Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning
Abstract
1. Introduction and the Environmental Impact of the Textile and Apparel Industry
2. Value Addition of Textile Waste Materials by Enzymatic Methods
3. Cotton—A Cellulosic Material
4. Cellulase
5. Pretreatment and Enzymatic Hydrolysis of Cellulose
5.1. Pretreatment
5.2. Acid Pretreatment
5.3. Alkali Pretreatment
5.4. Ionic Liquid Pretreatment
5.5. Supercritical Fluid Pretreatment
6. Fungal Cellulase Production from Textile Waste by Solid–State Fermentation
Utilization of Fungal Cellulase to Treat Textile Wastes
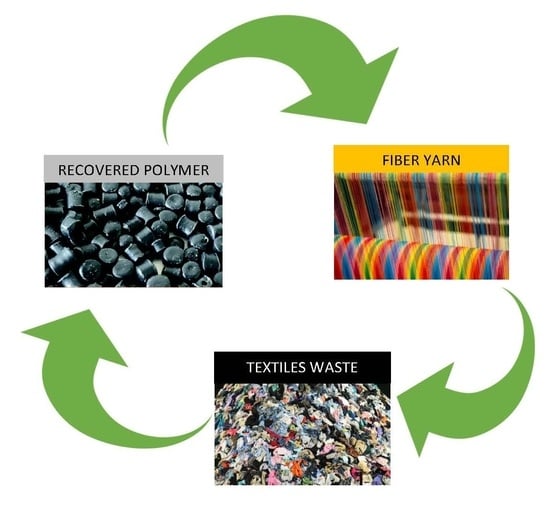
7. Regenerated Fibers from Waste Materials via Electrospinning
8. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okafor, C.C.; Madu, C.N.; Ajaero, C.C.; Ibekwe, J.C.; Nzekwe, C.A. Sustainable management of textile and clothing. Clean. Technol. Recycl. 2021, 1, 70–87. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Ding, X. Textile supply chain waste management in China. J. Clean. Prod. 2021, 289, 125147. [Google Scholar] [CrossRef]
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular Economy and Sustainability of the Clothing and Textile Industry. Mater. Circ. Econ. 2021, 3, 12. [Google Scholar] [CrossRef]
- Xu, C.-k.; Cheng, H.; Liao, Z.; Hu, H. An account of the textile waste policy in China (1991–2017). J. Clean. Prod. 2019, 234, 1459–1470. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A Review on Textile Recycling Practices and Challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Wojnowska-Baryla, I.; Bernat, K.; Zaborowska, M. Strategies of Recovery and Organic Recycling Used in Textile Waste Management. Int. J. Environ. Res. Public. Health 2022, 19, 5859. [Google Scholar] [CrossRef]
- Chavan, R.B. Environmental Sustainability through Textile Recycling. J. Text. Sci. Eng. S 2014, 2. [Google Scholar] [CrossRef]
- Tripa, S. Impact of the textile industry on the environment in the context of its sustainable development. Ann. Univ. Oradea Fasc. Text. Leatherwork 2013, 14, 171–176. [Google Scholar]
- Putting The Brakes on Fast Fashion in Story, Cities and Lifestyles; UN Environment Programme: Nairobi, Kenya, 2018.
- Pensupa, N. Chapter 12 Recycling of End-of-Life Clothes. In Sustainable Technologies for Fashion and Textiles; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wang, S. Brief Analysis on Closed-loop Ecosystem of Textile and Clothing Recycling. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 012058. [Google Scholar]
- Resta, B.; Gaiardelli, P.; Pinto, R.; Dotti, S. Enhancing environmental management in the textile sector: An Organisational-Life Cycle Assessment approach. J. Clean. Prod. 2016, 135, 620–632. [Google Scholar] [CrossRef]
- Pensupa, N.; Leu, S.Y.; Hu, Y.; Du, C.; Liu, H.; Jing, H.; Wang, H.; Lin, C.S.K. Recent Trends in Sustainable Textile Waste Recycling Methods: Current Situation and Future Prospects. Top. Curr. Chem. 2017, 375, 76. [Google Scholar] [CrossRef]
- Fortuna, L.M.; Diyamandoglu, V. Optimization of greenhouse gas emissions in second-hand consumer product recovery through reuse platforms. Waste Manag. 2017, 66, 178–189. [Google Scholar] [CrossRef]
- Belk, R. You are what you can access: Sharing and collaborative consumption online. J. Bus. Res. 2014, 67, 1595–1600. [Google Scholar] [CrossRef]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Porro, F.; Bedue, O.; Chanzy, H.; Heux, L. Solid-State 13C NMR Study of Na-Cellulose Complexes. Biomacromolecules 2007, 8, 2586–2593. [Google Scholar] [CrossRef]
- Schmidt, A. Gaining Benefits from Discarded Textiles: LCA of Different Treatment Pathways; Nordic Council of Ministers: Copenhagen, Denmark, 2016. [Google Scholar]
- Roos, S.; Sandin, G.; Zamani, B.; Peters, G.; Svanström, M. Will Clothing Be Sustainable? Clarifying Sustainable Fashion. In Textiles and Clothing Sustainability; Springer: Singapore, 2017; pp. 1–45. [Google Scholar] [CrossRef]
- Briga-Sá, A.; Nascimento, D.; Teixeira, N.; Pinto, J.; Caldeira, F.; Varum, H.; Paiva, A. Textile waste as an alternative thermal insulation building material solution. Constr. Build. Mater. 2013, 38, 155–160. [Google Scholar] [CrossRef]
- Gounni, A.; Mabrouk, M.T.; El Wazna, M.; Kheiri, A.; El Alami, M.; El Bouari, A.; Cherkaoui, O. Thermal and economic evaluation of new insulation materials for building envelope based on textile waste. Appl. Therm. Eng. 2019, 149, 475–483. [Google Scholar] [CrossRef]
- Barbero-Barrera, M.d.M.; Pombo, O.; Navacerrada, M.d.l.Á. Textile fibre waste bindered with natural hydraulic lime. Compos. Part. B Eng. 2016, 94, 26–33. [Google Scholar] [CrossRef]
- Vasconcelos, G.; Lourenço, P.B.; Camões, A.; Martins, A.; Cunha, S. Evaluation of the performance of recycled textile fibres in the mechanical behaviour of a gypsum and cork composite material. Cem. Concr. Compos. 2015, 58, 29–39. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of cellulose acetate using as raw material textile wastes. Mater. Today Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Wang, Y. Carpet Fiber Recycling Technologies. In Ecotextiles; Woodhead Publishing: Cambridge, UK, 2007; pp. 26–32. [Google Scholar] [CrossRef]
- Ranjithkumar, M.; Ravikumar, R.; Sankar, M.K.; Kumar, M.N.; Thanabal, V. An Effective Conversion of Cotton Waste Biomass to Ethanol: A Critical Review on Pretreatment Processes. Waste Biomass Valorization 2016, 8, 57–68. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Kliucininkas, L.; Lukošiūtė, S.-I.; Yan, L. Sustainable green technology for recovery of cotton fibers and polyester from textile waste. J. Clean. Prod. 2020, 254, 120078. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Du, C.; Lin, C.S.K. Recovery of Glucose and Polyester from Textile Waste by Enzymatic Hydrolysis. Waste Biomass Valorization 2018, 10, 3763–3772. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Xiang, H.; Xu, Y.; Li, Y. Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym. Degrad. Stab. 2002, 75, 185–191. [Google Scholar] [CrossRef]
- Mojsov, K. Microbial cellulases and their applications in textile processing. Int. J. Market. Technol. 2012, 2, 12–29. [Google Scholar]
- Brown, J.; Lu, C.L.; Coburn, J.; Kaplan, D.L. Impact of silk biomaterial structure on proteolysis. Acta Biomater. 2015, 11, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and biodegradation rate of poly(caprolactone)-starch blend and poly(butylene succinate) biodegradable polymer under aerobic and anaerobic environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Schnurer, A.; Schnurer, J. Fungal survival during anaerobic digestion of organic household waste. Waste Manag. 2006, 26, 1205–1211. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Degradable Polymers: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2022. [CrossRef]
- Gomez, J.G.; Méndez, B.S.; Nikel, P.I.; Pettinari, M.J.; Prieto, M.A.; Silva, L.F. Making Green Polymers Even Greener: Towards Sustainable Production of Polyhydroxyalkanoates from Agroindustrial By-Products. In Advances in Applied Biotechnology; Marian, P., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process. Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S. Processing strategies in bionanocomposites. Prog. Polym. Sci. 2013, 38, 1543–1589. [Google Scholar] [CrossRef]
- Steinbuchel, A. Non-biodegradable biopolymers from renewable resources: Perspectives and impacts. Curr. Opin. Biotechnol. 2005, 16, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Arora, M.; Minhas, J. Innovating Opportunities for Fashion Brands by Using Textile Waste for Better Fashion. In Recycling from Waste in Fashion and Textiles; Wiley Online: Hoboken, NJ, USA, 2020; pp. 101–121. [Google Scholar] [CrossRef]
- Shui, S. World Apparel Fibre Consumption Survey 2013. 2013. Available online: https://www.scribd.com/document/234350008/World-Apparel-Fiber-Consumption-FAO-2013 (accessed on 22 July 2023).
- Wilkins, T.A.; Arpat, A.B. The cotton fiber transcriptome. Physiol. Plant 2005, 124, 295–300. [Google Scholar] [CrossRef]
- Ryszard, M.K.O. Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and Cultivation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Huang, H.-D.; Liu, C.-Y.; Zhou, D.; Jiang, X.; Zhong, G.-J.; Yan, D.-X.; Li, Z.M. Cellulose composite aerogel for highly efficient electromagnetic interference shielding. J. Mater. Chem. A 2015, 3, 4983–4991. [Google Scholar] [CrossRef]
- Gordon, S.; Hsieh, Y. Cotton: Science and Technology; Woodhead Publishing: Sawston, UK, 2007. [Google Scholar]
- Quiroz-Castañeda, R.E.; Folch-Mallol, J.L. Hydrolysis of Biomass Mediated by Cellulases for the Production of Sugars. In Sustainable Degradation of Lignocellulosic Biomass—Techniques, Applications and Commercialization; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Ruel, K.; Nishiyama, Y.; Joseleau, J.P. Crystalline and amorphous cellulose in the secondary walls of Arabidopsis. Plant. Sci. 2012, 193–194, 48–61. [Google Scholar] [CrossRef]
- Babu, B.R.; Raghu, S.; Kumar, T.P. An Overview of Wastes Produced During Cotton Textile Processing and Effluent Treatment Methods. J. Cotton Sci. 2007, 11, 110–121. [Google Scholar]
- Babu, B.R.; Parande, A.K.; Raghu, S.; Kumar, T.P. Cotton Textile Processing: Waste Generation and Effluent Treatment. J. Cotton Sci. 2007, 11, 141–153. [Google Scholar]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Singh, P.; Ramteke, P.W. Application of Cellulases in Biofuels Industries: An Overview. J. Biofuels Bioenergy 2015, 1, 55–63. [Google Scholar] [CrossRef]
- Bisaria, V.S. Bioprocessing of Agro-Residues to Value Added Products; Springer Nature: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Pensupa, N.; Jin, M.; Kokolski, M.; Archer, D.B.; Du, C. A solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour. Technol. 2013, 149, 261–267. [Google Scholar] [CrossRef]
- Gusakov, A.V. Alternatives to Trichoderma reesei in biofuel production. Trends Biotechnol. 2011, 29, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.T.; Cherry, J. Progress and challenges in enzyme development for biomass utilization. Adv. Biochem. Eng. Biotechnol. 2007, 108, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, D. Production of cellulase by Trichoderma. Biotechnol. Bioeng. Symp. 1976, 6, 35–53. [Google Scholar]
- Dadheech, T.; Shah, R.; Pandit, R.; Hinsu, A.; Chauhan, P.S.; Jakhesara, S.; Kunjadiya, A.; Rank, D.; Joshi, C. Cloning, molecular modeling and characterization of acidic cellulase from buffalo rumen and its applicability in saccharification of lignocellulosic biomass. Int. J. Biol. Macromol. 2018, 113, 73–81. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Sohn, M.; Kang, Y.; Lee, J.H.; Realff, M.J. Protein engineering of cellulases. Curr. Opin. Biotechnol. 2014, 29, 139–145. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Leu, S.-Y.; Chen, S. Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuels Bioprod. Biorefining 2016, 10, 648–663. [Google Scholar] [CrossRef]
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.-Y.; Zhu, J.Y. Substrate-Related Factors Affecting Enzymatic Saccharification of Lignocelluloses: Our Recent Understanding. BioEnergy Res. 2012, 6, 405–415. [Google Scholar] [CrossRef]
- Markus Linder, T.T.T. The roles and function of cellulose-binding domains. J. Biotechnol. 1997, 57, 15–28. [Google Scholar] [CrossRef]
- Golan, A.E. (Ed.) Cellulase: Types and Action, Mechanism, and Uses; Nova Science Pub Inc.: Hauppauge, NY, USA, 2011. [Google Scholar]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Does change in accessibility with conversion depend on both the substrate and pretreatment technology? Bioresour. Technol. 2009, 100, 4193–4202. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y.; Wu, S.-Y.; Tsai, C.-Y.; Lin, C.-Y. Kinetics of cotton cellulose hydrolysis using concentrated acid and fermentative hydrogen production from hydrolysate. Int. J. Hydrogen Energy 2011, 36, 8743–8750. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Lin, P.-J.; Wu, Y.-Q.; Ye, L.-Y.; Yang, D.-J.; Shieh, C.-J.; Lee, C.-K. Simultaneous Saccharification and Fermentation of Waste Textiles for Ethanol Production. BioResources 2014, 9, 2866–2875. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Angayarkanni, J.; Rajendran, R.; Rajesh, R. Bioconversion of cotton waste from textile mills to bioethanol by microbial saccharification and fermentation. Ann. Biol. Res. 2011, 2, 380–388. [Google Scholar]
- Ouchi, A.; Toida, T.; Kumaresan, S.; Ando, W.; Kato, J. A new methodology to recycle polyester from fabric blends with cellulose. Cellulose 2009, 17, 215–222. [Google Scholar] [CrossRef]
- Shen, F.; Xiao, W.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013, 130, 248–255. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Taherzadeh, M.J. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 2009, 100, 1007–1010. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; Yu, C.W.; Dooley, T.M.; Jenkins, B.M.; VanderGheynst, J.S. Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotechnol. 2010, 162, 1768–1784. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Abdel-Fattah, Y.R.; Soliman, N.A.; Agblevor, F.A. Comparison of alkaline pulping with steam explosion for glucose production from rice straw. Carbohydr. Polym. 2011, 83, 720–726. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment. Bioresour. Technol. 2010, 101, 6718–6727. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Kumaran, J.; Amartey, S.; Maki, M.; Li, X.; Lu, F.; Qin, W. Biofuels and Bioproducts Produced through Microbial Conversion of Biomass. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 71–93. [Google Scholar] [CrossRef]
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective conversion of waste polyester–cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem. Eng. J. 2014, 253, 40–45. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zheng, Q.; Chen, L.; Huang, L.; Ni, Y.; Ouyang, X. Urea/NaOH system for enhancing the removal of hemicellulose from cellulosic fibers. Cellulose 2019, 26, 6393–6400. [Google Scholar] [CrossRef]
- Wang, H.; Kaur, G.; Pensupa, N.; Uisan, K.; Du, C.; Yang, X.; Lin, C.S.K. Textile waste valorization using submerged filamentous fungal fermentation. Process. Saf. Environ. Prot. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, C.L.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 139, 47–54. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Huddleston, J.G.; Holbrey, J.D.; Rogers, R.D. Ionic liquid salt-induced inactivation and unfolding of cellulase from Trichoderma reesei. Green. Chem. 2003, 5, 443–447. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.F.; Yang, G.; Jonsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Chen, Z.-Y.; Sun, J.-P.; Long, J.-J. Ecofriendly pretreatment of grey cotton fabric with enzymes in supercritical carbon dioxide fluid. J. Clean. Prod. 2016, 120, 85–94. [Google Scholar] [CrossRef]
- Schacht, C.; Zetzl, C.; Brunner, G. From plant materials to ethanol by means of supercritical fluid technology. J. Supercrit. Fluids 2008, 46, 299–321. [Google Scholar] [CrossRef]
- Gu, T.; Held, M.A.; Faik, A. Supercritical CO2 and ionic liquids for the pretreatment of lignocellulosic biomass in bioethanol production. Environ. Technol. 2013, 34, 1735–1749. [Google Scholar] [CrossRef]
- Saka, S.; Ueno, T. Chemical conversion of various celluloses to glucose and its derivatives in supercritical water. Cellulose 1999, 6, 177–191. [Google Scholar] [CrossRef]
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef]
- De Jong, E.; Jungmeier, G. Biorefinery Concepts in Comparison to Petrochemical Refineries. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar] [CrossRef]
- Brijwani, K.; Vadlani, P.V. Cellulolytic Enzymes Production via Solid-State Fermentation: Effect of Pretreatment Methods on Physicochemical Characteristics of Substrate. Enzym. Res. 2011, 2011, 860134. [Google Scholar] [CrossRef]
- Singhania, R.R.; Sukumaran, R.K.; Patel, A.K.; Larroche, C.; Pandey, A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzym. Microb. Technol. 2010, 46, 541–549. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Sanromán, M.A. Application of solid-state fermentation to ligninolytic enzyme production. Biochem. Eng. J. 2005, 22, 211–219. [Google Scholar] [CrossRef]
- Bansal, N.; Tewari, R.; Soni, R.; Soni, S.K. Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manag. 2012, 32, 1341–1346. [Google Scholar] [CrossRef]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Xin, F.; Geng, A. Horticultural waste as the substrate for cellulase and hemicellulase production by Trichoderma reesei under solid-state fermentation. Appl. Biochem. Biotechnol. 2010, 162, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, C.; Pensupa, N.; Lin, C.S.K. Optimisation of fungal cellulase production from textile waste using experimental design. Process. Saf. Environ. Prot. 2018, 118, 133–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wang, Y. Recent progress in cellulose-based electrospun nanofibers as multifunctional materials. Nanoscale Adv. 2021, 3, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Nayak, R. Review of Literature: Melt Electrospinning. In Polypropylene Nanofibers: Melt Electrospinning versus Meltblowing; Nayak, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–39. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Balogh, A.; Horváthová, T.; Fülöp, Z.; Loftsson, T.; Harasztos, A.H.; Marosi, G.; Nagy, Z.K. Electroblowing and electrospinning of fibrous diclofenac sodium-cyclodextrin complex-based reconstitution injection. J. Drug. Deliv. Sci. Technol. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Kerwald, J.; de Moura Junior, C.F.; Freitas, E.D.; de Moraes Segundo, J.D.D.P.; Vieira, R.S.; Beppu, M.M. Cellulose-based electrospun nanofibers: A review. Cellulose 2021, 29, 25–54. [Google Scholar] [CrossRef]
- Clemons, C. Nanocellulose in Spun Continuous Fibers: A Review and Future Outlook. J. Renew. Mater. 2016, 4, 327–339. [Google Scholar] [CrossRef]
- De Silva, R.; Vongsanga, K.; Wang, X.; Byrne, N. Understanding key wet spinning parameters in an ionic liquid spun regenerated cellulosic fibre. Cellulose 2016, 23, 2741–2751. [Google Scholar] [CrossRef]
- LeBlanc, R. Automated Textile Sorting for Recycling. Available online: https://www.liveabout.com/ (accessed on 29 January 2020).
- Nikolakopoulosa, A.; Barlaa, F.; Kokossisa, A. Design of Circular Economy Plants–The Case of the Textile Recycling Plant. In Proceedings of the 27th European Symposium on Computer Aided Process Engineering, Barcelona, Spain, 1–5 October 2017. [Google Scholar]


| Material Generation | Process | Solid Waste Generation | Composition of Waste Generated | Existing Methods of Recycling Material | Further Applications |
|---|---|---|---|---|---|
| Cotton fibers | Ginning | Fiber waste | Cellulose, protein, sugar, wax | Mechanical recycling | Textile products |
| Yarn preparation | Spinning | Fiber lint, yarn wastes | Cellulose, protein, sugar | Re-spinning and plying | Carpet and clothes |
| Yarn preparation for fabrics | Weaving preparation (warping, sizing) | Fibers, yarn wastes | Cellulose, protein, starch, sugar | Re-spinning and plying | Carpet and clothes |
| Fabrics | Weaving/knitting | Yarn, fabrics | Cellulose, starch, sugar | Mechanical and chemical recycling | New fabrics |
| Fabric pretreatment | Desizing | Fiber lint, yarn | Cellulose | Mechanical and chemical recycling | New fabrics |
| Fabric pretreatment | Scouring and bleaching | Few or very few wastes | - | Mechanical and chemical recycling | New fabrics |
| Dyed and printed fabrics | Dyeing and printing | Few or very few wastes | - | Mechanical and chemical recycling | New fabrics |
| Finishing | Fabric finishing | Torn fabrics | Cellulose | ||
| After use | Fabrics | Cellulose |
| Name of Strain | Optimum Moisture Condition (%) | Maximum Cellulase Activity (FPU/g) |
|---|---|---|
| A. niger CKB | 70–75 | 0.43 |
| A. niger N402 | 85 | 0.42 |
| T. reesei | 85 | 0.11 |
| R. variabilis | 65 | 0.20 |
| A. oryzae | 65–75 | 0.19 |
| T. longibrachiatum | 85 | 0.11 |
| Electrospinning Parameters | Effects on Fiber Morphology |
|---|---|
| Increased polymer concentration, i.e., increasing viscosity | Formation of longer fibers with increased fiber diameter |
| Increased conductivity | Formation of beaded fibers with decreased fiber diameter |
| Increased surface tension | Formation of beaded fibers |
| Increased flow rate | Formation of continuous fibers with decreased fiber diameter |
| Increased voltage | Formation of beaded fibers with increased fiber diameter |
| Increased distance between the spinneret and the collector | Decreased fiber diameter |
| Increased humidity | Overall decreased fiber diameter and caused uneven diameter distribution |
| Temperature | Had no direct effect on fiber morphology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suen, D.W.-S.; Chan, E.M.-H.; Lau, Y.-Y.; Lee, R.H.-P.; Tsang, P.W.-K.; Ouyang, S.; Tsang, C.-W. Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning. Sustainability 2023, 15, 11638. https://doi.org/10.3390/su151511638
Suen DW-S, Chan EM-H, Lau Y-Y, Lee RH-P, Tsang PW-K, Ouyang S, Tsang C-W. Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning. Sustainability. 2023; 15(15):11638. https://doi.org/10.3390/su151511638
Chicago/Turabian StyleSuen, Dawson Wai-Shun, Eve Man-Hin Chan, Yui-Yip Lau, Rachel Hiu-Pui Lee, Paul Wai-Kei Tsang, Shaobo Ouyang, and Chi-Wing Tsang. 2023. "Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning" Sustainability 15, no. 15: 11638. https://doi.org/10.3390/su151511638
APA StyleSuen, D. W.-S., Chan, E. M.-H., Lau, Y.-Y., Lee, R. H.-P., Tsang, P. W.-K., Ouyang, S., & Tsang, C.-W. (2023). Sustainable Textile Raw Materials: Review on Bioprocessing of Textile Waste via Electrospinning. Sustainability, 15(15), 11638. https://doi.org/10.3390/su151511638