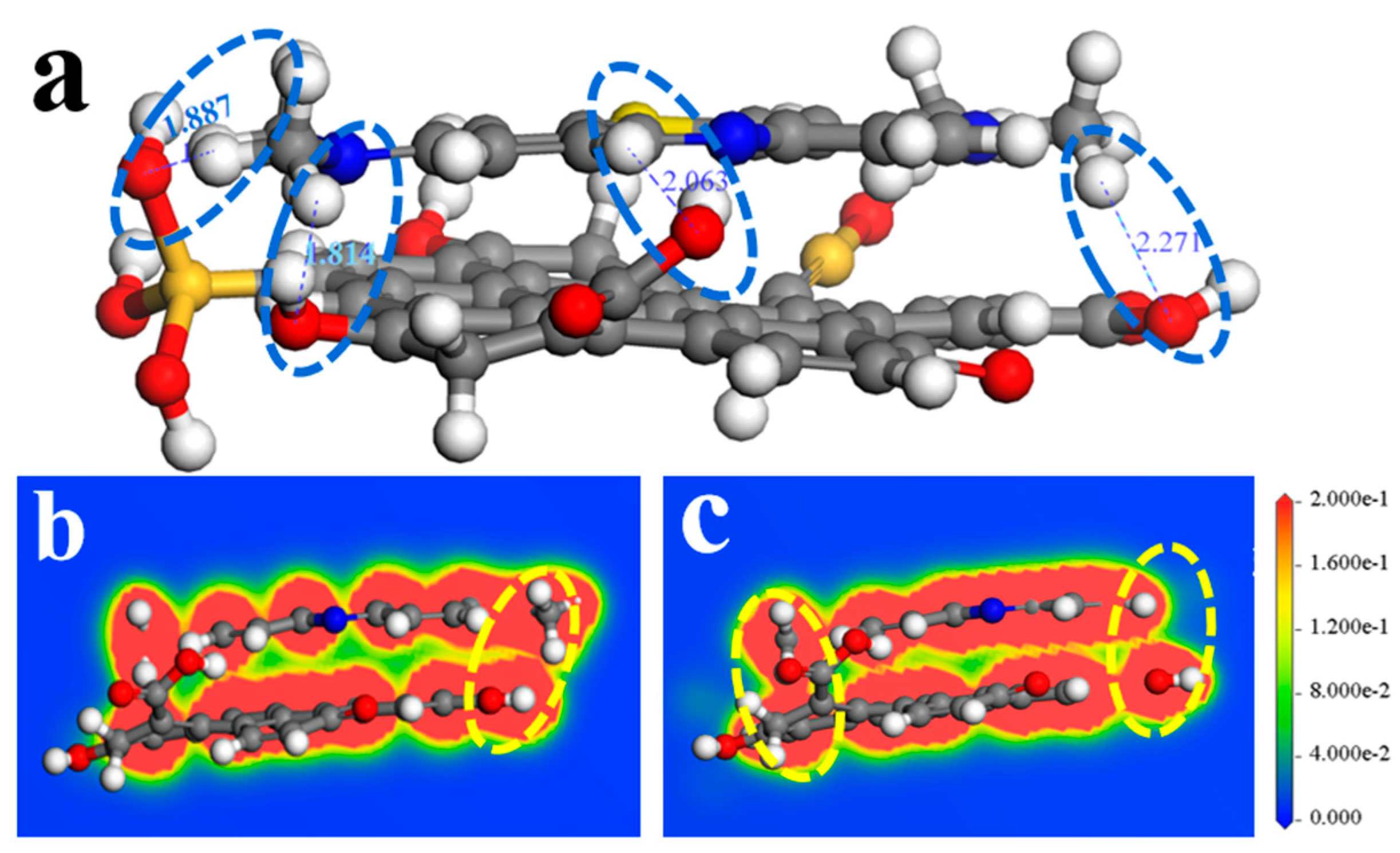
3.3.2. DFT Calculation
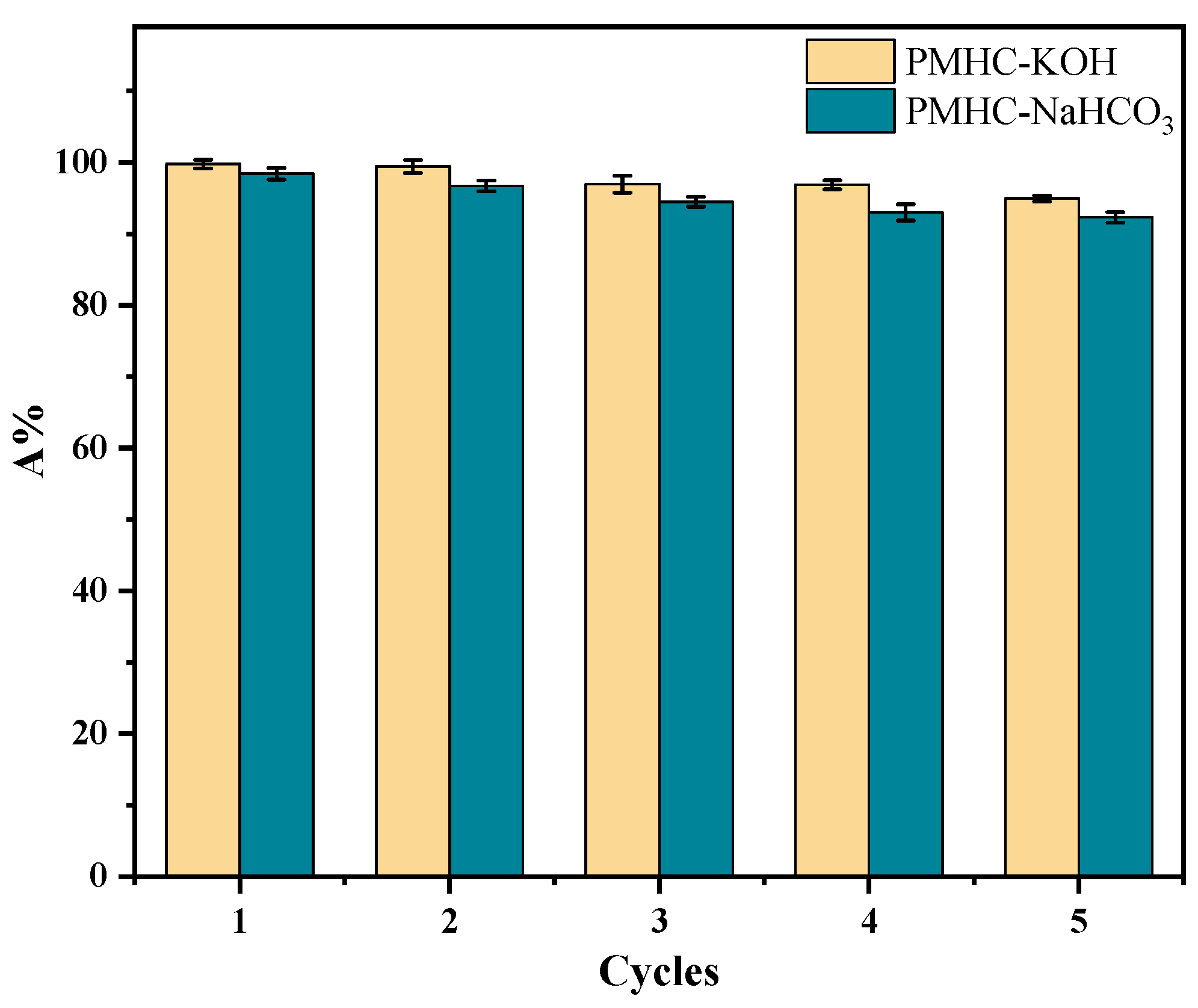
3.4. Regeneration of Modified Hydrochar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, J.; Pei, M.; He, Y.; Du, Y.; Guo, W.; Wang, L. Hydrothermal and activated synthesis of adsorbent montmorillonite supported porous carbon nanospheres for removal of methylene blue from waste water. RSC Adv. 2015, 5, 89839–89847. [Google Scholar] [CrossRef]
- Ai, L.; Li, L. Efficient removal of organic dyes from aqueous solution with ecofriendly biomass-derived carbon@montmorillonite nanocomposites by one-step hydrothermal process. Chem. Eng. J. 2013, 223, 688–695. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Namal, O.O.; Kalipci, E. Adsorption kinetics of methylene blue using alkali and microwave-modified apricot stones. Sep. Sci. Technol. 2019, 54, 1722–1738. [Google Scholar] [CrossRef]
- Wu, T.; Yang, G.; Cao, J.; Xu, Z.; Jiang, X. Activation and adsorption mechanisms of methylene blue removal by porous biochar adsorbent derived from eggshell membrane. Chem. Eng. Res. Des. 2022, 188, 330–341. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Z.; Li, S.; Li, Y.; Zhu, R. Adsorption performance and mechanism of methylene blue on chemically activated carbon spheres derived from hydrothermally-prepared poly(vinyl alcohol) microspheres. J. Mol. Liq. 2016, 220, 56–62. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Zhan, Y.; Zhang, L.; Pan, Y.; Zhang, C.; Yu, Z. Novel polyvinylidene fluoride nanofiltration membrane blended with functionalized halloysite nanotubes for dye and heavy metal ions removal. J. Hazard. Mater. 2016, 317, 60–72. [Google Scholar] [CrossRef]
- Tu, Y.; Shao, G.; Zhang, W.; Chen, J.; Qu, Y.; Zhang, F.; Tian, S.; Zhou, Z.; Ren, Z. The degradation of printing and dyeing wastewater by manganese-based catalysts. Sci. Total Environ. 2022, 828, 154390. [Google Scholar] [CrossRef]
- Mittal, Y.; Dash, S.; Srivastava, P.; Mishra, P.M.; Aminabhavi, T.M.; Yadav, A.K. Azo dye containing wastewater treatment in earthen membrane based unplanted two chambered constructed wetlands-microbial fuel cells: A new design for enhanced performance. Chem. Eng. J. 2022, 427, 131856. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, L.; Ren, P.; Zhong, M.; Ma, J.; Fan, X. Performances and Mechanism of Methyl Orange and Congo Red Adsorbed on the Magnetic Ion-Exchange Resin. J. Chem. Eng. Data 2020, 65, 725–736. [Google Scholar] [CrossRef]
- Abbasi, N.; Khan, S.A.; Khan, T.A. Response surface methodology mediated process optimization of Celestine blue B uptake by novel custard apple seeds activated carbon/FeMoO4 nanocomposite. J. Water Process Eng. 2021, 43, 102267. [Google Scholar] [CrossRef]
- Pai, S.; Kini, M.S.; Selvaraj, R. A review on adsorptive removal of dyes from wastewater by hydroxyapatite nanocomposites. Environ. Sci. Pollut. Res. 2021, 28, 11835–11849. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, J.; Wang, T.; Wang, P. Adsorption of toxic metal ion in agricultural wastewater by torrefaction biochar from bamboo shoot shell. J. Clean. Prod. 2022, 338, 130558. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Tong, D.S.; Wu, C.W.; Adebajo, M.O.; Jin, G.C.; Yu, W.H.; Ji, S.F.; Zhou, C.H. Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites. Appl. Clay Sci. 2018, 161, 256–264. [Google Scholar] [CrossRef]
- Wang, Y.; Wohlert, J.; Bergenstråhle-Wohlert, M.; Tu, Y.; Ågren, H. Molecular mechanisms for the adhesion of chitin and chitosan to montmorillonite clay. RSC Adv. 2015, 5, 54580–54588. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.M.; Tong, D.S.; Li, C.S.; Ji, S.F.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Xu, C.Y.; Yu, W.H.; Zhou, C.H. Insight into formation of montmorillonite-hydrochar nanocomposite under hydrothermal conditions. Appl. Clay Sci. 2016, 119, 116–125. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, K.; Sudibyo, H.; Tester, J.W.; Huang, G.; Han, L.; Goldfarb, J.L. Production of upgraded biocrude from hydrothermal liquefaction using clays as in situ catalysts. Energy Convers. Manag. 2021, 247, 114764. [Google Scholar] [CrossRef]
- Viglašová, E.; Galamboš, M.; Danková, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef]
- Jing, F.; Guan, J.; Tang, W.; Chen, J. Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ. Pollut. 2022, 310, 119881. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Li, L.; Tian, R.; Chen, J.; Ning, Q.; Wang, B.; Tang, L.; Zeng, G. Influence of feedstocks and modification methods on biochar’s capacity to activate hydrogen peroxide for tetracycline removal. Bioresour. Technol. 2019, 291, 121840. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour. Technol. Rep. 2019, 7, 100266. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Petrović, J.T.; Stojanović, M.D.; Milojković, J.V.; Petrović, M.S.; Šoštarić, T.D.; Laušević, M.D.; Mihajlović, M.L. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, Y.; Shao, Q. Facile modification of hydrochar derived from cotton straw with excellent sorption performance for antibiotics: Coupling DFT simulations with experiments. Sci. Total Environ. 2021, 760, 144124. [Google Scholar] [CrossRef]
- Güleç, F.; Riesco, L.M.G.; Williams, O.; Kostas, E.T.; Samson, A.; Lester, E. Hydrothermal conversion of different lignocellulosic biomass feedstocks—Effect of the process conditions on hydrochar structures. Fuel 2021, 302, 121166. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Jiao, K.; Ning, X.; Zhang, J.; Guo, X.; Li, J.; Wang, C. Conversion mechanism and gasification kinetics of biomass char during hydrothermal carbonization. Renew. Energy 2021, 173, 318–328. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, D.; Liu, R.; Ding, Y.; Dai, X. Effects of hydrothermal treatment on organic compositions, structural properties, dewatering and biogas production of raw and digested sludge. Sci. Total Environ. 2022, 848, 157618. [Google Scholar] [CrossRef] [PubMed]
- Zelaya Soulé, M.E.; Fernández, M.A.; Montes, M.L.; Suárez-García, F.; Torres Sánchez, R.M.; Tascón, J.M.D. Montmorillonite- hydrothermal carbon nanocomposites: Synthesis, characterization and evaluation of pesticides retention for potential treatment of agricultural wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124192. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, J.; Saleem, M.; Yang, Y.; Zhang, Q. Enhanced adsorptive removal of carbendazim from water by FeCl3-modified corn straw biochar as compared with pristine, HCl and NaOH modification. J. Environ. Chem. Eng. 2022, 10, 107024. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Zheng, Y.; He, Z.; Guan, P.; He, X.; Hui, L.; Dai, Y. Study of Schiff base formation between dialdehyde cellulose and proteins, and its application for the deproteinization of crude polysaccharide extracts. Ind. Crops Prod. 2018, 112, 532–540. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, Y.; Liu, X. Insights into interfacial interaction mechanism of dyes sorption on a novel hydrochar: Experimental and DFT study. Chem. Eng. Sci. 2021, 233, 116432. [Google Scholar] [CrossRef]
- Taher, T.; Munandar, A.; Mawaddah, N.; Wisnubroto, M.S.; Mega, P.; Bahar, S.; Siregar, N.; Rahayu, N.; Lesbani, A.; Gusti, Y. Synthesis and characterization of montmorillonite—Mixed metal oxide composite and its adsorption performance for anionic and cationic dyes removal. Inorg. Chem. Commun. 2023, 147, 110231. [Google Scholar] [CrossRef]
- Jawad, A.H.; Saud, A.; Surip, S.N.; Alothman, Z.A. Hybrid multifunctional biocomposite of chitosan grafted benzaldehyde/montmorillonite/algae for effective removal of brilliant green and reactive blue 19 dyes: Optimization and adsorption mechanism. J. Clean. Prod. 2023, 393, 136334. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, T.; Li, L.; Liu, B.; Wang, X.; Zhang, S.; Li, L.; Wang, B.; Zimmerman, A.R.; Gao, B. Hydrothermal carbonization of distillers grains with clay minerals for enhanced adsorption of phosphate and methylene blue. Bioresour. Technol. 2021, 340, 125725. [Google Scholar] [CrossRef]
- Li, H.Z.; Zhang, Y.N.; Guo, J.Z.; Lv, J.Q.; Huan, W.W.; Li, B. Preparation of hydrochar with high adsorption performance for methylene blue by co-hydrothermal carbonization of polyvinyl chloride and bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Hu, Q.; Lan, R.; He, L.; Liu, H.; Pei, X. A critical review of adsorption isotherm models for aqueous contaminants: Curve characteristics, site energy distribution and common controversies. J. Environ. Manag. 2023, 329, 117104. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, X.; Liu, Z.; Geng, L.; Zhang, X.l.; Niu, P.; Zhang, X.; Zhang, Y.; Zhang, D.; Hu, H.; et al. Combining hierarchical pores and unsaturated sites into Quasi-MIL-125(Ti) for ultra-fast and efficient adsorption of cationic dyes. Polyhedron 2023, 239, 116430. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Singh Baghel, R.; Kumar, M. Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chem. Eng. J. 2020, 381, 122713. [Google Scholar] [CrossRef]
- Jarrah, N. Competitive adsorption isotherms of rhodium 6G and methylene blue on activated carbon prepared from residual fuel oil. J. Environ. Chem. Eng. 2017, 5, 4319–4326. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Y.; Zeng, G.; Gong, J.; Tan, X.; Wen, J.; Liu, S.; Jiang, L.; Li, M.; Yin, Z. Adsorption of 17β-estradiol from aqueous solution by raw and direct/pre/post-KOH treated lotus seedpod biochar. J. Environ. Sci. 2020, 87, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Bian, Y.; Wang, F.; Jiang, X.; Ji, R.; Gu, C.; Yang, X.; Song, Y. Green conversion of crop residues into porous carbons and their application to efficiently remove polycyclic aromatic hydrocarbons from water: Sorption kinetics, isotherms and mechanism. Bioresour. Technol. 2019, 284, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-pot synthesis and characterization of engineered hydrochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere 2020, 254, 126866. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Dutta, K.; Yata, V.K.; Arunachalam, K. Valorization of waste pine needle biomass into biosorbents for the removal of methylene blue dye from water: Kinetics, equilibrium and thermodynamics study. Environ. Technol. Innov. 2022, 25, 102200. [Google Scholar] [CrossRef]
- Liu, J.L.; Qian, W.C.; Guo, J.Z.; Shen, Y.; Li, B. Selective removal of anionic and cationic dyes by magnetic Fe3O4-loaded amine-modified hydrochar. Bioresour. Technol. 2021, 320, 124374. [Google Scholar] [CrossRef]
- Da Silva Andrade, J.G.; Porto, C.E.; Moreira, W.M.; Batistela, V.R.; Scaliante, M.H.N.O. Production of hydrochars from Pinus caribaea for biosorption of methylene blue and tartrazine yellow dyes. Clean. Chem. Eng. 2023, 5, 100092. [Google Scholar] [CrossRef]
- Ranote, S.; Chauhan, S.; Kumar, K.; Chauhan, G.S. A simple protocol to functionalize whole pine needles biowaste for effective and selective methylene blue adsorption. Bioresour. Technol. Rep. 2023, 22, 101417. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Kurczewska, J. Chitosan-montmorillonite hydrogel beads for effective dye adsorption. J. Water Process Eng. 2022, 48, 102928. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; He, J.; Peng, W.; Cao, Y.; Liu, J.; Huang, Y.; Fan, G. Chitosan-induced self-assembly of montmorillonite nanosheets along the end-face for methylene blue removal from water. Int. J. Biol. Macromol. 2023, 227, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ni, W.X.; Li, B. Porous Organic Polymer Synthesized by Green Diazo-Coupling Reaction for Adsorptive Removal of Methylene Blue. ACS Omega 2021, 6, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Huang, D.; Zeng, G.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Li, J.; Liu, C.; et al. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene blue removal from water using the hydrogel beads of poly(vinyl alcohol)-sodium alginate-chitosan-montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef]
- Cao, D.; Chen, Y.; Jin, W.; Li, W.; Wang, R.; Wang, K.; Tang, A.; Zhu, L.; Kong, D. Non-porous covalent organic polymers enable ultrafast removal of cationic dyes via carbonyl/hydroxyl-synergetic electrostatic adsorption. Sep. Purif. Technol. 2023, 315, 123689. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Zhang, H.; Huang, Z.; Jia, Q.; Zhang, S. Adsorption mechanism of methylene blue on oxygen-containing functional groups modified graphitic carbon spheres: Experiment and DFT study. Appl. Surf. Sci. 2021, 540, 148386. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.L.; Zheng, L.; Chen, D.; Wu, Z.; Sun, C.; Bolan, N.; Zhao, H.; Peng, A.-a.; Fang, Z.; et al. Spectroscopic investigations and density functional theory calculations reveal differences in retention mechanisms of lead and copper on chemically-modified phytolith-rich biochars. Chemosphere 2022, 301, 134590. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, L.; Su, Y.; Meng, P.; Zhou, Q.; Zeng, H.; Zhang, T.; Yu, H. Competitive adsorption of Cd(II), Pb(II) and Cu(II) ions from acid mine drainage with zero-valent iron/phosphoric titanium dioxide: XPS qualitative analyses and DFT quantitative calculations. Chem. Eng. J. 2022, 445, 136778. [Google Scholar] [CrossRef]
- Ahangari, M.G.; Mashhadzadeh, A.H.; Fathalian, M.; Dadrasi, A.; Rostamiyan, Y.; Mallahi, A. Effect of various defects on mechanical and electronic properties of zinc-oxide graphene-like structure: A DFT study. Vacuum 2019, 165, 26–34. [Google Scholar] [CrossRef]
- Pakornchote, T.; Ektarawong, A.; Alling, B.; Pinsook, U.; Tancharakorn, S.; Busayaporn, W.; Bovornratanaraks, T. Phase stabilities and vibrational analysis of hydrogenated diamondized bilayer graphenes: A first principles investigation. Carbon N. Y. 2019, 146, 468–475. [Google Scholar] [CrossRef]
- Jiang, D.; Li, H.; Cheng, X.; Ling, Q.; Chen, H.; Barati, B.; Yao, Q.; Abomohra, A.; Hu, X.; Bartocci, P.; et al. A mechanism study of methylene blue adsorption on seaweed biomass derived carbon: From macroscopic to microscopic scale. Process Saf. Environ. Prot. 2023, 172, 1132–1143. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, L.; Yu, H.; Ren, J.; Peng, D.; Zhang, L.; Meng, P. Multiple adsorption systems and electron-scale insights into the high efficiency coadsorption of a novel assembled cellulose via experiments and DFT calculations. J. Hazard. Mater. 2021, 416, 125748. [Google Scholar] [CrossRef]
- Allangawi, A.; Aziz Aljar, M.A.; Ayub, K.; El-Fattah, A.A.; Mahmood, T. Removal of methylene blue by using sodium alginate-based hydrogel; validation of experimental findings via DFT calculations. J. Mol. Graph. Model. 2023, 122, 108468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, S.; Cheng, W.; Zhang, L.; Meng, P.; Zhang, T.; Yu, H.; Peng, D. Theoretical calculations, molecular dynamics simulations and experimental investigation of the adsorption of cadmium(ii) on amidoxime-chelating cellulose. J. Mater. Chem. A 2019, 7, 13714–13726. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, M.; Liu, H.; Ouyang, S.; Ding, N.; Zhang, P. Adsorption behavior and mechanism of acid orange 7 and methylene blue on self-assembled three-dimensional MgAl layered double hydroxide: Experimental and DFT investigation. Appl. Surf. Sci. 2020, 522, 146370. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Ge, W.; Shu, Q.; Min, F. Experimental investigation and DFT calculation of different amine/ammonium salts adsorption on oxidized coal. Chem. Phys. 2022, 561, 111598. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]






| Isotherm Model | Constants | |||
|---|---|---|---|---|
| PMHC-KOH | PMHC-NaHCO3 | |||
| Langmuir | 278.41 | 121.28 | ||
| 0.408 | 0.361 | |||
| 0.9719 | 0.9544 | |||
| Freundlich | 101.97 | 43.47 | ||
| n | 4.067 | 4.639 | ||
| 0.8718 | 0.9814 | |||
| Redlich–Peterson | 117.73 | 105.21 | ||
| 0.445 | 1.804 | |||
| g | 0.987 | 0.844 | ||
| 0.9723 | 0.9870 | |||
| Kinetic Model | Constants | |||
|---|---|---|---|---|
| PMHC-KOH | PMHC-NaHCO3 | |||
| Pseudo-first-order | 64.48 | 61.76 | ||
| k1 | 1.225 | 0.0212 | ||
| R2 | 0.9884 | 0.9802 | ||
| Pseudo-second-order | qe | 66.33 | 67.34 | |
| k2 | 0.0433 | 0.00041 | ||
| 0.9962 | 0.9949 | |||
| Elovich | α | 1.03943 × 1012 | 5.0013 | |
| 0.4779 | 0.0873 | |||
| 0.9645 | 0.9420 | |||
| No. | Adsorbents | Qm (mg/g) | k2 (g/mg/min) | References |
|---|---|---|---|---|
| 1 | ZnCl2 modified bamboo hydrochar | 47.30 | Not Given | [44] |
| 2 | native pine needle biochar (PNBC), weak Acid-treated biochar (WABC), strong acid-treated biochar (SABC) | 106.38 | 0.0022 | [45] |
| 113.63 | 0.0022 | |||
| 153.84 | 0.0073 | |||
| 3 | Fe3O4-loaded protonated amine-modified hydrochar (Fe3O4-PAMH) | 148.84 | 0.000436 | [46] |
| 4 | Hydrochar from wood residues of Pinus caribaea (PIN), combined with acid-base treatment (PIN-200-24-B) | 132.10 | 0.003 ± 0.001 | [47] |
| 149.00 | 0.033 ± 0.002 | |||
| 5 | Oxidized pine needles oxime (OPNoxime) | 169.21 | 0.00147 | [48] |
| 6 | Coconut shell waste hydrochar by NaOH impregnation (COSHTC). | 200.01 | 0.066 | [49] |
| 7 | PMHC-NaHCO3 | 121.28 | 0.00041 | This study |
| PMHC-KOH | 278.41 | 0.0433 |
| Model | |||||
|---|---|---|---|---|---|
| HC + MB | −3970.2024 | −2787.9787 | −1182.0940 | −0.12954 | −3.525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, N.; Gao, L.; Li, S.; Ma, Z.; Li, L.; Hu, M. Simple Alkali-Modified Persimmon Peel–Montmorillonite Composite Hydrochar for Rapid and Efficient Removal of Methylene Blue. Sustainability 2023, 15, 11867. https://doi.org/10.3390/su151511867
Chai N, Gao L, Li S, Ma Z, Li L, Hu M. Simple Alkali-Modified Persimmon Peel–Montmorillonite Composite Hydrochar for Rapid and Efficient Removal of Methylene Blue. Sustainability. 2023; 15(15):11867. https://doi.org/10.3390/su151511867
Chicago/Turabian StyleChai, Na, Lihui Gao, Shulei Li, Zilong Ma, Lingni Li, and Ming Hu. 2023. "Simple Alkali-Modified Persimmon Peel–Montmorillonite Composite Hydrochar for Rapid and Efficient Removal of Methylene Blue" Sustainability 15, no. 15: 11867. https://doi.org/10.3390/su151511867
APA StyleChai, N., Gao, L., Li, S., Ma, Z., Li, L., & Hu, M. (2023). Simple Alkali-Modified Persimmon Peel–Montmorillonite Composite Hydrochar for Rapid and Efficient Removal of Methylene Blue. Sustainability, 15(15), 11867. https://doi.org/10.3390/su151511867