Organically Cultivated Vine Varieties—Distinctive Qualities of the Oils Obtained from Grape Seeds
Abstract
1. Introduction
2. Results
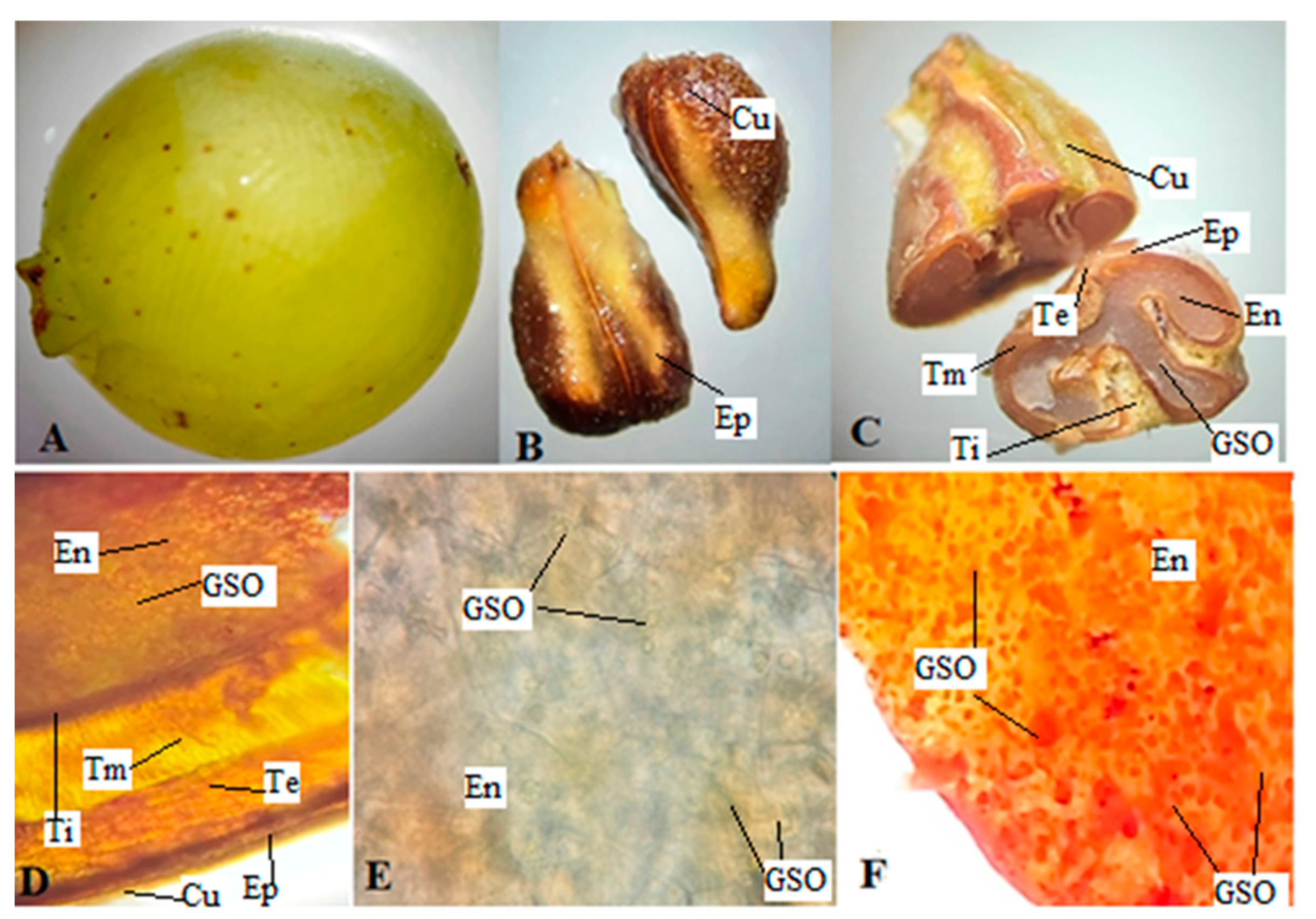
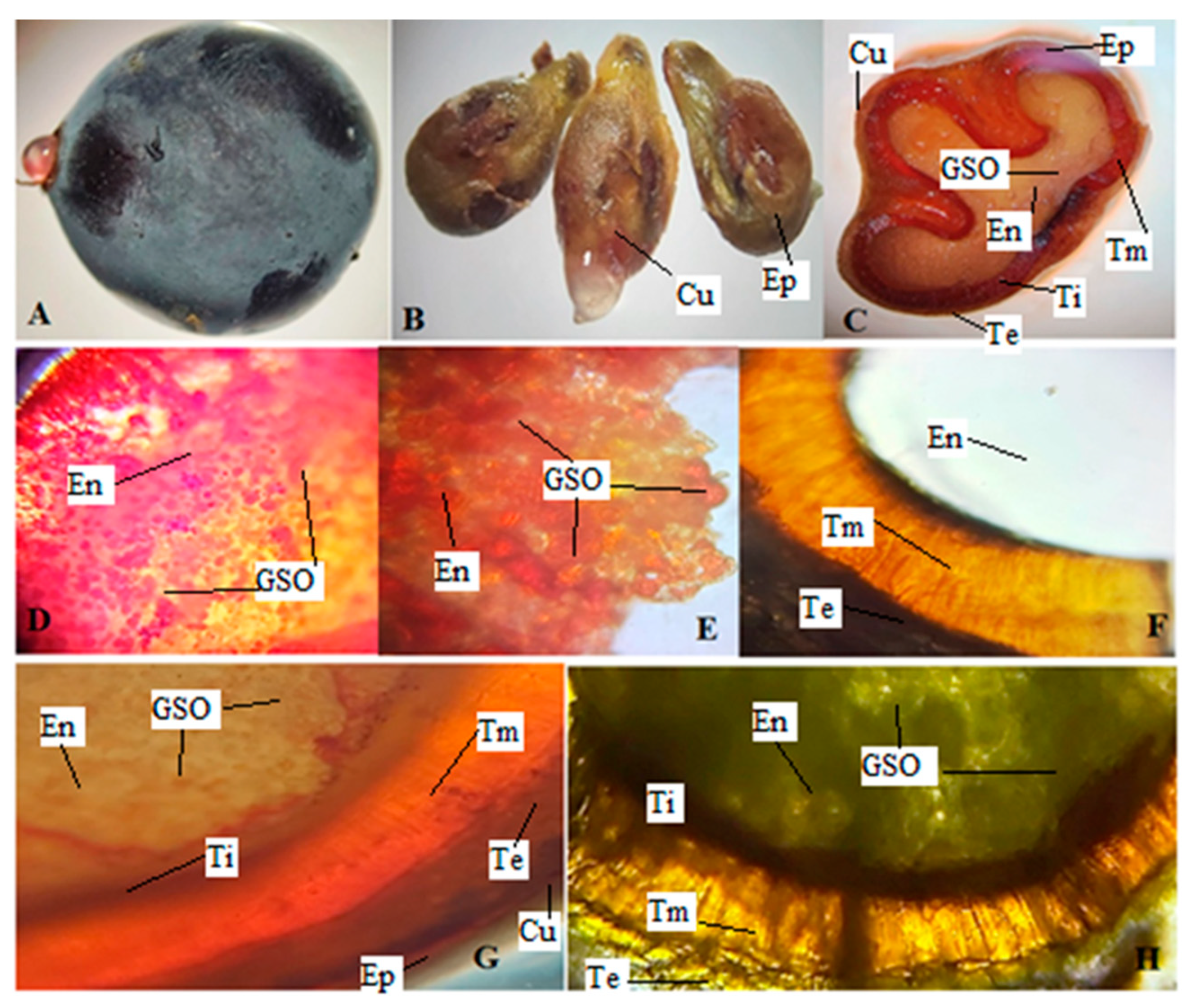
2.1. Macroscopic and Microscopic Analyses
2.2. Extraction Yield and Physical Indices
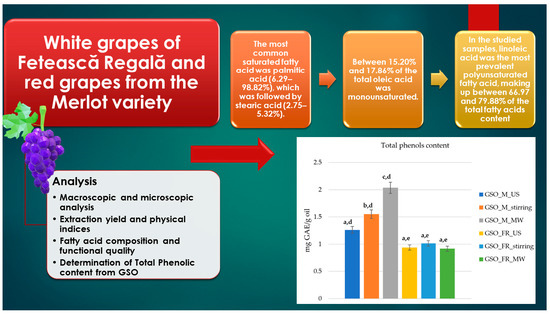
2.3. Fatty Acid Composition and Functional Quality
2.4. Determination of Total Phenolic Content
2.5. Antioxidant Capacity Determination of Grape Seed Oils
3. Discussion
4. Materials and Methods
4.1. Description of the Plant Material
4.2. Seed Samples
4.3. Macroscopic and Microscopic Analyses
4.4. Chemicals and Reagents
4.5. Extraction Techniques
4.5.1. Ultrasound-Assisted Extraction
4.5.2. Cold Extraction under Stirring
4.5.3. Microwave-Assisted Extraction
4.5.4. Extraction Yield and Physical Indices
4.6. Chemical Characterization of Grape Seed Oil
4.6.1. Fatty Acid Determination from GSO
4.6.2. Functional Quality
4.6.3. Determination of Total Phenolic Content
4.7. Antioxidant Capacity Determination of Grape Seed Oil
4.7.1. DPPH (2,2-Diphenyl-1-Picryl-Hydrazyl-Hydrate) Assay
4.7.2. FRAP (Ferric Reducing Antioxidant Power) Assay
4.7.3. ABTS (2,20-Azino-Bis [3-Ethylbenzothiazolin-6-Sulfonic Acid]) Assay
4.7.4. CUPRAC (Cupric Reducing Antioxidant Capacity) Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sargolzaei, M.; Rustioni, L.; Cola, G.; Ricciardi, V.; Bianco, P.A.; Maghradze, D.; Failla, O.; Quaglino, F.; Toffolatti, S.L.; De Lorenzis, G. Georgian Grapevine Cultivars: Ancient Biodiversity for Future Viticulture. Front. Plant Sci. 2021, 12, 630122. [Google Scholar] [CrossRef] [PubMed]
- Antoce, A.O.; Calugaru, L.L. Evolution of grapevine surfaces in Romania after accession to European Union—period 2007–2016. BIO Web Conf. 2017, 9, 1–7. [Google Scholar] [CrossRef]
- OIV: Organisation Internationale de la Vigne et du vin Global Economic Vitiviniculture Data. Available online: https://www.oiv.int/ (accessed on 21 March 2023).
- Oprea, O.; Apostol, L.; Bungau, S.B.; Cioca, G.; Samuel, A.D.; Badea, M.; Gaceu, L. Researches on the chemical composition and the rheological properties of wheat and grape epicarp flour mixes. Rev. Chim. 2018, 69, 70–75. [Google Scholar] [CrossRef]
- Badea, M.; di Modugno, F.; Floroian, L.; Tit, D.M.; Restani, P.; Bungau, S.; Iovan, C.; Badea, G.E.; Aleya, L. Electrochemical strategies for gallic acid detection: Potential for application in clinical, food or environmental analyses. Sci. Total Environ. 2019, 672, 129–140. [Google Scholar] [CrossRef]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape seed oil: A potential functional food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-Based Compounds from Grape Seeds: A Biorefinery Approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef]
- Argon, Z.U.; Celenk, V.U.; Gumus, Z.P. Cold pressed grape (Vitis vinifera) seed oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Academic Press: New York, NY, USA, 2020; pp. 39–52. [Google Scholar]
- Abdel-Daim, M.M.; Abo-EL-Sooud, K.; Aleya, L.; Bungau, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L.; Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: London, UK, 2020; pp. 1–26. [Google Scholar]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Kapcsándi, V.; Hanczné Lakatos, E.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Characterization of fatty acid, antioxidant, and polyphenol content of grape seed oil from different Vitis vinifera L. varieties. OCL 2021, 28, 1–6. [Google Scholar] [CrossRef]
- Galili, S.; Hovav, R. Determination of Polyphenols, Flavonoids, and Antioxidant Capacity in Dry Seeds. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Academic Press: New York, NY, USA, 2014; pp. 305–323. [Google Scholar]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Pallag, A.; Bungau, S.G.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 107–115. [Google Scholar]
- Duba, K.; Fiori, L. Supercritical CO2 extraction of grape seeds oil: Scale-up and economic analysis. Int. J. Food Sci. Technol. 2019, 54, 1306–1312. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of Microwave-Assisted Extraction of Phenolic Antioxidants from Grape Seeds (Vitis vinifera). Food Bioprocess Technol. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Makarova, N.V.; Valiulina, D.F.; Eremeeva, N.B. Comparative studies of extraction methods of biologically-active substances with antioxidant properties from grape seed (Vitis vinifera L.). Proc. Univ. Appl. Chem. Biotechnol. 2020, 10, 140–148. [Google Scholar] [CrossRef]
- Cristea, O.; Catana, L.; Bracacescu, C.; Baltatu, C.V.; Vladutoiu, L.; Grigore, I.; Ungureanu, N. Processing methods of wine by-products to obtain grape seed oil and grape seed flour as functional ingredients for food fortification. In Proceedings of the Actual Tasks on Agricultural Engineering, Zagreb, Croatia, 2–4 March 2021; pp. 445–453. [Google Scholar]
- Aziz, R.; Popescu, M.L.; Mihele, D. Contributions to the Pharmacognostical Study on Grapes Hamburg Cultivar. Farmacia 2008, 56, 571–576. [Google Scholar]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the Phytochemistry-Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef]
- Nowshehri, J.A.; Bhat, Z.A.; Shah, M.Y. Pharmacognostic Standardisation and Phytochemical Evaluation on the Seeds of Two Vitis Vinefera L. Varieties Grown in Kashmir Valley, India. Pharmacogn. J. 2016, 8, 465–470. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Gülcü, M.; Uslu, N. Geçgel Determination of Bioactive Compounds and Mineral Contents of Seedless Parts and Seeds of Grapes. S. Afr. J. Enol. Vitic. 2017, 38, 212–220. [Google Scholar]
- Tangolar, S.G.; Özoǧul, Y.; Tangolar, S.; Torun, A. Evaluation of fatty acid profiles and mineral content of grape seed oil of some grape genotypes. Int. J. Food Sci. Nutr. 2009, 60, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Al Juhaimi, F.; Geçgel; Gülcü, M.; Hamurcu, M.; Özcan, M.M. Bioactive Properties, Fatty Acid Composition and Mineral Contents of Grape Seed and Oils. S. Afr. J. Enol. Vitic. 2017, 38, 103–108. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.L. Optimization of oil yield and oil total phenolic content during grape seed cold screw pressing. Ind. Crops Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Li, H.; Fu, X.; Deng, G.; David, A.; Huang, L. Extraction of oil from grape seeds (Vitis vinifera L.) using recyclable CO2-expanded ethanol. Chem. Eng. Process.-Process Intensif. 2020, 157, 108147. [Google Scholar] [CrossRef]
- Nehdi, I.A. Cupressus sempervirens var. horizentalis seed oil: Chemical composition, physicochemical characteristics, and utilizations. Ind. Crops Prod. 2013, 41, 381–385. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Kamiloglu, O.; Demirkeser, O. Fatty Acid Composition, Total Phenolic Content and Antioxidant Activity of Grape Seed Oils Obtained by Cold- Pressed and Solvent Extraction. Indian J. Pharm. Educ. Res. 2019, 53, 144–150. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 2018, 8, 107–113. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Menezes, E.G.O.; Freitas, L.C.; Andrade, E.H.d.A.; Ribeiro-Costa, R.M.; Silva, J.O.C., Jr.; Carvalho, R.N., Jr. Supercritical CO2 extraction of uxi (Endopleura uchi) oil: Global yield isotherms, fatty acid profile, functional quality and thermal stability. J. Supercrit. Fluids 2020, 165, 104932. [Google Scholar] [CrossRef]
- De Souza, R.d.C.; Souza Machado, B.A.; de Abreu Barreto, G.; Leal, I.L.; dos Anjos, J.P.; Umsza-Guez, M.A. Effect of Experimental Parameters on the Extraction of Grape Seed Oil Obtained by Low Pressure and Supercritical Fluid Extraction. Molecules 2020, 25, 1634. [Google Scholar] [CrossRef]
- Mollica, A.; Scioli, G.; Valle, A.D.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; De Córdova, M.L.F.; Castillo-López, R.; et al. Phenolic Analysis and In Vitro Biological Activity of Red Wine, Pomace and Grape Seeds Oil Derived from Vitis Vinifera L. cv. Montepulciano d’Abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Google Map with the Grapevine Plantation. Available online: https://www.google.com/maps/place/47°16′10.6%22N+22°08′04.2%22E/@47.26962,22.1327663,578m/data=!3m2!1e3!4b1!4m13!1m8!3m7!1s0x4747ca07a65c2231:0x4fcaa48c15f119dc!2sSîniob+417192!3b1!8m2!3d47.2638987!4d22.128266!16s%2Fg%2F1q6j717xq!3m3!8m2!3d47.26962!4d22.1 (accessed on 8 April 2023).
- Dabetic, N.M.; Todorovic, V.M.; Djuricic, I.D.; Stankovic, J.A.A.; Basic, Z.N.; Vujovic, D.S.; Sobajic, S.S. Grape Seed Oil Characterization: A Novel Approach for Oil Quality Assessment. Eur. J. Lipid Sci. Technol. 2020, 122, 1900447. [Google Scholar] [CrossRef]
- Pharmacognostic determinations, Macroscopic and microscopic control of plant products. In Romanian Pharmacopoeia; Bucharest Medical Publishing House: Bucharest, Romania, 1993; pp. 1051–1053.
- Hong, N.; Yaylayan, V.A.; Vijaya Raghavan, G.S.; Paré, J.R.J.; Bélanger, J.M.R. Microwave-assisted Extraction of Phenolic Compounds from Grape Seed. Nat. Prod. Lett. 2001, 15, 197–204. [Google Scholar] [CrossRef]
- Bandici, L.; Teusdea, A.C.; Soproni, V.D.; Hathazi, F.I.; Arion, M.N.; Molnar, C.O.; Vicas, S.I. The Influence of Microwave Treatments on Bioactive Compounds and Antioxidant Capacity of Mentha piperita L. Materials 2022, 15, 7789. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; da Silva, M.P.; de Sousa, S.H.B.; Bezerra, P.D.N.; Menezes, E.G.O.; da Silva, N.J.N.; Banna, D.A.D.d.S.; Araújo, M.E.; de Carvalho, R.N., Jr. Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Eroglu, E.; Girgin, S.N. A unique phenolic extraction method from olive oil macerate of Hypericum perforatum using DMSO: Assessment of in vitro anticancer activity, LC-MS/MS profile, total phenolic content and antioxidant capacity. S. Afr. J. Bot. 2021, 139, 6–11. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; D’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in vitro Anti-inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef] [PubMed]




| GSO Sample | Amount of Seed/Solvent | Oil Quantity (g) | Oil Volume (mL) | Density (g/mL) | Yield (% w/w) |
|---|---|---|---|---|---|
| GSO_M_US | 30 g/250 mL | 2.707 ± 0.01 a | 3 ± 0.10 a | 0.9023 ± 0.02 a | 9.02 ± 0.05 a |
| GSO_M_stirring | 3.503 ± 0.12 b | 3.7 ± 0.20 b | 0.9467 ± 0.02 a | 11.67 ± 0.38 b | |
| GSO_M_MW | 20 g/400 mL | 2.157 ± 0.04 c | 2.3 ± 0.10 b | 0.937 ± 0.03 a | 10.79 ± 0.20 c |
| GSO_FR_US | 30 g/250 mL | 3.220 ± 0.08 a | 3.6 ± 0.10 a | 0.8944 ± 0.04 a | 10.73 ± 0.27 a |
| GSO_FR_stirring | 3.755 ± 0.16 b | 4.3 ± 0.10 b | 0.8732 ± 0.04 a | 12.52 ± 0.52 b | |
| GSO_FR_MW | 20 g/400 mL | 2.104 ± 0.10 c | 2.3 ± 0.10 c | 0.9147 ± 0.03 a | 10.52 ± 0.50 c |
| Fatty Acids | Rt | (M+) | GSO_M_US | GSO_M_ Stirring | GSO_M_ MW | GSO_FR_ US | GSO_FR_ Stirring | GSO_FR_ MW | |
|---|---|---|---|---|---|---|---|---|---|
| (6:0) | Hexanoic | 7.804 | 130 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.02 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.00 |
| (14:0) | Myristic | 18.308 | 242 | 0.03 ± 0.00 | 0.06 ± 0.01 | 0.02 ± 0.00 | 0.07 ± 0.01 | 0.05 ± 0.00 | 0.07 ± 0.01 |
| (16:0) | Palmitic | 21.224 | 270 | 8.26 ± 0.71 | 9.82 ± 0.10 | 6.29 ± 0.70 | 8.78 ± 0.9 | 8.69 ± 0.90 | 9.48 ± 0.75 |
| (18:0) | Stearic | 24.46 | 298 | 4.66 ± 0.38 | 5.32 ± 0.50 | 2.75 ± 0.30 | 4.07 ± 0.40 | 4.15 ± 0.03 | 4.04 ± 0.30 |
| (20:0) | Arachidic | 28.852 | 326 | 0.23 ± 0.01 | 0.23 ± 0.02 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.01 |
| (22:0) | Behenic | 35.515 | 354 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 |
| ΣSFAs | - | - | 13.30 ± 0.21 a | 15.53 ± 0.37 b | 9.26 ± 0.52 c | 13.15 ± 0.71 | 13.17 ± 0.45 | 13.90 ± 1.03 | |
| 16:1(n-9) | cis-7 hexadecenoic | 21.579 | 268 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.08 ± 0.00 | 0.05 ± 0.01 | 0.08 ± 0.01 |
| 16:1(n-7) | Palmitoleic | 21.674 | 268 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.20 ± 0.15 |
| 18:1(n-9) | Oleic | 24.97 | 296 | 15.84 ± 1.04 | 17.86 ± 1.46 | 15.20 ± 0.90 | 15.79 ± 1.40 | 15.58 ± 1.35 | 17.37 ± 1.50 |
| 18:1(n-7) | Vaccenic | 25.075 | 296 | 1.20 ± 0.95 | 1.29 ± 1.5 | 0.88 ± 0.05 | 0.81 ± 0.07 | 0.79 ± 0.15 | 0.89 ± 0.10 |
| 20:1(n-9) | 11-eicosenoic | 25.075 | 0.25 ± 0.01 | 0.27 ± 0.02 | 0.41 ± 0.03 | 0.24 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.01 | |
| ΣMUFAs | - | - | 17.45 ± 0.32 | 19.59 ± 0.77 | 16.64 ± 0.45 | 17.08 ± 0.41 | 16.75 ± 0.53 | 18.73 ± 0.16 | |
| 18:2(n-6) | Linoleic | 26.051 | 294 | 68.74 ± 5.30 | 79.88 ± 8.14 | 73.72 ± 6.40 | 69.40 ± 6.01 | 69.76 ± 5.60 | 66.97 ± 5.03 |
| 20:2(n-6) | Eicosadienoic | 31.088 | 322 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.00 |
| 18:3(n-3) | α-linolenic | 27.446 | 292 | 0.45 ± 0.01 | 0.40 ± 0.01 | 0.34 ± 0.02 | 0.33 ± 0.02 | 0.28 ± 0.01 | 0.34 ± 0.02 |
| - | ΣPUFAs | - | - | 69.25 ± 0.46 | 80.32 ± 0.51 | 74.10 ± 0.35 | 69.77 ± 0.12 | 70.09 ± 0.42 | 67.37 ± 0.26 |
| - | n-3 | - | - | 0.45 ± 0.01 | 0.40 ± 0.01 | 0.34 ± 0.02 | 0.33 ± 0.02 | 0.28 ± 0.01 | 0.34 ± 0.02 |
| - | n-6 | - | - | 68.80 ± 0.57 | 79.93 ± 0.30 | 73.75 ± 0.18 | 69.45 ± 0.32 | 69.81 ± 0.22 | 67.03 ± 0.30 |
| - | n-3/n-6 | - | - | 0.0065 | 0.005 | 0.0046 | 0.0047 | 0.004 | 0.005 |
| - | PUFAs/SFAs | - | - | 5.21 | 5.17 | 8.00 | 5.31 | 5.32 | 4.85 |
| Sample | H/H | AI | TI |
|---|---|---|---|
| GSO_M_US | 10.20 a | 0.381 a | 0.291 a |
| GSO_M_stirring | 9.89 b | 0.393 a | 0.298 a |
| GSO_M_MW | 14.09 c | 0.278 a | 0.196 a |
| GSO_FR_US | 9.63 a | 0.404 a | 0.219 a |
| GSO_FR_stirring | 9.76 a | 0.401 a | 0.292 a |
| GSO_FR_MW | 8.83 b | 0.440 a | 0.309 a |
| GSO Sample | DPPH (µmol TE/g Oil) | FRAP (µmol TE/g Oil) | CUPRAC (µmol TE/g Oil) | ABTS (µmol TE/g Oil) |
|---|---|---|---|---|
| GSO_M_US | 8.553 ± 0.076 a | 9.677 ± 0.913 a | 13.498 ± 2.849 b | 4.025 ± 0.205 a |
| GSO_M_stirring | 14.035 ± 0.554 b | 6.921 ± 0.193 b | 9.840 ± 2.799 b | 1.595 ± 0.079 b |
| GSO_M_MW | 10.307 ± 0.526 c | 13.387 ± 0.374 c | 19.799 ± 0.733 c | 1.908 ± 0.134 c |
| GSO_FR_US | 7.675 ± 0.080 a | 1.040 ± 0.270 a | 5.993 ± 0.441 a | 2.768 ± 0.107 a |
| GSO_FR_stirring | 8.443 ± 0.225 b | 6.129 ± 0.170 b | 14.636 ± 0.330 b | 1.124 ± 0.056 b |
| GSO_FR_MW | 7.905 ± 0.021 a | 6.981 ± 0.120 c | 8.125 ± 0.022 c | 1.255 ± 0.025 b |
| Antioxidant Capacity | Pearson Correlation | GSO_M_US | GSO_M_ Stirring | GSO_M_ MW | GSO_FR_ US | GSO_FR_ Stirring | GSO_FR_ MW |
|---|---|---|---|---|---|---|---|
| FRAP | r | 1.000 * | 1.000 * | 1.000 * | 1.000 * | 1.000 * | 0.991 |
| p | 0.002 | 0.008 | 0.003 | 0.019 | 0.010 | 0.085 | |
| DPPH | r | 0.993 | 1.000 * | 1.000 * | 1.000 * | 1.000 * | 0.524 |
| p | 0.076 | 0.005 | 0.003 | 0.017 | 0.006 | 0.649 | |
| CUPRAC | r | 1.000 * | 1.000 * | 1.000 * | 1.000 * | 0.871 | −0.989 |
| p | 0.003 | 0.005 | 0.002 | 0.019 | 0.327 | 0.096 | |
| ABTS | r | −0.712 | 0.222 | 0.453 | −0.978 | 0.196 | −0.704 |
| p | 0.495 | 0.857 | 0.700 | 0.134 | 0.874 | 0.503 |
| Sample | Extraction Technique | Process Condition |
|---|---|---|
| Red grape seeds Merlot (M) | ||
| GSO_M_US | Ultrasound-Assisted Extraction | Solvent: n-hexan, 37 kHz, 30 °C, 90 min |
| GSO_M_stirring | Conventional Extraction | Solvent: n-hexan, 90 min, room temperature |
| GSO_M_MW | Microwave-Assisted Extraction | Solvent: n-hexan, 300 W, 30 s ON, 8 min OFF, 40 min, 38 °C |
| White grape seeds Fetească Regală (FR) | ||
| GSO_FR_US | Ultrasound-Assisted Extraction | Solvent: n-hexan, 37 kHz, 30 °C, 90 min |
| GSO_FR_stirring | Conventional Extraction | Solvent: n-hexan, 90 min, room temperature |
| GSO_FR_MW | Microwave-Assisted Extraction | Solvent: n-hexan, 300 W, 30 s ON, 8 min OFF, 40 min, 38 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitea, M.A.; Gitea, D.; Mirela Tit, D.; Bungau, S.G.; Bogdan, M.A.; Radu, A.-F.; Dulf, F.V.; Pasca, M.B. Organically Cultivated Vine Varieties—Distinctive Qualities of the Oils Obtained from Grape Seeds. Sustainability 2023, 15, 11037. https://doi.org/10.3390/su151411037
Gitea MA, Gitea D, Mirela Tit D, Bungau SG, Bogdan MA, Radu A-F, Dulf FV, Pasca MB. Organically Cultivated Vine Varieties—Distinctive Qualities of the Oils Obtained from Grape Seeds. Sustainability. 2023; 15(14):11037. https://doi.org/10.3390/su151411037
Chicago/Turabian StyleGitea, Manuel Alexandru, Daniela Gitea, Delia Mirela Tit, Simona Gabriela Bungau, Mihaela Alexandra Bogdan, Andrei-Flavius Radu, Francisc Vasile Dulf, and Manuela Bianca Pasca. 2023. "Organically Cultivated Vine Varieties—Distinctive Qualities of the Oils Obtained from Grape Seeds" Sustainability 15, no. 14: 11037. https://doi.org/10.3390/su151411037
APA StyleGitea, M. A., Gitea, D., Mirela Tit, D., Bungau, S. G., Bogdan, M. A., Radu, A.-F., Dulf, F. V., & Pasca, M. B. (2023). Organically Cultivated Vine Varieties—Distinctive Qualities of the Oils Obtained from Grape Seeds. Sustainability, 15(14), 11037. https://doi.org/10.3390/su151411037