Abstract
Agroforestry home gardens are integrated arrangements of common vegetable plants near residences, resembling tropical forests. They cultivate multiple species to meet families’ basic needs, including food, medicine, and family activities. This study aimed to assess the composition and use of plant species in agroforestry home gardens in three municipalities in the western region of Pará. The study analyzed 119 home gardens in Belterra, Mojuí dos Campos, and Santarém in Pará, Brazilian Amazonia. These home gardens span peri-urban, urban, floodplain, indigenous, and tourist land zones. Data were collected through questionnaires and visits, surveying 5323 plants from 188 species and 62 plant families. The findings revealed that 80.5% of plants concentrated in just 18.6% of the species, with no significant difference in species per home garden across zones. Notably, food species, particularly fruit-bearing plants, dominated these home gardens. This indicates a potential emphasis on incentive programs and public policies. Most home gardens contained up to 17 plant species, while less than half included medicinal plants. Native and exotic species were equally represented, with native plants valued for shading benefits. Agroforestry home gardens are vital for conserving and cultivating food species. Their specific purposes influence species distribution and selection, showcasing their socioeconomic and environmental significance. Thus, recognizing and investing in these land-use systems is crucial to maintain and enhance regional benefits.
1. Introduction
Agroforestry home gardens are plants managed in traditional land-use systems in tropical countries, including trees, shrubs, herbaceous plants, and sometimes animals [1], as an activity usually practiced by families in areas close to homes [2]. The structure of a home garden can resemble that of tropical forests, with canopy strata formation, high plant diversity, and biomass and carbon storage [3,4].
Implementing these spaces promotes the cultivation of multiple species to ensure basic needs on the properties [5,6], such as providing food to families [7,8,9]. These environments provide food options that can be enjoyed in nature with different food supply options and food shortage situations [10]. Agroforestry home gardens expand opportunities for income complementation of traditional populations [11,12], although commercialization is not the primary motivating force for home garden maintenance [13].
These spaces, also known as backyard vegetable gardens, have great botanical richness [2,14] and have been seen as a way to expand production alternatives as they are types of agroforestry systems that can present agricultural and forest species in a consortium way [15] as well as presenting agrosilvopastoral characteristics [16] when the animal component is inserted in the interaction. Multistrata agroforestry systems, as, in fact, the home gardens are configured, stand out from other consortium systems for their more significant potential of providing an ecosystem service due to the similarity with natural environments of the secondary forests [17], such as home gardens in Belterra, Pará, with meliponiculture [18].
The family relationships established in these environments intensify the sharing of knowledge and interpersonal actions that accumulate knowledge and strengthen crops and soil management, gradually contributing to the perpetuation of local culture [19,20]. Both the accumulation of traditional knowledge [21] and the conservation of local biodiversity [2] are variable responses that indicate the consolidation of production. These land-use systems are also spaces for maintaining and conserving essential memories associated with family life. According to [22], there is relevance for tradition, values, customs, and rural habits, which confirms the need for appreciation beyond environmental and economic measurements.
The ecological and socioeconomic characteristics that are added to the home gardens, with the coexistence of numerous species in the same area, offer a variety of foods throughout the year, collaborating mainly with the food and nutritional security of the family unit [8,23,24] and increased family income with the sale of surplus production [19,25]. Polyculture environments, such as home gardens, can contribute to preventing the collapse of tropical biodiversity [26], potentially towards achieving the Sustainable Development Goals [27]. However, despite agroforestry home gardens’ economic, social, and ecological importance, only some initiatives and actions have been aimed at their valorization.
Knowing the diversity of species and understanding the breadth of agroforestry home gardens’ role in the family environment is a crucial issue to encourage and expand their adoption in the Amazon region. This study aimed to assess the composition and use of plant species in agroforestry home gardens in three municipalities in the western region of Pará. This assessment holds significance as it aims to systematize information about these environments to support initiatives that can strengthen natural resource management and promote individual and collective well-being in the region.
2. Materials and Methods
2.1. Definition and Delimitation of Agroforestry HomeGardens
For this research, home gardens (To facilitate the reading of the text, the name “home gardens” is considered the summarized form of “agroforestry home gardens”) were considered to be the environments where there is a combination of vegetal plants with or without animal husbandry, arranged in areas adjacent to the residence [28], where routine family, social, and productive activities are commonly carried out. The research universe was based on areas under family responsibility and administration, regardless of property ownership.
Information from the interviewees was used to indicate the edges of the home gardens for the physical delimitation of these spaces. It should be noted that in some areas surveyed, especially in peri-urban locations, floodplains, and indigenous lands, these limits were established by the way activities were handled in the place, with the residents defining the places where the handling of activities is more intensified, such as the realization of sweeping, cleaning or removal of waste, disposal of spaces for the drying of clothes, and for hammocks for the resting of the residents, among other activities commonly developed in these places.
2.2. Area of Study
The studied area is located in the mesoregion of the Lower Amazon and the microregion of Santarém. The survey was conducted in the municipalities of Belterra, Mojuí dos Campos, and Santarém, west of Pará, in Brazilian Amazonia. Based on demographic density, these municipalities are classified into predominantly urban (Santarém), adjacent rural (Belterra), and remote rural (Mojuí dos Campos), all with a population unit with a moderate or low degree of urbanization [29]. The decision to conduct the research in these municipalities was based on their geographical proximity, which provided logistical advantages, facilitating data collection and establishing effective communication with the participants. The geographical proximity contributed to greater accessibility for insertion and collaboration in the study. Furthermore, these municipalities have regional significance, highlighted by the presence of the Federal University of West Pará (UFOPA) in teaching, research, and extension activities.
According to [30], the increase in demographic density in the “gray” areas affects areas that are no longer strictly rural and not precisely urban, the so-called peri-urban areas, as they are adjacent to the urban centers of the municipalities. Thus, in the three mapped municipalities’ peri-urban areas, communities between 1.1 and 19.2 km from each urban center were selected.
The prevailing climate in the region is of the hot and humid type, with rainfall concentrated in the first half of the year, average annual temperature between 25° and 27 °C, average air humidity of 86%, average annual rainfall of 1920 mm, varying in terms of monthly rainfall between 170 mm and 60 mm [31], and falling within the climate subtype Am3 [32,33]. The predominant soils are classified as yellow latosol [34,35]. The predominant vegetation is typical of dense ombrophilous forest [36]. There are two well-marked seasons in the year, one where the rains are concentrated, between December and May, and the less-rainy period, between June and November.
For the survey, five different zones were considered in the three selected municipalities (Figure 1): i. peri-urban—in the communities of Cidade Alta, São José Operário, Cipoal, Garrafão, Cucurunã, Iruruma, Jacamim, Colônia São Jorge, and São Raimundo; ii. urban—in the central neighborhoods of the three municipalities; iii. floodplain—in the Alto Jari community, where the vegetation and residence infrastructure are subject to temporary flooding, with the interaction between the aquatic and terrestrial ecosystem for about four months; iv. indigenous land—in the indigenous villages of Muratuba and Paricatuba, located in the Tapajós-Arapiuns Extractive Reserve, belonging to the municipality of Santarém; and, finally, v. tourist—in the community of Alter do Chão, where there is intense visitation and the economy has a strong influence of the tourist sector, visitation, and natural leisure.
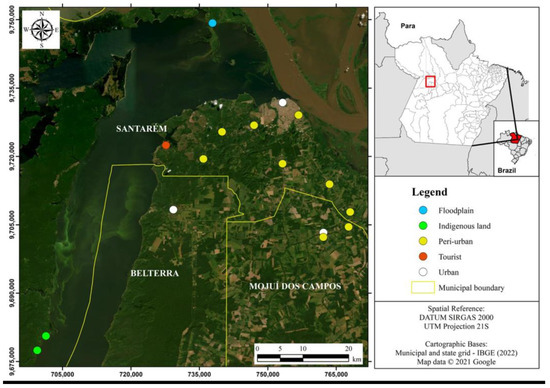
Figure 1.
Location map of the study area in three municipalities in the State of Pará, Brazil.
The sampling was carried out in 119 home gardens, arranged in the following zones: (a) peri-urban (40); (b) urban (28); (c) floodplain (21); (d) indigenous (20); and (e) tourist (10). Home gardens in isolated rural areas were not included due to non-compliance with the population density criterion or because they do not minimally configure a community agglomeration, except for home gardens in the floodplain area, usually with low demographic density.
2.3. Data Collection and Analysis
Data were obtained from semi-structured interviews, where the quantity and vernacular name of the plants that make up the home gardens and the use indicated by the interviewees (food, medicinal, ornamental, and shading) were recorded.
Sampling was carried out based on the consent of the owners, and the information was collected collaboratively with the interviewees through observation on a guided tour [37]. Only owners who provided verbal consent were included in the survey. Meetings were held with leaders in the indigenous area to discuss and clarify the study’s objectives. It was decided to research two of the twenty-one villages of the Tupinambá people since these served as a support base, facilitating the translocation of personnel and the logistics of collection.
To identify the species, life form, and origin, a consultation was carried out with specialized virtual sites to confirm the botanical nomenclature [38] and the parabotanist nomenclature of the Federal University of Western Pará. The research was registered with the Genetic Heritage Management Council and the National System for the Management of Genetic Heritage and Traditional Knowledge under access registers No. A6E86AF and A8E5DE0. In the case of indigenous lands, the leaders authorized the research, called “caciques” (chieftains).
2.4. Statistical Analysis
The study design was randomized entirely, and the collections covered the years 2016 to 2019. The analysis in different periods does not compromise the data quality since the home gardens’ character is not affected by environmental variations [39].
Data on a number of species (richness) and a number of individual plants in the home gardens, divided by the five zones (peri-urban, urban, floodplains, indigenous, and tourist), were submitted to the normality test through the Kolmogorov–Smirnov test, being considered with normal distribution since they presented a non-significant p-value at the level of 0.05 for the number of species and individual plants. Thus, the linear association analysis was performed using Pearson’s coefficient.
For the correlation analysis between several species and individual plants, considering all home gardens as a single sample, the Spearman coefficient was used as a function of the non-parametric distribution.
To verify the existence of statistical differences between the areas studied, analysis of variance (ANOVA) was used, with a subsequent test of comparison of means by Tukey’s test, adopting a significance level of 95%. All tests and statistical inferences were performed using Bioestat Software [40].
3. Results
3.1. General Patterns
The survey in the home gardens totaled 5323 individual plants of 188 species belonging to 62 botanical families. There are 13 (thirteen) botanical families (Arecaceae, Malvaceae, Rutaceae, Musaceae, Malpighiaceae, Anacardiaceae, Amaryllidaceae, Myrtaceae, Apiaceae, Fabaceae, Euphorbiaceae, Salicaceae, and Lecythidaceae, in ascending order) that represent 74.8% of the entire plant population of the sampled home gardens, corresponding to 3984 individual plants. The remaining 25.2% of the individual plants are distributed in 49 families. Among the 188 species identified, 88.5% occur in less than 15% of the home gardens, and yet, within this range of analysis, 56.6% of the species were observed in less than 2.5% of the home gardens studied.
The families Arecaceae, Malvaceae, Rutaceae, and Musaceae represented 37.8% of the individual plants. These families include species of regional importance for food consumption, such as cocoa (Theobroma cacao L.), cupuaçu (Theobroma grandiflorum (Willd. ex Spring), açaí (Euterpe oleracea Mart.), orange (Citrus sp.), and banana (Musa paradisiacal). The botanical families Malpighiaceae, Anacardiaceae, Amaryllidaceae, and Myrtaceae, responsible for 21.4% of the population, present prominent species in regional feeding and domestic use such as cashew (Anacardium occidentale L.), music (Byrsonima crassifolia (L.)), chives (Allium schoenoprasum L.), Jambo (Eugenia malaccensis L.), mango (Mangifera indica L.), and acerola (Malpighia emarginata L.). It is also noted that Apiaceae, Fabaceae, Euphorbiaceae, Salicaceae, and Lecythidaceae, which corresponded to 15.6% of the sampled individual plants, have important representatives, such as Brazil nuts (Bertholletia excelsa Bonpl.), cumaru (Dipteryx sp.), cilantro (Coriandrum sativum L.), and rubber tree (Hevea brasiliensis (Willd. ex A.Juss.) Müll.Arg.). It should be noted that 23.6% of the plants (1255) are distributed in the remaining 22 botanical families, with 22 to 106 individual plants per family.
On the other hand, evidencing the peculiarity of these home gardens, 2.9% of the population was distributed into 30 families with less than 20 individual plants in the sum of the areas evaluated, reinforcing the importance of these spaces in biodiversity conservation. In the inventory of home gardens, it was found that 80.5% of the individual plants (4284) are concentrated in 18.6% of the species (36). Likewise, the rule was also valid for the number of species and botanical families, with 81.0% of the individual plants (4311) concentrated in 25.8% of the families (16).
Figure 2 shows the arrangement of the data illustrating the number of plants and species for all the home gardens evaluated. Data in the five different zones revealed that the range of species in the environments ranged from 3 to 30, and the number of individual plants showed significant variation (5 to 140), resulting in a mean and standard deviation of 12.9 ± 5.3 species per home garden and 44.7 ± 29.1 individual plants in each property. Both variables with high heterogeneity, indicated by the value of the coefficient of variation (41.3 to 65.1%).
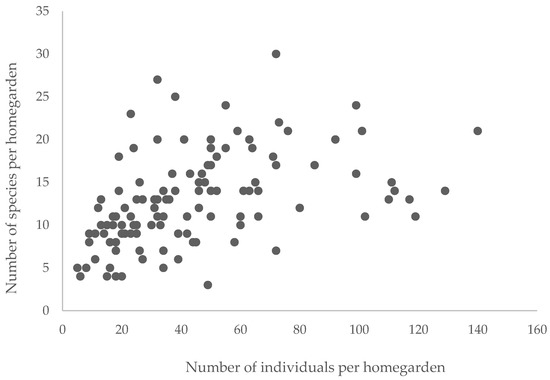
Figure 2.
Distribution of plant species and individual plants in agroforestry homegardens in Western Pará, Brazilian Amazon.
Considering all the home gardens evaluated, the Spearman correlation test indicated that there is a significance (p-value =< 0.0001) with a weak relationship (r: 0.438) between the number of species and the number of individual plants in the home gardens. Considering the different zones studied, moderate (r: 0.50 to 0.67), and significant (p-value < 0.05) correlations (Pearson’s coefficient) were identified for these two variables.
The range expressed in Table 1 shows that even in the different study zones, the minimum (4 to 6) and maximum (up to 30) values for the number of species are similar, except for the floodplain area where the maximum value was lower (16), revealing greater species richness in urban and peri-urban areas. The characteristic of the annual flooding of floodplain areas determines that, according to reports from residents, many crops are limited because they do not withstand flooding for several months.

Table 1.
Number of species (richness) and sum of number of vegetal individual plants in agroforestry homegardens, in different zones, in the western region of Pará, Brazilian Amazonia.
The average number of species per home garden ranged from 10.5 to 14.5 and showed no statistically significant difference (p-value = 0.06; F = 2.2770 and GL = 4) in the five zones evaluated. For the number of individual plants, the average was distinct between the areas, with values between 30.9 and 62.5, reflected in a statistically significant difference (p-value = 0.0005; F = 5.8099 and GL = 4), with emphasis on the floodplain and indigenous areas that differed from two other zones (peri-urban and tourist, respectively) and each other.
The distribution of absolute and relative frequency for the number of species per home garden (Figure 3A) showed that the highest concentration (67.2%) occurs in units with 6.8 to 16.5 species, observed in 70 of the 119 home gardens sampled, expressing that environments with greater diversity predominate in the sampling universe of this research. As for the sum of individual plants, it is noted that 39 home gardens have from 5 to 26.8 individual plants (Figure 3B), the most expressive class (32.8%) in the distribution of the study. The next class of this variable (26.8 to 49.7) is also relevant, covering 31.1% of the sampled home gardens, thus demonstrating that more than half of the sampled areas contain less than 49 plants in the environment.
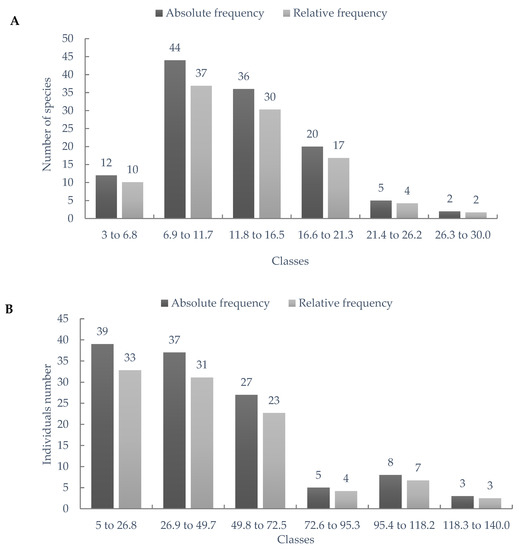
Figure 3.
Distribution by frequency classes for number of species (A) and sum of vegetal individual plants (B) in agroforestry homegardens in Western Pará, Brazilian Amazon.
3.2. Sociocultural Use of Species in Homegardens
The species found in home gardens have diverse purposes and can be food, medicinal, shading, ornamental, or even have dual use (food and medicinal). The cultivation of species mentioned solely for food purposes represented 84.5% of the individual plants found (4497), distributed in 104 species, while 4.5% (41 species) have medicinal and medicinal/food uses (2.9% and 1.6%, respectively), indicating that, mostly, the maintenance of plants in home gardens in the study areas is intended to meet the demands of self-consumption, food security, and immediate health care, which shows the multifunctionality of these environments.
Regarding medicinal use, species were found for this destination in 42.8% of the home gardens inventoried, with emphasis on six species—Ocimum basilicum L. (alfavaca), Carapa sp. (andiroba), Dipteryx sp. (cumaru), Crescentia cujete L. (cuisine), Zingiber officinale Roscoe. (ginger), and Jatropha gossypifolia L. (purple pawn)—which had a higher abundance.
3.3. Form of Life and Origin of Plants
Regarding the form of life or habit of the inventoried vegetal plants, there was a predominance (Figure 4) of individual arboreal plants (trees) distributed in 85 species. Next, the herb/herbaceous plants dominated with 50 species, palm trees with 11 species, and shrubs with 8.3% of the individual plants distributed in 22 species. Together, the plants listed in the categories of life form bamboo, liana, and tree/shrub were responsible for 0.7% of the total individual plants and 20 species.
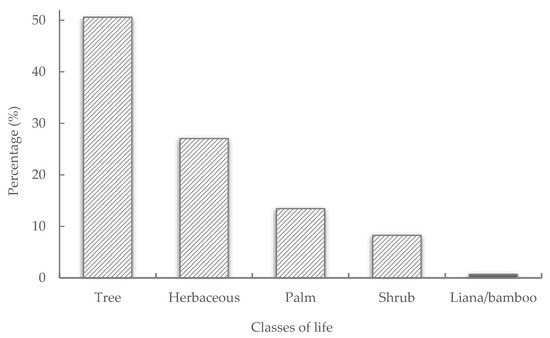
Figure 4.
Percentage of plants distributed classes of life form or habit in agroforestry homegardens in the west of Pará, Brazilian Amazonia.
Regarding the total number of plants found, approximately half of them have native origin in Brazil (49.5%), while the others (50.5%) are of exotic origin, with 40.4% cultivated and 10.1% naturalized, according to the classification of the Reflora program (Flora and Funga do Brasil, 2022). When considering the number of species, this proportion is maintained, with 103 species of native origin (54.8%) and 85 of exotic origin, subdivided into cultivated (31.4%) and naturalized (26.0%).
Still addressing the origin of the plants, it is noteworthy that for shading, exclusive use of native (100%) plants was identified, possibly by maintaining forest species when creating home gardens or even by managing local natural regeneration. This result is opposite to that found for species with a dual purpose (food/medicinal), in which all individual plants were of exotic origin (88.2%—cultivated and 11.8%—naturalized). It is pointed out, however, that species indicated for other uses than shading also result in the shade in home gardens, which, however, was not considered in this work, where the criterion for inference is the primary use pointed out by the maintainers of the home gardens. For ornamental use, plants of exotic origin stood out (57.3%), while for medicinal use, evidence is that 60.5% are of native origin. The food use category was the only one that presented a relatively equitable distribution for origin, where 44.3% are considered native, while 55.9% are classified as exotic (cultivated 44.1% and naturalized 11.6%).
3.4. Characteristics of Homegardens in the Five Zones
Evaluating the categories of plant use in the different zones/areas of the study, it is noted that in all of them, the use of plants for food use predominated (68.4 to 92.4% per area). In the second place, shading plants stood out in indigenous and floodplain areas (8.3% and 26.7%, respectively), while in peri-urban and urban areas, this place belonged to plants for medicinal use (2.9 to 3.9%). In the tourist area, ornamental plants (15.2%) appeared as the second prominence in the home gardens. Notably, ornamental use was indicated for 1.5% of all inventoried plants (89 individual plants distributed in 19 species).
It is also noted that, in the indigenous, peri-urban, and urban areas, the tree component (trees) dominated 45.6% to 65.6% of the individual plants. Palm trees stood out in the indigenous and peri-urban areas, occupying the second place in the number of individual plants (12.8 to 28.2%). In the tourist area, this dynamic was different, and all plants had a similar distribution between trees and herbs (35.9 to 38.9%).
Still, in this analysis, the floodplain area was the one that showed the most differentiated performance from the others, with a predominance of individual plants of herbaceous size (52.6%) stimulated by the use of suspended flower beds for the cultivation of vegetables and medicinal herbs, mainly.
Plants of exotic origin predominate in the peri-urban, urban, and tourist areas (53.8%, 57.9%, and 60.5%, respectively). In the peri-urban area, exotic species are mainly used for food purposes, while in the tourist zone, they are intended for both food and ornamental use. In the indigenous area, the highest percentage is of native origin (62.0%), driven by plants for food use and shading. Finally, in the floodplain area, the distribution was mathematically similar (50.1% and 49.9%) for this approach.
3.5. Featured Species in Homegardens
The twenty most frequent species in the home gardens have food purposes (Table 2), with a major emphasis on the fruit-bearing trees of permanent cultivation, such as mango, cupuaçu, and orange, present in more than half of the areas, evidencing the essentiality of the production of fruits in home gardens.

Table 2.
Popular name and percentages of frequency and abundance of the twenty species with the highest frequency value in agroforestry homegardens in the west of Pará, Brazilian Amazonia.
The twenty most abundant species represented 66.5% of all inventoried individual plants, while the 10 most abundant represented 47.9%. Cupuaçu, banana, and açaí stand out with the highest sum of individual plants (414, 352, and 308, respectively).
4. Discussion
4.1. General Patterns
The maintenance and inclusion of unique, endemic, or little-known species may indicate a reserve of crucial species that still reach future markets in addition to being configured as a stock of genetic materials [8,41,42] in addition to the need for a systematic effort to improve the conservation and availability of wild relatives of crops for use in plant breeding [43].
The survey’s distribution of individual plants and species confirmed the Pareto principle, which predicts that about 80% of the consequences are produced by 20% of the causes [44]. Also called the power law, this concept was observed in real data and is considered an auxiliary tool to understand the behavior and parameters of distributions, with potential application in several natural situations [45].
The correlations suggest that, despite a linear and partial relationship between the number of species and individual plants in the backyards, factors related to space management, family dynamics of area use, and management of controlled units determine the composition of the backyards. The conditions described show that each yard has particularities, compositions, and structures that are different from each other and locations [41]. In the context of backyards in Santarém/PA, the circulation of plants and plant material maintains a high agrobiodiversity in the backyards [45], showing an intense dynamic of modification. In addition, the origin of the people who manage home gardens can also influence the composition of these land-use systems in the Amazon [46].
Notably, the indigenous home gardens were superior to the peri-urban ones in terms of the number of individual plants. This may be because indigenous areas are more extensive than peri-urban areas. Even in such a distinct environment, as to the location and environmental characteristics, the maintenance and cultivation of individual vegetable plants in home gardens are maintained in familiar environments. Floristic composition and species selection in home gardens are strongly influenced by the decisions of the owners aiming at food security [13].
Evaluating the size of the home garden, information not available for all zones in this analysis, and its relations with the number of individual plants and species could bring more effective answers about the relationships established in these spaces and the choices for cultivation and maintenance since it is likely that in indigenous areas, due to the availability of space, there would be a greater total number of plants in the home gardens. In the floodplain, despite being a rural area with large extensions, there is a limitation of cultivated area for reasons of the annual flood regime. A study published with data from the indigenous zone showed that among 20 home gardens evaluated, there is a great diversity of use of the areas (ten categories of use), where the average size of the home gardens was 6049.3 ± 5295.8 m2 [47]. Also, these places are intended for productive practices, with greater frequency of fruit species, and thus are of great importance for family food sovereignty and income promotion. As for data from the flooding zone, the average size of home gardens was 3300 m2, with a substantial presence of native tree species typical of this environment [48].
The research also made it possible to identify home gardens in different environments in the municipalities so that the five zones evaluated have an evident distinction between themselves in occupation, demonstrated by the statistically significant differences in the number of individual plants. Differences in agrobiodiversity in home gardens arise through the interaction of human action, plant responses, and unique soil properties in relation to socioeconomic and historical trajectories over time [49]. These differences were also expressed by the coefficient of variation between the areas (Table 1), which ranged from 34.7 to 50.3% for the number of species and from 39.5 to 68.2% for the number of individual plants, according to [50], indicating high heterogeneity (CV > 30%) in the samples.
4.2. Sociocultural Use of Species in Homegardens
The emphasis on the food use of products from home gardens may indicate an economic impact on the income of the keepers through the reduction in costs with the acquisition of external products due to the supplies provided. The cultivation of food species in home gardens is an important feature of this type of production system since it is characterized as an accessible and safe source of food and inputs, contributing to a more diverse and healthy family diet [15,51], expressing the direct and indirect relationships between home gardens and food security through evidence of a supplementary source of food [10].
The fact that food use stands out in this analysis reinforces the importance and the relationship between human beings and the diversity and periodicity of food supply. The act of eating is also developed according to social rules, individual trajectories, and group values [52]. This connection is also expressed in the literature, such as the example of the work of Rubem Alves that discusses food as affection and identity [53]. Thus, food, as a cultural product, is surrounded by traditions, rituals, and affective relationships that preserve traditional elements [54], highlighting the importance of home gardens in the identity of communities.
Another aspect inherent in the discussion of the food use of plant species in home gardens since the beginning of the twenty-first century is that about 400,000 species from biodiversity and agrobiodiversity could serve as human food in the world [55], and a long history of plant domestication has influenced Amazonian tree communities [56], and some species have been domesticated in home gardens [57]. However, about 15 species currently account for 90% of the world’s human diet [58], and some species account for more than 50% of the global supply of calories [59].
For the Brazilian conditions, ref. [60] highlights that despite the richness and potential of Brazilian biodiversity, its use as food is still neglected and little known. In this regard, home gardens can be constituted as a space of resistance or a barrier, as opposed to the tendency to simplify food, as they concentrate countless individual plants that provide biomass of human interest (mainly food), which in natural environments, would have a lower density of plants for use through extractivism.
On the other hand, regarding the Brazilian production of non-timber forest products, the food category corresponds to 96% of all production [61]. As an example, species such as bacaba (Oenocarpus bacaba Mart.), pupunha (Bactris gasipaes Mart.), sapucaia chestnut (Lecythis pisonis Cambess.), tucumã (Astrocaryum aculeatum G.Mey.), açaí (Euterpe oleracea Mart.), and uxi (Endopleura uchi) (Huber) Cuatrec), were identified in the home gardens studied and have acceptance and preference in the local daily life, peculiar flavors, and are fruit-bearing plants maintained or cultivated to supplement household food. It should be noted that the frequency of food consumption in 10 years (2008 to 2018) showed an increase in the consumption of açaí in Brazil, in urban and rural areas, especially in the northern region, with an increase from 9.0% to 12.4% [62], covering all income groups. Still, considering this report, in the northern region, there was a reduction in the consumption of fruits such as bananas, mangoes, and oranges, and in the national scenario, the consumption of fruits, vegetables, and legumes showed a reduction, being below the recommended level.
At the same time, data from the Food and Nutrition Surveillance System [63] show that for adults in the municipalities of Santarém and Mojuí dos Campos, fruit consumption in 2021 was 43 to 79%, while the consumption of vegetables was more emphatic, with 71 to 83%. This same source also indicates that 58 to 63% of the adults evaluated in the three municipalities of this study are overweight or have some degree of obesity. These findings point to the importance of maintaining home gardens to contribute to improving nutrition as a possible strategy of diversification and alternation in the availability of fruits, mainly. Since food sovereignty is associated with sustainable systems as a way to reduce hunger and malnutrition [64], home gardens could play a leading role, given their richness of species and food potential.
On the other hand, a report on food and nutrition security (2018 to 2020) in Latin America [65] indicated that in 23.5% of the Brazilian population, moderate or severe food insecurity is prevalent and that 7.5 million people face severe food insecurity. Food and nutritional security, as expressed in Law No. 11346/2006, consists of the realization of the right of all to regular and permanent access to quality food in sufficient quantity [66]. Studies with adolescents in the urban area of municipalities in Brazilian Amazonia showed a prevalence of food and nutritional insecurity in households and low consumption of vegetables, fruits, and legumes [52].
The number of species used for medicinal purposes (42.8%) indicates the strong importance of popular medicine in the daily life and well-being of the populations in the studied areas. Other studies of backyards in the Amazon have also shown the predominance of plants intended for medicinal use [41], where the choice and maintenance of species are generally assumed by women [67,68].
In this context, the commercial production at the national level of medicinal products could be more expressive and presents a low variety of product supply [61]. Thus, the cultivation of species for medicinal purposes in home gardens holds great importance for the health of maintainers. Added to this is the distance between the areas surveyed and the urban centers, which are common sources of access to conventional medicines originating in pharmacies. In the case of floodplain areas, the dynamics of flooding and drought impose additional difficulties to moving to urban centers, making the use of medicinal products a necessary measure that is sometimes reinforced by the traditional practices of riverside populations [69,70].
Still, addressing the use indicated for plants in home gardens, the cultivation/maintenance for shading stands out, pointing to 9.4% (29 species—502 individual plants). The shaded areas in home gardens are often used for activities such as leisure, rest, and work [8], also providing an important microclimate to the development of different plant and animal species [71] where they constitute the places of preference for coexistence by families due to the sensations of environmental comfort [72], including strengthening more resilient green cities [73].
4.3. Characteristics of Homegardens in the Five Zones
For the floodplain, it was reported during the research that there are difficulties in the cultivation of tree species in the areas subjected to flooding, which restricts the insertion of the tree component. Thus, native species with the capacity to adapt to the seasonal regime are prioritized [74], whereas home garden owners demand less effort to insert perennial crop species in their home gardens, opting to resort to spontaneous species of native origin [73]. In this environment, potted cultivation is recurrent due to the ease of transportation between homes, a typical activity due to periodic floods.
4.4. Featured Species in Homegardens
Diagnosis with more than 500 families of farmers in the Santarém plateau showed that 70% of farmers had expanded their crops with a focus on fruit production [74]. This pattern was also observed in the evaluation of home gardens in several places in the country [75] and in studies in home gardens in Amazonia [25,76,77], where fruit-bearing trees such as açaí, cupuaçu, and banana are the more frequent species. Among the ten most frequent species (34.5 to 54.6%), exotic species are predominant. The most abundant, but not the most frequent, were the mercury species (Laetia corymbose Spruce ex Benth.), chicory (Eryngium sp.), sapucaia chestnut (Lecythis prisons Cambess.), rubber tree (Hevea brasiliensis (Willd. ex A.Juss.) Müll. Arg), pineapple (Ananas sp.), and cilantro (Coriandrum sativum L.).
Among the twenty most frequent species, fourteen—mango, cupuaçu, orange, coconut, banana, açaí, lemon, cashew, murici, pupunha, papaya, tangerine and chives—stand out, in ascending order, with 55 to 178 individual plants. In this ranking, the prominence of chives can be attributed to the wide use of this species as a spice in regional cuisine in the daily eating routine. Chives, as well as several tropical spices, have numerous nutritional properties [78]. Considering the possibility of forming vegetal gardens in these spaces, it is noteworthy that this practice can serve as an indication of food security, an important item of agrobiodiversity [57]. The inclusion of new species in the diet will certainly increase the food security of Brazilians [60].
Cupuaçu, occurring in 53.8% of the home gardens, has productive regional prominence in the municipalities of the West of Pará. Data from the Agricultural Census indicated the production of 109 tons of cupuaçu fruit in the Lower Amazonia region in 2016 from a planted area of 54 hectares, corresponding to 2096 kg per hectare that year [79]. In the urban area of Belterra, great potential for commercialization of cupuaçu pulp from home gardens was estimated [80].
This study also revealed that producing fruits such as banana, coconut, guava, lemon, papaya, and pupunha stood out for having at least one specimen in each area evaluated. However, most of the species (42.1%) were counted in only one of the studied areas, reflecting the unique behavior and diversity in cultivating the home gardens. This fact shows that these environments are distinct and peculiar, probably due to the circumstantial introduction or cultivation of species not commonly found in most home gardens. These findings reveal that the conservation of these spaces will indicate the conservation of species of little occurrence in the environment, which can contribute to the local biodiversity. The promotion and expansion of these environments can result in a strategy to increase diversity and genetic exchanges and favor local wildlife.
4.5. Importance and Perspectives for Agroforestry Homegardens
The local dynamics, stimulated by small crops such as those carried out in home gardens, are altered and interface with the national and international dynamics of food distribution. In this sense, a document from the Pan American Health Organization, in an approach to Brazilian strategies to combat hunger and poverty, indicated the implementation of productive home gardens as a strategy to minimize food shortages.
Home gardens, with their diversity of species and purposes, pointed out in this study, can be a source of autonomy and contribute to the sovereignty and food security of families by assisting mainly in the availability and variety of food. This entails providing year-round food security at a low cost while sustaining numerous ecosystem services [10]. A study in municipalities of Legal Amazonia indicated that, due to the situation of food and nutritional insecurity, policies should encourage administrators, media, and civil society to include in their agenda and seek actions of the public power related to the greater supply of healthy foods [52].
The results reveal that in the home gardens evaluated, there is a predominance of species of food use, primarily fruit-bearing, showing a direction for incentive actions and/or for ordering public policies and programs aimed at these family spaces. The sale of manufactured products obtained from these home gardens, such as pulps, jellies, sweets, and handicrafts, among others, could mean a form of exploitation and income generation. Local productive initiatives already recognize the potential of these environments, such as, for example, the agroecological fair for the commercialization of products from productive urban home gardens [81] in the Pérola do Maicá neighborhood in Santarém [82]. A study in this region also showed that home gardens managed by women generate income and produce food to ensure food security [83] and because they are a small-scale system, they can facilitate the participation of women and other marginalized groups [27].
The commercialization of products or surpluses from these home gardens, mainly due to the potential expressed in this survey for fruit species, could strengthen the so-called short commercialization chains or alternative food circuits that are based on an agri-food system that aims to strengthen the relationship between consumer and producer, promoting the reconnection between those involved and incorporating collective social and cultural values [84,85,86].
The cultivation or maintenance of vegetal plants in home gardens has varied dynamics, and this inclusion is performed in order for the opportunities and experiences stimulated by exchanges, visits, and social interactions. In this sense, home gardens promote agrobiodiversity, understood as the processes of natural selection and the careful selection and inventive developments of farmers over time [57]. Thus, the home gardens addressed in this analysis were shown as mixed agroecosystems, with species expressing cultural and local knowledge of diversity.
The home gardens denoted complex structures, with interconnection between the elements that make up the environment; despite the quantitative similarities identified in the evaluated areas, it has become clear that these environments have a dependent dynamic based on the influence of different social, economic, and ecological factors. The diversity expressed in home gardens shows possible resilience to facing economic and climate cycles.
Despite the relevance above, there are no records of policies or projects aimed at managing the organic waste generated by these home gardens, such as leaves, fruits, and branches. The municipal laws of Santarém, specifically laws 17.894/2004 and 20.534/2018, prohibit the open burning of materials that may cause negative environmental impacts. Furthermore, these laws establish the requirement for proper waste disposal [87]; however, the practice is still widely used, evidenced by statistics of fires and urban occurrences [88], which can lead to conflict and inconveniences in community coexistence. In this sense, payment for environmental services could be an alternative to reward service providers [89] if it were linked, for example, to the proper disposal of waste as strategies for valuing home gardens. The transformation of organic materials from these environments, via composting, with integrated and synchronized collections by the government, could also result in the maintenance of individual tree plants in the home gardens.
This research highlights the importance of making systematic efforts to improve the conservation and availability of plant species in yards, especially those that are unique, endemic, or little known. Carrying out a qualitative analysis of a complex system, such as home gardens, involving different levels, such as the areas selected in this study, requires admitting that the inferences are limited to the “cuts” produced by the data presented. Thus, to represent, in a more in-depth way, the complexity of the insertion or maintenance of vegetal plants in these environments, a multidisciplinary weighting would be necessary to point out the direction and causality of the choices made by the owners of the home gardens.
The analysis of home gardens can bring visibility to these spaces, reducing vulnerability and neglect about recognizing their socioeconomic and environmental importance, to which they are subject, to meet the demands of real estate expansion. Since home gardens are considered important and socially relevant ecosystems and not on the margins of conservation policies and actions, these environments could expand and improve their conditions.
5. Conclusions
The evaluated backyards exhibit a diversity of plant species, totaling 188 species belonging to 62 botanical families. It was also observed that the majority of species (88.5%) occur in less than 15% of the backyards, and over half of the species (56.6%) were observed in less than 2.5% of the studied backyards. This indicates a significant variability in species distribution. The data also reveal that the composition of the backyards is influenced by factors such as space management, family dynamics in land use, and property management. Each backyard has its own particularities and distinct structures, which are linked to the owners’ decisions concerning food security and the specific environmental characteristics of each locality.
The results obtained in this study can provide an overview of the species cultivated in backyards in the Amazon region, especially regarding the species of greater abundance and frequency (Mangifera indica L.; Theobroma grandiflorum (Willd. ex Sprng); Citrus sp.; Cocos nucifera L. and Musa paradiasiaca). These spaces present a great diversity of cultivated species, with a similar distribution between exotic and native species, but with variations in composition depending on the environment in which they are located.
Considering the data from this study, it is encouraging to state that, for conservation projects and/or technical assistance, priority should be given to the species that represent 80% of the individuals and those most found in the study, especially the native ones. This distribution pattern can be a useful tool for understanding distribution behaviors. Thus, efforts can be directed to already familiar crops suitable for residents, enabling scale production. In the same way, it is advisable that the efforts of social or cooperative organizations aiming at the production or aggregation of value consider the species listed in this study as priorities to guide actions or projects. In this research, we evaluated three regions in different municipalities in the Amazon; however, future research with a greater number of sampled regions to validate the results found in this study is necessary.
Author Contributions
Methodology, L.S.d.S.L.; Validation, D.P. and M.P.d.M.B.; Formal analysis, L.S.d.S.L., T.A.V., T.G.d.S.O., V.S.d.S. and Á.F.d.S.; Investigation, D.P., T.G.d.S.O., V.S.d.S., Á.F.d.S. and P.d.S.F.d.L.; Data curation, P.d.S.F.d.L. and I.V.P.G.; Writing—original draft, L.S.d.S.L.; Writing—review & editing, D.P., L.G.M., M.P.d.M.B., T.A.V. and A.S.T.; Supervision, D.P. and L.G.M.; Project administration, L.S.d.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Federal University of Western Pará within the scope of the institution’s academic and scientific activities. Additionally, the payment of article processing charges (APC) was funded through resources provided by the Call for Proposals 03/2022/PROPPIT/UFOPA-PAPCIQ, Program for Support of Qualified Scientific Publications.
Institutional Review Board Statement
Informed consent was obtained verbally from all owners of agroforestry backyards for the conduction of the floristic composition research.
Informed Consent Statement
The research was registered with the Genetic Heritage Management Council and the National System for the Management of Genetic Heritage and Traditional Knowledge under access registers No. A6E86AF and A8E5DE0.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their thanks to the owners as well as to the home garden maintainers who kindly allowed the collection of information in their homes and collaborated with this research. Also, gratitude is extended to the students and technicians involved in the academic actions that resulted in the database used for the writing of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. An Introduction to Agroforestry: Four Decades of Scientific Developments; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Almeida, L.S.; Gama, J.R.V. Quintais agroflorestais: Estrutura, composição florística e aspectos socioambientais em área de assentamento rural na Amazônia brasileira. Ciênc. Florest. 2014, 24, 1041–1053. [Google Scholar] [CrossRef]
- Farrell, J.G.; Altieri, M.A. Sistema agroflorestais. In Agroecologia: Bases Cientifica para Uma Agricultura Sustentável, 3rd ed.; Altieri, M.A., Ed.; Expressão popular: São Paulo, Brazil; AS-PTA: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Lowe, W.A.M.; Silva, G.L.L.P.; Pushpakumara, D.K.N.G. Homegardens as a modern carbon storage: Assessment of tree diversity and above-ground biomass of homegardens in Matale district, Sri Lanka. Urban For. Urban Green. 2022, 74, 127671. [Google Scholar] [CrossRef]
- Duffy, C.; Toth, G.G.; Hagan, R.P.O.; McKeown, P.C.; Rahman, S.A.; Widyaningsuh, Y.; Sunderland, T.C.H.; Spillane, C. Agroforestry contributions to smallholder farmer food security in Indonesia. Agrofor. Syst. 2021, 95, 1109–1124. [Google Scholar] [CrossRef]
- Pereira, C.N.; Maneschy, R.Q.; Oliveira, P.D.; De Souza Oliveira, I.K. Caracterização de quintais agroflorestais no projeto de assentamento Belo Horizonte I, São Domingos do Araguaia, Pará. Rev. Agroecossist. 2013, 2, 73–81. [Google Scholar] [CrossRef]
- Santos, D.W.S.; Vieira, T.A. Plant diversity of tree and shrub strata of agroforestry homegardens in an agro-extractive settlement, Monte Alegre, Pará. Ciênc. Nat. 2021, 43, e1. [Google Scholar] [CrossRef]
- Rayol, B.P.; Miranda, I.S. Quintais agroflorestais na Amazônia Central: Caracterização, importância social e agrobiodiversidade. Ciênc. Florest. 2019, 29, 1614–1629. [Google Scholar] [CrossRef]
- Vieira, T.A.; Rosa, L.S.; Santos, M.M.L.S. Condições socioeconômicas para o manejo de quintais agroflorestais em Bonito, Pará. Rev. Bras. Ciênc. Agrár. 2013, 8, 458–463. [Google Scholar] [CrossRef]
- Mattsson, E.; Ostwald, M.; Nissanka, S.P. What is good about Sri Lankan homegardens with regards to food security? A synthesis of the current scientific knowledge of a multifunctional land-use system. Agrofor. Syst. 2018, 92, 1469–1484. [Google Scholar] [CrossRef]
- Suwardi, A.B.; Navia, Z.I.; Mubarak, A.; Mardudi, M. Diversity of home garden plants and their contribution to promoting sustainable livelihoods for local communities living near Serbajadi protected forest in Aceh Timur region, Indonesia. Biol. Agric. Hortic. 2023, 39, 1–13. [Google Scholar] [CrossRef]
- Bortoluzzi, R.N.; Moreira, L.L.; Vieira, C.R. Diversidade de plantas alimentares em quintais agroflorestais de Cuiabá e Várzea Grande, Mato Grosso, Brasil. Interações 2021, 22, 295–307. [Google Scholar] [CrossRef]
- Rayol, B.P.; Do Vale, I.; Miranda, I.S. Tree and palm diversity in homegardens in the Central Amazon. Agrofor. Syst. 2019, 93, 515–529. [Google Scholar] [CrossRef]
- Ramli, M.R.; Malek, S.; Milow, P. Diversity, structure, and application of self-organizing map on plant species in homegardens. Trop. Ecol. 2022, 1–16. [Google Scholar] [CrossRef]
- Yinebeb, M.; Lulekal, E.; Bekele, T.; Lemessa, D. Homegardens plant species richness and their use types have positive associations across agricultural landscapes of Northwest Ethiopia. Glob. Ecol. Conserv. 2022, 40, e02342. [Google Scholar] [CrossRef]
- Silva, I.C. Sistemas Agroflorestais: Conceitos e Métodos; SBSAF: Itabuna, Brazil, 2013. [Google Scholar]
- De Vasconcellos, R.C.; Beltrão, N.E.S. Avaliação de prestação de serviços ecossistêmicos em sistemas agroflorestais através de indicadores ambientais. Interações 2018, 19, 209–220. [Google Scholar] [CrossRef]
- Viana, A.P.S.; Pauletto, D.; Gama, J.R.V.; Pires, A.P.; Freitas, H.H.; Pacheco, A. Meliponicultura em sistemas agroflorestais em Belterra, Pará. ACTA Apic. Bras. 2021, 9, e7913. [Google Scholar] [CrossRef]
- Guimarães, A.S. Quintais Agroflorestais e Estado Nutricional Familiar na Comunidade Santa Maria, Santarém-PA. Ph.D. Thesis, Universidade Federal do Oeste do Pará, Santarém, Brazil, 2015. [Google Scholar]
- Getachew, E.; Lemessa, D.; Leulekal, E. Socio-ecological determinants of species composition of crops in homegardens of southern Ethiopia. Agrofor. Syst. 2022, 96, 11–21. [Google Scholar] [CrossRef]
- Matos Filho, J.R.; Costa Moraes, L.L.; da Luz Freitas, J.; Cruz Junior, F.O.; Santos, A.C. Quintais agroflorestais em uma comunidade rural no vale do Rio Araguari, Amazônia Oriental. Rev. Ibero-Am. Ciênc. Ambient. 2021, 12, 47–62. [Google Scholar] [CrossRef]
- Silva, A.C.G.F.; Anjos, M.C.R.; Anjos, A. Quintais produtivos: Para além do acesso à alimentação saudável, um espaço de resgate do ser. Guaju 2016, 2, 77–101. [Google Scholar] [CrossRef]
- Kabashima, Y.; Andrade, M.L.; Gandara, F.B.; Tomas, F.L. Sistemas agroflorestais em áreas urbanas. Rev. Soc. Bras. Arborização Urbana 2009, 4, 01–20. [Google Scholar] [CrossRef]
- Gervazio, W. Agrobiodiversidade e Qualidade do Solo em Quintais Agroflorestais Urbanos na Cidade de Alta Floresta–MT. 2015.136 f. Ph.D. Thesis, Universidade do Estado de Mato Grosso, Cáceres, Brazil, 2015. [Google Scholar]
- Vieira, T.A.; Rosa, L.S.; Santos, M.M.L.S. Agrobiodiversidade de quintais agroflorestais no município de Bonito, Estado do Pará. Rev. Ciênc. Agrár. 2012, 55, 159–166. [Google Scholar] [CrossRef]
- Barlow, J.; França, F.; Gardner, T.A.; Hicks, C.C.; Lennox, G.D.; Berenguer, E.; Castello, L.; Economo, E.P.; Ferreira, J.; Guénard, B.; et al. The future of hyperdiverse tropical ecosystems. Nature 2018, 559, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mina, U.; Kumar, B.M. Homegarden agroforestry systems in achievement of Sustainable Development Goals. A review. Agron. Sustain. Dev. 2022, 42, 44. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.G.R.; Carmuça, A.M.; Esmeraldo, G.G.S.L.; Sousa, N.R. Quintais produtivos: Contribuição à segurança alimentar e ao desenvolvimento sustentável local na perspectiva da agricultura familiar (o caso do Assentamento Alegre, no município de Quixeramobim/CE). Rev. Bras. Agroecol. 2013, 8, 135–147. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística—Ibge. Classificação e Caracterização dos Espaços Rurais e Urbanos do Brasil: Uma Primeira Aproximação; Instituto Brasileiro de Geografia e Estatística—Ibge: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Veiga, J.E. Nem tudo é urbano. Ciênc. Cult. 2004, 56, 26–29. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Martorano, L.G.; Nechet, D.; Pereira, L.C. Tipologia climática do Estado do Pará: Adaptação do método de Köppen. Bol. Geogr. Teor. 1993, 23, 45–46. [Google Scholar]
- Martorano, L.G.; Soares, W.B.; Moraes, J.R.D.S.C.D.; Nascimento, W.; Aparecido, L.E.D.O.; Villa, P.M. Climatology of Air Temperature in Belterra: Thermal Regulation Ecosystem Services Provided by the Tapajós National Forest in the Amazon. Rev. Bras. Meteorol. 2021, 36, 327–337. [Google Scholar] [CrossRef]
- Oliveira Júnior, R.C.; Corrêa, J.R.V. Caracterização Dos Solos do Município de Belterra, Estado do Pará; Embrapa Amazônia Oriental: Belém, Brazil, 2001; Volume 88, pp. 1–39. [Google Scholar]
- Rodrigues, T.E.; Santos, P.L.; Oliveira Júnior, R.C.; Valente, M.A.; Silva, J.M.L.; Cardoso Júnior, E.Q. Caracterização Dos Solos da Área do Planalto de Belterra, Município de Santarém, Estado do Pará; Embrapa Amazônia Oriental: Belém, Brazil, 2001; Volume 15, pp. 1–54. [Google Scholar]
- Ferreira Neto, H.G.; Pereira, C.A.; Almeida, E.N. Dinâmica da produção de alimentos na região de Santarém, Oeste do Pará. Rev. Terceira Margem Amaz. 2019, 4, 12. [Google Scholar]
- Bernard, H.R. Research Methods in Anthropology: Qualitative and Quantitative Approaches; AltaMira Press: Lanham, MD, USA, 2005. [Google Scholar]
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 30 August 2022).
- Silva, V.; Nascimento, V.T.; Soldati, G.T.; Medeiros, M.F.T.; de Albuquerqueb, U.P. Métodos e Técnicas na Pesquisa Etnobiológica e Etnoecológica; NUPEEA: Recife, Brazil, 2010; pp. 189–206. [Google Scholar]
- Ayres, M.; Ayres Júnior, M.; Ayres, D.L.; Santos, A.D.A. Aplicações Estatísticas Nas Áreas das Ciências Bio-Médicas; Instituto Mamirauá: Belém, Brazil, 2007; Volume 364, Available online: https://www.mamiraua.org.br/downloads/programas/ (accessed on 2 May 2022).
- Carvalho, T.K.N.; de Oliveira Abreu, D.B.; de Lucena, C.M.; Pedrosa, K.M.; Neto, C.F.A.V.; Alves, C.A.B.; Félix, L.P.; Florentino, A.T.N.; Alves, R.R.N.; Andrade, L.A.; et al. Structure and floristics of home gardens in an altitudinal marsh in northeastern Brazil. Ethnobot. Res. Appl. 2013, 11, 29–47. [Google Scholar]
- Quaresma, A.P.; Almeida, R.H.C.; Oliveira, C.M.; Kato, O.R. Composição florística e faunística de quintais agroflorestais da agricultura familiar no nordeste paraense. Rev. Verde Agroecol. Desenvolv. Sustent. 2015, 10, 76–84. [Google Scholar] [CrossRef]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- Dunford, R.; Su, Q.; Tamang, E. The Pareto Principle. Plymouth Stud. Sci. 2014, 7, 140–148. [Google Scholar]
- Newman, M.E. Power laws, Pareto distributions and Zipf’s law. Contemp. Phys. 2005, 46, 323–351. [Google Scholar] [CrossRef]
- Garcia, B.N.R.; Vieira, T.A.; Oliveira, F.A. Tree and shrub diversity in agroforestry homegardens in rural community in Eastern Amazon. Floresta 2017, 47, 543–552. [Google Scholar] [CrossRef]
- Da Silva, A.M.; Pauletto, D.; da Silva, A.F.; Sousa, V.S.; de Sousa Oliveira, T.G.; da Silva, A.F.; Rego, J.F.M.; Vieira, T.A.; da Silva Santos, A. Diversidade e multifuncionalidade de quintais agroflorestais em aldeias da Terra Indígena Tupinambá, Santarém, Pará. Biodiversidade 2022, 21, 4. [Google Scholar]
- Pauletto, D.; Machado, L.; Figueira, N.; Cardoso, G. Caracterização de quintais agroflorestais da Várzea: Estudo de caso na comunidade Alto Jari em Santarém–Pará. Cad. Agroecol. 2020, 15, 2. [Google Scholar]
- Fraser, J.A.; Junqueira, A.B.; Clement, C.R. Homegardens on Amazonian dark earths, non-anthropogenic upland, and floodplain soils along the Brazilian middle Madeira River exhibit diverging agrobiodiversity. Econ. Bot. 2011, 65, 1–12. [Google Scholar] [CrossRef]
- Garcia, C.H. Tabelas para Classificação do Coeficiente de Variação; IPEF: Washington, DC, USA, 1989; Volume 171, p. 11. [Google Scholar]
- Garcia, B.N.R.; Vieira, T.A.; Oliveira, F.A. Aspectos socioeconômicos de manejadores de quintais agroflorestais: O caso de uma comunidade rural na Amazônia. Rev. Contrib. Cienc. Soc. 2017, 1, 1–10. [Google Scholar]
- Guerra, L.D.D.S.; Espinosa, M.M.; Bezerra, A.C.D.; Guimarães, L.V.; Martins, M.S.A.S. Desafios para a Segurança Alimentar e Nutricional na Amazônia: Disponibilidade e consumo em domicílios com adolescentes. Ciênc. Saúde Colet. 2018, 23, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Bauer, C. Está na Mesa: Receitas com Pitadas Literárias; Papirus Editora: Campinas, Brazil, 2005. [Google Scholar]
- Zuin, L.F.S.; Zuin, P.B. Alimentação é cultura: Aspectos históricos e culturais que envolvem a alimentação e o ato de se alimentar: [revisão]. Nutr. Rev. Soc. Bras. Aliment. Nutr. 2009, 34, 225–241. [Google Scholar]
- Santilli, J. Agrobiodiversidade e Direitos dos Agricultores; Editora Peirópolis LTDA: Sao Paulo, Brazil, 2009. [Google Scholar]
- Levis, C.; Costa, F.R.C.; Bongers, F.; Peña-Claros, M.; Clement, C.R.; Junqueira, A.B.; Neves, E.G.; Tamanaha, E.K.; Figueiredo, F.O.G.; Salomão, R.P.; et al. Persistent effects of pre-Columbian plant domestication on Amazonian Forest composition. Science 2017, 355, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations—FAO. Training Building on Gender, Agrobiodiversity and Local Knowledge; FAO: Rome, Italy, 2005. [Google Scholar]
- Paterniani, E. Agricultura sustentável nos trópicos. Estud. Av. 2001, 15, 303–326. [Google Scholar] [CrossRef]
- Palombi, L.; Sessa, R. Climate-Smart Agriculture: Sourcebook; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Tuler, A.C.; Peixoto, A.L.; da Silva, N.C.B. Plantas alimentícias não convencionais (PANC) na comunidade rural de São José da Figueira, Durandé, Minas Gerais, Brasil. Rodriguésia 2019, 70, e01142018. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística—IBGE. Produção da Extração Vegetal e da Silvicultura (PEVS); Instituto Brasileiro de Geografia e Estatística—IBGE: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2017–2018: Análise do Consumo Alimentar Pessoal no Brasil; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Sistema de Vigilância Alimentar e Nutricional (Sisvan). Relatórios de Acesso Público. Ministério da Saúde. Available online: https://sisaps.saude.gov.br/sisvan/relatoriopublico/index (accessed on 31 August 2022).
- Maluf, R.S. Segurança Alimentar e Nutricional; Vozes: Petrópolis, Brazil, 2007; 176p. [Google Scholar]
- FAO; FIDA; OPS; WFP; UNICEF. América Latina y el Caribe—Panorama Regional de la Seguridad Alimentaria y Nutricional 2021: Estadísticas y Tendencias; FAO: Santiago, Chile, 2021. [Google Scholar] [CrossRef]
- Brasil. Lei nº 11.326, de 24 de Julho de 2006. Diário Oficial da União: Seção 1, Brasília, DF, ano 2009, n. 128, p. 1–72, 8 jul. 2009. Estabelece as Diretrizes para a Formulação da Política Nacional da Agricultura Familiar e Empreendimentos Familiares Rurais. Available online: https://www.planalto.gov.br/ccivil_03/_ato2004-2006/2006/lei/l11326.htm (accessed on 1 March 2023).
- Florentino, A.T.N.; Araújo, E.L.; Albuquerque, U.P. Contribuição de quintais agroflorestais na conservação de plantas da Caatinga, Município de Caruaru, PE, Brasil. Acta Bot. Bras. 2007, 21, 37–47. [Google Scholar] [CrossRef]
- Siviero, A.; Lin, C.M.; Silveira, M.; Daly, D.C.; Wallace, R.H. Etnobotânica e Botânica Econômica do Acre; Edufac: Penetanguishene, ON, Canada, 2016. [Google Scholar]
- Moura, E.A.F.; do Nascimento, A.C.S.; Corrêa, D.S.S.; Alencar, E.F.; de Sousa, I.S. Sociodemografia da Reserva de Desenvolvimento Sustentável Mamirauá; IDSM: Gau-Odernheim, Germany, 2016. [Google Scholar]
- Nunes, J.G.S.; de Sousa Vale, J.; dos Reis, N.M.; do Nascimento, D.P. População Ribeirinha e Promoção da Saúde. Revista Científica da Faculdade de Educação e Meio Ambiente 2022, 13. [Google Scholar]
- Palheta, I.C. Quintais Urbanos e Plantas Medicinais: Um Estudo Etnobotânico no Bairro São Sebastião, Abaetetuba-PA. Ph.D. Thesis, Universidade do Estado de Pará, Belém, Brazil, 2015. [Google Scholar]
- Lobato, G.J.M.; Martorano, L.G.; Lucas, F.C.A.; Tavares-Martins, A.C.C.; Jardim, M.A.G. Condições Térmico-Hídricas E Percepções de Conforto Ambiental em Quintais Urbanos de Abaetetuba, Pará, Brasil. Revista Raega-O Espaço Geográfico em Análise 2016, 38, 245. [Google Scholar] [CrossRef]
- Ferreira-Alves, E.; Rayol, B. Diversidade das Espécies Arbóreas em Quintais de Várzea da Ilha Saracá, Limoeiro do Ajuru, Pará. Espaço Aberto 2021, 11, 63–80. [Google Scholar] [CrossRef]
- Gama, J.R.V.; Souza, A.L.; Martins, S.V.; Souza, D.R. Comparação entre florestas de várzea e de terra firme do Estado do Pará. Rev. Árvore 2005, 29, 607–616. [Google Scholar] [CrossRef]
- Biassio, A. Agrobiodiversidade em Escala Familiar nos Municípios de Antonina e Morretes (PR): Base para Sustentabilidade Socioeconômica e Ambiental. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2011. [Google Scholar]
- Pereira, A.G.; Alcantara, L.C.S.; De Oliveira, R.E.; Sais, A.C. Plantas com potencial medicinal em quintais agroflorestais: Diversidade entre comunidades rurais do Portal da Amazônia-Mato Grosso, Brasil. Res. Soc. Dev. 2021, 10, e59010615713. [Google Scholar] [CrossRef]
- Souza, A.M.B. Aspectos da segurança alimentar com base em quintais agroflorestais na comunidade rural de Santa Luzia do Induá no município de Capitão Poço, PA. Rev. Agroecossist. 2018, 9, 275–287. [Google Scholar] [CrossRef]
- Silva, A.P.G.D.; Borges, C.D.; Miguel, A.C.A.; Jacomino, A.P.; Mendonça, C.R.B. Características físico-químicas de cebolinhas comum e europeia. Braz. J. Food Technol. 2015, 18, 293–298. [Google Scholar] [CrossRef]
- Censo Agropecuário. 2017. Online. Cupuaçu. Pará. Available online: https://censoagro2017.ibge.gov.br/templates/censo_agro/resultadosagro/agricultura.html?localidade=15&tema=78248> (accessed on 19 January 2023).
- De Matos Rebêlo, A.G.; Capucho, H.L.V.; Pauletto, D.; da Silva, G.R.; dos Santos, M.J.C. Quintais agroflorestais urbanos em Belterra, PA: Importância ecológica e econômica. Terceira Margem Amazônia 2019, 4, 12. [Google Scholar]
- Associação Pérola do Maicá (APM). Rede Social Facebook; Associação Perola do Maicá (APM): Santarém, Brazil, 2022. [Google Scholar]
- Organização Pan-Americana da Saúde (OPAS). Sistemas Alimentares e Nutrição: A Experiência Brasileira para Enfrentar Todas as Formas de má Nutrição; Organização Pan-Americana da Saúde (OPAS): Brasília, Brazil, 2017. [Google Scholar]
- Sousa, W.L.; Santos, A.O.; Serrão, E.M.; Gama, A.S.P.; Vieira, T.A. Quintais agroflorestais e trabalho da mulher em espaço periurbano: Um estudo de caso em Santarém, Pará, Brasil. Res. Soc. Dev. 2020, 9, e8691210792. [Google Scholar] [CrossRef]
- Cepal-FAO-IICA. Fomento de Circuitos Cortos Como Alternativa para la Promoción de la Agricultura Familiar; Comisión Económica para América Latina y el Caribe, Organización de las Naciones Unidas para la Alimentación-FAO, Instituto Interamericano de Cooperación para la Agricultura: Buenos Aires, Argentina, 2014. [Google Scholar]
- Goodman, D. Espaço e lugar nas redes alimentares alternativas: Conectando produção e consumo. In Cadeias Curtas e Redes Agroalimentares Alternativas—Negócios e Mercados da Agricultura Familiar; Cirad: Paris, France, 2017; Volume 1. [Google Scholar]
- Souza Amaral, L.; Santos, C.J.; Souza, C.R.; Penha, T.A.M.; Araújo, J.P. O papel das Cadeias Curtas de Comercialização na construção de um modelo de desenvolvimento rural sustentável no semiárido nordestino: O caso da Central de Comercialização da Agricultura Familiar do Rio Grande do Norte (CECAFES). Desenvolv. Meio Ambiente 2020, 55. [Google Scholar] [CrossRef]
- Câmara Municipal de Santarém (CMS). Pará. Interlegis. Sistema de Apoio ao Processo Legislativo. Lei nº 17.894, de 15 de Dezembro de 2004. Available online: https://sapl.santarem.pa.leg.br/ (accessed on 5 August 2022).
- Lima, A.C.; Pauletto, D.; de Andrade, J.C.G.; da Silva, J.M. Registros de incêndios na região metropolitana de Santarém/PA no período de 2012 a 2016. Rev. Ibero-Am. Ciênc. Ambient. 2020, 11, 9–18. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Nogueira, C.M. Pagamentos por Serviços Ambientais: Uma abordagem conceitual, regulatória e os limites de sua expansão no Brasil. Extensão Rural 2021, 28, e13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).