Abstract
Synthetic chemical surfactants (SCSs) are a versatile group of amphiphilic chemical compounds synthesized from fossil fuel precursors which have found use in various industrial applications. Their global usage is estimated to be over 15 million tons annually, which has resulted in unabated environmental damage and potential toxicological effects to humans and other organisms. Current societal challenges to ensure environmental protection and reduce reliance on finite resources have led to an increased demand for sustainable and environmentally friendly alternatives, such as biosurfactants, to replace these toxic pollutants. Biosurfactants are biodegradable, non-toxic, and generally environmentally compatible amphiphilic compounds. Although there is enormous potential for microbial biosurfactants to replace SCSs, the key challenge limiting their commercialization relates to their low yields and substantial cost for production compared to that for the SCSs. In this review, we discuss the release of SCSs, with wastewater treatment plants (WWTPs) as the major point source of their release into the ocean, and we then delve into the consequences of these pollutants on marine organisms and humans. We then explore microbial biosurfactants as a replacement for SCSs, with a focus on rhamnolipids, and end with some perspectives on current and future work for commercializing microbial biosurfactants.
1. Introduction
Synthetic chemical surfactants (SCSs) are a broad group of chemical compounds that are widely used in the pulp and paper industry, in oil recovery, as antimicrobial agents for fruit and vegetable preservation, in the manufacture of paints, pesticides, fungicides and herbicides, in the treatment and dyeing of fabrics and leather, as additives in lubricating oils, cosmetics and personal hygiene products, as well as in domestic and industrial cleaning products, amongst other applications [1,2,3,4]. According to a report by Allied Market Research [5], the surfactant market in 2019 was estimated at USD 41 billion and the expected Compound Annual Growth Rate (CAGR) was 5.3% from 2020 to 2027, reaching USD 58.5 billion. The report also states that the group of anionic surfactants are the market leaders, with its main representative being linear dodecylbenzene sulfonates (LAS), followed by non-ionic surfactants whose main representatives are nonylphenol ethoxylates (NPEO) and linear alcohol ethoxylates (LAE). The entry of SCSs into the environment occurs from a range of sources, the most important of which are wastewater treatment plants (WWTP) [6,7] where these chemicals, depending on their susceptibility to microbial degradation, may be partially degraded into by-products [8]. Global surfactant usage is estimated to be over 15 million tons annually. Since numerous consumer products are marketed as down-the-drain disposable, most end up in WWTPs [9]. However, WWTPs do not effectively remove SCSs, mainly because these compounds and their by-products are not readily agglomerated and are subsequently sedimented out into the sludge fraction. Whilst LAS are mostly removed in WWTPs via effective microbial biodegradation processes, NPEOs are less biodegradable. NPEOs themselves show little toxicity, but their byproducts, mainly nonyl and octyl phenols, are toxic and can be readily absorbed by suspended soils [10]. This results in the unmitigated release of large quantities of SCSs from WWTP discharge points where these compounds and their by-products find their way into rivers and coastal areas and spread further out to sea [11,12,13,14]. These SCSs are potentially some of the most concerning pollutants that are released into the environment from WWTPs, which will be discussed in this review together with their partial degradation by-products. Since many types of SCSs are toxic and can remain in the environment undegraded for many years, their impact on marine ecosystems remains largely unknown. There is currently no effective technology available that can be implemented into a WWTP to remove SCSs, and as such this pollution problem continues unabated.
Growing public awareness about marine pollution and the risks it can pose to humans and other life has led to increased interest toward the use of naturally derived, biological surfactants (i.e., biosurfactants) which are commonly associated with low toxicity, high biodegradability, better environmental compatibility, and may be sustainably sourced compared to SCSs which are produced via organo-chemical synthesis in a laboratory or industrial chemical plant [15]. Despite the advantages of biosurfactants over SCSs, their commercialization for use in consumer products has been limited, mainly due to their cost of production and the relatively low yields produced in comparison to the production of SCSs. The most extensively studied biosurfactant for biotechnological applications are rhamnolipids (RL), due to their physiochemical properties and potential to reach high fermentation titers [16], but their entry into the market is still constrained by low yields and the fact they are derived from pathogenic bacterial species.
In this review, we discuss WWTPs as a major point source for the release of SCSs into the ocean, as well as some of the most important types of SCSs based on the risks they pose to the environment and to humans. We explore biosurfactants as replacements for SCSs as a way to help mitigate the risks, with a focus on RLs for applications in marine-safe personal care products, and also discuss the pathways of current and future work for increasing the uptake of this important biosurfactant class.
2. Priority SCSs of Concern and Pathways of Entry to the Ocean
The pathway for entry of SCSs into the environment is generally the same almost anywhere in the world, regardless of climate, geographical location, or social and cultural habits. The process begins with the industrial manufacture of the SCSs, followed by their use and then discarding them by some form or another (commonly down roadside or household drains). These pollutants find their way to a WWTP or, if such infrastructure is not available, into streams and rivers [17,18]. Whilst a fraction of SCSs and their by-products end their journey on land, the final destination for the bulk of these chemicals is the ocean (Figure 1), mainly from the effluents of WWTPs [19,20,21,22]. Direct discharge of SCSs into the environment is also possible, for example, as in the case of pesticide application to agricultural land, and the spraying of synthetic chemical dispersants on the sea surface to combat oil spillage.
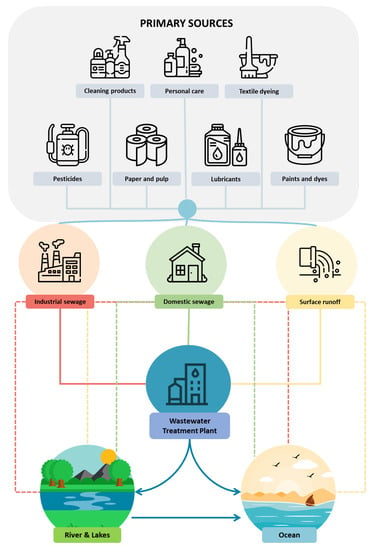
Figure 1.
Pathways for entry of synthetic chemical surfactants (SCSs) into the oceans. Solid lines represent treated discharge through a WWTP; dotted lined represent direct discharge of untreated source SCSs into the environment.
2.1. LAS, NPEO, and Their By-Products in WWTPs
The first widely commercialized class of SCS was linear alkylbenzene sulfonates (LAS) (Figure 2), which has increased in use over the years [23]. Due to extensive use for many years, there is concern regarding its long-term presence in the environment and its toxic effects to a range of organisms. LAS are a class of anionic surfactants consisting of a hydrophilic sulfonate head-group and a hydrophobic alkylbenzene tail. First introduced in the 1930s in the form of branched alkylbenzene sulfonates (BAS), LAS is one of the oldest and most widely used SCSs. It is used in numerous consumer goods that include personal care products (e.g., toothpaste, shampoos, soaps) and household care products (e.g., dishwashing liquids, laundry detergents, spray cleaners) [24]. Following environmental concerns, BAS were replaced with LAS during the 1960s [25], and since then production of LAS has increased significantly, from approximately 1 million tons in 1980 to 3.5 million tons in 2016, making them the most produced anionic surfactant after soaps.
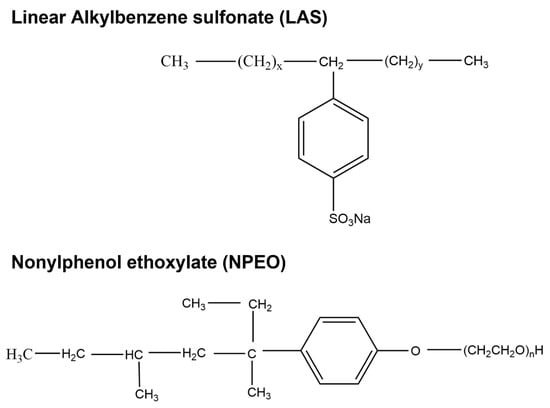
Figure 2.
Chemical structure of Linear Alkylbenzene sulfonate (LAS) and Nonylphenol ethoxylate (NPEO).
Since the beginning of the commercial introduction of synthetic surfactants, they have since been detected in the environment at increasing concentrations. Decades following its commercial introduction, several researchers reported the occurrence of LAS in different environmental matrices. For instance, McAvoy et al. [26] monitored fifty sewage treatment plants in the USA and found that the average concentration of influent LAS was 5 mg L−1, with an average removal of 77% to 99%. Tabor and Barber [27] evaluated the occurrence of LAS in the Mississippi River (USA), which is the largest river in North America and its basin covers 41% of the US territory; the concentration of LAS along the river ranged from 0.1 to 28.2 μg L−1. In a study by González-Mazo et al. [28], the behavior of LAS was evaluated in a coastal zone of the Bay of Cádiz (southwest Spain) under the effect of strong tidal currents and directly receiving untreated wastewater discharge from a population of approximately 100,000 inhabitants, and the occurrence of dissolved LAS was reported to range from 2.7 to 1687.2 μg L−1, while the LAS adsorbed to suspended solids ranged from 14.4 to 5941.0 μg L−1. Sakai et al. [29] reported the occurrence of LAS in two rivers in Malaysia, with concentrations ranging from 3.3 to 2406.8 μg L−1 in the Langat River, and from 1.9 to 1044.8 μg L−1 on the Selangor River.
Another important group of SCSs that are prevalently in WWTP effluent discharge and of concern are the nonylphenol ethoxylates (NPEO) (Figure 2). These are commercially important, non-ionic surfactants which are used in detergents, paints, pesticides, personal care products, plastics, and a variety of other products and applications. The European Union prohibits their use due to their effects on human health and to the environment [30,31]. The main issue regarding this group of SCSs is that during their biodegradation, some recalcitrant by-products are generated, such as 4-nonylphenol (4-NP) and 4-octylphenol (4-OP), which can act as endocrine disrupters [32,33,34]. The occurrence of 4-NP has been reported in tap water [35,36] and across various environmental matrices, such as in rain and snow [37], and in waters and sediments of rivers [38,39,40,41], lakes [42], estuaries [43,44], and marine environments [45]. Concentrations differ depending on the discharge source and location. 4-NP has been detected in rivers at 0.015–2.25 μg L−1 [46] to 15 μg L−1 [19], whereas in river sediments concentrations can be higher, reaching 5100 mg kg−1 [47]. In 2021, Lalonde and Garron [48] assessed the freshwater environment at 35 sites in Canada and reported concentrations of 4-NP, nonylphenol monoethoxylate (NP1EO), nonylphenol diethoxylate (NP2EO), and octylphenols ranging from 1.29 to 477.22 ng L−1. Based on the dominant activities present upstream in their watersheds, the sampling sites were categorized into the following groups: mixed use, municipal wastewater treatment plant (MWWTP)-associated, textile mill, urban, and reference sites. The study found that 4-NP and 4-OP activities were detected more frequently in sites associated with urban and effluent launch from WWTP.
Surface runoff can also carry SCSs (and other pollutants) from urban areas, due to the use of these compounds in a diverse range of products for use outdoors, such as lubricants used for motor vehicles [49]. In 2021, Zhao et al. [50] investigated the occurrence of 4-NP and 4-OP in Pearl River in rainfall runoff, and the maximum 4-NP concentrations found were 14.5 μg L−1 in surface water and 3.1 μg g−1 in sediments. According to their study, the mass loads from runoff rainfall were 3–62 times higher than those from WWTP effluents, suggesting runoff from rainfall is an important source of SCSs into receiving waters. In a study by Salgueiro-González et al. [39], the concentrations of 4-NP and 4-OP were measured in water and sediment collected along the Minho River estuary in Portugal. It was reported that these NPEO degradation by-products were present in almost all samples and was directly related to the release of treated effluent into the river from a WWTP. The concentrations of 4-NP and 4-OP ranged, from 0.05 μg L−1 to 0.888 μg L−1 in liquid samples, and from 13 ng g−1 to 4536 ng g−1 dry weight in sediment samples.
Compared to anionic and non-ionic surfactants, cationic surfactants represent a smaller class of surfactants. It is worth noting that cationic surfactants such as quaternary ammonium compounds (QACs) have been identified as emerging contaminants in sewage sludge and estuarine sediments with values above 100 µg g−1 [51]. Although cationic surfactants are not widely used, their higher sorption capacity and poor anaerobic biodegradation are the reasons for such high concentrations in sewage-impacted estuarine sediments [52]. Cationic surfactants are used in personal care products such as hair conditioners, as quaternary ammoniums are positively charged which attracts to negatively charged hair providing a very effective binding to allow absorption of conditioning compounds to act [53]. Since such products are disposed down the drain, cationic surfactants can accumulate and become persistent in the environment considering the increased consumer use of such products. More research is necessary to monitor the accumulation of cationic surfactants compared to anionic and non-ionic surfactants since their levels are rising [51].
2.2. Fate of SCSs through WWTPs
Due to the widespread and extensive use of SCSs in many products and their applications worldwide, their presence in sewage effluent is omnipresent. As such, assessing pollutant loads in effluent treatment processes needs to account for the presence of these chemicals. The aim of a WWTP is to significantly reduce the quantity of carbonaceous (organic; predominantly determined as biological oxygen demand (BOD)) materials, as well as pathogens and, where sensitive waters are involved, nitrogen (N) and phosphorus (P) compounds prior to being discharged into receiving systems [54,55]. If these materials are not removed, this will result in their release in large quantities into the environment which can have deleterious effects on dissolved oxygen concentrations, as well as the trophic state and ultimately the well-being of the flora and fauna in receiving waters [56]. There are technologies capable of effectively eliminating these compounds, but this requires high investment, increased operating costs, and specialized staff. The most promising technologies include advanced oxidative processes (AOP), ozonation associated with hydrogen peroxide, photocatalysis, micellar enhanced ultra-filtration (MEUF), as well as more current methods such as adsorption on activated carbon, nanofiltration in membranes, reverse osmosis, chlorination, and reactors with UV lamps [3]. Implementation of such technologies occurs at a much slower rate compared to the increasing industrial manufacture, commercial and consumer use of SCSs.
A WWTP works as a “factory” that, by established microbial consortia, executes a diversity of metabolic pathways that direct energy from organic matter to a central metabolic pathway, ending up in products such as gases (methane and carbon dioxide) and new biomass. An in-depth description on the workings of a WWTP is outside the scope of this review, so the reader is referred to other works that focus on this topic (e.g., [57]). In this WWTP “factory”, the consortia of microbial “employees” prioritize the use of simpler and easily metabolizable substrates over more complex ones, such as SCSs, that metabolically require more energy to break them down by biological systems, as can be seen principally in microorganisms. For example, with respect to LAS, the most widely used SCS, its biodegradation begins with the hydroxylation of the alkyl chain attached to the aromatic ring [58] with successive β-oxidations, followed by desulfonation, and finally the cleavage of the ring [59]. This process is dependent on oxygen which, under anaerobic conditions, is generated from water molecules via dioxygenases which are subsequently added to the alkyl chain via hydroxylases. As an alternative to this route, Lara-Martín et al. [60] reported that the degradation of alkylphenols can be initiated by the addition of fumarate to the alkyl chain instead of via hydroxylation. The following steps proceed via the conventional β-oxidations leading to the formation of benzoyl-CoA and cleavage of the aromatic ring. These degradation steps are specific for each type of SCS chemical and may vary according to the type of WWTP since each treatment facility will vary in microbial consortia, as well as geochemical and abiotic conditions. Due to these reasons, it is difficult to remove SCSs in conventional WWTP, and the majority of these chemicals end up passing through wastewater facilities and are released into receiving waters.
The average influent and effluent LAS concentrations going through WWTPs have been monitored in different countries, as summarized in Figure 3. Whilst the majority of LAS is removed in WWTPs, a proportion of effluent LAS passes through WWTP and ultimately enters the ocean. Considering the scale of products containing LAS, and although concentrations of effluent LAS appear to be minimal, the volume of effluent LAS released into the oceans each year around the world is staggering. Considering its very slow rate of biodegradation under environmental conditions, its environmental accumulation will be expected to result in deleterious consequences to marine life. It is worth bearing in mind that the figures are approximate values since treatment types vary from one WWTP to another, due to different methods and country legislations. These values are likely to be a great underestimate in comparison to the actual amounts, emphasizing the greater concern of SCS effluents into the ocean and the consequences and ramifications associated with this. In effect, it is largely not possible to remove SCSs using conventional WWT systems to curb their release into the environment. Additional infrastructure is required to be implemented to a plant in order to more effectively capture these chemicals, either by physically separating them out of the wastewater, or chemically or biologically breaking them down into innocuous end products before they are released into effluent streams. However, this increases the operational complexity and cost of the treatment process, which is a major impediment toward this end. To circumvent this problem, one way is to address the problem at the source. For example, recent years have seen manufacturers of SCSs investing into less harmful (i.e., biosurfactants) replacements for these chemicals. Consumer awareness about the impacts of SCSs (and other anthropogenic pollutants) to the environment and to human health has spurred manufacturers to invest in biosurfactants (discussed below).
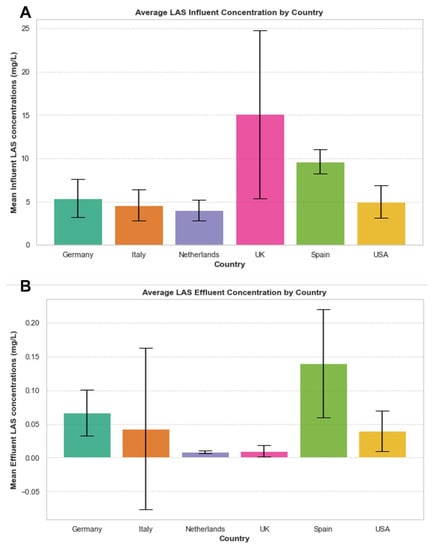
Figure 3.
Mean influent (A) and effluent (B) values of LAS concentration (mg/L) in WWTPs across different countries (Germany, Italy, The Netherlands, UK, Spain, and USA). Error bars represent standard deviation. Values are taken from Feijtel et al., 1996 [61] and McAvoy et al., 1993 [26]. Values are approximate and will vary from one WWTP to another as well as country. Negative standard deviation error bar in (B) Italy is likely due to a lack of data in day one of the monitoring study due to major plant disturbance from data obtained by Di Corcia et al., 1994 [62].
3. Environmental Risks of SCSs on Aquatic Organisms and Humans
SCSs and their degradation by-products have been shown to exhibit toxicological effects on humans and upon the full trophic range of aquatic organisms. Once in the environment, these chemicals can cause deleterious consequences to living cells, such as impairing cell membrane function [63]. Table 1 presents some of the reported effects of LAS, which is the most widely used and arguably the most concerning SCS, and 4-NP which is the most common SCS degradation by-product detected in WWTPs and released into receiving waters. An important concern relates to WWTPs located upstream of aquaculture farms, considering the ease at which by-products of some types of SCSs, like 4-NP, become transported through the trophic chain and may end up on a plate for human consumption.

Table 1.
Effects of LAS and 4-NP on aquatic species.
LAS has been shown to directly affect biological activity by binding with enzymes, proteins, and phospholipids, altering their function and, consequently, triggering undesirable biochemical reactions and promoting metabolic imbalance [79]. Kusk and Petersen [64] evaluated the acute toxicity of LAS for the crustacean species Acartia tonsa (during 48 h and 18% salinity) and verified a mortality rate of 50% (LC50) at a LAS concentration of 2.1 mg L−1. The authors also found that a concentration of 0.54 mg L−1 caused a 50% larval mortality rate and a reduced development rate. Hofer et al. [65] studied the chronic effects of LAS in rainbow trout (Oncorhynchus mykiss) at a concentration of 0.2 mg L−1 over 54 days and observed hypertrophy of the lamellar gill epithelium and reduction of swimming ability.
As mentioned before, the degradation by-products of SCSs can pose a greater risk to the environment as they are more toxic than their respective precursor parent compound. This has been reported in the cases of 4-NP and 4-OP [69,70,71,75,80], which are generated from the degradation of the surfactant NPEO [7,30,47,81,82,83,84]. NPEO is a non-ionic surfactant widely used in the production of detergents, lubricants, antistatic agents, high-performance textile cleaning agents, pesticides, antioxidants for rubber production, and lubricating oil additives [85]. The alkylphenols are a group of SCSs with an enormous diversity of compounds, each with distinct characteristics [86,87]. Their degradation produces by-products that are more harmful than their respective parent compound [88], and have a high capacity for bioaccumulation [42,43,89], allowing them to be easily transported through the trophic chain and to potentially end up in food for human consumption. NPEO has nine carbons attached to the aromatic ring in the para position in relation to the n-ethoxylated group. When these NPOEs reach a WWTP, they may be partially degraded by microorganisms, forming 4-NP and 4-OP. In addition, genotoxic effects have been reported in test organisms like fish [34,42,69,70,71], birds [90], plants [46,91,92], rats [93,94,95,96], and human cells [33,97,98,99]. The diffusion of these compounds in the environment is so high that levels of these by-products have also been detected in umbilical cord blood samples [99] and urine [100,101], and they have been associated with increased childhood obesity and early puberty.
Concerns about NPEO emerged when Soto et al. [102] observed that 4-NF, generated from the degradation of NPEO, was able to mimic estrogen and act as an endocrine disruptor, inducing the proliferation of mammary tumor cells. This motivated several other research groups to investigate the xenobiotic effects of 4-NP, leading several research groups to confirm the ability of this degradation by-product to mimic the female hormone 17β-estradiol [30,47,88,97,103,104]. Furthermore, it was observed that the recalcitrance of 4-NP is greater with the decrease in the number of ethoxylated groups [30,83,105]. 17β-estradiol is a natural hormone that influences the development and maintenance of female sex characteristics [106]. Because of this, 4-NP is expected to trigger a variety of reactions in exposed organisms. 4-NP can bind to the same receptors as the hormone 17β-estradiol, generating competition for binding to the estrogen receptor due to similarities in its chemical structure [30]. The same functional regions for protein synthesis are activated by both compounds. However, different biological responses are provoked, such as reduction of hemoglobin and blood erythrocytes [70], oocyte atrophy [72], increased frequency of reproductive stages [73], testicular damage and reduced sperm density [76], and apoptosis and DNA damage [69,77]. Some of the effects of 4-NP on aquatic organisms are presented in Table 1.
Shirdel et al. [71] evaluated the effects of 4-NP (1–100 µg L−1) on the levels of plasma reproductive hormones and liver antioxidant enzymes, as well as the histopathology of reproductive and non-reproductive organs of brown trout (Salmo trutta Caspius). After 21 days of exposure, the authors found an increase in the concentration of estradiol in the plasma of both male and female organisms and a reduction in antioxidant enzymes in the liver. Also observed were histopathological lesions in liver and kidney tissues, such as congestion, cytoplasmic degeneration, hypertrophy, necrosis, nuclear degeneration, pyknosis, and vacuolar degeneration. In male organisms, different levels of hyperemia in the testes, a decrease in seminiferous lobe thickness, and spermatogonial degeneration were observed.
The effects of SCS by-products in humans (in vivo) and on human cell lines have been reported and have highlighted some concerning effects that included decreased secretion of oxytocin [97], increased proliferation of aberrant cells [33], childhood obesity [100], and increase in inflammatory processes [107] (Table 2). Bechi et al. [97] studied the effects of SCS by-products in human placental cells and observed that, even at low concentrations (0.022–220 ng L−1 of 4-NP), there was a reduction in oxytocin secretion, which inferred the potential of these chemicals to lead to pregnancy interruption and other complications. Forte et al. [33] analyzed the effects of NPEO on lymph node carcinoma of the prostate (LNCaP) cell line and found that it induced proliferation of LNCaP and increased the expression of estrogen receptor α (ERα) and its translocation from the cytoplasm to the nucleus. Moreover, the authors found NPEO also resulted in up-regulation of key target genes involved in the cell cycle and inflammation processes.

Table 2.
Effects of 4-Nonylphenol (4-NP) and 4-Nonylphenol (4-NP) on human and in vitro human cells.
Consumption of SCSs by humans is a common occurrence, especially in societies in which plastic containers and packaging are usually used for storage of food and drink, and is related to certain symptoms, diseases, and defects in adults, children, and unborn babies. SCSs are used in the synthesis of polymers for the manufacture of plastic containers and packaging and, as such, they can be released into food and water and in turn ingested by humans. Loyo-Rosales et al. [108] evaluated the migration of NPEOs in plastic bottles used to store drinking water. The authors observed concentrations ranging from 180 to 300 ng L−1 in drinking water stored in plastic containers made of HDPE (high-density polyethylene), PET (polyethylene terephthalate), and PVC (polyvinyl chloride) bottles purchased at a supermarket. In a study by Chen et al. [99], the authors reported 1.82 to 211 ng of 4-NP g−1 plasma in cord blood samples from 174 human fetuses, with the highest concentrations detected in samples from pregnant women residing in metropolitan areas. The authors posited that through repeated consumption of food contained in plastic containers, fetuses of pregnant women are more likely to encounter high exposure levels to SCSs due to transplacental absorption, and the accumulation of these chemicals due to inefficient detoxification mechanisms in the fetus. In another study, Choi et al. [100] studied the association between obesity in young girls and several endocrine-disrupting chemicals (EDCs), including 4-NP. The concentration of 4-NP (and other EDCs tested) was lower in serum and urine in the control group compared to that in the obese group, and the results showed a statistically significant relationship to childhood obesity. Similarly, Hou et al. [101] investigated the exposure effects of NP on obesity and pubertal maturity to compare the body sizes of general adolescents in Taiwan. Urine samples were analyzed from 270 children aged 6 to 15 years old, and the study showed that 4-NP exposure was positively correlated with abdominal obesity, including skinfold thickness, waist circumference, waist-to-height and waist-to-hip ratios, and indicated a dose–response relationship. The pathway of SCSs affecting human and aquatic organisms has been summarized in Figure 4.
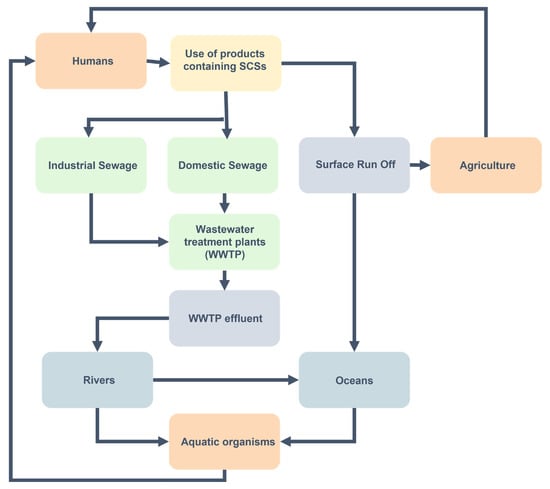
Figure 4.
Flowchart showing the pathway of SCSs to the oceans, humans, and aquatic organisms. Orange represents sources; yellow represents the use of products containing SCSs as the source activity, green represents wastewater containing SCSs and treatment of wastewater, blue represents water bodies where SCSs are present.
Consumer products, including personal care products like shampoo, are important sources of human exposure to SCSs and harmful chemicals including volatile organic chemicals (VOCs). Diethanolamine (DEA) is widely used to precipitate diethanolamides and diethanolamine salts of long-chain fatty acids used in soaps and surfactants in various products including shampoos, conditioners, and cosmetics. This chemical is banned in the EU and Canada in cosmetics due to its ability to react with other ingredients in products to form carcinogenic chemicals [109]. In the study by Knox et al., 2023 [109], it was found that many consumer products sold in California contained chemicals associated with cancer, reproductive harm, and developmental harm. Considering the extortionate usage of personal care products, these chemicals will accumulate individually as well as in combination which could be very harmful. These findings emphasize the need for safer products for consumers to ensure humans are not exposed to such risks. Safer products not only have to be safe for humans, but also marine organisms. Not only will suitable replacements need to be safe and non-toxic, but also function as suitable replacements for the purpose of the product, at the same extent if not better. In a study by BIOWAYS assessing public perception of biobased products, 50.2% of respondents consider biobased products to be as good as conventional products and 53.1% were willing to pay more for a biobased product if they have the same functionality and properties of a fossil-fuel-derived product [110]. There is clearly a market for biobased personal care products, but such products will need to be suitable alternatives to conventional ones already being used.
4. The Need for Greater Biosurfactant Uptake by Industry: Toward a Turn in the Tide for Reducing the Entry of SCSs into the Ocean
As noted earlier, the volume of SCSs entering the marine environment annually is enormous, and their toxic effects to aquatic organisms (including terrestrial model organisms) has been extensively researched and documented. Since enormous quantities of healthcare products (soaps, shampoos, detergents, cosmetics etc.) are flushed down domestic drainage systems, WWTPs are a significant point source of SCS release to the oceans due to their inability to capture and remove these chemicals [111]. To-date, most of the research to improve the capture and removal of SCSs in WWTPs has focused on installing new infrastructure for the treatment of wastewater. However, this approach is somewhat synonymous with the age-old idiom “treat the symptom, not the disease”. It would be far more effective to begin to address the problem at the root cause, in this case to reduce the quantities of SCSs used in commercial and industrial products by replacing them with innocuous or, at least, less toxic alternatives that do not harm marine life and accumulate in the food chain where they could find a route to human consumption.
There are countless reviews on biosurfactants (also referred to more generally as surface-active agents) which also include their higher-molecular-weight sister group called the bioemulsifiers. As such, we will not dwell or go into any lengthy description about these molecules in this review, except to provide a glance-over on the basic nature of these molecules which will become useful when we discuss a special class of biosurfactants; the RLs.
Briefly, biosurfactants are like SCSs in that they are surface-active and amphipathic compounds, but the difference is that the former are derived from biological sources, principally microorganisms and plants, but also animals [112]. Because of their amphipathic nature, biosurfactants can dissolve in both polar and non-polar solvents, and as such they are able to interface between aqueous and non-aqueous solvents/substances [113]. The effectiveness of these chemicals is determined by their ability to lower the surface tension (ST) of water, or to lower the interfacial tension (IFT) between two immiscible phases (e.g., of a non-polar and polar liquid) [113]. The ST is a measure of the energy (per unit area) that is required to increase the surface area of a liquid by lowering of the intermolecular forces between water molecules. An effective biosurfactant is able to lower the ST of water from 72 to <35 mN/m, whereas the IFT should be lowered from 40 to 1 mN/m in the case of a water and non-aqueous (e.g., n-hexadecane) mixtures [114]. Further to the description on what defines an efficient biosurfactant, it is one that has a low critical micelle concentration (CMC)—the CMC is the minimum concentration required to initiate micelle formation and generally correlates with the ST and IFT [114]. A low CMC means that less biosurfactant is required to reduce the ST or IFT. Biosurfactants are biodegradable, less toxic, and highly specific in comparison to SCSs. Most biosurfactants are thermostable and pH resistant. For example, a psychrophilic strain of Arthrobacter protophormiae produced a biosurfactant that was resistant to temperatures over the range of 30–100 °C and resistant to high sodium chloride concentrations of 100 gL−1 [115]. Biosurfactants are also biodegradable under aerobic, anaerobic, and anoxic conditions. The biodegradation of biosurfactants occurs in two stages: firstly, the hydrocarbon chains are broken causing a structural change and thereby the loss the amphiphilicity of the biosurfactant and the second stage is the conversion of the byproducts in the first stage into water, minerals, and CO2 [116]. Biosurfactants have a higher degradation potential than SCSs and also display the capacity to reduce the risk of their accumulation in the environment [117]. They also display high specificity due to their complex structures and specific functional groups, allowing them to be used in highly specific applications. The combination of these characteristics distinguishes biosurfactants from SCSs, allowing them to have wider applications across multiple industrial processes. Various biosurfactants and their associated applications, advantages, and disadvantages have been summarized in Table 3.

Table 3.
Various microbial biosurfactants and their associated applications, advantages, and challenges.
Owing largely to their enormous genomic diversity, microorganisms are recognized as the most sustainable and commercially promising source for biosurfactants. To-date, bacteria and yeast have been the main focus of this enterprise as many species of these organisms have been shown to produce biosurfactants with different structures, chemistries, and functional qualities for potential commercial application, and indeed a few have reached a commercial endpoint. These compounds are found forming structural components of microbial cells, such as of cell walls, or they are excreted extracellularly out of the cell [131]. With respect to their structure and chemical composition, biosurfactants vary greatly depending on the producing microbial species, and, broadly speaking, they are classified based on their chemical charge (i.e., anionic, non-ionic, or cationic) or molecular weight [132,133,134]. Based on the latter, biosurfactants are classified as either low molecular-weight (LMW) surfactants, which reduce surface tension between two immiscible liquids, or high molecular weight (HMW) emulsifiers, which enable the formation of oil-in-water or water-in-oil emulsions and are also referred to as polymeric surfactants (or bioemulsifiers) that are commonly composed of exopolysaccharides (EPS) with/without protein and lipid.
There is a major market opportunity, societal need, and increasing consumer pull for scientifically proven, eco-compatible products. As a case example, in the personal care market, no products in the USD 29 billion shampoo global market can claim to be safe to marine life based on robust multi-species ecotoxicity testing. Current marketing and product labelling by many industries of their personal care products is flooded with ‘green washing’, with claims such as “reef safe”, but where the evidence is lacking. As the world becomes increasingly conscious of the damage inflicted on marine life by pollutants, including plastics from fishnets, bags, and other sources, liquid pollutants, such as SCSs, have become a focus of significant attention [3,135]. Up to 82,000 personal care ingredients flow into the ocean, even after wastewater treatment—shampoos account for a significant proportion of that outflow by virtue of their frequent use by the world’s population [135,136]. Significant quantities of shampoo-derived chemicals enter the oceans from coastal populations through so-called ‘grey water’ outflows, even after water treatment [3]. Fossil fuel-based surfactants are used most often in shampoos as active ingredients, and have been demonstrated to be damaging to marine life [17,137,138]. Yet, to-date, no commercial retailer or supplier has provided substantiated evidence of the true eco-compatibility of surfactant ingredients used in shampoos, let alone for the other ingredients in shampoo formulations, such as by adopting ecotoxicity testing with different, and relevant, model species.
One of the key challenges for the 21st century is to reduce dependence on finite supplies of fossil fuels (oil, coal, gas) by moving toward the use of renewable and sustainable sources to supply our energy needs and the wide range of materials and fine chemicals that are largely still derived from crude oil and its derivatives. Under current climate change scenarios, even plant and animal sources used for supplying industrial materials and fine chemicals are non-sustainable since they can be seriously affected by political upheavals and meteorological events. As noted earlier, SCSs are synthesized from organo-chemical synthesis using fossil fuel-based precursors [139], which is problematic, not only because they are derived from a non-renewable resource, but also because of their proven or perceived toxicological effects to humans and to the environment [140] compared with their biogenic counterparts, the biosurfactants [141]. Biosurfactants, which are of biological origin, have gained increasing interest in recent years, mainly driven by changing government legislation requiring a shift toward industrial use of renewable and less toxic compounds, and an increasing consumer demand for natural and ‘environmentally-friendly’ ingredients [142]. An important trend in the household products, personal care products, food, and healthcare industries is the adoption of ‘natural’ ingredients that are eco-compatible (i.e., biodegradable and non-toxic to life) and, where possible, with perceived benefits to the wellbeing of consumers.
For commercial exploitation, microorganisms offer a reliable and sustainable alternative for producing biosurfactants compared to surfactants derived via organo-chemical synthesis, or deriving these chemicals from plant or animal sources [143]. In contrast to biosurfactants derived from plant and animal sources, which can be hampered by limited production (e.g., low crop yields), political constraints, and increasing energy and transport costs, biosurfactants produced by microorganisms offer a much more sustainable source. Additionally, microorganisms possess enormous genetic diversity, which offers considerable promise in identifying novel compounds, including biosurfactants. The peer review literature and worldwide patent databases contain a plethora of reports and descriptions of biosurfactants produced by many different species of microorganisms, yet notably less than a handful have ever reached the market. In other words, the uptake of microbial-produced biosurfactants by industry still significantly falls short compared to that for the SCSs, and this is largely due to the economics of producing these chemicals—specifically, upscaling the process for biosurfactant production is rarely economically viable. The price of microbial-produced biosurfactants is approximately USD 20–25 per kg−1, which is 20–30% more expensive in comparison to their synthetic counterparts at USD 1–3 per kg−1 [144]. There are two major reasons for this, the first of which relates to insufficient production, as, quite often, yields from microbial cells fall short of satisfying industrial demand [145]. While it is well established that growth parameters (e.g., pH, temperature, aeration, agitation, O2/CO2 levels, C:N ratios, substrate composition/concentration etc.) affect biosurfactant production during fermentation [146], a major reason preventing their commercialization is still the low final quantities that are produced per liter volume of fermentation broth—for example, in the case of the RLs, often anywhere from mg/L to <2 g/L of fermentation medium, even under optimized fermentation conditions [147].
The other major reason is that in many cases, the biosurfactant-producing organisms are pathogenic, or they can become pathogenic under certain conditions, which prevents their usage in a range of applications. For example, high water content cosmetic products, such as shampoos, shower gels, creams, and dental products, are susceptible to microbial contamination of pathogenic microorganisms, including P. aeruginosa and Burkholderia species, which in turn pose potential harm to the user [148]. Recently in the UK, a batch of Vernacare personal cleaning products were recalled due to P. aeruginosa contamination and resulted in a stoppage of their production until the issue had been resolved [149]. With these concerns regarding natural microbial contamination of pathogenic strains in cosmetics products, there is more incentive to not implement the use of pathogenic biosurfactant-producing organisms in commercial products destined for human use or consumption. Attempts also to modify genetic/physiological processes, such as quorum sensing to upregulate biosurfactant production of RLs [150], have to a point been successful, but have not been able to yield the quantities required to supply the market to replace fossil fuel-based surfactants, or biosurfactants sourced from non-sustainable sources. It is important to note that microbial biosurfactants are and will remain more expensive than conventional fossil fuel-derived SCSs for the foreseeable future. Currently, their use can therefore only be justified on the basis of added functionality, synergistic interaction with other ingredients, or PR value. All easily produced microbial biosurfactants are commercialized (Table 4). Hence, the most important limitation in getting a microbial biosurfactant commercialized has been the economics associated with their production.

Table 4.
Microbial biosurfactant-producing companies and their applications.
More so than environments on terra firma, including freshwater, the marine environment remains a highly promising source for the discovery of biosurfactants and other novel natural products, particularly from extreme habitats, such as hydrothermal vents, polar regions, and the deep sea [151]. Due to its vastness (>70% of the Earth’s surface area), variable depth (<1 m down to ca. 10 km), and physical and chemical conditions, scientists and explorers across the many fields in oceanography still consider the oceans a frontier for discovery. This has much to do with the wider range of abiotic conditions found in marine environments than on land. Genetic diversity is also enormous in the oceans, as microbial cell abundances in seawater are estimated to average 105 cells/mL with an average taxonomic diversity of 1000 species/mL [152]. Oil-degrading microorganisms, in particular, are recognized for producing these types of surface-active compounds, principally as a way to gain access to utilizable hydrocarbons from oil as a source of their carbon and energy. In this way, many species of microorganisms thrive in oil spills, producing biosurfactants (or bioemulsifiers) that reduce the interfacial tension of the oil, and resulting in smaller oil droplet sizes, as well as increasing the solubility of hydrocarbon species in the oil [153,154]. Bioemulsifiers, which are excreted by many species of marine bacteria, are high-molecular-weight macromolecules, commonly exopolysaccharides (EPS) with/without other associated chemical groups, and which have amphiphilic qualities that contribute to many functions in the marine environment [155]. Like biosurfactants, bioemulsifiers can increase the bioavailability of poorly soluble hydrophobic substrates, which in the event of an oil spill will facilitate oil hydrocarbon dispersion, emulsification, and ultimately biodegradation [156]. Marine environments are a particularly rich source for discovering biosurfactant- and bioemulsifier-producing microorganisms (Table 5). Despite this, the rate of interest for exploring the marine environment for microbial biosurfactants had only increased in recent years, as there are considerably far fewer publications on this compared to biosurfactants from terrestrial microbes [154].

Table 5.
Microbial biosurfactant-producing bacteria from marine environments. The high-molecular-weight bioemulsifiers are included since these types of compounds have amphipathic properties that can serve as ingredients in personal care products.
Gene transfer is highly frequent amongst marine microbial communities, and as such these organisms are provisioned with a higher genomic flexibility that allows them to adapt to varying environmental conditions [174]. This high gene transfer frequency could allow unique biochemistry and novel characteristics, ideal for biotechnological applications. For example, biosurfactants from psychrophilic microorganisms have been explored in formulations for washing products since they can work efficiently at lower temperatures [175]. Particularly as consumer goods companies are aiming for products to be energy saving and reduce environmental impact, such characteristics are beneficial to meet such criteria [175]. Marine bacteria are generally mesophilic, which could be advantageous to industrial production since less energy is required and does not require high-temperature production. In addition, it has been noted that biosurfactants produced from mesophiles have high levels of thermo-stability [154], a characteristic that could be advantageous in industrial applications. Marine-derived biosurfactants have also been shown to have antimicrobial, anti-adhesive, and biofilm disrupting properties, and for these reasons could be suitable for cosmetics and personal care products [176]. As such, biosurfactants are considered viable replacements to SCSs, yet the marine environment remains relatively unexplored for microorganisms that produce these molecules.
5. The Rhamnolipids: Speeding Up the Turn in the Tide?
Rhamnolipids (RLs) are glycolipids, one of the major categories of biosurfactants. Global interest in RL production has been on the rise due to their broad range of applications in various industries and their excellent physicochemical properties [16,177]. RLs are low-molecular-weight compounds of relatively simple molecular structure—i.e., they are composed of one or two rhamnose sugars with up to two ß-hydroxy fatty acid tails ranging in chain length (from 8 to 16 carbons). Thus, their synthesis is achieved using either just two or three separate enzymes (RhlA and RhlB, and/or RhlC), as shown in Figure 5. The first two enzymes are encoded by the genes rhlA and rhlB, respectively, and are located on a single operon alongside an AHL-mediated quorum sensing system (rhlR, rhlRI), whereas the third enzyme is encoded by rhlC, and is located ca. 2.5 Mb downstream of the rhl operon. In comparison to other glycolipid surfactants, RLs are unique as they contain a hydrophilic group consisting of either one or two (L)-rhamnose molecules with a glycosidic link to a hydrophobic group made up of one or two β-hydroxy fatty acids. RLs have two forms dependent on the presence of one or two rhamnose molecules (Figure 5) and are known as mono-RLs and di-RLs, respectively [178].
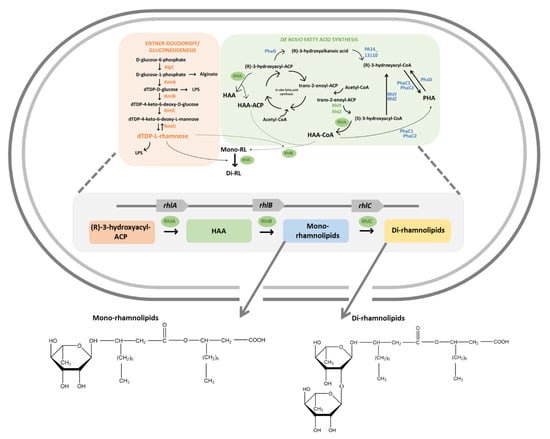
Figure 5.
Pathway for the synthesis of mono-and di-rhamnolipids.
Among the various different types of biosurfactants, RLs stand apart [177]. This is because high yields of production can be achieved after relatively short incubation periods and since the primary producer is P. aeruginosa, it is easy to cultivate and is a well-understood bacterial species [179]. Compared to other biosurfactants (tannins, saponin, lecithin), rhamnolipids showed the best emulsifying properties in distilled water and seawater [180]. They are widely recognized in industry as eco-compatible, ‘green’ replacements of the classic fossil fuel-based surfactants. A major and recent advancement in the commercialization of RLs was the partnership between Evonik and Unilever to build the world’s first industrial-scale production plant for RLs [181]. The partnership dated back several years, when Unilever developed the first household cleaning product, Quix, containing biosurfactants which was distributed in Chile in 2019. The success of Quix led to another pilot release of a biosurfactant-based hand dishwash in Vietnam. Following this, both companies intended to distribute these on a global scale, leading to a large investment between EVONIK and Unilever in the construction of a new million-euro production plant in Slovakia for, largely, RLs production. This demonstrates significant industry-led commitment to these biomolecules by major global players.
Other companies around the world that have also invested into research for producing RLs are TeeGene Biotech (Teeside, UK), AGAE Technologies (Corvallis, OR, USA), Jeneil (Saukville, WI, USA), and Paradigm Biomedical Inc. (New York, NY, USA) Table 4). However, production yields of RLs by these companies is limited to approximately a couple of grams per L of fermentation volume, as the producing strains have been stretched as far as they can be optimized for maximal RL synthesis, and because genetic engineering attempts to obtain overexpressing strains have been unsuccessful. Quite recently, only EVONIK has been successful in cloning the RL synthesis pathway from P. aeruginosa into the overexpression host Escherichia coli.
It also accomplished RL-overexpression using the chassis Pseudomonas putida, but only overexpression of RL-producing genes in the host E. coli resulted in market entry for these compounds. For other companies to source this RL product, this comes with a cost higher than from having this ingredient autonomy via inhouse production. From a company’s financial perspective, vertical integration and supply line control are valuable and preferred over depending on other suppliers.
RLs have found usage in enhanced oil recovery (EOR) [182], agriculture [183], pharmaceuticals and therapeutics [184], detergent compositions, laundry products, shampoos and soaps [185], cosmetics and skin treatments [186], and show low toxicity and antimicrobial activities against both Gram-positive and Gram-negative bacteria [187], and as such show promise for uses in pharmaceuticals and therapeutics [184]. The antimicrobial activities of RLs are also a favorable property as it acts as a preservative, thereby removing the requirement to add additional preservative ingredients to extend the shelf-life of a product. The RL market is expected to reach over USD 145 million by 2026 with a compound annual growth rate of >5% [188].
Until recently, RLs were known to be produced by principally P. aeruginosa, with some strains producing mono- or di-RLs, or both. Historically, RLs were first described just over 70 years ago from P. aeruginosa [189], thereafter becoming the key species in RL research. Despite the vast number of reports in the literature describing RL-producing bacteria [190], it is important to note that some publications misinform on the production of RLs from “non-Pseudomonas” strains, either because the strains were incorrectly identified or their produced biosurfactants were misidentified as RLs [191]. The second major microbial RL-producers comprise species of Burkholderia [192,193,194,195], and a few other members of the class Gammaproteobacteria, mainly species of Pseudoxanthomonas [196], Pantoea [197], and Acinetobacter and Enterobacter [198], and have since been identified as RL producers. In addition to varying, albeit low, quantities of RLs produced by these organisms, their potential as pathogens in humans, animals, or plants has made them unappealing for commercial development in various applications, especially where human/animal contact or ingestion is involved [190,199].
Whilst RL-producing microorganisms are ubiquitous across different environments, generally they have been isolated from terrestrial habitats. In two recent keystone publications, RLs were found to be produced by two new strains of marine bacteria that are not related to pathogenic P. aeruginosa. 16S rRNA gene sequencing identified one of the strains to represent a new species of Pseudomonas (strain MCTG214(3b1)) that is unrelated to pathogenic P. aeruginosa [172]. The strain was found to express RL synthesis genes rhlA and rhlB, although no rhlC homologue was identified. The study also found the strain produced a significantly high level of short-chain di-RLs, the first to have been described in a non-pathogenic marine Pseudomonas species [172]. The other study identified a RL-producing Marinobacter species, and optimization of culturing conditions to optimize RL production found that it could be cultured in cheap and renewable feedstocks [168]. In these studies, RL synthesis by each of the two strains was confirmed by analytical methods (e.g., NMR and GC/MS) and bio-molecular tools (e.g., qPCR) [168,172], and their produced RLs were subsequently shown to be non-cytotoxic or mutagenic [200]. These characteristics present these two strains as excellent candidates for the development of a microbial chassis for the heterologous overexpression of RLs. These RL-producing strains, which were isolated from the marine environment, represent the first non-pathogenic RL-producers, as P. aeruginosa and the other above-named RL-producers are known pathogens or can turn pathogenic under certain conditions [168]. This opens up a huge opportunity to develop the overexpression of these biomolecules for use in applications whose doors have for so long been closed off because the producing organisms, or the native sources of RLs, display pathogenicity. The overexpression of RL production using the genetic pathway encoded by organisms other than pathogenic P. aeruginosa (cf. production of RLs by EVONIK in collaboration with UNILEVER) has not been completed before, thus representing a unique opportunity (discussed in the next section below) to attempt this and which could open new opportunities for using RLs in a much wider field of applications than is currently happening.
6. Genetic Engineering: The Most Promising Road to Market?
Microbial biosurfactants have enormous potential in commercial products, and many published reports proclaim biosurfactants from microorganisms as viable alternatives to replacing fossil fuel-derived SCSs. However, the major setback to their commercialization is the cost competitiveness compared to SCSs which, quite often, the latter are significantly cheaper to produce. All current commercially available microbial biosurfactants—i.e., sophorolipids, mannosylerythritol lipids, RLs, and surfactin—are derived from industrial fermentation based on renewable raw materials. These four biosurfactant types currently occupy only small market niches because their upscaled production, via mainly optimization of the fermentation conditions, has been marginal compared to the non-optimized strain. To obtain higher yields (from mg/L to g/L of biosurfactant per L of fermentation medium), history shows that genetic engineering, such as cloning of the respective biosynthetic pathway in a competent host, is duly necessary to circumvent the limitations that are inherent in the wild-type producing strain. However, the expertise in genetic engineering and funding to support this is commonly a limiting factor to achieving this.
Before delving into the genetic engineering possibilities, we would like to summarize some of the options that have commonly been attempted for upscaling the production of biosurfactants, including that of the RLs, as these options are generally cheaper and systematic for almost any lab around the world to use. The biggest limitation in introducing biosurfactants into consumer products is cost, and this can only be achieved when profit is achieved. In general, studies have determined mg/L yields of biosurfactant, which is extortionately low compared to g/L yields of SCSs [154]. Microbial production of biosurfactants can be increased by optimization of growth conditions, but the cost of raw materials for RL fermentation is estimated to be approximately 50% of the total production costs, and as such this makes it difficult for these and other biosurfactant classes to compete economically with SCSs [144]. Many studies have investigated the use of waste and renewable substrates as a carbon source (e.g., mannitol, glucose, glycerol, n-paraffin, n-alkane, polycyclic aromatic hydrocarbons, or vegetable oils) to reduce the production cost of RLs [201,202,203,204]. The use of such waste materials has other benefits, such as in circularity of economics and waste management. Despite this, not much progress has been made on commercial upscaling of biosurfactant production using waste feedstocks. It is also noteworthy that whilst many published reports on biosurfactants note that the cost of raw or waste feedstocks contribute a significant cost to the overall economics of production, this is possibly still a small fraction compared to the costs of energy required for prolonged periods, as when running a multi-day fermentation.
Another way of reducing total production costs is by addressing the downstream process as this has the largest impact, accounting for approximately 80% of the total production costs [205]. The recovery and purification of RLs usually involves the use of acid precipitation and organic solvent extraction [147,205]—chemicals which, in large volumes, contribute a significant expense from their purchase and also for their appropriate disposal. In addition, the use of such chemicals can result in reduced biosurfactant activity and undesirable product aggregation [206], as may be affected by the biosurfactant molecule’s ionic charge, water solubility, and location of the product [15]. How much processing is involved will of course depend on the intended application of the respective biosurfactant, and as such affect the cost of production [207]. For low-cost applications which do not require a high-purity product, such as in oil recovery, RLs can be recovered directly from fermentation broth, and there is research exploring the use of a cell-free fermentation broth in place of purified RLs for industrial applications [208]. A low-cost, integrated foam fractionation process has also been proposed, resulting in relatively high-purity RLs [209].
Of all avenues to tackle the problem of production costs, genetic engineering is unquestionably the most promising, and history holds testament to this by the fact that most antibiotics, therapeutics, pharmaceuticals, and other compounds are today produced by a recombinant overexpression system. As noted earlier, P. aeruginosa is the most studied RL-producing strain, and as such this species has been the focus for increasing RL yields by genetic engineering. The two main approaches in this respect have involved either the introduction of genes that promote RL production, or the deletion of genes that inhibit RL production. The introduction of the strong promoter of the oprL gene to the RL genes rhlAB increased the copy number of rhlAB genes and the engineered strain produced a higher yield of RLs [210]. Alternatively, it was found that deletion of the cplX and lon genes, which expresses the negative regulators of quorum sensing in P. aeruginosa, was found to increase RL production since RL biosynthesis genes are closely linked to the quorum-sensing mechanism in this organism [211]. Whilst these are promising approaches, as they have led to reported increases in RL yields, there is still the problem relating to the pathogenicity of the strain which can severely limit its application, especially in certain industrial sectors. Yet even with non-pathogenic strains of marine or terrestrial origin, quite often the biosurfactants they produce have not reached a commercial endpoint due to low yields, as well as insufficient structural elucidation and uncharacterized genes [154]. One method of tackling this could be by removing the pathogenic trait of the strain [16]. The synthesis of pyocyanin, a secondary metabolite of P. aeruginosa which interferes with multiple cellular functions, is the cause of its pathogenicity [212] and inhibition of its synthesis is suggested to remove its pathogenic trait. This could be achieved by inhibiting the synthesis of the precursors involved in the expression of phzABCDEFG and phzHMS, which are the genes involved in the synthesis of pyocyanin [213]. More research would be required to assess the effects of removing the pathogenicity of the strain on RL production for the recombinant strain to have potential in industrial applications.
E. coli is one of the most characterized and commonly used chassis in recombinant technology. Undoubtedly, it has played a pivotal role in developing the tools we use today in synthetic biology since E. coli was one of the primary species these tools were built upon [214]. For the recombinant production of RLs, as a microbial chassis E. coli could be suitable since it has been highly characterized and proven to be useful for many industrial biotechnological applications. However, there is an apparent requirement for new chassis to advance metabolic engineering across numerous applications. In recent decades, bacterial species, including P. putida and Bacillus subtilis, have been extensively characterized with many defined genetic toolkits developed for these species for metabolic engineering [215,216]. For example, P. putida KCTC1067 expressing both rhlAB and rhlI was reported to produce 7.3 g of RL per L of fermentation medium [217]. Although, it should be noted that it has been found to be difficult to overproduce RLs in heterologous hosts. This was made evident when comparing the results of one study aiming to express rhlAB to produce mono-RLs and HAAs, which resulted in 0.25 g/L using an E. coli chassis, and 0.6 g/L with Pseudomonas fluorescens [218], to another study where both rhlAB and rhlBDAC were introduced into E. coli, and a yield of 120.6 mg/L was achieved [219]. Since the precursors to RL biosynthesis are derived from central metabolic pathways native to P. aeruginosa, heterologous hosts may not have the essential genes required to synthesize these precursors, and as such it is more difficult to overproduce RLs. Considering these limitations, a highly characterized pseudomonad would be a more suitable option for the heterologous expression of RLs compared to E. coli, and other non-pseudomonad species, since the central metabolic pathways will have more similarity or compatibility. Once a suitable candidate for heterologous expression of RL genes has been identified and with more research optimizing the strain for RL production, it could be possible to develop an industrially viable process for high RL production in the future [220].
7. Conclusions
The abundance of products and widespread applications of SCSs means their presence in sewage effluent is omnipresent. In the Mississippi River alone, concentrations of LAS along the river ranged from 0.1 to 28.2 μg L−1 [27] indicating the volume of SCSs entering the marine environment is extensive and is even likely to be undervalued. SCSs are detrimental to aquatic organisms, human health, and the environment in general, as they can cause complications in pregnancy, obesity, and cancer [97,100,107]. The occurrence of these chemicals in the environment is mainly associated with their incomplete removal at WWTPs, leading to their unmitigated release, including that of their by-products, into the oceans. Proper removal of SCSs at WWTPs is one approach to tackle this problem, but it would be far more effective to replace SCSs with a greener and environmentally friendly alternative(s), particularly with increasing public demand for this considering these pollutants are recognized to inflict detrimental damage to marine life and ultimately find a route to human consumption. As well as the toxicity of SCSs, there is a worldwide push to move away from a dependence on finite resources, including fossil fuels, to produce materials and chemicals like SCSs. Surfactants from biogenic sources (biosurfactants), and in particular the RLs, are seen as promising alternatives to SCSs since they are biodegradable, non-toxic, and can be produced from renewable resources—ideal characteristics toward implementing more eco-conscious products into industrial and domestic usage.
From a production standpoint, microbial biosurfactants are more sustainable than those from other sources. Microorganisms are also more genetically diverse than, for example, higher animals and plants, and different species of microbes have been shown to be excellent sources of novel biosurfactants. Despite this, the uptake of microbial biosurfactants by industry falls significantly short compared to their synthetic chemical counterparts, due mainly to the costs of production and low yields, making them economically uncompetitive compared to SCSs. In order for microbial biosurfactants to reach a commercial endpoint, production costs and yield must be optimized. To achieve this, marine microorganisms have been considered a highly promising source for the discovery of biosurfactants, as well as other types of biomolecules. Whilst there are several methods that are currently being explored to reduce production costs, such as via the use of waste and renewable substrates/feedstocks as a carbon source for the producing organisms, or by optimizing the downstream process, the most promising approach going forward in this respect is considered to be genetic engineering. Various genetic engineering approaches, particularly for microbial RLs production, have been explored in order to improve yield, such as via the deletion of non-competitive genes or the introduction of genes to promote RLs synthesis. However, much more research is needed for achieving the overexpression of RLs to meet commercial demand. The heterologous expression of RL genes derived from marine organisms could be a promising solution to the limitations of microbial biosurfactant production, but a highly characterized synthetic biology chassis will need to be identified for an industrially viable process for high RL production. Advancements in synthetic biology coupled with the growing demand for biosurfactants will undoubtedly see RLs become more commercially viable, perhaps not so far into the future.
Author Contributions
K.C.L.F. and T.G. conceived the idea for writing this review, and together with H.S.D. wrote the paper, with contributions from M.B.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript contains work conducted during a PhD study undertaken as part of the Industrial Biotechnology Innovation Centre (IBioIC) Collaborative Training Partnership (CTP). It is sponsored by a PhD studentship funded by the Biotechnology and Biological Sciences Research Council (BBSRC) awarded to K.C.L.F., and a PhD studentship awarded to H.S.D. by the São Paulo Research Foundation (FAPESP, process 2021/14384-1), whose support is gratefully acknowledged.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank Ian Fotheringham for invaluable advice on microbial overexpression systems and genetic engineering approaches, and Allard Marx for providing invaluable insight on ingredients used, and the products produced, by the personal care industry.
Conflicts of Interest
The authors declare no conflict or competing financial interest.
References
- Chowdhury, S.; Shrivastava, S.; Kakati, A.; Sangwai, J.S. Comprehensive Review on the Role of Surfactants in the Chemical Enhanced Oil Recovery Process. Ind. Eng. Chem. Res. 2022, 61, 21–64. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of Surfactant from Industrial Wastewaters by Coagulation Flocculation Process. Int. J. Environ. Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef]
- Palmer, M.; Hatley, H. The Role of Surfactants in Wastewater Treatment: Impact, Removal and Future Techniques: A Critical Review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of Pearl Necklace-like ZIF-8@chitosan/PVA Nanofiber with Synergistic Effect for Recycling Aqueous Dye Removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef] [PubMed]
- Allied Market Research Surfactants Market—Global Opportunity Analysis and Industry Forecast, 2020–2027. Available online: https://www.alliedmarketresearch.com/surfactant-market (accessed on 20 January 2023).
- González, M.M.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Risk Assessment of Nonylphenol and Nonylphenol Ethoxylates in Sewage Sludge from Different Conventional Treatment Processes. Sci. Total Environ. 2010, 408, 563–570. [Google Scholar] [CrossRef]
- Bina, B.; Mohammadi, F.; Amin, M.M.; Pourzamani, H.R.; Yavari, Z. Determination of 4-Nonylphenol and 4-Tert-Octylphenol Compounds in Various Types of Wastewater and Their Removal Rates in Different Treatment Processes in Nine Wastewater Treatment Plants of Iran. Chin. J. Chem. Eng. 2018, 26, 183–190. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Guan, J.; Li, Y.; Ren, N. Removal of Surfactants Nonylphenol Ethoxylates from Municipal Sewage-Comparison of an A/O Process and Biological Aerated Filters. Chemosphere 2014, 97, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Ying, G.G.; Williams, B.; Kookana, R. Environmental Fate of Alkylphenols and Alkylphenol Ethoxylates—A Review. Environ. Int. 2002, 28, 215–226. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; Combi, T.; Corada-Fernández, C.; González-Mazo, E.; Lara-Martín, P.A. Occurrence and Spatial Distribution of Legacy and Emerging Organic Pollutants in Marine Sediments from the Atlantic Coast (Andalusia, SW Spain). Sci. Total Environ. 2017, 605–606, 980–994. [Google Scholar] [CrossRef]
- Lara-Martín, P.A.; Gómez-Parra, A.; Köchling, T.; Sanz, J.L.; Amils, R.; González-Mazo, E. Anaerobic Degradation of Linear Alkylbenzene Sulfonates in Coastal Marine Sediments. Environ. Sci. Technol. 2007, 41, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Alsalahi, M.A.; Latif, M.T.; Ali, M.M.; Magam, S.M.; Wahid, N.B.A.; Khan, M.F.; Suratman, S. Distribution of Surfactants along the Estuarine Area of Selangor River, Malaysia. Mar. Pollut. Bull. 2014, 80, 344–350. [Google Scholar] [CrossRef]
- Petrovic, M.; Fernández-Alba, A.R.; Borrull, F.; Marce, R.M.; Mazo, E.G.; Barceló, D. Occurrence and Distribution of Nonionic Surfactants, Their Degradation Products, and Linear Alkylbenzene Sulfonates in Coastal Waters and Sediments in Spain. Environ. Toxicol. Chem. 2002, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.D.; Banat, I.M. Microbial Production of Surfactants and Their Commercial Potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Li, Q. Microbial Production of Rhamnolipids: Opportunities, Challenges and Strategies. Microb. Cell Fact. 2017, 16, 137. [Google Scholar] [CrossRef]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in Aquatic and Terrestrial Environment: Occurrence, Behavior, and Treatment Processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Langford, K.H.; Lester, J.N. Fate and Behaviour of Endocrine Disrupters in Wastewater Treatment Processes. In Endocrine Disrupters in Wastewater and Sludge Treatment Processes; Birkett, J.W., Lester, J.N., Eds.; CRC Press: London, UK, 2002; pp. 103–145. ISBN 978-1-56670-601-8. [Google Scholar]
- Petrovic, M.; Solé, M.; López de Alda, M.J.; Barceló, D. Endocrine Disruptors in Sewage Treatment Plants, Receiving River Waters, and Sediments: Integration of Chemical Analysis and Biological Effects on Feral Carp. Environ. Toxicol. Chem. 2002, 21, 2146–2156. [Google Scholar] [CrossRef]
- Corsi, S.R.; Zitomer, D.H.; Field, J.A.; Cancilla, D.A. Nonylphenol Ethoxylates and Other Additives in Aircraft Deicers, Antiicers, and Waters Receiving Airport Runoff. Environ. Sci. Technol. 2003, 37, 4031–4037. [Google Scholar] [CrossRef]
- Corvini, P.F.X.; Schäffer, A.; Schlosser, D. Microbial Degradation of Nonylphenol and Other Alkylphenols—Our Evolving View. Appl. Microbiol. Biotechnol. 2006, 72, 223–243. [Google Scholar] [CrossRef]
- Valbonesi, P.; Profita, M.; Vasumini, I.; Fabbri, E. Contaminants of Emerging Concern in Drinking Water: Quality Assessment by Combining Chemical and Biological Analysis. Sci. Total Environ. 2021, 758, 143624. [Google Scholar] [CrossRef]
- Hallmann, E.; Tomczak-Wandzel, R.; Mędrzycka, K. Fate of LAS Surfactant in WWTPS Based on Measured Concentrations in Wastewater and Sludge. In Proceedings of the Research and Application of New Technologies in Wastewater Treatment and Municipal Solid Waste, Polish-Swedish-Ukrainian Seminar, Krakow, Poland, 17–19 October 2011. [Google Scholar]
- HERA LAS, Linear Alkzlbenzene Sulphonate. Available online: https://www.heraproject.com/files/HERA-LAS%20revised%20April%202013%20Final1.pdf (accessed on 1 March 2023).
- Kocal, J.A.; Vora, B.V.; Imai, T. Production of Linear Alkylbenzenes. Appl. Catal. A Gen. 2001, 221, 295–301. [Google Scholar] [CrossRef]
- McAvoy, D.C.; Eckhoff, W.S.; Rapaport, R.A. Fate of Linear Alkylbenzene Sulfonate in the Environment. Environ. Toxicol. Chem. 1993, 12, 977–987. [Google Scholar] [CrossRef]
- Tabor, C.F.; Barber, L.B. Fate of Linear Alkylbenzene Sulfonate in the Mississippi River. Environ. Sci. Technol. 1996, 30, 161–171. [Google Scholar] [CrossRef]
- González-Mazo, E.; Forja, J.M.; Gómez-Parra, A. Fate and Distribution of Linear Alkylbenzene Sulfonates in the Littoral Environment. Environ. Sci. Technol. 1998, 32, 1636–1641. [Google Scholar] [CrossRef]
- Sakai, N.; Shirasaka, J.; Matsui, Y.; Ramli, M.R.; Yoshida, K.; Ali Mohd, M.; Yoneda, M. Occurrence, Fate and Environmental Risk of Linear Alkylbenzene Sulfonate in the Langat and Selangor River Basins, Malaysia. Chemosphere 2017, 172, 234–241. [Google Scholar] [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the Environment: A Critical Review on Occurrence, Fate, Toxicity and Treatment in Wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef]
- European Union. European Union Directive 2003/53/EC of the European Parliament and of the Council of 18 June 2003; European Union: Brussels, Belgium, 2003; pp. 24–27. [Google Scholar]
- Acir, I.; Guenther, K. Endocrine-Disrupting Metabolites of Alkylphenol Ethoxylates—A Critical Review of Analytical Methods, Environmental Occurrences, Toxicity, and Regulation. Sci. Total Environ. 2018, 635, 1530–1546. [Google Scholar] [CrossRef]
- Forte, M.; Di Lorenzo, M.; Carrizzo, A.; Valiante, S.; Vecchione, C.; Laforgia, V.; De Falco, M. Nonylphenol Effects on Human Prostate Non Tumorigenic Cells. Toxicology 2016, 357–358, 21–32. [Google Scholar] [CrossRef]
- Shirdel, I.; Kalbassi, M.R. Effects of Nonylphenol on Key Hormonal Balances and Histopathology of the Endangered Caspian Brown Trout (Salmo Trutta Caspius). Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2016, 183–184, 28–35. [Google Scholar] [CrossRef]
- Montagner, C.C.; Sodré, F.F.; Acayaba, R.D.; Vidal, C.; Campestrini, I.; Locatelli, M.A.; Pescara, I.C.; Albuquerque, A.F.; Umbuzeiro, G.A.; Jardim, W.F. Ten Years-Snapshot of the Occurrence of Emerging Contaminants in Drinking, Surface and Ground Waters and Wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2019, 30, 614–632. [Google Scholar] [CrossRef]
- Machado, K.C.; Grassi, M.T.; Vidal, C.; Pescara, I.C.; Jardim, W.F.; Fernandes, A.N.; Sodré, F.F.; Almeida, F.V.; Santana, J.S.; Canela, M.C.; et al. A Preliminary Nationwide Survey of the Presence of Emerging Contaminants in Drinking and Source Waters in Brazil. Sci. Total Environ. 2016, 572, 138–146. [Google Scholar] [CrossRef]
- Fries, E.; Püttmann, W. Occurrence of 4-Nonylphenol in Rain and Snow. Atmos. Environ. 2004, 38, 2013–2016. [Google Scholar] [CrossRef]
- Bennie, D.T.; Sullivan, C.A.; Lee, H.B.; Peart, T.E.; Maguire, R.J. Occurrence of Alkylphenols and Alkylphenol Mono- and Diethoxylates in Natural Waters of the Laurentian Great Lakes Basin and the Upper St. Lawrence River. Sci. Total Environ. 1997, 193, 263–275. [Google Scholar] [CrossRef]
- Salgueiro-González, N.; Turnes-Carou, I.; Besada, V.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Occurrence, Distribution and Bioaccumulation of Endocrine Disrupting Compounds in Water, Sediment and Biota Samples from a European River Basin. Sci. Total Environ. 2015, 529, 121–130. [Google Scholar] [CrossRef]
- Lee, C.C.; Jiang, L.Y.; Kuo, Y.L.; Hsieh, C.Y.; Chen, C.S.; Tien, C.J. The Potential Role of Water Quality Parameters on Occurrence of Nonylphenol and Bisphenol A and Identification of Their Discharge Sources in the River Ecosystems. Chemosphere 2013, 91, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.J.; Pan, C.G.; Zhang, M.; Zhang, N.S.; Windfeld, R.; Salvito, D.; Selck, H.; Van den Brink, P.J.; Ying, G.G. Occurrence and Ecological Risk Assessment of Emerging Organic Chemicals in Urban Rivers: Guangzhou as a Case Study in China. Sci. Total Environ. 2017, 589, 46–55. [Google Scholar] [CrossRef]
- Dan, L.; Wu, S.; Xu, H.; Zhang, Q.; Zhang, S.; Shi, L.; Yao, C.; Liu, Y.; Cheng, J. Distribution and Bioaccumulation of Endocrine Disrupting Chemicals in Water, Sediment and Fishes in a Shallow Chinese Freshwater Lake: Implications for Ecological and Human Health Risks. Ecotoxicol. Environ. Saf. 2017, 140, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic Endocrine-Disrupting Compounds in the Pearl River Estuary: Occurrence, Bioaccumulation and Risk Assessment. Sci. Total Environ. 2017, 584–585, 1100–1107. [Google Scholar] [CrossRef]
- Jackson, M.; Eadsforth, C.; Schowanek, D.; Delfosse, T.; Riddle, A.; Budgen, N. Comprehensive Review of Several Surfactants in Marine Environments: Fate and Ecotoxicity. Environ. Toxicol. Chem. 2016, 35, 1077–1086. [Google Scholar] [CrossRef]
- Cantarero, S.; Camino-Sánchez, F.J.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A.; Vílchez, J.L.; Verge, C.; Reis, M.S.; Saraiva, P.M. Evaluation of the Presence of Major Anionic Surfactants in Marine Sediments. Mar. Pollut. Bull. 2012, 64, 587–594. [Google Scholar] [CrossRef]
- Esteban, S.; Llamas, P.M.; García-Cortés, H.; Catalá, M. The Endocrine Disruptor Nonylphenol Induces Sublethal Toxicity in Vascular Plant Development at Environmental Concentrations: A Risk for Riparian Plants and Irrigated Crops? Environ. Pollut. 2016, 216, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Ahel, M.; Giger, W.; Schaffner, C. Behaviour of Alkylphenol Polyethoxylate Surfactants in the Aquatic Environment-II. Occurrence and Transformation in Rivers. Water Res. 1994, 28, 1143–1152. [Google Scholar] [CrossRef]
- Lalonde, B.; Garron, C. Nonylphenol, Octylphenol, and Nonylphenol Ethoxylates Dissemination in the Canadian Freshwater Environment. Arch. Environ. Contam. Toxicol. 2021, 80, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Benfaremo, N.; Liu, C. Crankcase Engine Oil Additives. Lubrication 1990, 76, 1–12. [Google Scholar]
- Zhao, J.L.; Huang, Z.; Zhang, Q.Q.; Ying-He, L.; Wang, T.T.; Yang, Y.Y.; Ying, G.G. Distribution and Mass Loads of Xenoestrogens Bisphenol a, 4-Nonylphenol, and 4-Tert-Octylphenol in Rainfall Runoff from Highly Urbanized Regions: A Comparison with Point Sources of Wastewater. J. Hazard. Mater. 2021, 401, 123747. [Google Scholar] [CrossRef]
- Li, X.; Doherty, A.C.; Brownawell, B.; Lara-Martin, P.A. Distribution and Diagenetic Fate of Synthetic Surfactants and Their Metabolites in Sewage-Impacted Estuarine Sediments. Environ. Pollut. 2018, 242, 209–218. [Google Scholar] [CrossRef]
- García, M.T.; Campos, E.; Sánchez-Leal, J.; Ribosa, I. Anaerobic Degradation and Toxicity of Commercial Cationic Surfactants in Anaerobic Screening Tests. Chemosphere 2000, 41, 705–710. [Google Scholar] [CrossRef]
- Robbins, C. Interactions of Shampoo and Conditioner Ingredients with Hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; pp. 329–443. ISBN 9783642256103. [Google Scholar]
- Gray, N. Biology of Wastewater Treatment, 2nd ed.; Imperial College Oress: London, UK, 2004. [Google Scholar]
- Grady, C., Jr.; Daigger, G.; Love, N.; CDM, F. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- UNESCO. The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO Publishing: Paris, France, 2015. [Google Scholar]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Gabriel, F.L.P.; Heidlberger, A.; Rentsch, D.; Giger, W.; Guenther, K.; Kohler, H.P.E. A Novel Metabolic Pathway for Degradation of 4-Nonylphenol Environmental Contaminants by Sphingomonas Xenophaga Bayram: Ipso-Hydroxylation and Intramolecular Rearrangement. J. Biol. Chem. 2005, 280, 15526–15533. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial Degradation of Aromatic Compounds—From One Strategy to Four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef]
- Lara-Martín, P.A.; Gómez-Parra, A.; Sanz, J.L.; González-Mazo, E. Anaerobic Degradation Pathway of Linear Alkylbenzene Sulfonates (LAS) in Sulfate-Reducing Marine Sediments. Environ. Sci. Technol. 2010, 44, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Feijtel, T.; Vits, H.; Murray-Smith, R.; Van Wijk, R.; Koch, V.; Schröder, R.; Birch, R.; Ten Berge, W. Fate of LAS in Activated Sludge Wastewater Treatment Plants: A Model Verification Study. Chemosphere 1996, 32, 1413–1426. [Google Scholar] [CrossRef]
- Di Corcia, A.; Samperi, R.; Bellioni, A.; Marcomini, A.; Zanette, M.; Lemnr, K.; Cavalli, L. AIS/CESIO Environmental Surfactant Monitoring Programme. Part 1: LAS Pilot Study at the Roma-Nord Sewage Treatment Plant and in the Tiber River. Riv. Ital. Delle Sostanze Grasse 1994, 71, 467–475. [Google Scholar]
- Parsi, K. Interaction of Detergent Sclerosants with Cell Membranes. Phlebology 2015, 30, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Kusk, K.O.; Petersen, S. Acute and Chronic Toxicity of Tributyltin and Linear Alkylbenzene Sulfonate to the Marine Copepod Acartia Tonsa. Environ. Toxicol. Chem. 1997, 16, 1629–1633. [Google Scholar] [CrossRef]
- Hofer, R.; Jeney, Z.; Bucher, F. Chronic Effects of Linear Alkylbenzene Sulfonate (LAS) and Ammonia on Rainbow Trout (Oncorhynchus Mykiss) Fry at Water Criteria Limits. Water Res. 1995, 29, 2725–2729. [Google Scholar] [CrossRef]
- Syberg, K.; Elleby, A.; Pedersen, H.; Cedergreen, N.; Forbes, V.E. Mixture Toxicity of Three Toxicants with Similar and Dissimilar Modes of Action to Daphnia Magna. Ecotoxicol. Environ. Saf. 2008, 69, 428–436. [Google Scholar] [CrossRef]
- Franco-Belussi, L.; Jones-Costa, M.; Salla, R.F.; Souza, B.F.S.; Pinto-Vidal, F.A.; Oliveira, C.R.; Silva-Zacarin, E.C.M.; Abdalla, F.C.; Duarte, I.C.S.; De Oliveira, C. Hepatotoxicity of the Anionic Surfactant Linear Alkylbenzene Sulphonate (LAS) in Bullfrog Tadpoles. Chemosphere 2021, 266, 129014. [Google Scholar] [CrossRef]
- Jones-Costa, M.; Franco-Belussi, L.; Vidal, F.A.P.; Gongora, N.P.; Castanho, L.M.; dos Santos Carvalho, C.; Silva-Zacarin, E.C.M.; Abdalla, F.C.; Duarte, I.C.S.; De Oliveira, C.; et al. Cardiac Biomarkers as Sensitive Tools to Evaluate the Impact of Xenobiotics on Amphibians: The Effects of Anionic Surfactant Linear Alkylbenzene Sulfonate (LAS). Ecotoxicol. Environ. Saf. 2018, 151, 184–190. [Google Scholar] [CrossRef]
- Sharma, M.; Chadha, P. Widely Used Non-Ionic Surfactant 4-Nonylphenol: Showing Genotoxic Effects in Various Tissues of Channa Punctatus. Environ. Sci. Pollut. Res. 2017, 24, 11331–11339. [Google Scholar] [CrossRef]
- Karmakar, S.; Karmakar, S.; Jana, P.; Chhaba, B.; Das, S.A.; Rout, S.K. Nonylphenol Exposure in Labeo Rohita (Ham.): Evaluation of Behavioural Response, Histological, Haematological and Enzymatic Alterations. Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2021, 247, 109058. [Google Scholar] [CrossRef] [PubMed]
- Shirdel, I.; Kalbassi, M.R.; Esmaeilbeigi, M.; Tinoush, B. Disruptive Effects of Nonylphenol on Reproductive Hormones, Antioxidant Enzymes, and Histology of Liver, Kidney and Gonads in Caspian Trout Smolts. Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2020, 232, 108756. [Google Scholar] [CrossRef] [PubMed]
- Scaia, M.F.; de Gregorio, L.S.; Franco-Belussi, L.; Succi-Domingues, M.; de Oliveira, C. Gonadal, Body Color, and Genotoxic Alterations in Lithobates Catesbeianus Tadpoles Exposed to Nonylphenol. Environ. Sci. Pollut. Res. 2019, 26, 22209–22219. [Google Scholar] [CrossRef] [PubMed]
- Rivero, C.L.G.; Barbosa, A.C.; Ferreira, M.F.N.; Dorea, J.G.; Grisolia, C.K. Evaluation of Genotoxicity and Effects on Reproduction of Nonylphenol in Oreochromis Niloticus (Pisces: Cichlidae). Ecotoxicology 2008, 17, 732–737. [Google Scholar] [CrossRef]
- Bin-Dohaish, E.A. The Effects of 4-Nonylphenol Contamination on Livers of Tilapia Fish (Oreochromus Spilurs) in Jeddah. Biol. Res. 2012, 45, 15–20. [Google Scholar] [CrossRef]
- Gautam, G.J.; Chaube, R.; Joy, K. Toxicity and Tissue Accumulation of 4-Nonylphenol in the Catfish Heteropneustes Fossilis with a Note on Prevalence of 4-NP in Water Samples Toxicity and Tissue Accumulation of 4-Nonylphenol in the Cat Fi Sh Heteropneustes Fossilis with a Note on Prevalenc. Endocr. Disruptors ISSN 2015, 3, e981442. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Sun, D.; Qian, C.W.; Chen, Q.; Duan, S.S.; Sun, S.Y. Lycium Barbarum Polysaccharide Alleviates Nonylphenol Exposure Induced Testicular Injury in Juvenile Zebrafish. Int. J. Biol. Macromol. 2017, 104, 618–623. [Google Scholar] [CrossRef]
- Sayed, A.E.D.H.; Hamed, H.S. Induction of Apoptosis and DNA Damage by 4-Nonylphenol in African Catfish (Clarias Gariepinus) and the Antioxidant Role of Cydonia Oblonga. Ecotoxicol. Environ. Saf. 2017, 139, 97–101. [Google Scholar] [CrossRef]
- Gregorio, L.S.; Franco-Belussi, L.; De Oliveira, C. Genotoxic Effects of 4-Nonylphenol and Cyproterone Acetate on Rana Catesbeiana (Anura)Tadpoles and Juveniles. Environ. Pollut. 2019, 251, 879–884. [Google Scholar] [CrossRef]
- Cserháti, T.; Forgács, E.; Oros, G. Biological Activity and Environmental Impact of Anionic Surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- Giger, W.; Brunner, P.H.; Schaffner, C. 4-Nonylphenol in Sewage Sludge: Accumulation of Toxic Metabolites from Nonionic Surfactants. Science 1984, 225, 623–625. [Google Scholar] [CrossRef]
- Fujita, M.; Ike, M.; Mori, K.; Kaku, H.; Sakaguchi, Y.; Asano, M.; Maki, H.; Nishihara, T. Behaviour of Nonylphenol Ethoxylates in Sewage Treatment Plants in Japan—Biotransformation and Ecotoxicity. Water Sci. Technol. 2000, 42, 23–30. [Google Scholar] [CrossRef]
- Nasu, M.; Goto, M.; Kato, H.; Oshima, Y.; Tanaka, H. Study on Endocrine Disrupting Chemicals in Wastewater Treatment Plants. Water Sci. Technol. 2001, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Hu, J.; Yang, M. Nonylphenol Ethoxylates and Their Biodegradation Intermediates in Water and Sludge of a Sewage Treatment Plant. Bull. Environ. Contam. Toxicol. 2003, 70, 527–532. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Guan, J.; Liang, H. Seasonal Variations in the Concentration and Removal of Nonylphenol Ethoxylates from the Wastewater of a Sewage Treatment Plant. J. Environ. Sci. 2017, 54, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Brooke, L.T.; Thursby, G.B. Aquatic Life Ambient Water Quality Criteria Nonylphenol; EPA: Washington, DC, USA, 2005.
- Priac, A.; Morin-Crini, N.; Druart, C.; Gavoille, S.; Bradu, C.; Lagarrigue, C.; Torri, G.; Winterton, P.; Crini, G. Alkylphenol and Alkylphenol Polyethoxylates in Water and Wastewater: A Review of Options for Their Elimination. Arab. J. Chem. 2017, 10, S3749–S3773. [Google Scholar] [CrossRef]
- Kim, Y.S.; Katase, T.; Sekine, S.; Inoue, T.; Makino, M.; Uchiyama, T.; Fujimoto, Y.; Yamashita, N. Variation in Estrogenic Activity among Fractions of a Commercial Nonylphenol by High Performance Liquid Chromatography. Chemosphere 2004, 54, 1127–1134. [Google Scholar] [CrossRef]
- Bonefeld-Jørgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-Disrupting Potential of Bisphenol A, Bisphenol A Dimethacrylate, 4-n-Nonylphenol, and 4-n-Octylphenol in Vitro: New Data and a Brief Review. Environ. Health Perspect. 2007, 115, 69–76. [Google Scholar] [CrossRef]
- Noori, L.; Ghanemi, K. Selective Extraction of Bisphenol A and 4-Nonylphenol from Canned Tuna and Marine Fish Tissues Using Choline-Based Deep Eutectic Solvents. Chem. Pap. 2019, 73, 301–308. [Google Scholar] [CrossRef]
- Cheng, Y.; Shan, Z.; Zhou, J.; Bu, Y.; Li, P.; Lu, S. Effects of 4-Nonylphenol in Drinking Water on the Reproductive Capacity of Japanese Quails (Coturnix Japonica). Chemosphere 2017, 175, 219–227. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Uptake and Distribution of Bisphenol A and Nonylphenol in Vegetable Crops Irrigated with Reclaimed Water. J. Hazard. Mater. 2015, 283, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, Y.; Zhang, Y.; Liu, Y.; Pan, B.; Wang, B.; Lin, Y. Accumulation and Toxicological Effects of Nonylphenol in Tomato (Solanum lycopersicum L.) Plants. Sci. Rep. 2019, 9, 7022. [Google Scholar] [CrossRef] [PubMed]
- El-Hefnawy, T.; Hernandez, C.; Stabile, L.P. The Endocrine Disrupting Alkylphenols and 4,4′-DDT Interfere with Estrogen Conversion and Clearance by Mouse Liver Cytosol. Reprod. Biol. 2017, 17, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, Q.; Sun, H.; Li, J.; Lin, Q.; Wu, H.; Liu, C. Effects of Separate or Combined Exposure of Nonylphenol and Octylphenol on Central 5-HT System and Related Learning and Memory in the Rats. Ecotoxicol. Environ. Saf. 2019, 172, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, X.; Luo, Y.; Yang, X.; Yang, M.; Yang, J.; Zhou, J.; Gao, F.; He, L.; Xu, J. Adverse Effects of Chronic Exposure to Nonylphenol on Non-Alcoholic Fatty Liver Disease in Male Rats. PLoS ONE 2017, 12, e0180218. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Wang, K.L.; Wang, S.W.; Hwang, G.S.; Mao, I.F.; Chen, M.L.; Wang, P.S. Differential Effects of Nonylphenol on Testosterone Secretion in Rat Leydig Cells. Toxicology 2010, 268, 1–7. [Google Scholar] [CrossRef]
- Bechi, N.; Ietta, F.; Romagnoli, R.; Jantra, S.; Cencini, M.; Galassi, G.; Serchi, T.; Corsi, I.; Focardi, S.; Paulesu, L. Environmental Levels of Para-Nonylphenol Are Able to Affect Cytokine Secretion in Human Placenta. Environ. Health Perspect. 2010, 118, 427–431. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef]
- Chen, M.L.; Chang, C.C.; Shen, Y.J.; Hung, J.H.; Guo, B.R.; Chuang, H.Y.; Mao, I.F. Quantification of Prenatal Exposure and Maternal-Fetal Transfer of Nonylphenol. Chemosphere 2008, 73, S239–S245. [Google Scholar] [CrossRef]
- Choi, J.; Eom, J.; Kim, J.; Lee, S.; Kim, Y. Association between Some Endocrine-Disrupting Chemicals and Childhood Obesity in Biological Samples of Young Girls: A Cross-Sectional Study. Environ. Toxicol. Pharmacol. 2014, 38, 51–57. [Google Scholar] [CrossRef]
- Hou, J.W.; Lin, C.L.; Tsai, Y.A.; Chang, C.H.; Liao, K.W.; Yu, C.J.; Yang, W.; Lee, M.J.; Huang, P.C.; Sun, C.W.; et al. The Effects of Phthalate and Nonylphenol Exposure on Body Size and Secondary Sexual Characteristics during Puberty. Int. J. Hyg. Environ. Health 2015, 218, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Justicia, H.; Wray, J.W.; Sonnenschein, C. P-Nonyl-Phenol: An Estrogenic Xenobiotic Released from “modified” Polystyrene. Environ. Health Perspect. 1991, 92, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Danzo, B.J. Environmental Xenobiotics May Disrupt Normal Endocrine Function by Interfering with the Binding of Physiological Ligands to Steroid Receptors and Binding Proteins. Environ. Health Perspect. 1997, 105, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, P.; François, F.; Comunale, F.; Fenet, H.; Boussioux, A.M.; Pons, M.; Nicolas, J.C.; Casellas, C. Reporter Cell Lines to Study the Estrogenic Effects of Xenoestrogens. Sci. Total Environ. 1999, 233, 47–56. [Google Scholar] [CrossRef]
- Uchiyama, T.; Makino, M.; Saito, H.; Katase, T.; Fujimoto, Y. Syntheses and Estrogenic Activity of 4-Nonylphenol Isomers. Chemosphere 2008, 73, 60–65. [Google Scholar] [CrossRef]
- Arevalo, M.; Azcoitia, I.; Garcia-segura, L.M. The Neuroprotective Actions of Oestradiol and Oestrogen Receptors. Nat. Rev. Neurosci. 2015, 16, 17–29. [Google Scholar] [CrossRef]
- Forte, M.; Di Lorenzo, M.; Iachetta, G.; Mita, D.G.; Laforgia, V.; De Falco, M. Nonylphenol Acts on Prostate Adenocarcinoma Cells via Estrogen Molecular Pathways. Ecotoxicol. Environ. Saf. 2019, 180, 412–419. [Google Scholar] [CrossRef]
- Loyo-Rosales, J.E.; Rosales-Rivera, G.C.; Lynch, A.M.; Rice, C.P.; Torrents, A. Migration of Nonylphenol from Plastic Containers to Water and a Milk Surrogate. J. Agric. Food Chem. 2004, 52, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Knox, K.E.; Dodson, R.E.; Rudel, R.A.; Polsky, C.; Schwarzman, M.R. Identifying Toxic Consumer Products: A Novel Data Set Reveals Air Emissions of Potent Carcinogens, Reproductive Toxicants, and Developmental Toxicants. Environ. Sci. Technol. 2023, 57, 7454–7465. [Google Scholar] [CrossRef]
- Delioglanis, I.; Tzagkaraki, E.; Karachaliou, E. Public Perception of Bio-Based Products—Societal Needs and Concerns (Updated Version). Available online: https://www.bioways.eu/download.php?f=307&l=en&key=f1d76fb7f2ae06b3ee3d4372a896d977 (accessed on 15 May 2023).
- Scott, M.J.; Jones, M.N. The Biodegradation of Surfactants in the Environment. Biochim. Biophys. Acta—Biomembr. 2000, 1508, 235–251. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A Review on Natural Surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N. Recent Advances in the Environmental Applications of Biosurfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 372–378. [Google Scholar] [CrossRef]
- Pruthi, V.; Cameotra, S.S. Production and Properties of a Biosurfactant Synthesized by Arthrobacter protophormiae—An Antarctic Strain. World J. Microbiol. Biotechnol. 1997, 13, 137–139. [Google Scholar] [CrossRef]
- Garcia, M.T.; Campos, E.; Dalmau, M.; Illán, P.; Sánchez-Leal, J. Inhibition of Biogas Production by Alkyl Benzene Sulfonates (LAS) in a Screening Test for Anaerobic Biodegradability. Biodegradation 2006, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.R.; Borges, A.C. Biodegradability of Bacterial Surfactants. Biodegradation 2011, 22, 585–592. [Google Scholar] [CrossRef]
- Kumar, R.; Das, A.J. Industrial Applications of Rhamnolipid: An Innovative Green Technology for Industry. In Rhamnolipid Biosurfactant: Recent Trends in Production and Application; Springer: Singapore, 2018; pp. 65–77. ISBN 9789811312892. [Google Scholar]
- Mnif, I.; Ellouz-Chaabouni, S.; Ghribi, D. Glycolipid Biosurfactants, Main Classes, Functional Properties and Related Potential Applications in Environmental Biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Roelants, S.; Solaiman, D.K.Y.; Ashby, R.D.; Lodens, S.; Lisa, V.R.; Soetaert, W. Production and Applications of Sophorolipids. In Biobased Surfactants: Synthesis, Properties, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 65–119. ISBN 9780128127056. [Google Scholar]
- Pal, S.; Chatterjee, N.; Das, A.K.; McClements, D.J.; Dhar, P. Sophorolipids: A Comprehensive Review on Properties and Applications. Adv. Colloid Interface Sci. 2023, 313, 102856. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Bestetti, G.; Smyth, T.J.P.; Banat, I.M. Production and Applications of Trehalose Lipid Biosurfactants. Eur. J. Lipid Sci. Technol. 2010, 112, 617–627. [Google Scholar] [CrossRef]
- Bages-Estopa, S.; White, D.A.; Winterburn, J.B.; Webb, C.; Martin, P.J. Production and Separation of a Trehalolipid Biosurfactant. Biochem. Eng. J. 2018, 139, 85–94. [Google Scholar] [CrossRef]
- Zhen, C.; Ge, X.-F.; Lu, Y.-T.; Liu, W.-Z. Chemical Structure, Properties and Potential Applications of Surfactin, as Well as Advanced Strategies for Improving Its Microbial Production. AIMS Microbiol. 2023, 9, 195–217. [Google Scholar] [CrossRef]
- Zhi, Y.; Wu, Q.; Xu, Y. Genome and Transcriptome Analysis of Surfactin Biosynthesis in Bacillus Amyloliquefaciens MT45. Sci. Rep. 2017, 7, 40976. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zuo, P.; Sheng, J.; Liu, Q.; Qin, X.; Ke, C. Characterization and Production of a Biosurfactant Viscosin from Pseudomonas sp. HN11 and Its Application on Enhanced Oil Recovery During Oily Sludge Cleaning. Appl. Biochem. Biotechnol. 2023, 1–17. [Google Scholar] [CrossRef]
- Saini, H.S.; Barragán-Huerta, B.E.; Lebrón-Paler, A.; Pemberton, J.E.; Vázquez, R.R.; Burns, A.M.; Marron, M.T.; Seliga, C.J.; Gunatilaka, A.A.L.; Maier, R.M. Efficient Purification of the Biosurfactant Viscosin from Pseudomonas Libanensis Strain M9-3 and Its Physicochemical and Biological Properties. J. Nat. Prod. 2008, 71, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Gürkök, S.; Özdal, M. Microbial Biosurfactants: Properties, Types, and Production. Anatol. J. Biol. 2021, 2, 7–12. [Google Scholar]
- Gautam, K.K.; Tyagi, V.K. Microbial Surfactants: A Review. J. Oleo Sci. 2006, 55, 155–166. [Google Scholar] [CrossRef]
- Raffa, P.; Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef]
- Shahaliyan, F.; Safahieh, A.; Abyar, H. Evaluation of Emulsification Index in Marine Bacteria Pseudomonas sp. and Bacillus sp. Arab. J. Sci. Eng. 2015, 40, 1849–1854. [Google Scholar] [CrossRef]
- Salaguer, J.-L. Surfactants, Types and Uses. Lab. Formul. Interfaces Rheol. Process. 2002, 2, 1–49. [Google Scholar]
- Ying, G.-G. Fate, Behavior and Effects of Surfactants and Their Degradation Products in the Environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, Remediation and Green Surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Wong, D.; Ntchantcho, R.; Pizarro, J.; Mart, J.; Echeverr, S.; et al. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Kookana, R.S.; Williams, M.; Boxall, A.B.A.; Larsson, D.G.J.; Gaw, S.; Choi, K.; Yamamoto, H.; Thatikonda, S.; Zhu, Y.G.; Carriquiriborde, P. Potential Ecological Footprints of Active Pharmaceutical Ingredients: An Examination of Risk Factors in Low-, Middle- and High-Income Countries. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130586. [Google Scholar] [CrossRef]
- Pavlić, Ž.; Vidaković-Cifrek, Ž.; Puntarić, D. Toxicity of Surfactants to Green Microalgae Pseudokirchneriella Subcapitata and Scenedesmus Subspicatus and to Marine Diatoms Phaeodactylum Tricornutum and Skeletonema Costatum. Chemosphere 2005, 61, 1061–1068. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, X.; Sun, M.; Wei, Z.; Wang, Y.; Gao, A.; Chen, D.; Zhao, X.; Feng, X. Exploring the Effects of Different Types of Surfactants on Zebrafish Embryos and Larvae. Sci. Rep. 2015, 5, 10107. [Google Scholar] [CrossRef]
- Dreja, M.; Vockenroth, I.; Plath, N. Biosurfactants—Exotic Specialties or Ready for Application? Tenside Surfactant Deterg. 2012, 49, 10–17. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Raimondi, C.; Bestetti, G.; Banat, I.M.; Smyth, T.J.; Papacchini, M.; Cavallo, M.; Fracchia, L. Environmental Fate, Toxicity, Characteristics and Potential Applications of Novel Bioemulsifiers Produced by Variovorax Paradoxus 7bCT5. Bioresour. Technol. 2012, 108, 245–251. [Google Scholar] [CrossRef]
- Fracchia, L.; Cavallo, M.; Martinotti, M.G.; Banat, I.M. Biosurfactants and Bioemulsifiers Biomedical and Related Applications—Present Status and Future Potentials. Biomed. Sci. Eng. Technol. 2012, 14, 1–49. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Saravanan, V. Biosurfactants-Types, Sources and Applications. Res. J. Microbiol. 2015, 10, 181–192. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in Utilization of Renewable Substrates for Biosurfactant Production. AMB Express 2011, 1, 5. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Ebadipour, N.; Roostaazad, R. Evaluation of a Recycling Bioreactor for Biosurfactant Production by Pseudomonas Aeruginosa MR01 Using Soybean Oil Waste. J. Chem. Technol. Biotechnol. 2016, 91, 1368–1377. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards Commercial Production of Microbial Surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.; Del Moral, A.; Ferrer, M.R.; Tallon, R.; Quesada, E.; Béjar, V. Mauran, an Exopolysaccharide Produced by the Halophilic Bacterium Halomonas Maura, with a Novel Composition and Interesting Properties for Biotechnology. Extremophiles 2003, 7, 319–326. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost Effective Technologies and Renewable Substrates for Biosurfactants’ Production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [PubMed]
- Lundov, M.; Moesby, L.; Zachariae, C.; Johansen, J. Contamination versus Preservation of Cosmetics: A Review on Legislation, Usage, Infections, and Contact Allergy. Contact Dermat. 2009, 60, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Vernacare Vernacare Issues Voluntary Cosmetic Product Recall. Available online: https://www.vernacare.com/news-hub/news/Vernacare-issues-voluntary-recall-across-all-cosmetic-products (accessed on 1 March 2023).
- Ahmed, S.A.K.S.; Rudden, M.; Smyth, T.J.; Dooley, J.S.G.; Marchant, R.; Banat, I.M. Natural Quorum Sensing Inhibitors Effectively Downregulate Gene Expression of Pseudomonas Aeruginosa Virulence Factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- Fenical, W. Chemical Studies of Marine Bacteria: Developing a New Resource. Chem. Rev. 1993, 93, 1673–1683. [Google Scholar] [CrossRef]
- Whitman, W.; Coleman, D.; WJ, W. Prokaryotes: The Unseen Majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Marine Hydrocarbon-Degrading Bacteria: Their Role and Application in Oil-Spill Response and Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323854559. [Google Scholar]
- Tripathi, L.; Irorere, V.U.; Marchant, R.; Banat, I.M. Marine Derived Biosurfactants: A Vast Potential Future Resource. Biotechnol. Lett. 2018, 40, 1441–1457. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 922. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sivapathasekaran, C.; Sen, R. Microbial Surfactants of Marine Origin: Potentials and Prospects. In Biosurfactants. Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010. [Google Scholar]
- Yakimov, M.M.; Golyshin, P.N.; Lang, S.; Moore, E.R.B.; Abraham, W.; Lunsdorf, H.; Timmis, K.N. A New, Hydrocarbon-Degrading and Surfactant-Producing Marine Bacterium. Int. J. Syst. Bacteriol. 1972, 48, 339–348. [Google Scholar] [CrossRef]
- Hassanshahian, M.; Emtiazi, G.; Cappello, S. Isolation and Characterization of Crude-Oil-Degrading Bacteria from the Persian Gulf and the Caspian Sea. Mar. Pollut. Bull. 2012, 64, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Raguénès, G.H.C.; Peres, A.; Ruimy, R.; Pignet, P.; Christen, R.; Loaec, M.; Rougeaux, H.; Barbier, G.; Guezennec, J.G. Alteromonas Infernus sp. nov., a New Polysaccharideproducing Bacterium Isolated from a Deep-Sea Hydrothermal Vent. J. Appl. Microbiol. 1997, 82, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Raguenes, G.; Pignet, P.; Gauthier, G.; Peres, A.; Christen, R.; Rougeaux, H.; Barbier, G.; Guezennec, J. Description of a New Polymer-Secreting Bacterium from a Deep-Sea Hydrothermal Vent, Alteromonas Macleodii subsp. Fijiensis, and Preliminary Characterization of the Polymer. Appl. Environ. Microbiol. 1996, 62, 67–73. [Google Scholar] [CrossRef]
- Ibacache-Quiroga, C.; Ojeda, J.; Espinoza-Vergara, G.; Olivero, P.; Cuellar, M.; Dinamarca, M.A. The Hydrocarbon-Degrading Marine Bacterium Cobetia sp. Strain MM1IDA2H-1 Produces a Biosurfactant That Interferes with Quorum Sensing of Fish Pathogens by Signal Hijacking. Microb. Biotechnol. 2013, 6, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Ståhle, J.; Parrilli, E.; Sannino, F.; Mitchell, D.E.; Pieretti, G.; Gibson, M.I.; Marino, G.; Lanzetta, R.; Parrilli, M.; et al. Structural Characterization of an All-Aminosugar-Containing Capsular Polysaccharide from Colwellia Psychrerythraea 34H. Int. J. Gen. Mol. Microbiol. 2017, 110, 1377–1387. [Google Scholar] [CrossRef]
- Iyer, A.; Mody, K.; Jha, B. Emulsifying Properties of a Marine Bacterial Exopolysaccharide. Enzyme Microb. Technol. 2006, 38, 220–222. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Leo, V.V.; Walker, G.M.; Green, D.H. Emulsifying Properties of a Glycoprotein Extract Produced by a Marine Flexibacter Species Strain TG382. Enzyme Microb. Technol. 2009, 45, 53–57. [Google Scholar] [CrossRef]
- Hao, D.H.; Lin, J.Q.; Song, X.; Lin, J.Q.; Su, Y.J.; Qu, Y.B. Isolation, Identification, and Performance Studies of a Novel Paraffin-Degrading Bacterium of Gordonia Amicalis LH3. Biotechnol. Bioprocess Eng. 2008, 13, 61–68. [Google Scholar] [CrossRef]
- Calvo, C.; Martínez-Checa, F.; Toledo, F.; Porcel, J.; Quesada, E. Characteristics of Bioemulsifiers Synthesised in Crude Oil Media by Halomonas Eurihalina and Their Effectiveness in the Isolation of Bacteria Able to Grow in the Presence of Hydrocarbons. Appl. Microbiol. Biotechnol. 2002, 60, 347–351. [Google Scholar] [CrossRef]
- Malavenda, R.; Rizzo, C.; Michaud, L.; Gerçe, B.; Bruni, V.; Syldatk, C.; Hausmann, R.; Lo Giudice, A. Biosurfactant Production by Arctic and Antarctic Bacteria Growing on Hydrocarbons. Polar Biol. 2015, 38, 1565–1574. [Google Scholar] [CrossRef]
- Tripathi, L.; Twigg, M.S.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Biosynthesis of Rhamnolipid by a Marinobacter Species Expands the Paradigm of Biosurfactant Synthesis to a New Genus of the Marine Microflora. Microb. Cell Fact. 2019, 18, 164. [Google Scholar] [CrossRef]
- Vasileva-Tonkova, E.; Gesheva, V. Biosurfactant Production by Antarctic Facultative Anaerobe Pantoea sp. during Growth on Hydrocarbons. Curr. Microbiol. 2007, 54, 136–141. [Google Scholar] [CrossRef]
- Xu, M.; Fu, X.; Gao, Y.; Duan, L.; Xu, C.; Sun, W.; Li, Y.; Meng, X.; Xiao, X. Characterization of a Biosurfactant-Producing Bacteria Isolated from Marine Environment: Surface Activity, Chemical Characterization and Biodegradation. J. Environ. Chem. Eng. 2020, 8, 104277. [Google Scholar] [CrossRef]
- Guo, P.; Xu, W.; Tang, S.; Cao, B.; Wei, D.; Zhang, M.; Lin, J.; Li, W. Isolation and Characterization of a Biosurfactant Producing Strain Planococcus sp. XW-1 from the Cold Marine Environment. Int. J. Environ. Res. Public Health 2022, 19, 782. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and Characterisation of Short Chain Rhamnolipid Production in a Previously Uninvestigated, Non-Pathogenic Marine Pseudomonad. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. [Google Scholar] [CrossRef]
- Raguénès, G.; Christen, R.; Guezennec, J.; Pignet, P.; Barbier, G. Vibrio diabolicus sp. nov., a New Polysaccharide-Secreting Organism Isolated from a Deep-Sea Hydrothermal Vent Polychaete Annelid, Alvinella pompejana. Int. J. Syst. Bacteriol. 1997, 47, 989–995. [Google Scholar] [CrossRef]
- Jackson, S.A.; Borchert, E.; Gara, F.O.; Dobson, A.D.W. Metagenomics for the Discovery of Novel Biosurfactants of Environmental Interest from Marine Ecosystems. Curr. Opin. Biotechnol. 2015, 33, 176–182. [Google Scholar] [CrossRef]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going Green and Cold: Biosurfactants from Low-Temperature Environments to Biotechnology Applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in Cosmetic Formulations: Trends and Challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef]
- Randhawa, K.K.S.; Rahman, P.K.S.M. Rhamnolipid Biosurfactants-Past, Present, and Future Scenario of Global Market. Front. Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; Lépine, F.; Déziel, E. Production of Rhamnolipids by Pseudomonas Aeruginosa. Appl. Microbiol. Biotechnol. 2005, 68, 718–725. [Google Scholar] [CrossRef]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent Advancements in the Production of Rhamnolipid Biosurfactants By Pseudomonas Aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef]
- Pekdemir, T.; Çopur, M.; Urum, K. Emulsification of Crude Oil-Water Systems Using Biosurfactants. Process Saf. Environ. Prot. 2005, 83, 38–46. [Google Scholar] [CrossRef]
- Evonik. Evonik Builds World’s First Industrial-Scale Production Plant for Rhamnolipids; Forward-Looking, Triple-Digit Million Investment in Slovakia. Strategic Partnership with Unilever. Innovation for Sustainability: Fermentation of Sugar Replaces Petrochemical Raw Materials; Evonik Industries AG: Essen, Germany, 2022; pp. 1–3. [Google Scholar]
- Rahman, K.S.M.; Rahman, T.J.; Kourkoutas, Y.; Petsas, I.; Marchant, R.; Banat, I.M. Enhanced Bioremediation of n-Alkane in Petroleum Sludge Using Bacterial Consortium Amended with Rhamnolipid and Micronutrients. Bioresour. Technol. 2003, 90, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.; Nitschke, M. Antimicrobial Activity of Rhamnolipids against Listeria Monocytogenes and Their Synergistic Interaction with Nisin. Food Control 2013, 29, 138–142. [Google Scholar] [CrossRef]
- Parry, A.; Parry, N.; Peilow, A.; Stevenson, P. Combinations of Rhamonlipids and Enzymes for Improved Cleaning. European Patent, EP2596087A1, 29 May 2013. [Google Scholar]
- Piljac, T.; Piljac, G. Use of Rhamnolipids as Cosmetics. European Patent EP1056462, 25 July 2007. pp. 1–9. [Google Scholar]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced Healing of Full-Thickness Burn Wounds Using Di-Rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef]
- Mordor Intelligence. Rhamnolipids Market—Growth Trends, COVID-19 Impact, and Forecasts (2021–2026); Mordor Intelligence: Hyderabad, India, 2021. [Google Scholar]
- Jarvis, F.G.; Johnson, M.J.; Jarvis, B.F.G. A Glyco-Lipide Produced by Pseudomonas Aeruginosa. J. Am. Chem. Soc. 1949, 71, 4124–4126. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of Structures, Microbial Origins and Roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial Biosurfactant Research: Time to Improve the Rigour in the Reporting of Synthesis, Functional Characterization and Process Development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Dubeau, D.; Déziel, E.; Woods, D.E.; Lépine, F. Burkholderia Thailandensis Harbors Two Identical Rhl Gene Clusters Responsible for the Biosynthesis of Rhamnolipids. BMC Microbiol. 2009, 9, 263. [Google Scholar] [CrossRef]
- Hörmann, B.; Müller, M.M.; Syldatk, C.; Hausmann, R. Rhamnolipid Production by Burkholderia Plantarii DSM 9509T. Eur. J. Lipid Sci. Technol. 2010, 112, 674–680. [Google Scholar] [CrossRef]
- Häußler, S.; Nimtz, M.; Domke, T.; Wray, V.; Steinmetz, I. Purification and Characterization of a Cytotoxic Exolipid of Burkholderia Pseudomallei. Infect. Immun. 1998, 66, 1588–1593. [Google Scholar] [CrossRef]
- Tavares, L.F.D.; Silva, P.M.; Junqueira, M.; Mariano, D.C.O.; Nogueira, F.C.S.; Domont, G.B.; Freire, D.M.G.; Neves, B.C. Characterization of Rhamnolipids Produced by Wild-Type and Engineered Burkholderia Kururiensis. Appl. Microbiol. Biotechnol. 2013, 97, 1909–1921. [Google Scholar] [CrossRef]
- Nayak, A.S.; Vijaykumar, M.H.; Karegoudar, T.B. Characterization of Biosurfactant Produced by Pseudoxanthomonas sp. PNK-04 and Its Application in Bioremediation. Int. Biodeterior. Biodegrad. 2009, 63, 73–79. [Google Scholar] [CrossRef]
- Rooney, A.P.; Price, N.P.J.; Ray, K.J.; Kuo, T.M. Isolation and Characterization of Rhamnolipid-Producing Bacterial Strains from a Biodiesel Facility. FEMS Microbiol. Lett. 2009, 295, 82–87. [Google Scholar] [CrossRef]
- HoŠková, M.; Schreiberová, O.; JeŽdík, R.; Chudoba, J.; Masák, J.; Sigler, K.; Řezanka, T. Characterization of Rhamnolipids Produced by Non-Pathogenic Acinetobacter and Enterobacter Bacteria. Bioresour. Technol. 2013, 130, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Seiler, T.B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-Specific and Whole-Organism Ecotoxicity of Mono-Rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Voulgaridou, G.P.; Mantso, T.; Anestopoulos, I.; Klavaris, A.; Katzastra, C.; Kiousi, D.E.; Mantela, M.; Galanis, A.; Gardikis, K.; Banat, I.M.; et al. Toxicity Profiling of Biosurfactants Produced by Novel Marine Bacterial Strains. Int. J. Mol. Sci. 2021, 22, 2383. [Google Scholar] [CrossRef]
- Nalini, S.; Parthasarathi, R. Biosurfactant Production by Serratia Rubidaea SNAU02 Isolated from Hydrocarbon Contaminated Soil and Its Physico-Chemical Characterization. Bioresour. Technol. 2013, 147, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Sakthipriya, N.; Doble, M.; Sangwai, J.S. Biosurfactant from Pseudomonas Species with Waxes as Carbon Source—Their Production, Modeling and Properties. J. Ind. Eng. Chem. 2015, 31, 100–111. [Google Scholar] [CrossRef]
- Sharma, D.; Ansari, M.J.; Al-Ghamdi, A.; Adgaba, N.; Khan, K.A.; Pruthi, V.; Al-Waili, N. Biosurfactant Production by Pseudomonas Aeruginosa DSVP20 Isolated from Petroleum Hydrocarbon-Contaminated Soil and Its Physicochemical Characterization. Environ. Sci. Pollut. Res. 2015, 22, 17636–17643. [Google Scholar] [CrossRef]
- Santos, A.S.; Sampaio, A.P.W.; Vasquez, G.S.; Santa Anna, L.M.; Pereira, N.; Freire, D.M.G. Evaluation of Different Carbon and Nitrogen Sources in Production of Rhamnolipids by a Strain of Pseudomonas Aeruginosa. Appl. Biochem. Biotechnol.—Part A Enzym. Eng. Biotechnol. 2002, 98–100, 1025–1035. [Google Scholar] [CrossRef]
- Najmi, Z.; Ebrahimipour, G.; Franzetti, A.; Banat, I.M. In Situ Downstream Strategies for Cost-Effective Bio/Surfactant Recovery. Biotechnol. Appl. Biochem. 2018, 65, 523–532. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Gibbs, B.F. Recovery of Biosurfactants by Ultrafiltration. J. Chem. Technol. Biotechnol. 1990, 47, 23–29. [Google Scholar] [CrossRef]
- Müller, M.M.; Kügler, J.H.; Henkel, M.; Gerlitzki, M.; Hörmann, B.; Pöhnlein, M.; Syldatk, C.; Hausmann, R. Rhamnolipids-Next Generation Surfactants? J. Biotechnol. 2012, 162, 366–380. [Google Scholar] [CrossRef]
- Sha, R.; Jiang, L.; Meng, Q.; Zhang, G.; Song, Z. Producing Cell-Free Culture Broth of Rhamnolipids as a Cost-Effective Fungicide against Plant Pathogens. J. Basic Microbiol. 2012, 52, 458–466. [Google Scholar] [CrossRef]
- Beuker, J.; Steier, A.; Wittgens, A.; Rosenau, F.; Henkel, M.; Hausmann, R. Integrated Foam Fractionation for Heterologous Rhamnolipid Production with Recombinant Pseudomonas Putida in a Bioreactor. AMB Express 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cui, Q.; Han, S.; Dong, H.; Zhang, J.; Ma, F.; Zhang, Y. Enhanced Rhamnolipid Production of Pseudomonas Aeruginosa SG by Increasing Copy Number of RhlAB Genes with Modified Promoter. RSC Adv. 2015, 5, 70546–70552. [Google Scholar] [CrossRef]
- Yang, N.; Ding, S.; Chen, F.; Zhang, X.; Xia, Y.; Di, H.; Cao, Q.; Deng, X.; Wu, M.; Wong, C.C.L.; et al. The Crc Protein Participates in Down-Regulation of the Lon Gene to Promote Rhamnolipid Production and Rhl Quorum Sensing in Pseudomonas Aeruginosa. Mol. Microbiol. 2015, 96, 526–547. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.; Hassett, D.J.; Lau, G.W. Human Targets of Pseudomonas Aeruginosa Pyocyanin. Proc. Natl. Acad. Sci. USA 2003, 100, 14315–14320. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Phillips, G.; Thomashow, L.S. Functional Analysis of Genes for Biosynthesis of Pyocyanin and Phenazine-1-Carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.L. The Next Generation of Synthetic Biology Chassis: Moving Synthetic Biology from the Laboratory to the Field. ACS Synth. Biol. 2016, 5, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Nikel, P.I.; de Lorenzo, V. Pseudomonas Putida as a Functional Chassis for Industrial Biocatalysis: From Native Biochemistry to Trans-Metabolism. Metab. Eng. 2018, 50, 142–155. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus Subtilis: A Universal Cell Factory for Industry, Agriculture, Biomaterials and Medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Cha, M.; Lee, N.; Kim, M.; Kim, M.; Lee, S. Heterologous Production of Pseudomonas Aeruginosa EMS1 Biosurfactant in Pseudomonas Putida. Bioresour. Technol. 2008, 99, 2192–2199. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Reiser, J.; Fiechter, A.; Witholt, B. Production of Pseudomonas Aeruginosa Rhamnolipid Biosurfactants in Heterologous Hosts. Appl. Environ. Microbiol. 1995, 61, 3503–3506. [Google Scholar] [CrossRef]
- Cabrera-Valladares, N.; Richardson, A.P.; Olvera, C.; Treviño, L.G.; Déziel, E.; Lépine, F.; Soberón-Chávez, G. Monorhamnolipids and 3-(3-Hydroxyalkanoyloxy) Alkanoic Acids (HAAs) Production Using Escherichia Coli as a Heterologous Host. Appl. Microbiol. Biotechnol. 2006, 73, 187–194. [Google Scholar] [CrossRef]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Wichmann, R.; Küpper, B.; Zwick, M.; Wilhelm, S.; et al. Growth Independent Rhamnolipid Production from Glucose Using the Non-Pathogenic Pseudomonas Putida KT2440. Microb. Cell Fact. 2011, 10, 80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).