Effectiveness of Polyvinylidene Fluoride Fibers (PVDF) in the Diffusion and Adsorption Processes of Atrazine in a Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
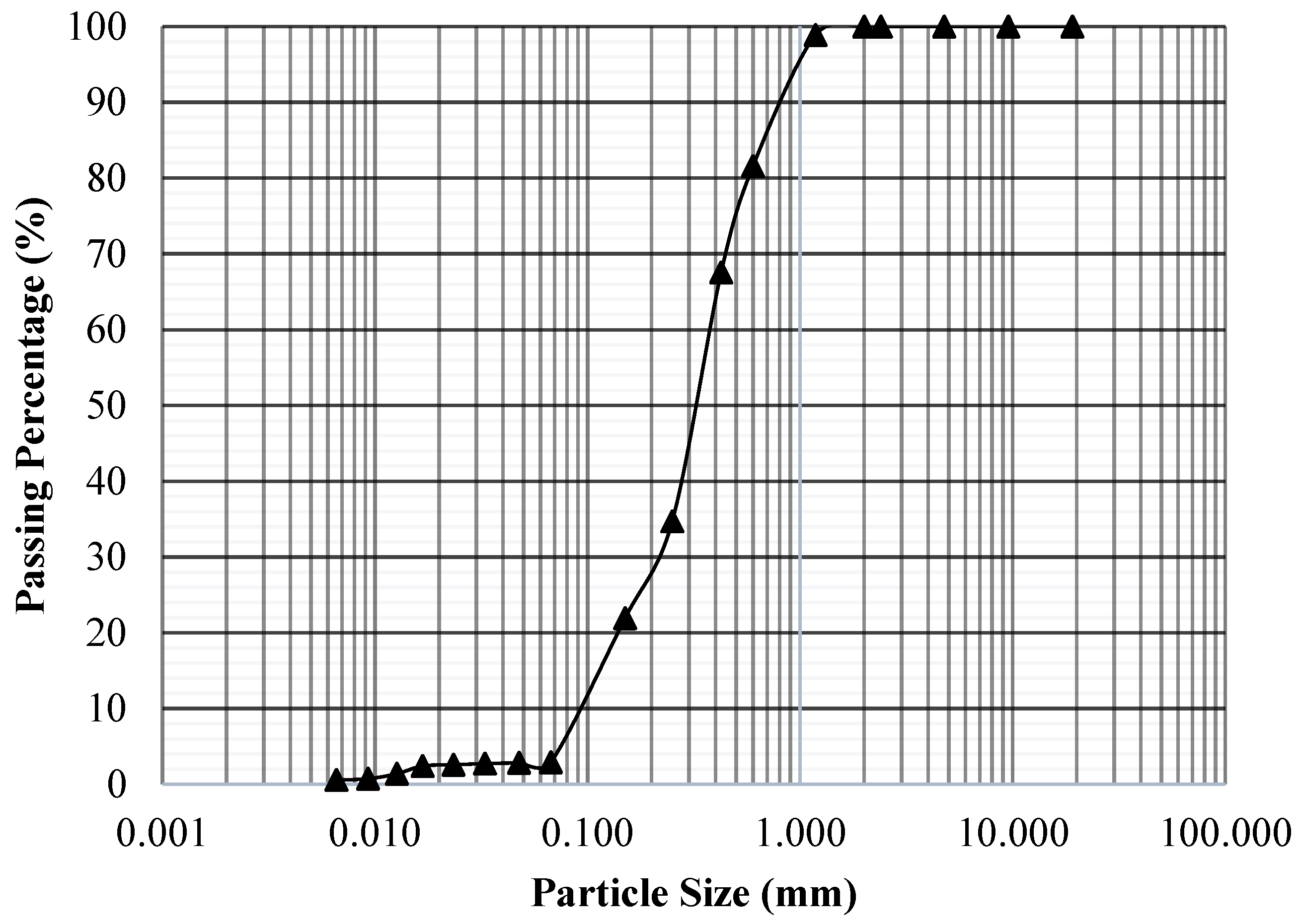
2.1. Materials
2.2. Experimental Study
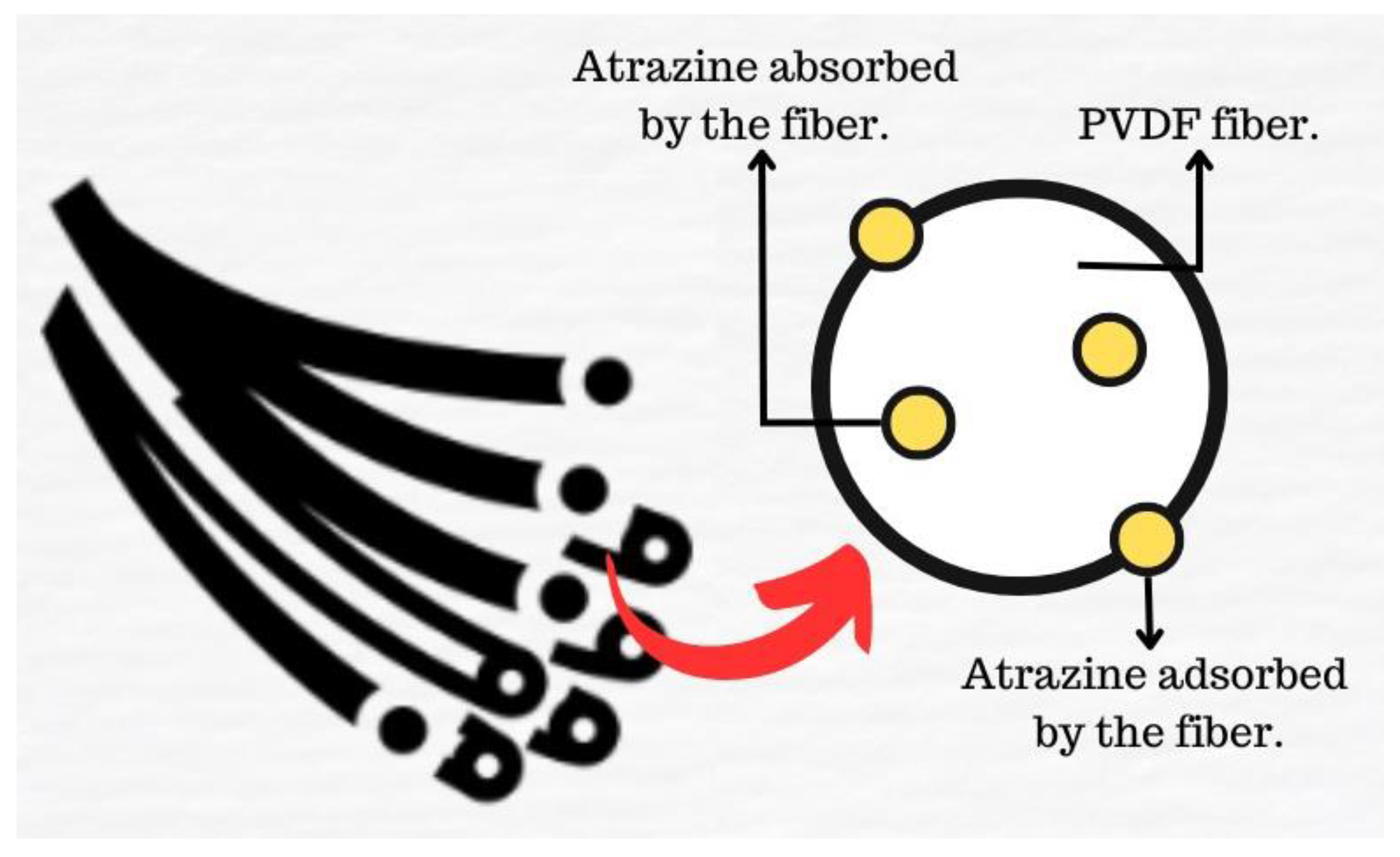
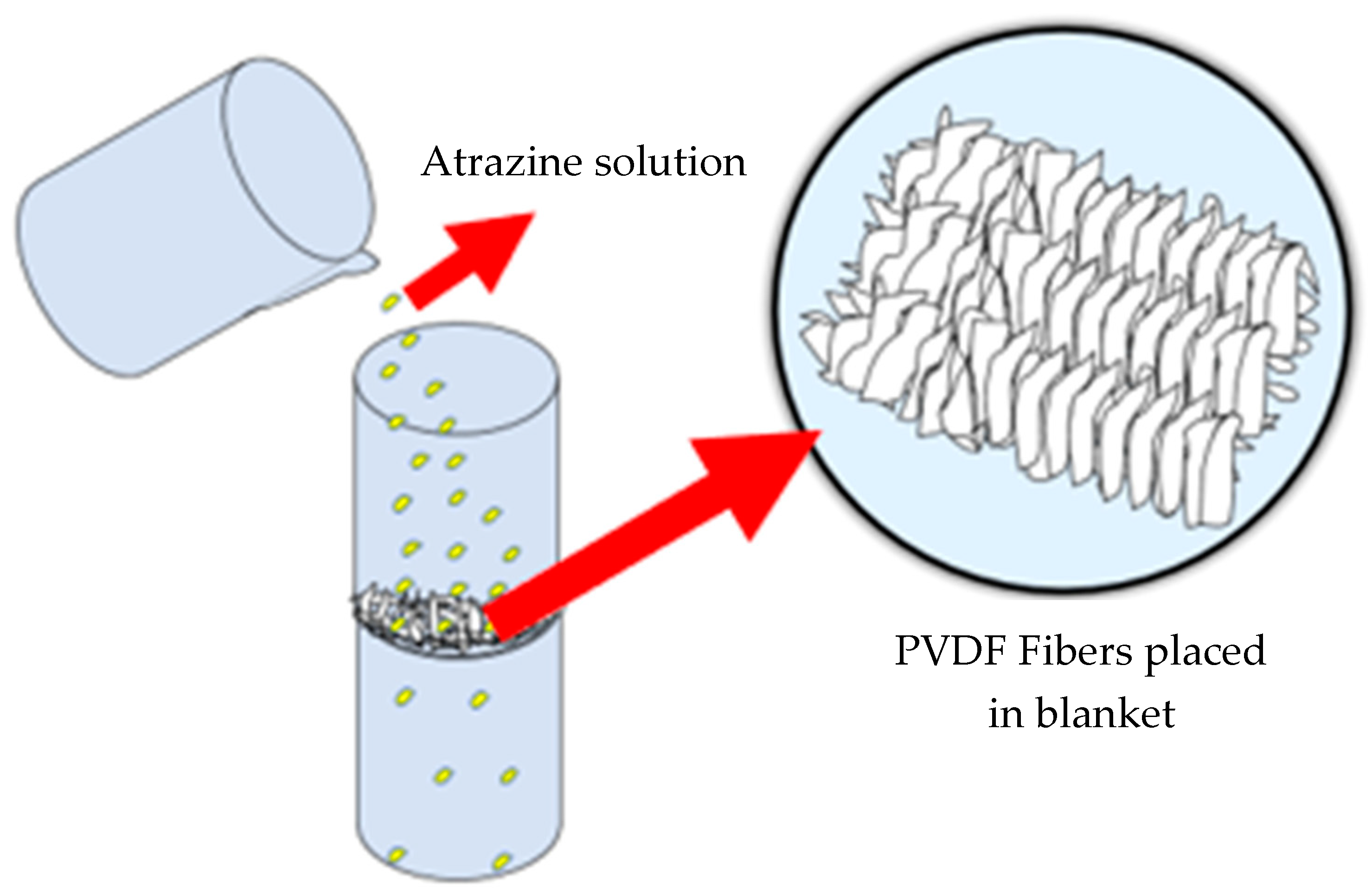
2.2.1. Absorption Test
2.2.2. Adsorption Test
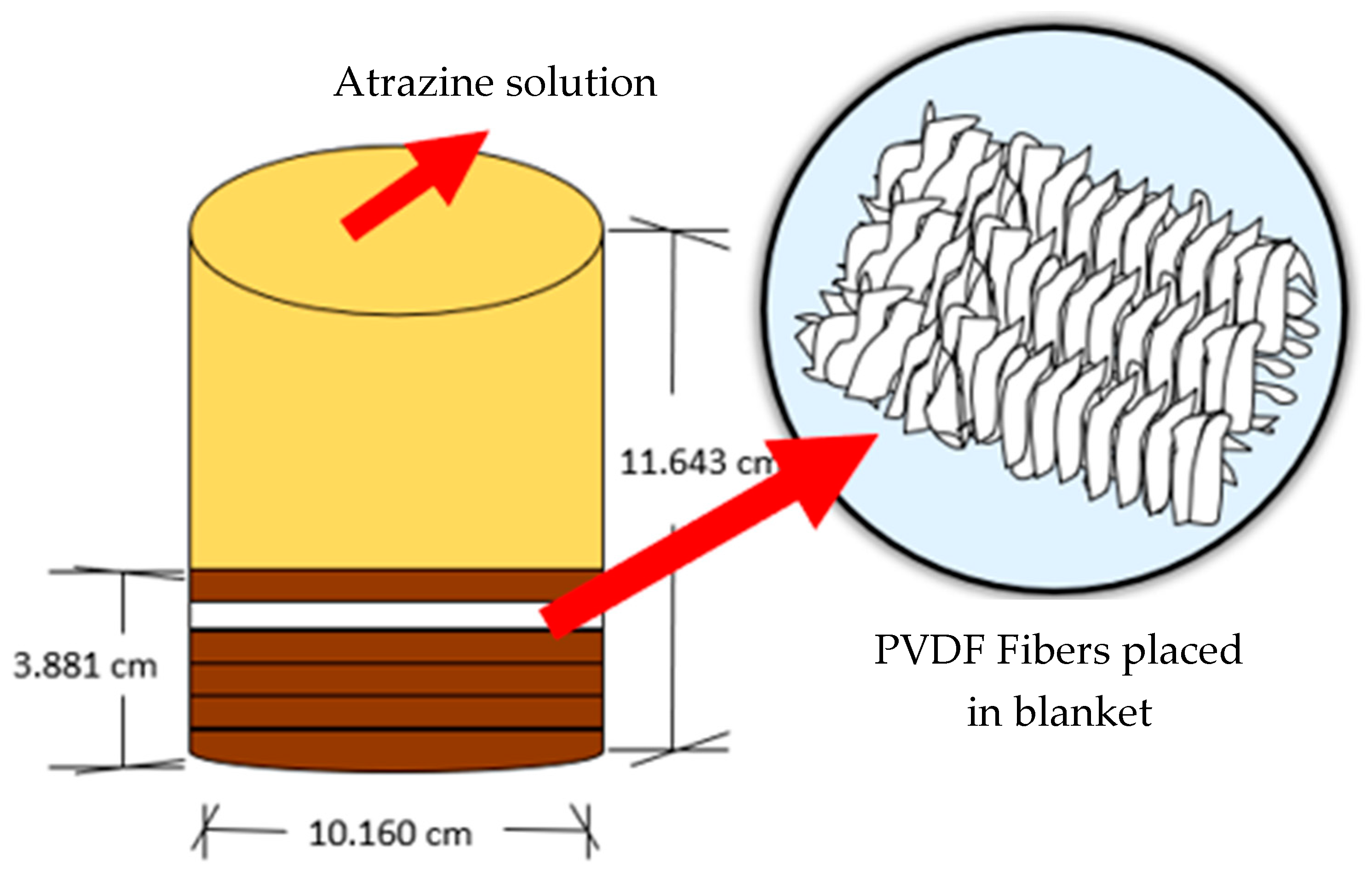
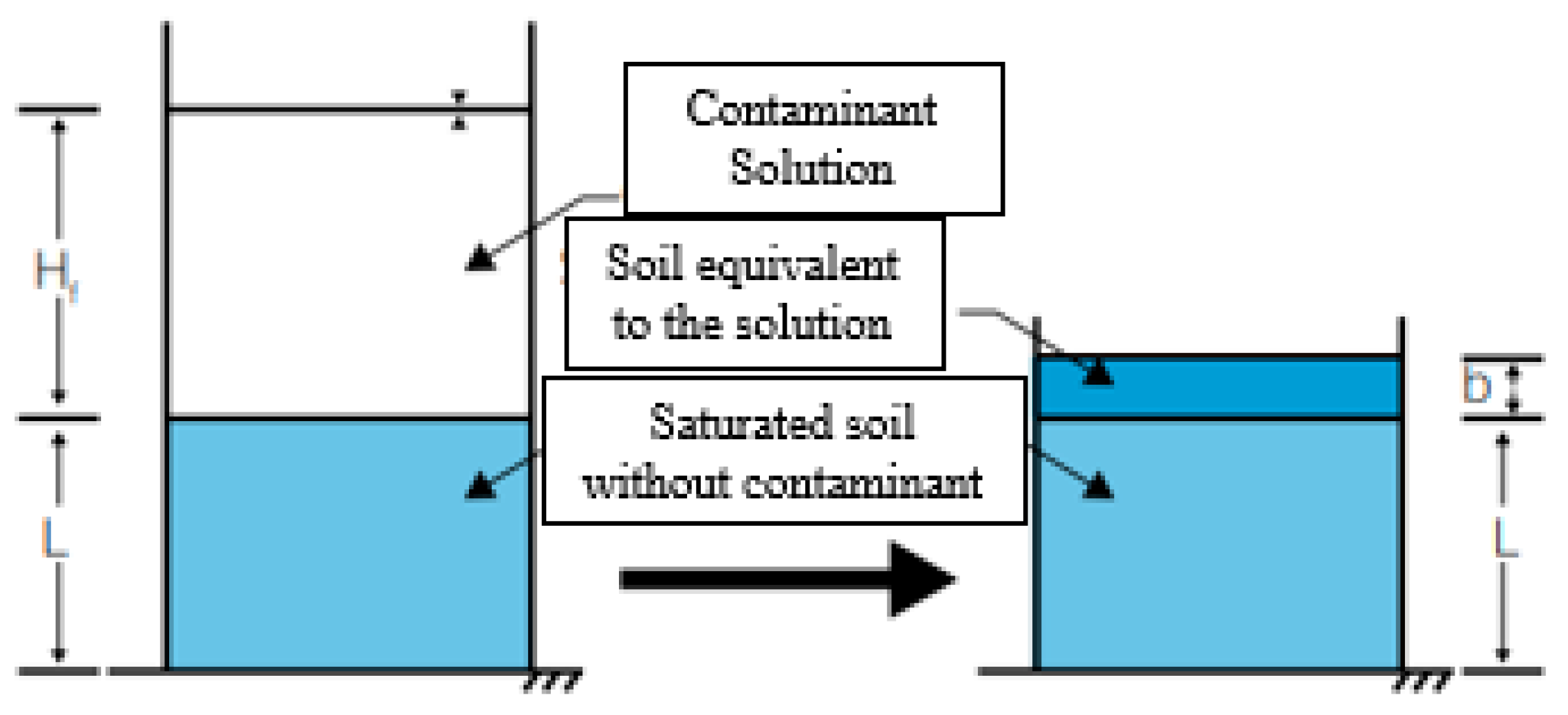
2.2.3. Diffusion Test
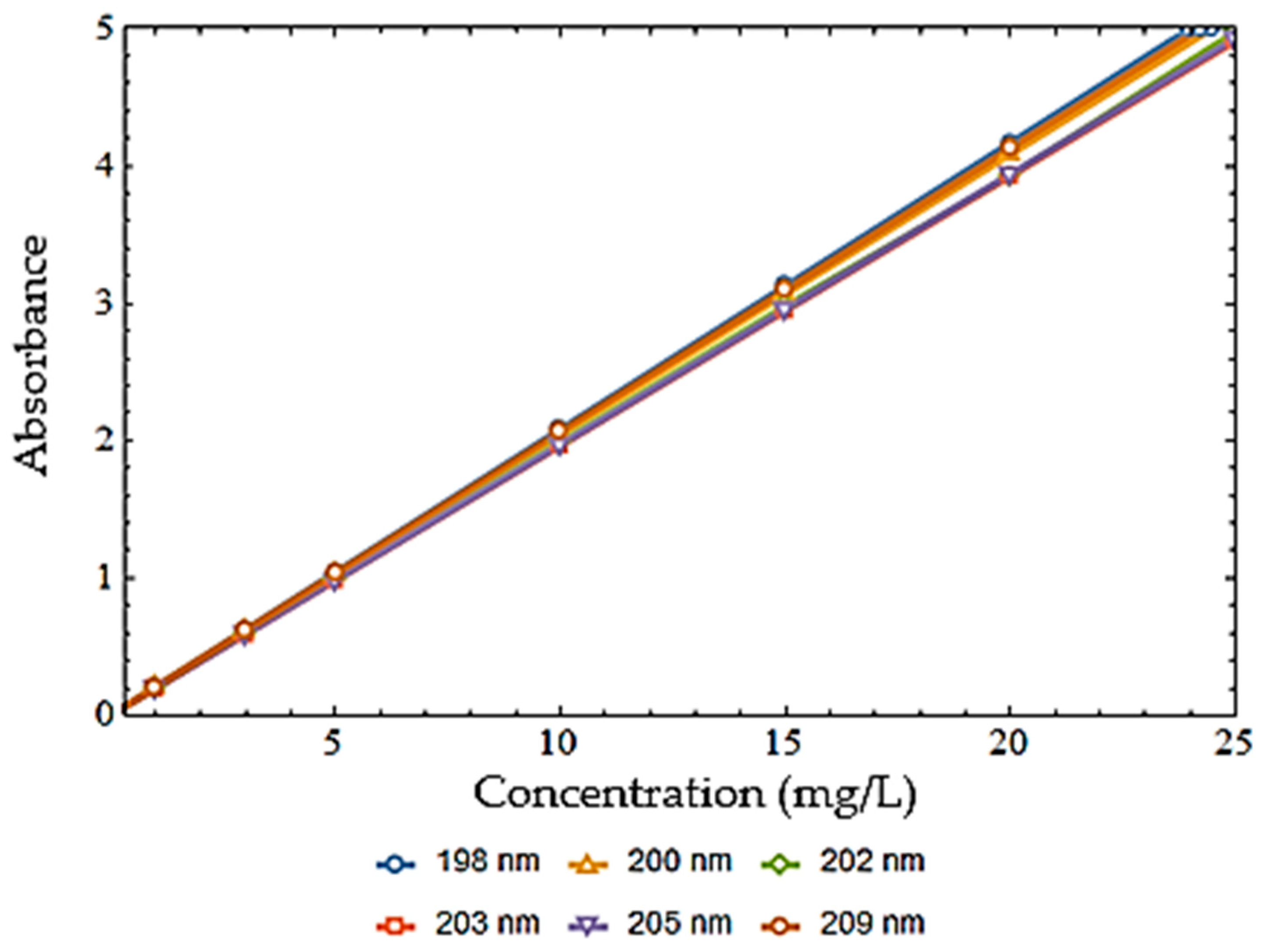
2.3. Chemical Analysis
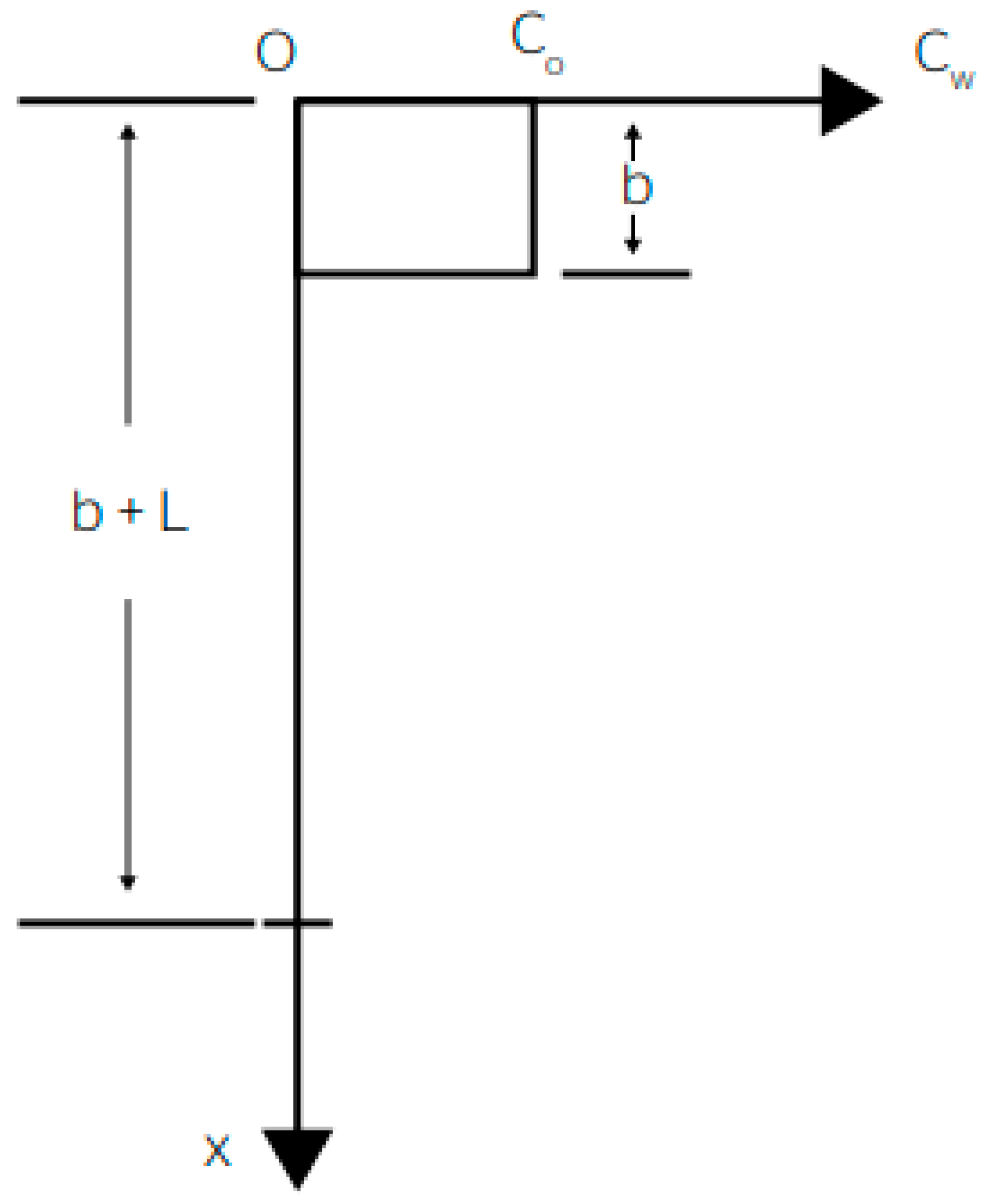
2.4. Computational Analysis
3. Results
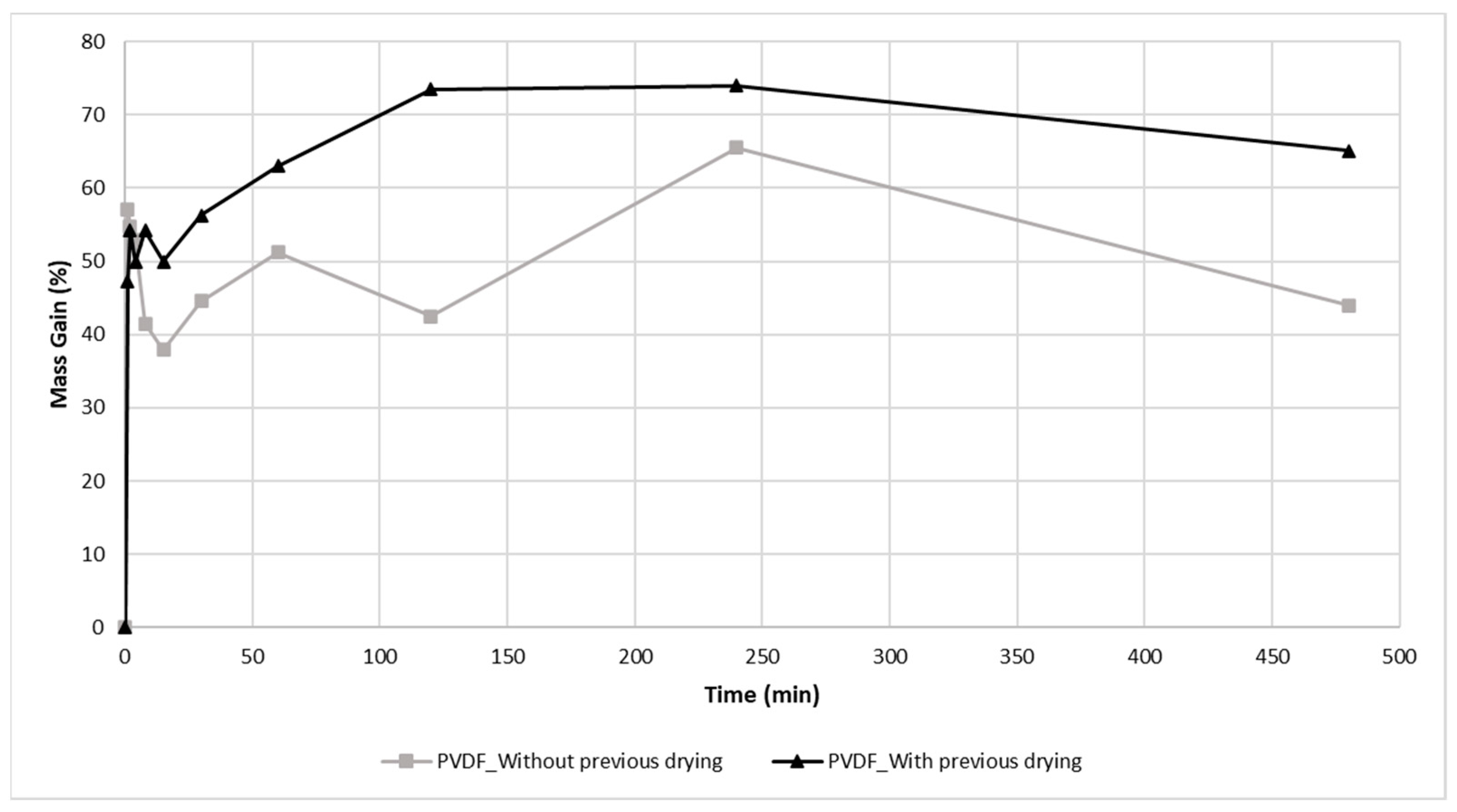
3.1. Absorption Test
3.2. Adsorption Test
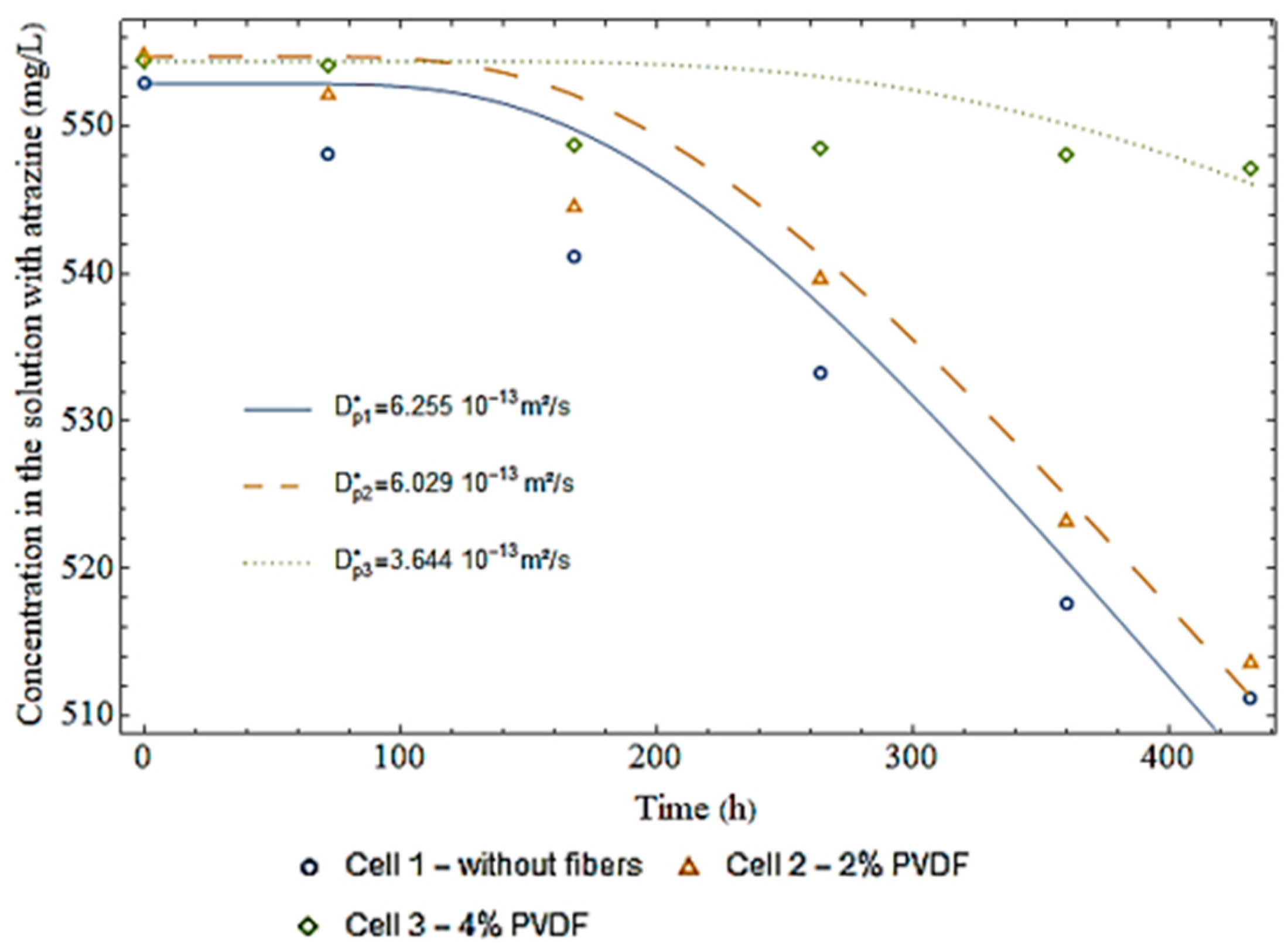
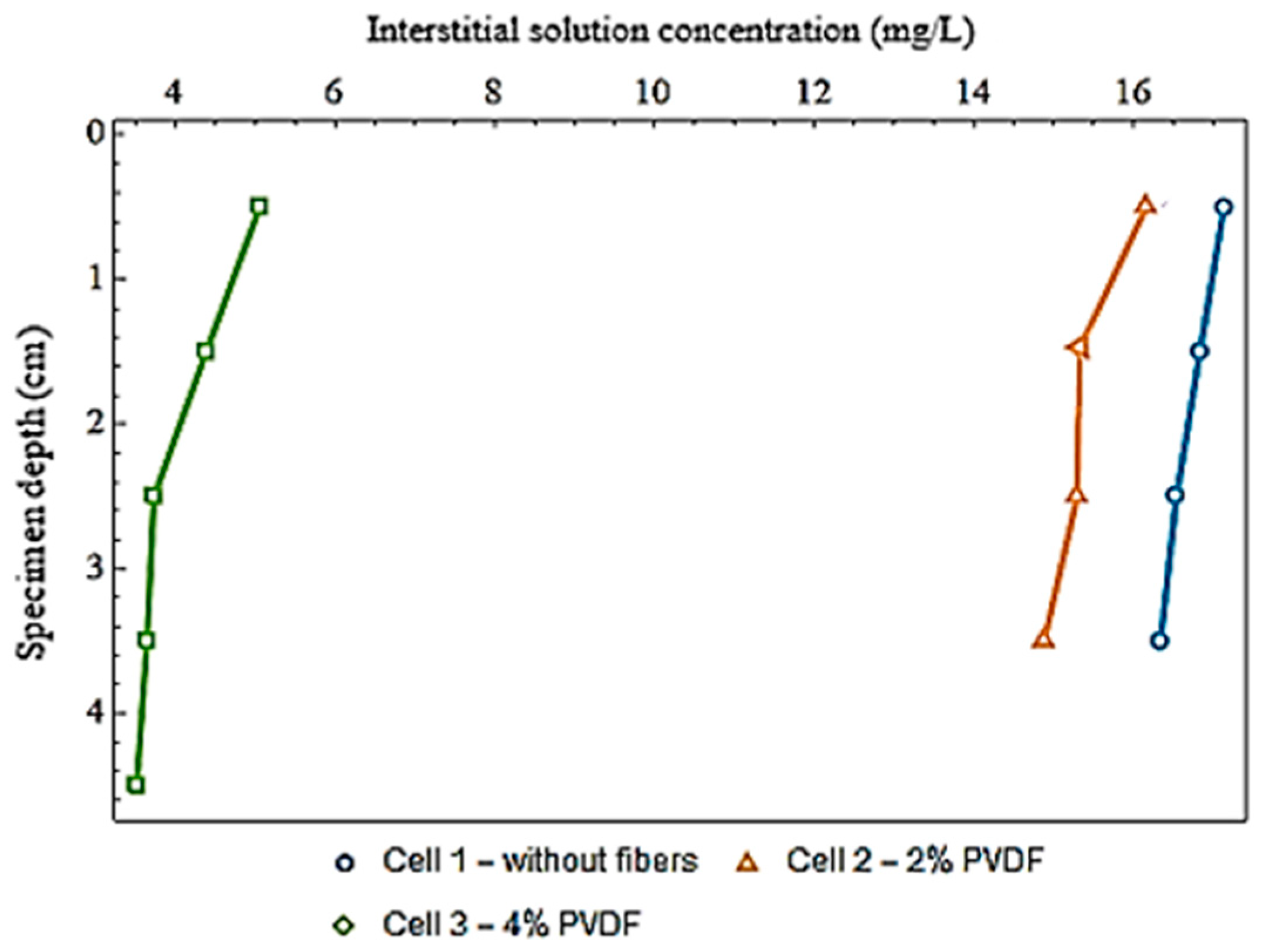
3.3. Diffusion Test
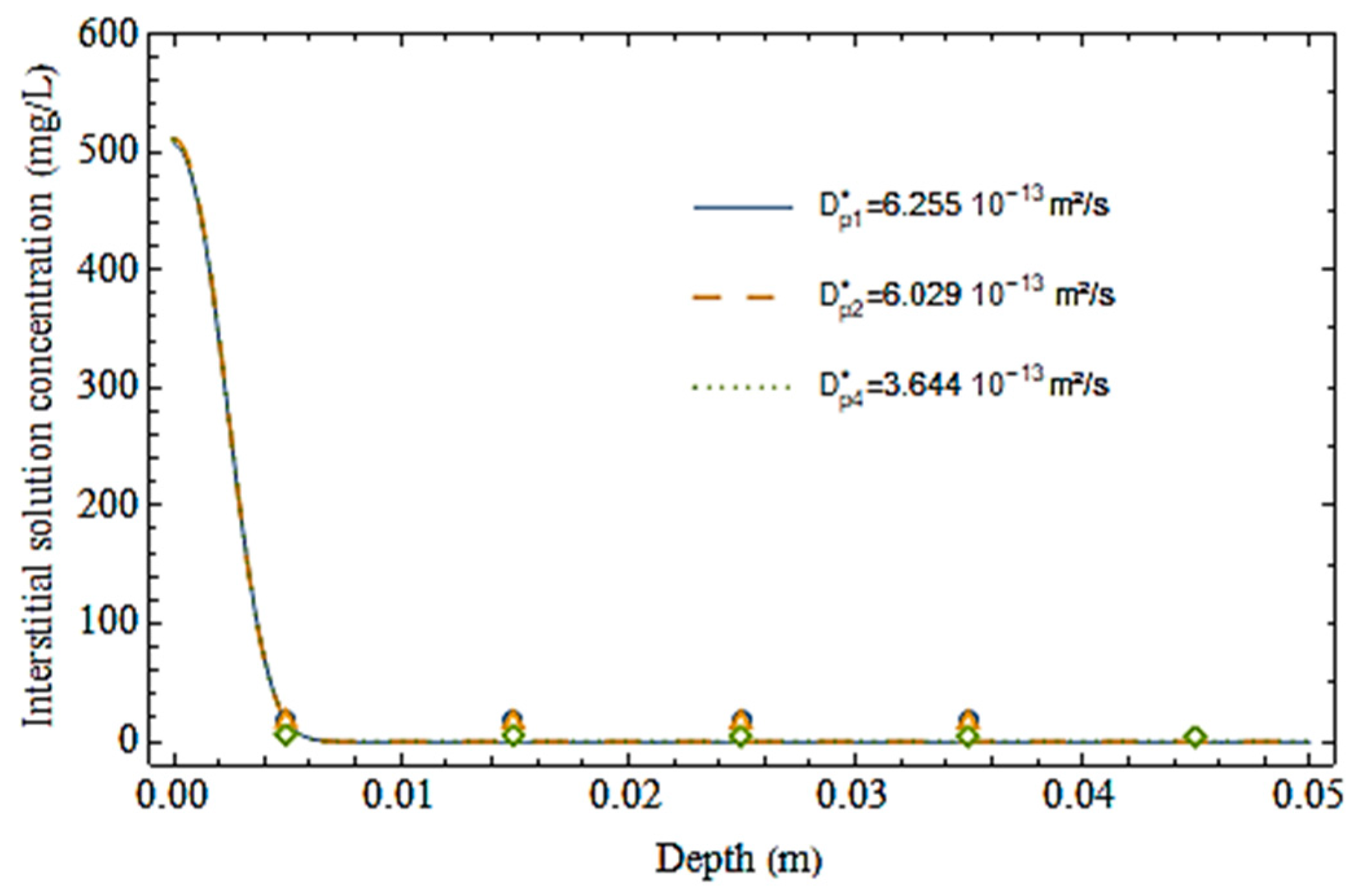
3.4. Computational Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA). 2021. Available online: http://www.ibama.gov.br/phocadownload/qualidadeambiental/relatorios/2019/Os_10_IAs_vendidos_2019.xls (accessed on 16 November 2022).
- Mencoboni, M.; Lerza, R.; Bogliolo, G.; Flego, G.; Pannacciulli, I. Effect of atrazine on hemopoietic system. In Vivo 1992, 6, 41–44. [Google Scholar]
- Cooper, R.L.; Goldman, J.M.; Stoker, T.E. Neuroendocrine and reproductive effects of contemporary-use pesticides. Toxicol. Ind. Health 1999, 15, 26–36. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Letcher, R.J.; Heneweer, M.; Giesy, J.P.; Vandenberg, M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Health Perspect. 2001, 109, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.M.; Thiruchelvam, M.; Cory-Slechta, D.A. Sustained exposure to the widely used herbicide atrazine: Altered function and loss of neurons in brain monoamine systems. Environ. Health Perspect. 2005, 113, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.M.; Brundage, K.M.; Barnett, J.B. In vitro atrazine exposure inhibits human natural killer cell lytic granule release. Toxicol. Appl. Pharmacol. 2007, 221, 179–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brazil—Ministry of the Environment, Conama Resolution No. 357. Classificação das Águas, Doce, Salobra e Salgada do Território Nacional. D.O.U. nº 053: Brasília, Brazil, 2005; pp. 58–63. Available online: https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Resolucao/2005/res_conama_357_2005_classificacao_corpos_agua_rtfcda_altrd_res_393_2007_397_2008_410_2009_430_2011.pdf (accessed on 16 November 2022).
- Salmazzo, G.R.; Falcão, E.A.; Caires, A.R.L.; Garcia, D.; Eiras, J.A.; Botero, E.R. Síntese e caracterização estrutural de compósitos a base de poli(fluoreto de vinilideno)/(Pb0.91La0.09)(Zr0.65Ti0.35)0.98O3. Cerâmica 2014, 60, 83–87. [Google Scholar] [CrossRef][Green Version]
- Bentini, R.; Pola, A.; Rizzi, L.G.; Athanassiou, A.; Fragouli, D. A highly porous solvent free PVDF/expanded graphite foam for oil/water separation. Chem. Eng. J. 2019, 372, 1174–1182. [Google Scholar] [CrossRef]
- Kong, J.; Li, K. Oil removal from oil-in-water emulsions using PVDF membranes. Sep. Purif. Technol. 1999, 16, 83–93. [Google Scholar] [CrossRef]
- Meng, Y.; Yi, W. Application of a PVDF-based stress gauge in determining dynamic stress-strain curves of concrete under impact testing. Smart Mater. Struct. 2011, 20, 065004. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Zhao, L. Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes. Membranes 2014, 4, 81–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Liu, J.; Zhao, L. Preparation and preliminary dialysis performance research of polyvinylidene fluoride hollow fiber membranes. Membranes 2015, 5, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.T.; Chang, C.C.; Yang, Y.S.; Yen, C.K.; Kao, Y.H.; Shiue, Y.L. Development of MMG sensors using PVDF piezoelectric electrospinning for lower limb rehabilitation exoskeleton. Sens. Actuators A Phys. 2019, 301, 111708. [Google Scholar] [CrossRef]
- Soriano, I. 3D Characterization of a Fiber-Reinforced Sand Using X-ray Tomography and Image Analysis. Master’s Thesis, Université Joseph Fourier, Grenoble, France, 2015; 54p. [Google Scholar]
- NBR ISSO 7181; Solo—Análise Granulométrica de Solos. Brazilian Association of Technical Standards. ABNT: Rio de Janeiro, Brazil, 2016; 7p.
- NBR ISO 12051; Solo—Determinação do Índice de Vazios Mínimo de Solos Não-Coesivos. ABNT: Rio de Janeiro, Brazil, 1991; 15p.
- NBR ISO 12004; Solo—Determinação do Índice de Vazios Máximo de Solos Não Coesivos. ABNT: Rio de Janeiro, Brazil, 1990; 6p.
- Zaffani, A.G.; Figueiredo, C.M.; Anache, J.A.A.; Wendland, E.C.; Crestana, S. Transporte do herbicida atrazina em diferentes solos brasileiros. In Proceedings of the IX Congresso Brasileiro de Geotecnia Ambiental (REGEO 2019), São Carlos, Brazil, 14–16 August 2019. [Google Scholar]
- Picanço, M.S. Compósitos Cimentícios Reforçados com Fibras de Curauá. Master’s Thesis, Pontifícia Universidade Católica do Rio de Janeiro (PUC Rio), Rio de Janeiro, Brazil, 2005; 101p. [Google Scholar]
- Silveira, L.R. Aspectos Geotécnicos e Ambientais para a Disposição Adequada de Lodo de Esgoto. Ph.D. Thesis, Department of Civil and Environmental Engineering, University of Brasília, Brasília, Brazil, 2014; 193p. [Google Scholar]
- NBR 9778; Argamassa e Concreto Endurecidos—Determinação da Absorção de Água, Índice de Vazios e Massa Específica. ABNT: Rio de Janeiro, Brazil, 2009; 4p.
- Barone, F.S.; Yanful, E.K.; Quigley, R.M.; Rowe, R.K. Effect of multiple contaminant migration on diffusion and adsorption of some domestic waste contaminants in a natural clayey soil. Can. Geotech. J. 1989, 26, 189–198. [Google Scholar] [CrossRef]
- Fonseca, M.C.; Ritter, E.; Cavalcante, A.L.B. Application of semi-analytical solution for modeling sorption and pure diffusion assays with leachate. Eng. Sanit. Ambient. 2019, 24, 965–973. (In Portuguese) [Google Scholar] [CrossRef]
- Baran, C.T.; Cavalcante, A.L.B. Application of a physical-mathematical model of leachate ion diffusion through compacted soils from diffusion tests. In Challenges and Innovations in Geomechanics: Proceedings of the 16th International Conference of IACMAG, Turin, Italy, 30 August–2 September 2021; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 2, pp. 932–940. [Google Scholar]
- Bouguer, P. Essai d’Optique sur la Gradation de la Lumière, 1st ed.; Hachette Livre Bnf: Paris, France, 1729; 214p. [Google Scholar]
- Shackelford, C.; Daniel, D.; Liljestrand, H.M. Diffusion of inorganic chemical species in compacted clay soil. J. Contam. Hydrol. 1989, 4, 241–273. [Google Scholar] [CrossRef]
- Barone, F.; Rowe, R.; Quigley, R. A laboratory estimation of diffusion and adsorption coefficients for several volatile organics in a natural clayey soil. J. Contam. Hydrol. 1992, 10, 225–250. [Google Scholar] [CrossRef]
- Shackelford, C.; Daniel, D. Diffusion in saturated soil. i: Background. J. Geotech. Eng. 1991, 117, 467–484. [Google Scholar] [CrossRef]
- Boscov, M.E.G. Contribuição ao Projeto de Sistemas de Contenção de Resíduos Perigosos Utilizando Solos Lateríticos. Ph.D. Thesis, Escola Politecnica University of Sao Paulo, Sao Paulo, Brazil, 1997. [Google Scholar]
- Gurjão, C. Estimativa de Propriedade Geoambientais de Camadas Impermeabilizantes de Solos Tropicais. Ph.D. Thesis, University of New Brunswick, Brasilia, Brazil, 2005. [Google Scholar]
- Rowe, R.K.; Booker, J.R. Pollute Version 7 Reference Guide. User Guide; GAEA Technologies Ltd.: Whitby, ON, Canada, 2004; Available online: http://www.gaea.ca/public/Pollute7_Users_Guide.pdf (accessed on 15 September 2022).
- Rowe, R.K.; Booker, J.R. 1-d pollutant migration in soils of finite depth. J. Geotech. Eng. 1985, 111, 479–499. [Google Scholar] [CrossRef]
- Rowe, R.K.; Caers, C.J.; Barone, F. Laboratory determination of diffusion and distribution coefficients of contaminants using undisturbed clayey soils. Can. Geotech. J. 1988, 25, 108–118. [Google Scholar] [CrossRef]
- Cavalcante, A.L.B.; Farias, M.M. Alternative solution for advective-dispersive flow of reagent solutes in clay liners. Int. J. Geomech. 2013, 13, 49–56. [Google Scholar] [CrossRef]
- Ogata, A.; Banks, R.B. A Solution of the Differential Equation of Longitudinal Dispersion in Porous Media; Report, U.S. Geological Survey Professional Paper; United States Government Printing Office: Washignton, DC, USA, 1961; pp. A1–A7.
- Ozelim, L.C.S.M.; Cavalcante, A.L.B. Integral and closed-form analytical solutions to the transport contaminant equation considering 3D advection and dispersion. Int. J. Geomech. 2013, 13, 686–691. [Google Scholar] [CrossRef]
- Paz, Y.P.L. Solução Semi-Analítica para Modelagem das Condições Inicial e de Contorno Aplicáveis ao Ensaio de Difusão Pura. Master’s Thesis, Departamento de Engenharia Civil e Ambiental, Universidade de Brasília, Brasília, Brazil, 2015; p. 139f. [Google Scholar]
- Ozelim, L.C.S.M.; Paz, Y.P.L.; Cunha, L.S.; Cavalcante, A.L.B. Enhanced landfill’s characterization by using an alternative analytical model for diffusion tests. Environ. Monit. Assess. 2021, 193, 739. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Selim, H.M. Evaluation of disequilibrium models for predicting atrazine transport in soils. Soil Sci. Soc. Am. J. 1997, 61, 1299. [Google Scholar] [CrossRef]
- Mao, M.; Ren, L. Simulating Nonequilibrium Transport of Atrazine through Saturated Soil. Groudwater 2004, 42, 500–508. [Google Scholar] [CrossRef]
- Celestino Ladu, J.L.; Zhang, D. Modeling atrazine transport in soil columns with HYDRUS-1D. Water Sci. Eng. 2001, 4, 258–269. [Google Scholar]
- Park, J.-H.; Feng, Y.; Pingsheng, J.; Voice, T.C.; Boyd, S.A. Assessment of bioavailability of soil-sorbed atrazine. Appl. Environ. Microb. 2003, 69, 3288–3298. [Google Scholar] [CrossRef]
- Abu-Zreig, M.; Abu-Ashour, J. Chloride and atrazine transport trough saturated soil columns. Toxicol. Environ. Chem. 2004, 86, 181–190. [Google Scholar] [CrossRef]
- Karickhoff, S.W. Organic Pollutant Sorption in Aquatic Systems. J. Hydraul. Eng. 1984, 110, 707–735. [Google Scholar] [CrossRef]
- Beltran, J.; Gerritse, R.G.; Hernandez, F. Effect of flow rate on the adsorption and desorption of glyphosate, simazine and atrazine in columns of sandy soils. Eur. J. Soil Sci. 1998, 49, 149–156. [Google Scholar] [CrossRef]
- Clausen, L.; Fabricius, I.; Madsen, L. Adsorption of Pesticides onto Quartz, Calcite, Kaolinite and α-Alumina. J. Environ. Qual. 2001, 30, 846–857. [Google Scholar] [CrossRef]
- Sadeghi, A.M.; Isensee, A.R.; Shirmonhammadi, A. Influence of soil texture and tillage on herbicide transport. Chemosphere 2000, 41, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Cosoli, P.; Fermeglia, M.; Ferrone, M. Molecular Simulation of Atrazine Adhesion and Diffusion in a Saturated Sand Model. Soil Sediment Contam. 2009, 19, 72–87. [Google Scholar] [CrossRef]

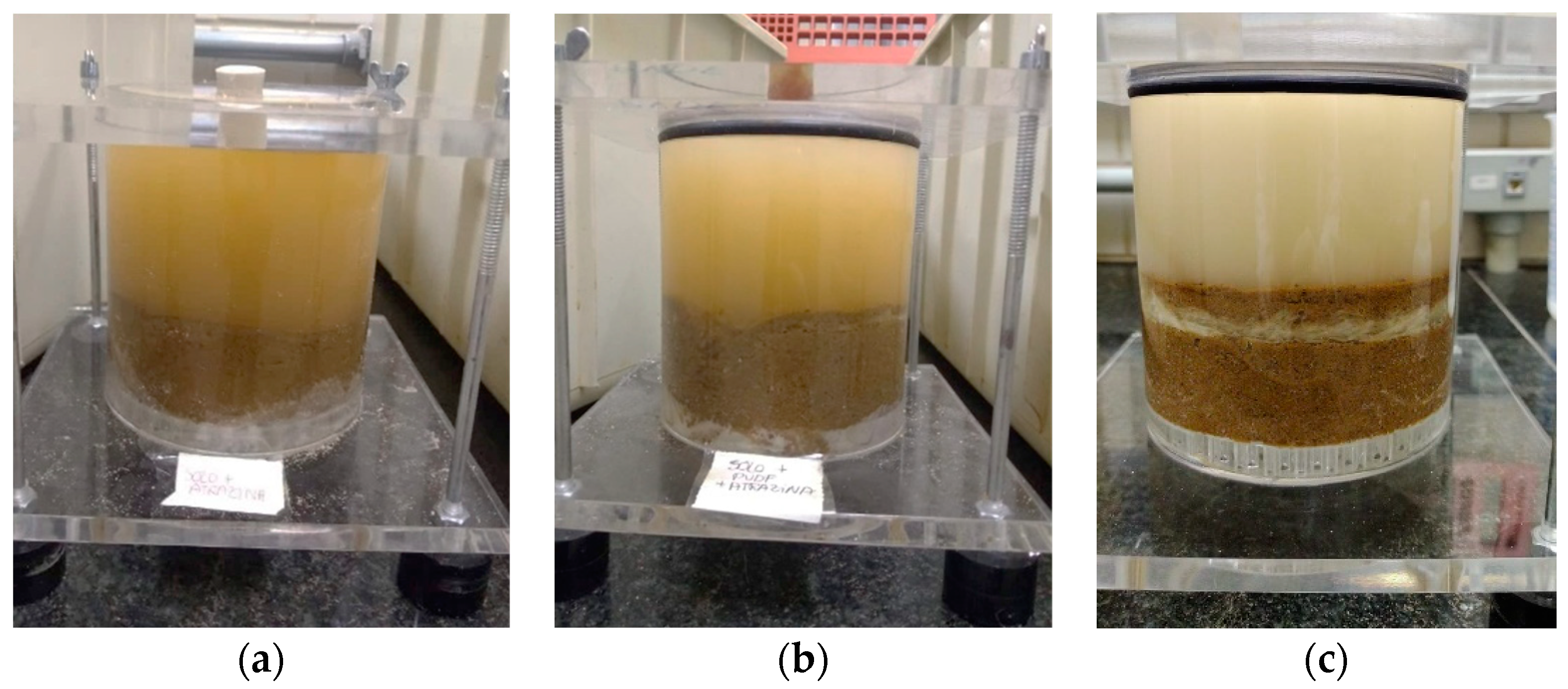
| Tests | Amount of Solutions (g) | Transport Time (s) | Flow Rate (m3/s) | Retained Amount (g) | Passing Amount (g) |
|---|---|---|---|---|---|
| Without fibers | 50.0 | 7.0 | 7.1 × 10−6 | 0.0 | 50.0 |
| 1.5 g of PVDF fibers | 50.0 | 25.0 | 2.0 × 10−6 | 5.7 | 43.5 |
| 4 g of PVDF fibers | 50.0 | 28.0 | 1.8 × 10−6 | 10.9 | 37.8 |
| Diffusion Test | Wavelength [nm] | Estimated Concentration [ppm]—Test Hours | |||||
|---|---|---|---|---|---|---|---|
| 0 | 72 | 168 | 264 | 360 | 432 | ||
| Cell 1—Without fibers | 205 | 552.9 | 548.1 | 541.1 | 533.2 | 517.5 | 511.2 |
| Cell 2—2% PVDF fibers | 203 | 554.7 | 552.1 | 544.5 | 539.6 | 523.1 | 513.5 |
| Cell 3—4% PVDF fibers | 198 | 554.4 | 554.1 | 548.7 | 548.5 | 548.0 | 547.1 |
| Diffusion Test | Wavelength [nm] | Estimated Concentration [ppm]—Depth [cm] | ||||
|---|---|---|---|---|---|---|
| 0.5 | 1.5 | 2.5 | 3.5 | 4.5 | ||
| Cell 1—Without fibers | 198 | 17.1 | 16.8 | 16.5 | 16.3 | - |
| Cell 2—2% PVDF fibers | 198 | 16.2 | 15.3 | 15.3 | 14.9 | - |
| Cell 3—4% PVDF fibers | 200 | 5.1 | 4.4 | 3.7 | 3.6 | 3.5 |
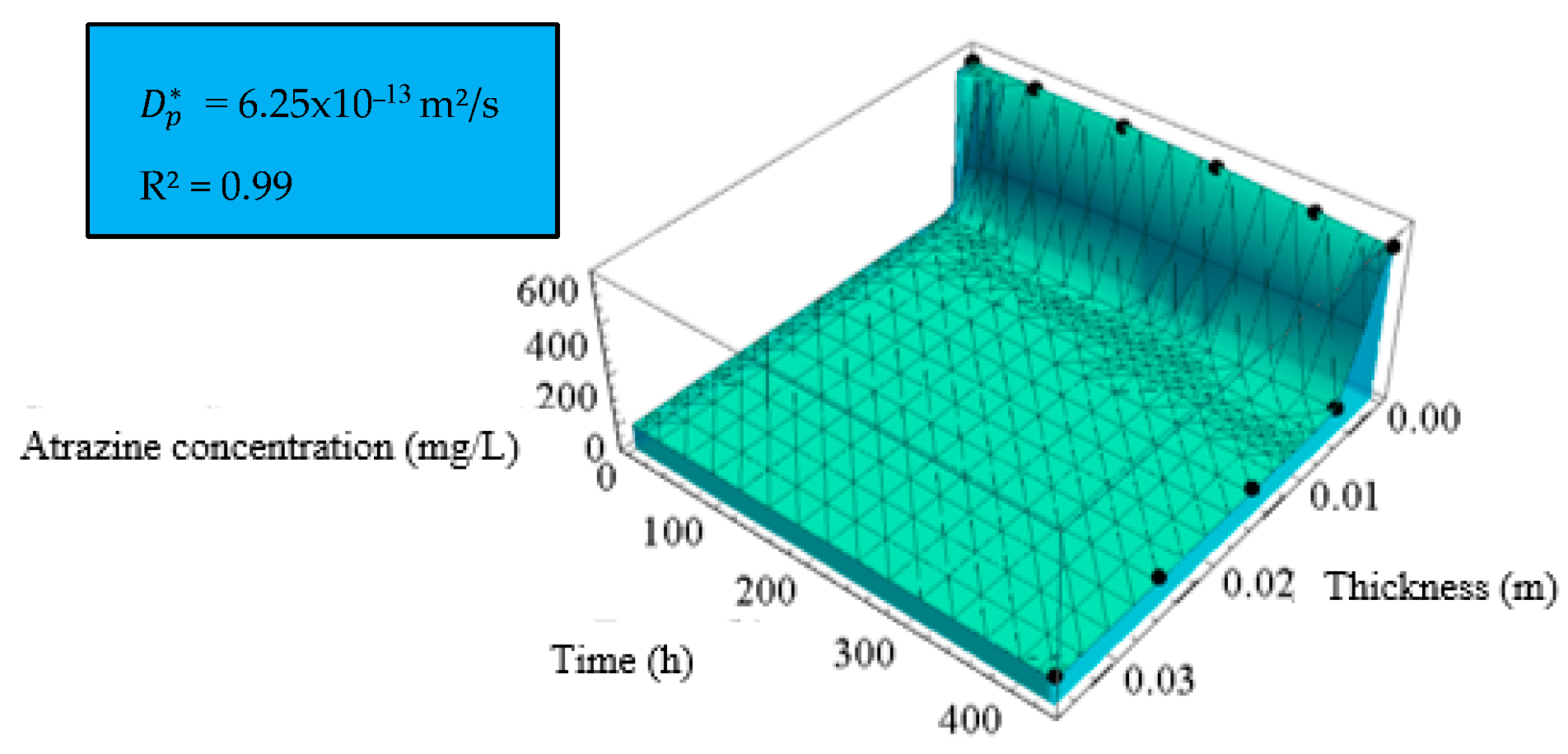
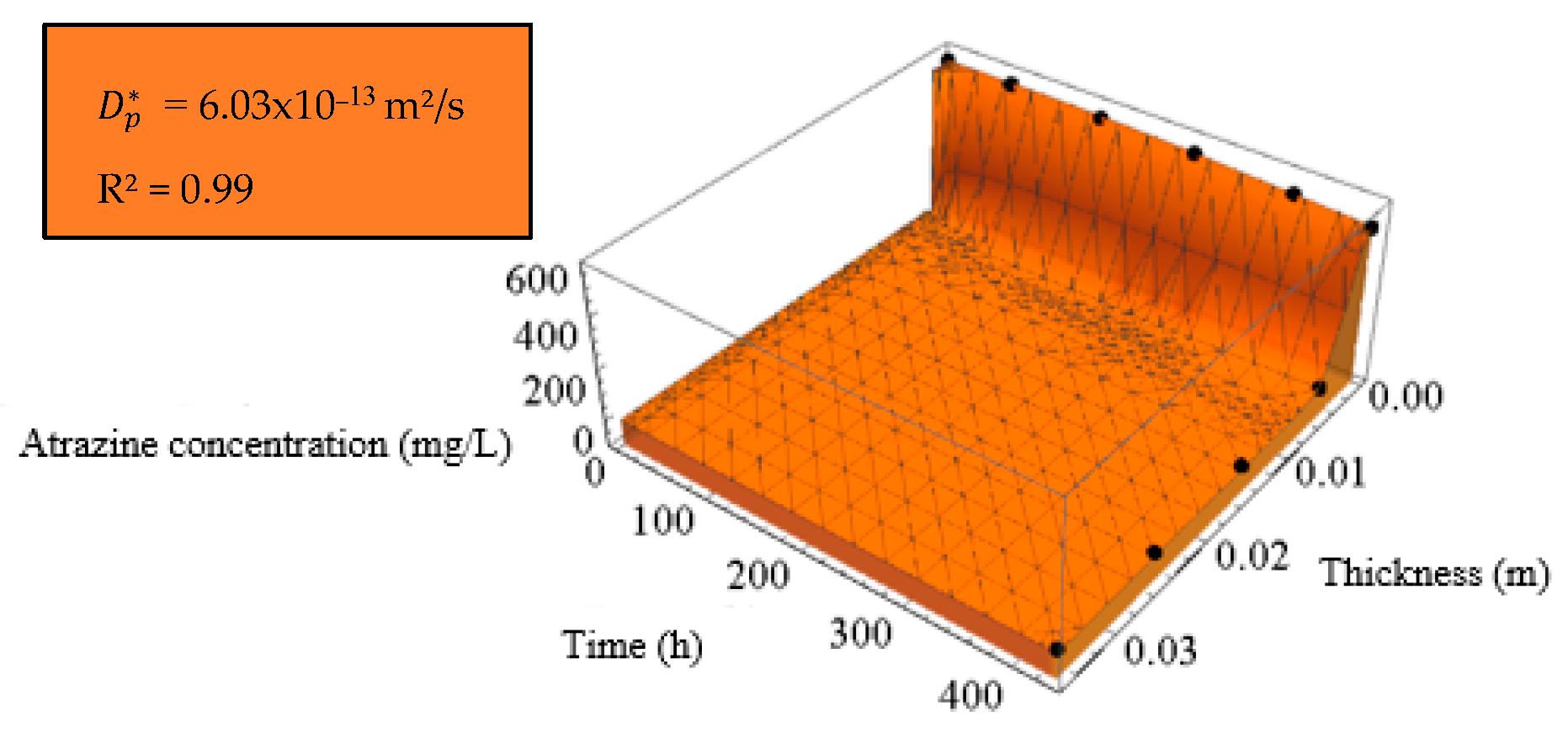
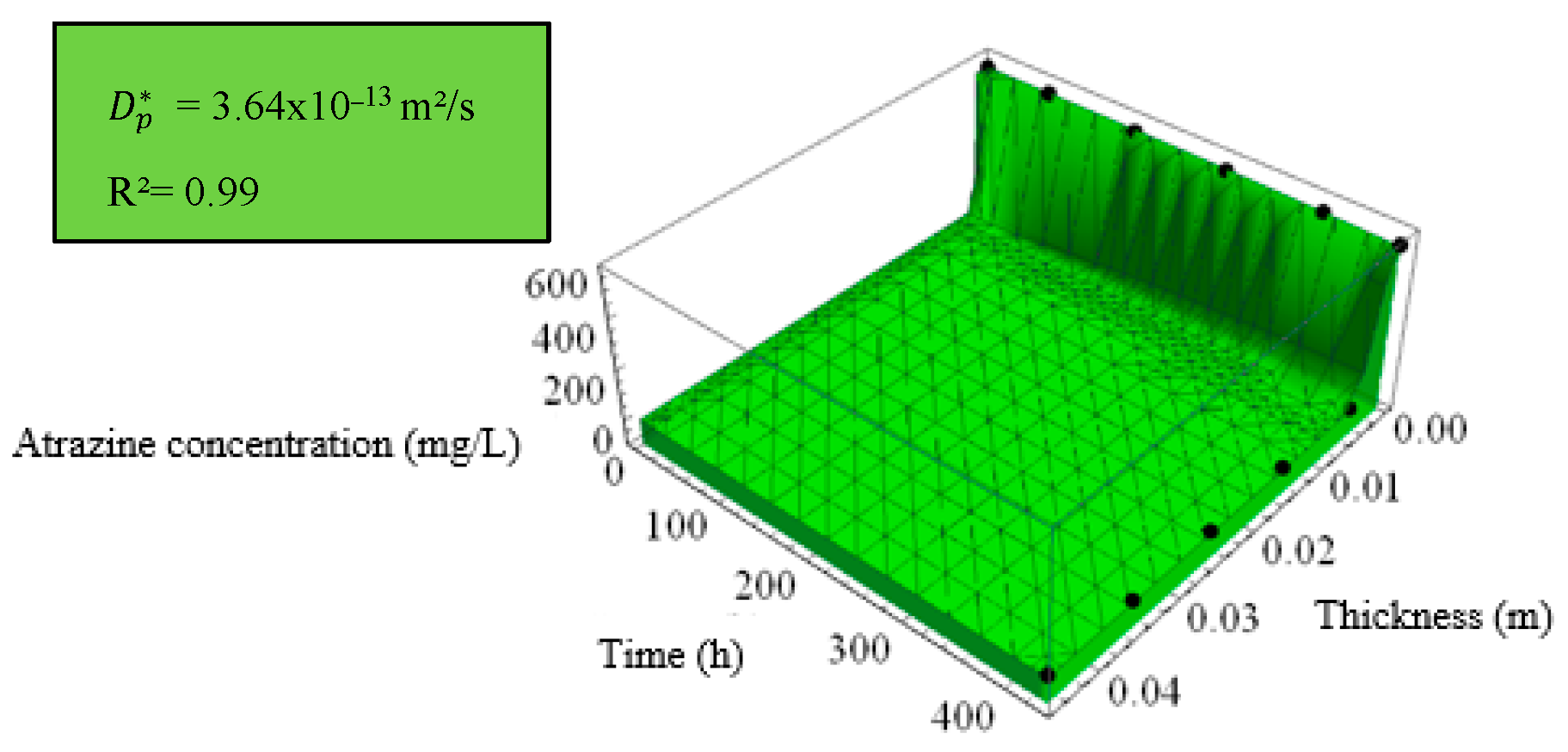
| Diffusion Test | [10–13 m2/s] | b [m] | R2 |
|---|---|---|---|
| Cell 1—Without fibers | 6.25 | 0.0024 | 0.99 |
| Cell 2—2% PVDF fibers | 6.03 | 0.0024 | 0.99 |
| Cell 3—4% PVDF fibers | 3.64 | 0.0026 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Deus Vieira, G.M.; da Costa Lemos, M.A.; Cavalcante, A.L.B.; Dal Toé Casagrande, M. Effectiveness of Polyvinylidene Fluoride Fibers (PVDF) in the Diffusion and Adsorption Processes of Atrazine in a Sandy Soil. Sustainability 2023, 15, 11729. https://doi.org/10.3390/su151511729
de Deus Vieira GM, da Costa Lemos MA, Cavalcante ALB, Dal Toé Casagrande M. Effectiveness of Polyvinylidene Fluoride Fibers (PVDF) in the Diffusion and Adsorption Processes of Atrazine in a Sandy Soil. Sustainability. 2023; 15(15):11729. https://doi.org/10.3390/su151511729
Chicago/Turabian Stylede Deus Vieira, Gabriella Melo, Moisés Antônio da Costa Lemos, André Luís Brasil Cavalcante, and Michéle Dal Toé Casagrande. 2023. "Effectiveness of Polyvinylidene Fluoride Fibers (PVDF) in the Diffusion and Adsorption Processes of Atrazine in a Sandy Soil" Sustainability 15, no. 15: 11729. https://doi.org/10.3390/su151511729
APA Stylede Deus Vieira, G. M., da Costa Lemos, M. A., Cavalcante, A. L. B., & Dal Toé Casagrande, M. (2023). Effectiveness of Polyvinylidene Fluoride Fibers (PVDF) in the Diffusion and Adsorption Processes of Atrazine in a Sandy Soil. Sustainability, 15(15), 11729. https://doi.org/10.3390/su151511729








