Preference of Major Stored Product Insects in Fortified Rice with Basil
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Rice Fortification
2.3. Insecticidal Application
2.4. Choice Tests
2.4.1. Test 1—Untreated Choice Tests
2.4.2. Test 2—Treated Choice Tests
2.5. Statistical Analysis
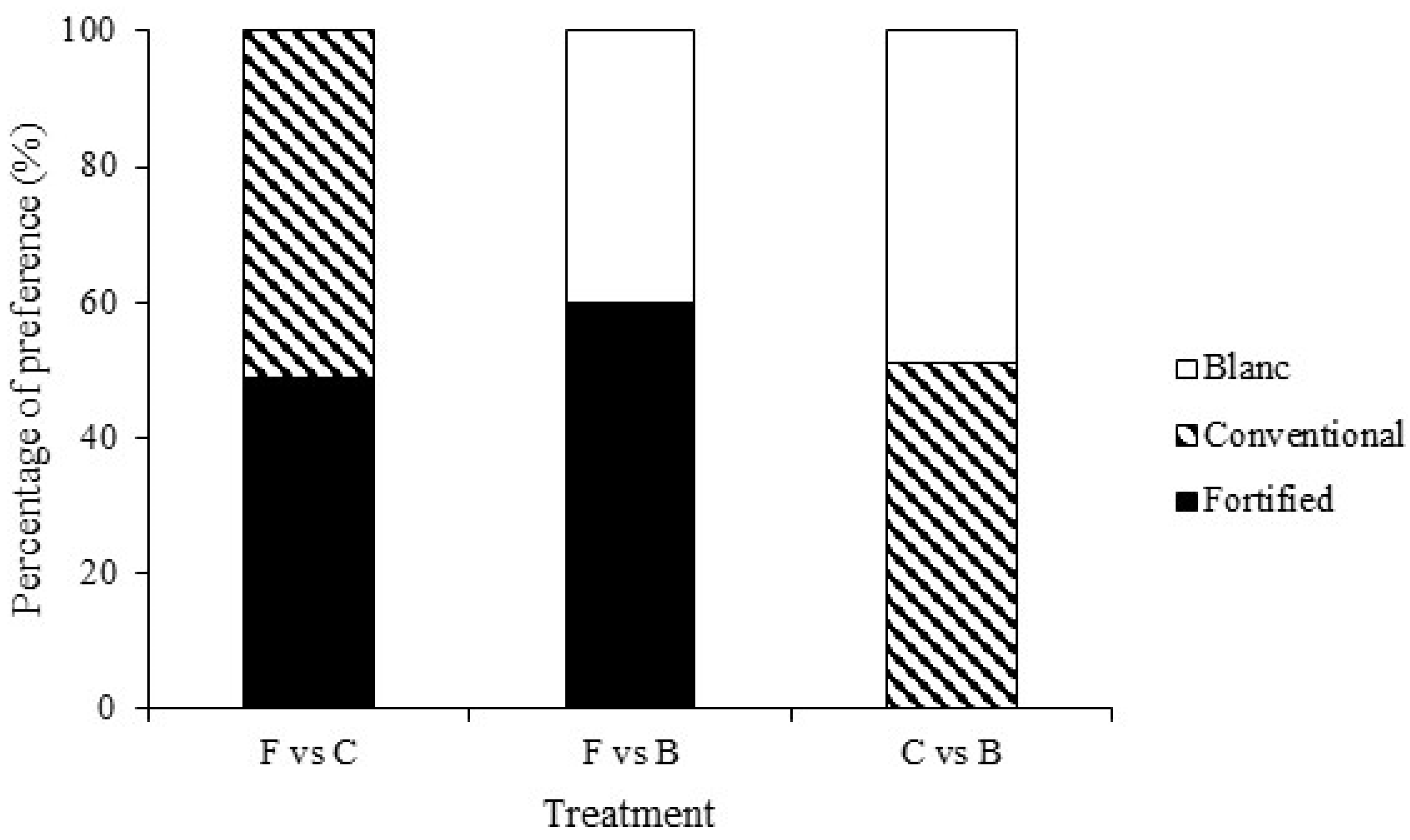
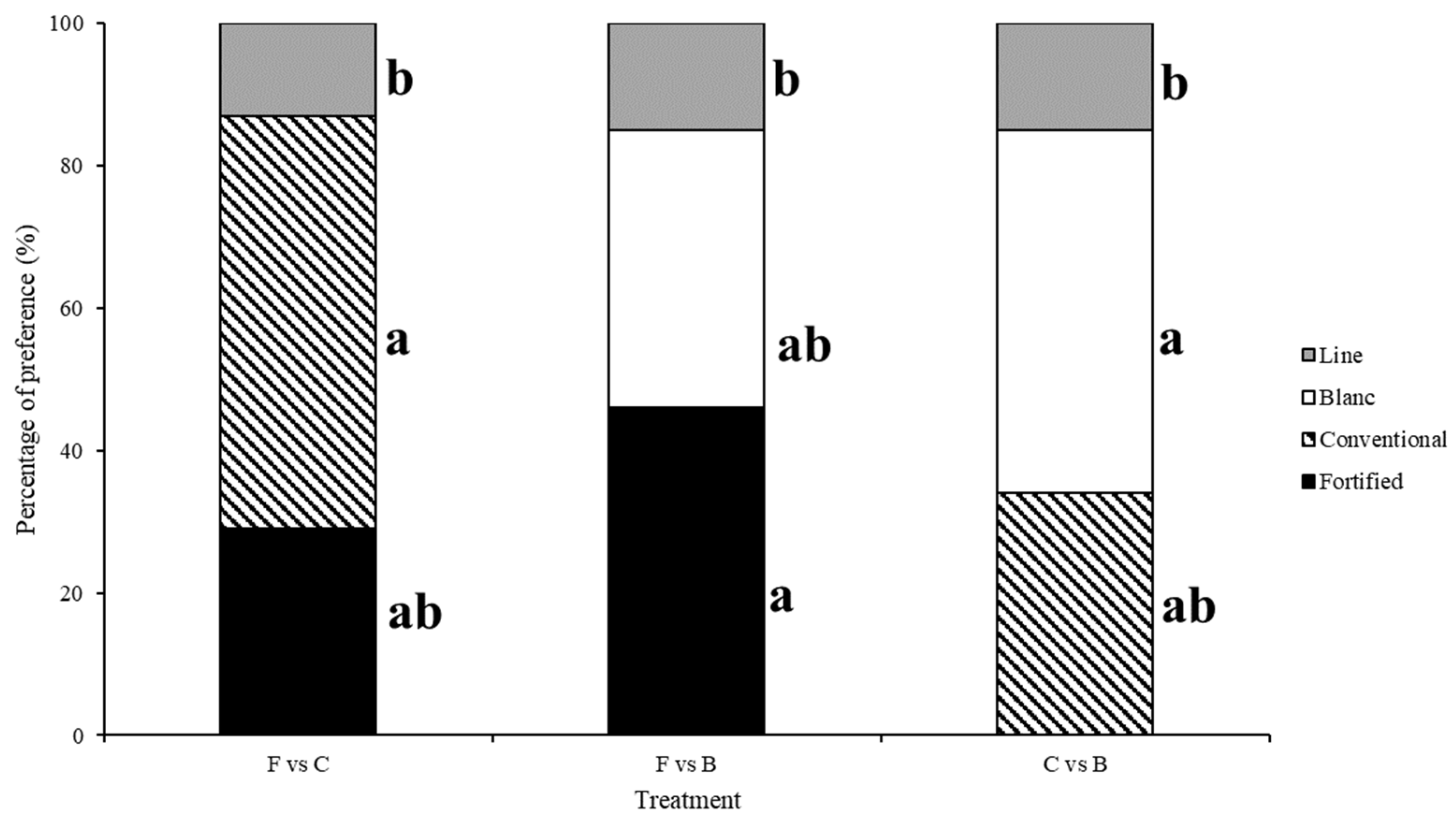
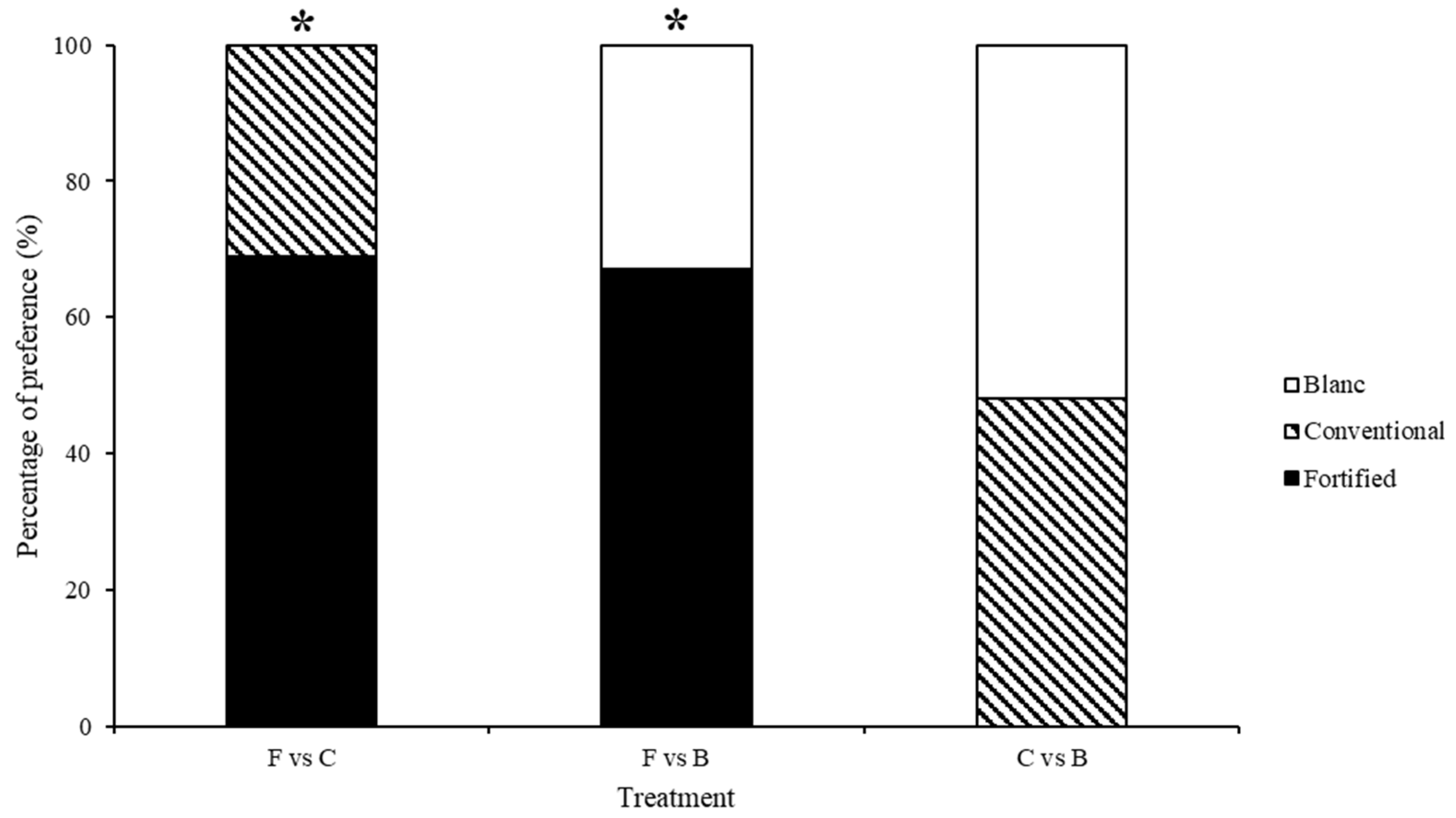
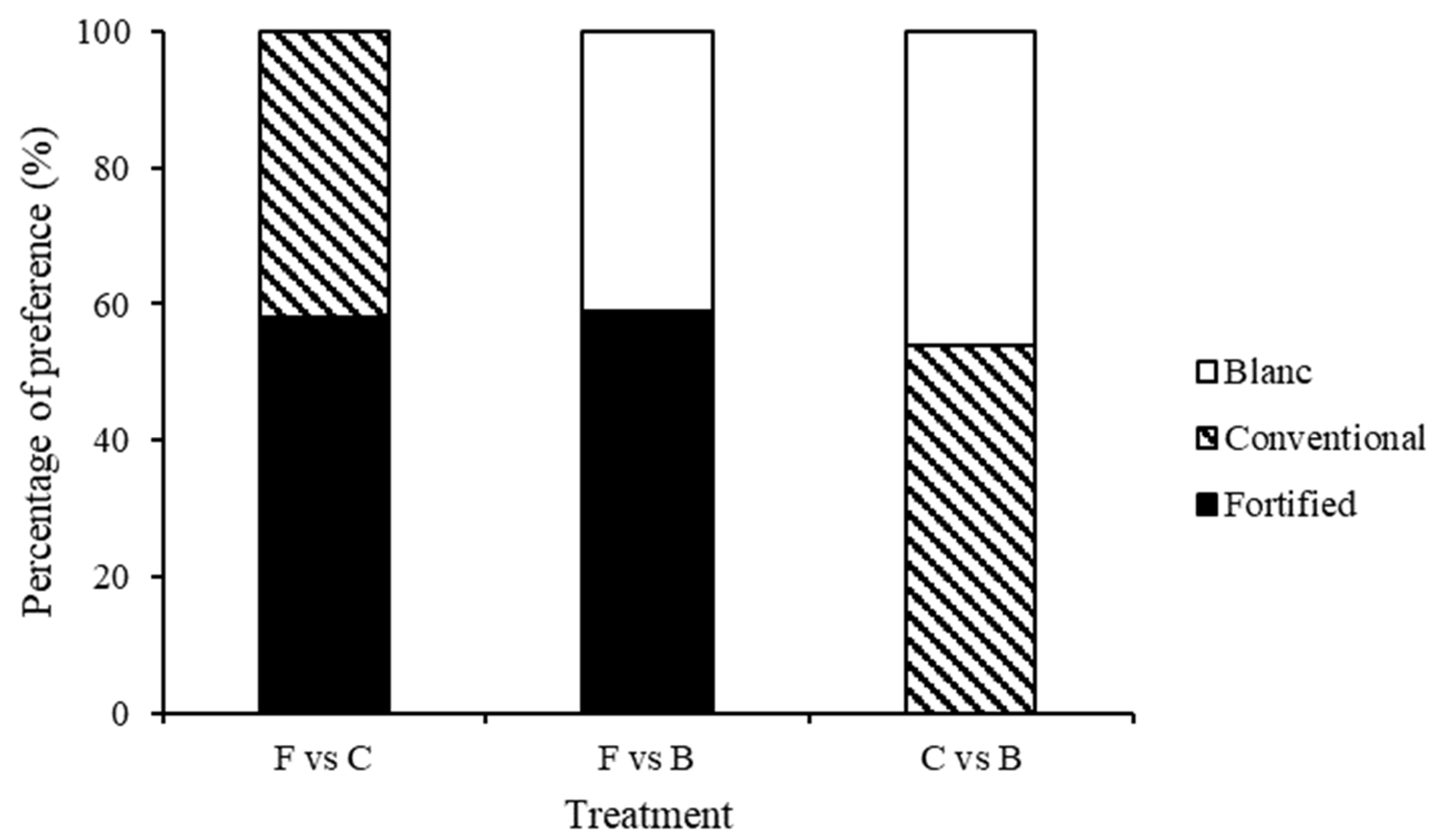
3. Results
3.1. Test 1—Untreated Choice Tests
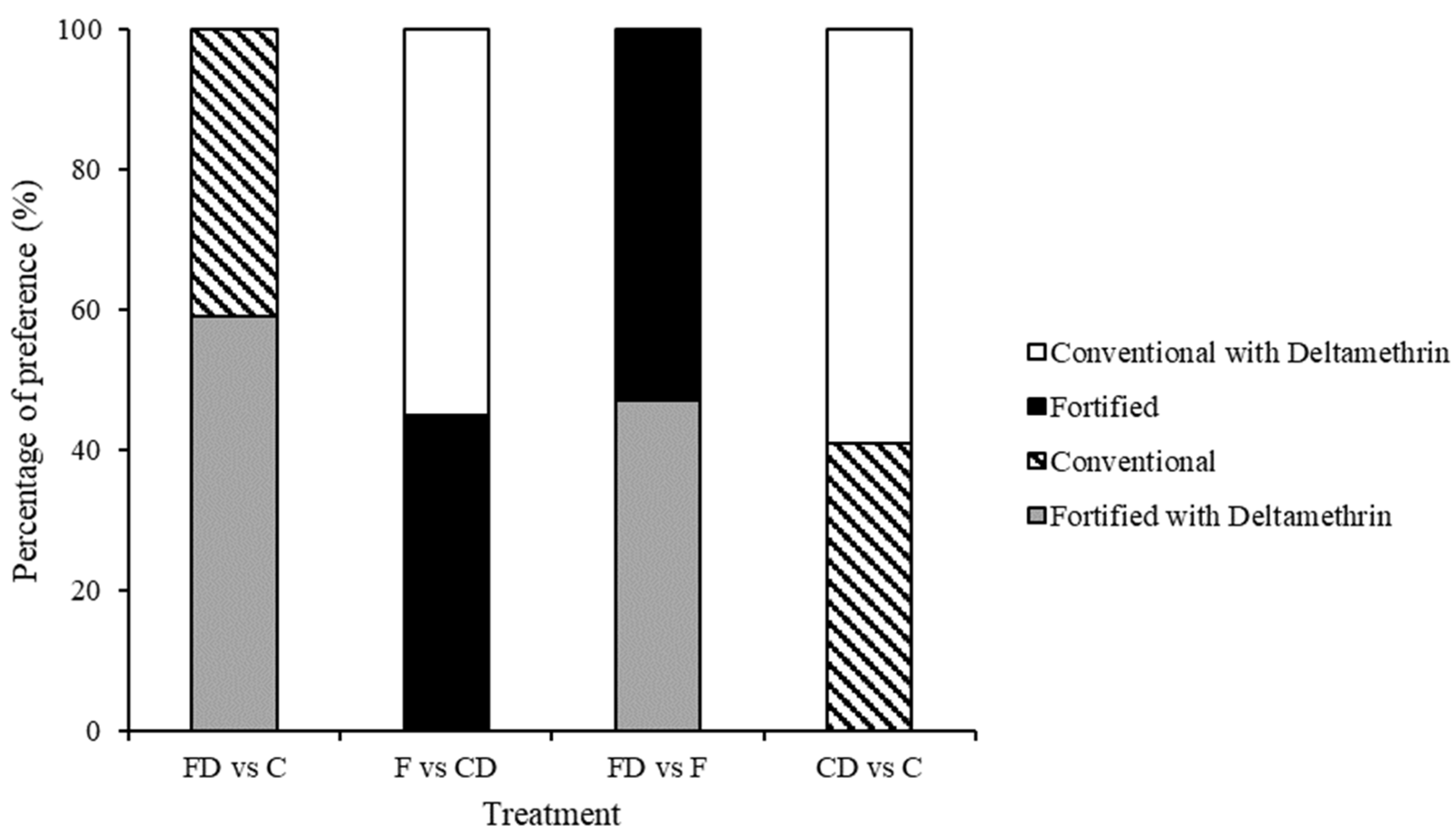
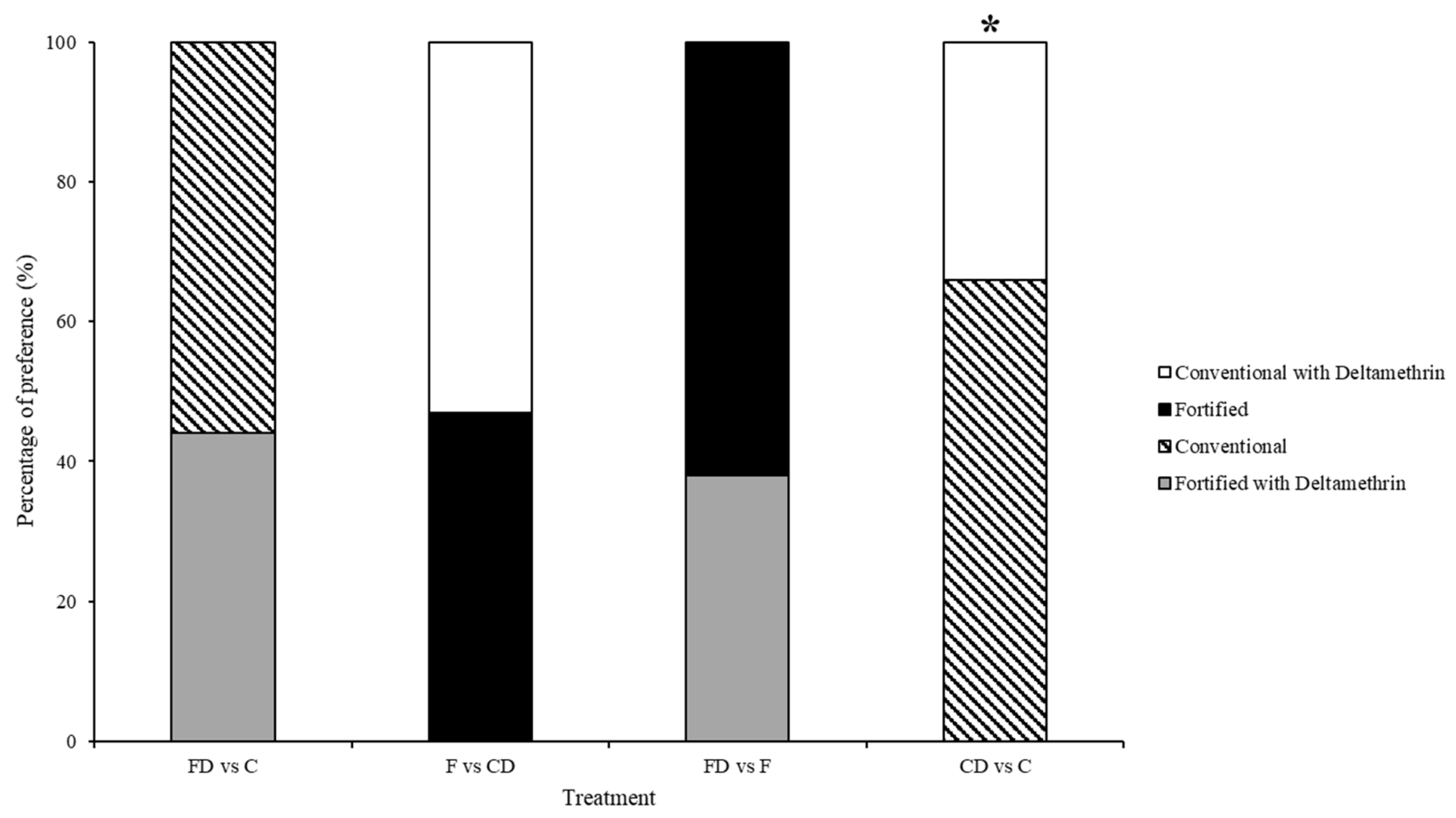
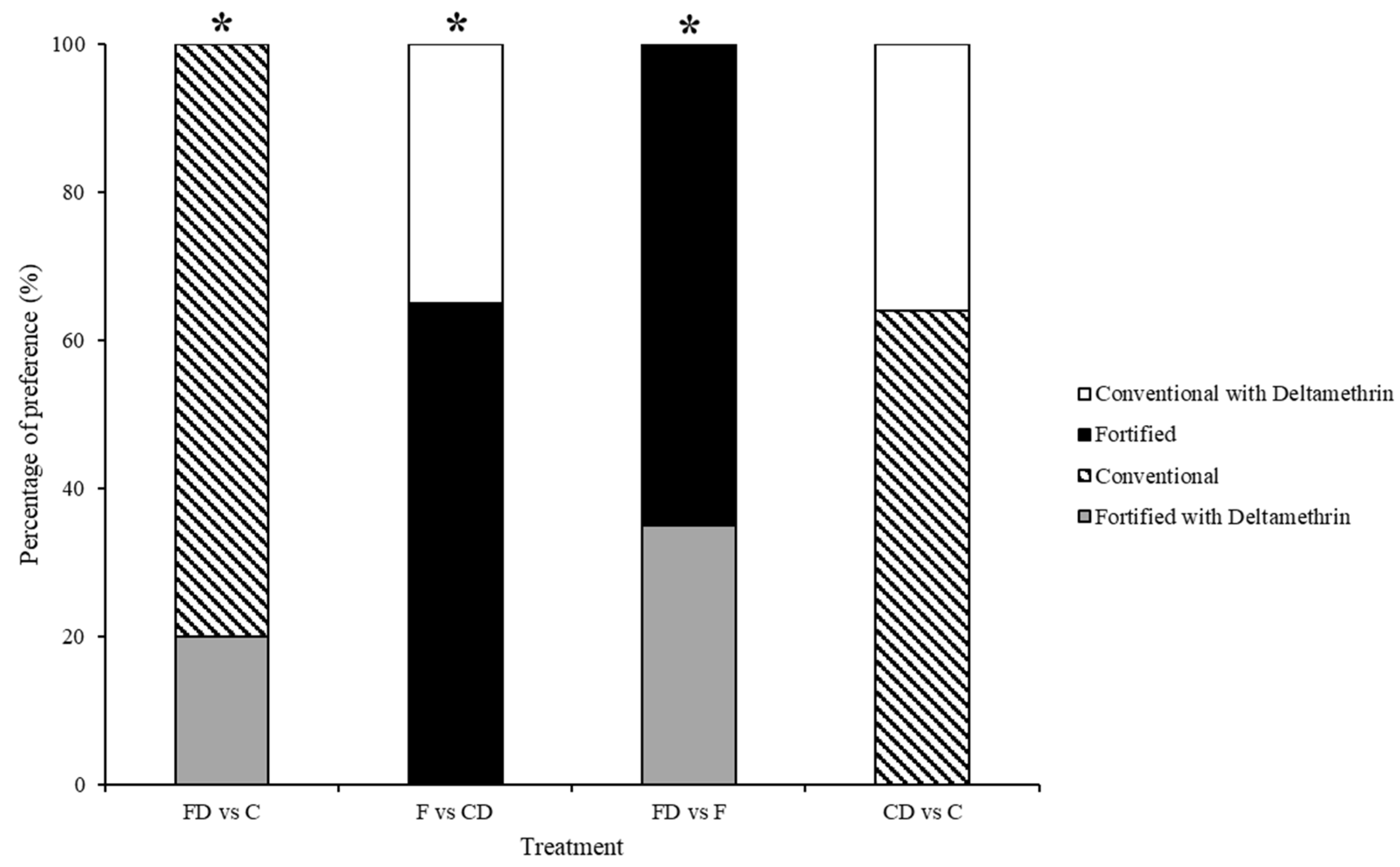
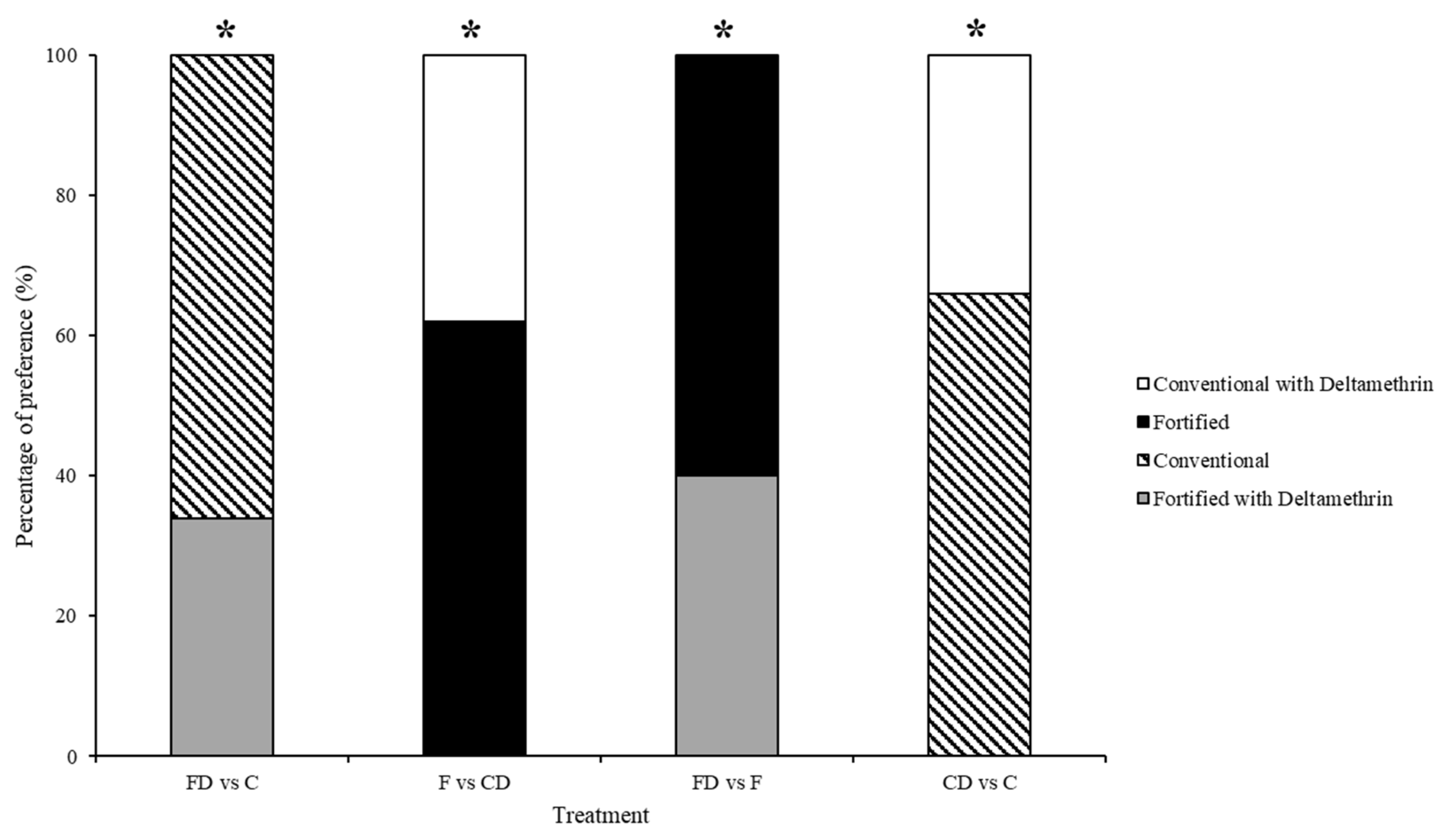
3.2. Test 2—Treated Choice Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2012. [Google Scholar]
- Bond, M.; Meacham, T.; Bhunnoo, R.; Benton, T.G. Food Waste within Global Food Systems. A Global Food Security Report. 2013. Available online: www.foodsecurity.ac.uk (accessed on 1 September 2013).
- FAO; IFAD; UNICEF; WFP; WHO. Repurposing food and agricultural policies to make healthy diets more affordable. In Brief to the State of Food Security and Nutrition in the World; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J. Importance of Stored Product Insects. In Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–17. [Google Scholar]
- Le Mouël, C.; Forslund, A. How can we feed the world in 2050? A review of the responses from global scenario studies. Eur. Rev. Agric. Econ. 2017, 44, 541–591. [Google Scholar] [CrossRef]
- Singh, P.K. A decentralized and holistic approach for grain management in India. Curr. Sci. 2010, 99, 279–1180. [Google Scholar]
- Nagpal, M.; Kumar, A. Grain losses in India and government policies. In Quality Assurance and Safety of Crops and Foods; 1st ICC India Grains Conference, in partnership with ICRISAT, India; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 4, p. 143. [Google Scholar]
- Boxall, R.A. Post-harvest losses to insects—A world overview. Int. Biodeterior. Biodegrad. 2001, 48, 137–152. [Google Scholar] [CrossRef]
- Mason, L.J.; McDonald, M. Biology, behaviour and ecology of stored grain and legume insects. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Agricultural Experiment Station and Cooperative Extension Service, Kansas State University: Manhattan, KS, USA, 2012; pp. 7–20. [Google Scholar]
- Sodhi, G.K.; Saxena, S. Plant growth promotion and abiotic stress mitigation in rice using endophytic fungi: Advances made in the last decade. Environ. Exp. Bot. 2023, 209, 105312. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Cereal Supply and Demand Brief. 2019. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 21 April 2021).
- Wong, J.; Huang, Y. China’s Food Security and Its Global Implications. China Int. J. 2012, 10, 113–124. [Google Scholar] [CrossRef]
- Akter, T.; Jahan, M.; Bhuiyan, M.S.I. Biology of the Angoumois grain moth, Sitotroga cerealella (Oliver) on stored rice grain in laboratory condition. J. Asiat. Soc. Bangladesh Sci. 2013, 39, 61–67. [Google Scholar] [CrossRef]
- James, F.C.; Frank, H.A.; Michael, A.M. Insect management in food processing facilities. Adv. Food Nutr. Res. 2004, 48, 239–295. [Google Scholar]
- Kumar, A.; Gowda, G.B.; Sah, R.P.; Sahu, C.; Biswal, M.; Nayak, S.; Kumar, S.; Swain, P.; Sharma, S. Status of glycemic index of paddy rice grain (Oryza sativa L.) on infestation by storage pest Sitotroga cerealella. J. Stored Prod. Res. 2020, 89, 101697. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F. Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Daglish, G.J.; Nayak, M.K.; Arthur, F.H.; Athanassiou, C.G. Insect pest management in stored grain. In Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 45–63. [Google Scholar]
- Dubey, N.K.; Shukla, R.; Kumar, A.; Singh, P.; Prakash, B. Prospects of botanical pesticides in sustainable agriculture. Curr. Sci. 2010, 98, 479–480. [Google Scholar]
- Stejskal, V.; Hubert, J.; Li, Z. Human health problems and accidents associated with occurrence and control of storage arthropods and rodents. Insect pest management in stored grain. In Recent Advances in Stored Product Protection; Athanassiou, C., Arthur, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 19–43. [Google Scholar]
- Singh, R.; Rup, P.J.; Koul, O. Bioefficacy of Eucalyptus camaldulensis var. obtusa and Luvunga scandens essential oils against Spodoptera litura (Lepidoptera: Nocutidae). Biopest. Int. 2008, 4, 128–137. [Google Scholar]
- Ditzen, M.; Pellegrino, M.; Vosshall, L.B. Insect odorant receptors are molecular targets of the insect repellent deet. Science 2008, 319, 1838–1842. [Google Scholar] [CrossRef]
- Kokwaro, J.O. Medicinal Plants of East Africa; East African Literature Bureau (General Printers Limited): Nairobi, Kenya, 1976; p. 384. [Google Scholar]
- Paton, A. A synopsis of Ocimum (Lablatae) in Africa. Kew Bull. 1991, 47, 403–435. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Yatoo, M.I.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum basilicum. Food Rev. Int. 2023, 39, 119–147. [Google Scholar] [CrossRef]
- Bora, K.S.; Arora, S.; Shri, R. Role of Ocimum Basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. J. Ethnopharmacol. 2011, 137, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Selvakkumar, C.; Gayathri, B.; Vinaykumar, K.S.; Lakshmi, B.S.; Balakrishnan, A. Potential anti-inflammatory properties of crude alcoholic extract of Ocimum basilicum L. in human peripheral blood mononuclear cells. J. Health Sci. 2007, 53, 500–505. [Google Scholar] [CrossRef]
- Chokechaijaroenporn, O.; Bunyapraphatsara, N.; Kongchuensin, S. Mosquito repellent activities of ocimum volatile oils. Phytomedicine 1994, 1, 135–139. [Google Scholar] [CrossRef]
- Palsson, K.; Jaenson, T.G.T. Plant products used as mosquito repellents in Guinea Bissau, West Africa. Acta Trop. 1999, 72, 39–52. [Google Scholar] [CrossRef]
- Tawatsin, A.; Wratten, S.D.; Scott, R.R.; Thavara, U.; Techandamrongsin, Y. Repellency of volatile oils from plants against three mosquito vectors. J. Vector Ecol. 2001, 26, 76–82. [Google Scholar]
- Zaridah, M.Z.; Nor Azah, M.A.; Abu Said, A.; Mohd Faridz, Z.P. Larvicidal properties of citronellal and Cymbopogon nardus essential oils from two different localities. Trop. Biomed. 2003, 20, 169–174. [Google Scholar]
- Hassanli, A.; Iwande, W.; Ole-Sitayo, N.; Moreka, L.; Nokoe, S. Weevil repellent constituents of Ocimum suave leaves and Eugenia caryophyllata cloves used as grain protectant in parts of Eastern Africa. Discov. Innovat. 1990, 2, 91–95. [Google Scholar]
- Obeng-Ofori, D.; Reichmuth, C.H.; Bekele, J.; Hassanali, A. Biological activity of 1,8 cineole, a major component of essential oil of Ucimum kenyense (Ayobangira) against stored product beetles. J. Appl. Ent. 1997, 121, 237–243. [Google Scholar] [CrossRef]
- Owusu, E.O. Effect of some Ghanaian plant components on control of two stored-product insect pests of cereals. J. Stored Prod. Res. 2001, 37, 85–91. [Google Scholar] [CrossRef]
- Popovic, Z.; Kostic, M.; Popovic, S.; Skoric, S. Bioactivities of essential oils from basil and sage to Sitophilus oryzae L. Biotechnol. Biotechnol. Equip. 2006, 20, 36–40. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.M.; Wong, S. Potential of Ocimum basilicum L. and Pandanus tectorius Parkinson from the ecology of Al-makhwah, Saudi Arabia in controlling Sitophilus oryzae (L.) and Tribolium castaneum (Herbst). Life Sci. J. 2013, 10, 2996–3000. [Google Scholar]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Hassan, E.; Gökçe, A. Production and Consumption of Biopesticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: New Delhi, India, 2014; pp. 361–379. [Google Scholar]
- Utono, I.M.; Coote, C.; Gibson, G. Field study of the repellent activity of ‘Lem-ocimum’-treated double bags against the insect pests of stored sorghum, Tribolium castaneum and Rhyzopertha dominica, in northern Nigeria. J. Stored Prod. Res. 2014, 59, 222–230. [Google Scholar] [CrossRef]
- Al-Harbi, N.A.; Al Attar, N.M.; Hikal, D.M.; Mohamed, S.E.; Abdel Latef, A.A.H.; Ibrahim, A.A.; Abdein, M.A. Evaluation of insecticidal effects of plants essential oils extracted from basil, black seeds and lavender against Sitophilus oryzae. Plants 2021, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Agrafioti, P.; Lampiri, E.; Igoumenidis, P.E.; Karathanos, V.T.; Perdikaris, A.; Athanassiou, C.G. Population growth changes in major stored product insects on rice fortified with spearmint and basil. Agronomy 2022, 12, 2088. [Google Scholar] [CrossRef]
- Igoumenidis, P.E.; Karathanos, V.T. Diffusion and thermal stability of phenolic compounds during fortified rice rehydration process. J. Food Eng. 2016, 174, 1–7. [Google Scholar] [CrossRef]
- Bekele, A.J.; Obeng-Ofori, D.; Hassanali, A. Evaluation of Ocimum suave (Willd) as a source of repellents, toxicants and protectants in storage against three stored product insect pests. Int. J. Pest Manag. 1996, 42, 139–142. [Google Scholar] [CrossRef]
- Ngassoum, M.B.; Ngamo Tinkeu, L.S.; Ngatanko, L.; Tapondjou, L.A.; Lognay, G.; Malaisse, F.; Hance, T. Chemical composition, insecticidal effect and repellent activity of essential oils of three aromatic plants, alone and in combination, towards Sitophilus oryzae L. (Coleoptera: Curculionidae). Nat. Prod. Commun. 2007, 2, 1229–1232. [Google Scholar] [CrossRef]
- Ogendo, J.O.; Kostyukovsky, M.; Ravid, U.; Matasyoh, J.C.; Deng, A.L.; Omolo, E.O.; Shaaya, E. Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J. Stored Prod. Res. 2008, 44, 328–334. [Google Scholar] [CrossRef]
- Akob, C.A.; Ewete, F.K. Laboratory evaluation of bioactivity of ethanolic extracts of plants used for protection of stored maize against Sitophilus zeamais Motschulsky in Cameroon. Afr. Entomol. 2009, 17, 90–94. [Google Scholar] [CrossRef]
- Pugazhvendan, S.R.; Ross, P.R.; Elumalai, K. Insecticidal and repellant activities of plants oil against stored grain pest, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Asian Pac. J. Trop. Dis. 2012, 2, 412–415. [Google Scholar] [CrossRef]
- Kathirvelu, C.; Mangayarkarsi, S.; Kanagaraj, B. Repellency effect of synthetic volatiles and essential oils against Callosobruchus maculatus and Tribolium castaneum. Int. J. Emerg. Technol. Eng. Res. 2020, 11, 174–180. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Vayias, B.J.; Tsakiri, J.B.; Mikeli, N.H.; Meletsis, C.M.; Tomanović, Ž. Persistence and efficacy of Metarhizium anisopliae (Metschnikoff) Sorokin (Deuteromycotina: Hyphomycetes) and diatomaceous earth against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) on wheat and maize. Crop Prot. 2008, 27, 1303–1311. [Google Scholar]
- Shivaramu, S.; Parepally, S.K.; Byregowda, V.Y.; Damodaram, K.J.P.; Bhatnagar, A.; Naga, K.C.; Sharma, S.; Kumar, M.; Kempraj, V. Estragole, a potential attractant of the winged melon aphid, Aphis gossypii. Pest Manag. Sci. 2023, 79, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Moawad, S.S.; Ebadah, I.M.A. Assessment of some botanical oils impact on biology of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Wulfenia 2021, 28, 3. [Google Scholar]
- Hagstrum, D.W.; Phillips, T.W. Evolution of stored-product entomology: Protecting the world food supply. Annu. Rev. Entomol. 2017, 62, 379–397. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Aggarwal, K.K.; Kukreja, A.K.; Dwivedi, S.; Singh, A.K. Effects of volatile oil constituents of Mentha species against stored grain pests, Callosobruchus maculatus and Tribolium castaneum. J. Med. Aromat. Plant Sci. 2000, 22, 549–556. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G. Insecticidal effect and adherence of PyriSec in different grain commodities. Crop Prot. 2005, 27, 703–710. [Google Scholar] [CrossRef]
- Isman, M. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Liang, G.W.; Xu, Y.J.; Lu, Y.Y.; Zeng, L. Diatomaceous earth enhances the toxicity of garlic, Allium sativum, essential oil against stored-product pests. J. Stored Prod. Res. 2010, 46, 118–123. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Evergetis, E.; Katsoula, A.M.; Haroutounian, S.A. Insecticidal efficacy of silica gel with Juniperus oxycedrus ssp. Oxycedrus (Pinales: Cupressaceae) essential oil against Sitophilus oryzae (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2013, 106, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Arena, J.S.; Omarini, A.B.; Zunino, M.P.; Peschiutta, M.L.; Defagó, M.T.; Zygadlo, J.A. Essential oils from Dysphania ambrosioides and Tagetes minuta enhance the toxicity of a conventional insecticide against Alphitobius diaperinus. Ind. Crops Prod. 2018, 122, 190–194. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Bedini, S.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Bocchino, R.; Girardi, J.; Giannotti, P.; Ferroni, G.; Conti, B. Sensory quality of essential oils and their synergistic effect with diatomaceous earth for the control of stored grain insects. Insects 2019, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.D.; Achimón, F.; Pizzolitto, R.P.; Ramírez Sánchez, A.; Góez Torres, E.A.; Zygadlo, J.A.; Zunino, M.P. An alternative to reduce the use of the synthetic insecticide against the maize weevil Sitophilus zeamais through the synergistic action of Pimenta racemosa and Citrus sinensis essential oils with chlorpyrifos. J. Pest. Sci. 2021, 94, 409–421. [Google Scholar] [CrossRef]
- Boukraa, N.; Ladjel, S.; Benlamoudi, W.; Goudjil, M.B.; Berrekbia, M.; Eddoud, A. Insecticidal and repellent activities of Artemisia herba alba Asso, Juniperus phoenicea L and Rosmarinus officinalis L essential oils in synergized combinations against adults of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Biocatal. Agric. Biotechnol. 2022, 45, 102513. [Google Scholar] [CrossRef]
- Lampiri, E.; Agrafioti, P.; Vagelas, I.; Athanassiou, C.G. Insecticidal effect of an enhanced attapulgite for the control of four stored-product beetle species. Agronomy 2022, 12, 1495. [Google Scholar] [CrossRef]
- Silva, S.; Cunha, J.; Carvalho, S.; Zandonadi, C.; Martins, R.; Chang, R. Ocimum basilicum essential oil combined with deltamethrin to improve the management of Spodoptera frugiperda. Ciênc. Agrotec. 2017, 41, 665–675. [Google Scholar] [CrossRef]
- Suwannayod, S.; Sukontason, K.L.; Pitasawat, B.; Junkum, A.; Limsopatham, K.; Jones, M.K.; Somboon, P.; Leksomboon, R.; Chareonviriyaphap, T.; Tawatsin, A.; et al. Synergistic toxicity of plant essential oils combined with pyrethroid insecticides against blow flies and the house fly. Insects 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Lorini, I.; Galley, D.J. Relative effectiveness of topical, filter paper and grain applications of deltamethrin, and associated behaviour of Rhyzopertha dominica (F.) strains. J. Stored Prod. Res. 1998, 34, 377–383. [Google Scholar] [CrossRef]
- Diotaiuti, L.; Penido, C.M.; Araújo, H.S.; Schofield, C.J.; Pinto, C.T. Excito-repellency effect of deltamethrin on triatomines under laboratory conditions. Rev. Soc. Bras. Med. Trop. 2000, 33, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Chareonviriyaphap, T.; Prabaripai, A.; Bangs, M.J. Excito-repellency of deltamethrin on the malaria vectors, Anopheles minimus, Anopheles dirus, Anopheles swadiwongporni, and Anopheles maculatus, in Thailand. J. Am. Mosq. Control Assoc. 2004, 20, 45–54. [Google Scholar]
- Velki, M.; Plavsin, I.; Dragojevic, J.; Hackenberger, B.K. Toxicity and repellency of dimethoate, pirimiphos-methyl and deltamethrin against Tribolium castaneum (Herbst) using different exposure methods. J. Stored Prod. Res. 2014, 59, 36–41. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Sparks, T.C.; Story, R.N. Evolution of insect resistance to insecticides: A reevaluation of the roles of physiology and behavior. Bull. Entomol. Soc. Am. 1984, 30, 41–51. [Google Scholar] [CrossRef]


| Adults | Larvae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sitophilus oryzae | Rhyzopertha dominica | Tribolium castaneum | Oryzaephilus surinamensis | Tribolium castaneum | ||||||
| Combinations | X2 | p | X2 | p | X2 | p | X2 | p | X2 | p |
| C vs. F | 2.161 | 0.706 | 11.830 | 0.159 | 1.642 | 0.990 | 5.330 | 0.255 | 0.657 | 0.957 |
| F vs. B | 2.083 | 0.720 | 1.765 | 0.987 | 1.634 | 0.990 | 13.388 | 0.010 | 0.579 | 0.965 |
| C vs. B | 1.761 | 0.780 | 8.255 | 0.409 | 3.406 | 0.906 | 1.042 | 0.903 | 1.616 | 0.806 |
| Adults | Larvae | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sitophilus oryzae | Rhyzopertha dominica | Tribolium castaneum | Oryzaephilus surinamensis | Tribolium castaneum | ||||||
| Combinations | X2 | p | X2 | p | X2 | p | X2 | p | X2 | p |
| FD vs. C | 3.473 | 0.482 | 2.576 | 0.631 | 0.568 | 0.967 | 3.125 | 0.537 | 0.624 | 0.960 |
| F vs. CD | 0.404 | 0.982 | 1.067 | 0.899 | 1.445 | 0.836 | 0.879 | 0.928 | 1.104 | 0.894 |
| FD vs. F | 1.044 | 0.903 | 1.761 | 0.780 | 1.952 | 0.745 | 4.835 | 0.305 | 1.250 | 0.870 |
| CD vs. C | 3.059 | 0.548 | 3.890 | 0.421 | 0.624 | 0.960 | 4.948 | 0.293 | 1.515 | 0.824 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampiri, E.; Agrafioti, P.; Rumbos, C.I.; Athanassiou, C.G. Preference of Major Stored Product Insects in Fortified Rice with Basil. Sustainability 2023, 15, 11379. https://doi.org/10.3390/su151411379
Lampiri E, Agrafioti P, Rumbos CI, Athanassiou CG. Preference of Major Stored Product Insects in Fortified Rice with Basil. Sustainability. 2023; 15(14):11379. https://doi.org/10.3390/su151411379
Chicago/Turabian StyleLampiri, Evagelia, Paraskevi Agrafioti, Christos I. Rumbos, and Christos G. Athanassiou. 2023. "Preference of Major Stored Product Insects in Fortified Rice with Basil" Sustainability 15, no. 14: 11379. https://doi.org/10.3390/su151411379
APA StyleLampiri, E., Agrafioti, P., Rumbos, C. I., & Athanassiou, C. G. (2023). Preference of Major Stored Product Insects in Fortified Rice with Basil. Sustainability, 15(14), 11379. https://doi.org/10.3390/su151411379









