Abstract
Persistent organic pollutants (POPs) usually originate from human activities and have been released into the environment for several decades. They are highly resistant to natural decomposition and can accumulate in an organism’s tissues and in all environmental components. Due to their unique characteristics, they have an ability to bio-magnify and bio-accumulate in animals, through the food chain and via inhalation, severely endangering the health of people. As reported, the exposure of humans to POPs causes various health problems such as cancers, diabetes, birth defects, endocrine disruption, cardiovascular diseases and dysfunctional immune and reproductive systems. The residents of South Korea are likely to face a high risk of diseases because of the existence of POPs in the environment. For instance, South Korea’s atmosphere has been reported as a hotspot for POP pollution. Besides, South Koreans’ high amount of seafood consumption is considered another source of POPs. Therefore, this article reviews the status of POP contamination in food and the health impact of POPs in South Korea. Based on the findings, the most-reported diseases were obesity and diabetes, which positively correlated to age, food habits, body index, and level of exposure to POPs. In addition, cancer and metabolic diseases are at an alarming level. Therefore, the public health impacts of POPs need continuous assessment in South Korea over the next decade.
1. Introduction
Persistent organic pollutants (POPs) encompass a group of chemical compounds defined by the Stockholm Convention in 2001, as characterized by four key attributes [1]. These substances exhibit persistence, meaning they resist degradation in the environment [2]. Additionally, they are bioaccumulative, meaning that they build up in living organisms over time. POPs are also known to possess toxicity and mobility. Primarily originating from human activities, these pollutants have been continuously emitted into the environment for several decades. Notably, they have been found to contain numerous carcinogens as well as compounds that disrupt the endocrine system [3].
The environment contains three distinct categories of POPs: (1) pesticides, specifically organochlorine pesticides (OCPs) such as dichlorodiphenyltrichloroethane (DDT) and its byproducts; (2) industrial and technical chemicals comprising polychlorinated biphenyls (PCBs), perfluorooctanesulfonate (PFOS) and polybrominated diphenyl ethers (PBDEs); (3) by-products resulting from industrial processes, such as polyaromatic hydrocarbons (PAHs), polychlorinated dibenzofurans (PCDFs) and polychlorinated dibenzo-p-dioxins (PCDDs) [4]. Another study has classified the various types of POPs into four groups: those subjected to the elimination of production and usage, those with restricted production and usage, unintentionally produced substances, and chemicals currently under investigation. Chemically, POPs can be categorized as brominated, chlorinated, or fluorinated compounds [5].
POPs exhibit remarkable resilience to natural degradation within the environment, persisting in aquatic environments, soils, food chains, and ultimately, within the human body for prolonged periods, even after production has ceased. Possessing lipophilic characteristics, these pollutants can accumulate in various environmental elements and organisms’ tissues, and can also be transported through the atmosphere across substantial distances [6]. These attributes enable them to undergo biomagnification and bioaccumulation within animals, posing significant threats to both human health and the integrity of natural ecosystems [7].
These contaminants interrupt the food chain, thus threatening the survival of all humans and wildlife on Earth in the long term. Populations worldwide, including humans and animals, face potential prolonged exposure to POPs. These pollutants can accumulate within the fatty tissues of living organisms, leading to their increased concentration as they progress through the food chain [4]. Scientific evidence confirms the detrimental impact of POPs on human health. Exposure to these contaminants can give rise to a wide range of health issues, including endocrine disruption, cardiovascular diseases, cancer, diabetes, birth defects and impairments in immune and reproductive systems’ functionalities [4,7,8,9].
A recently published review on the distribution pattern of POPs in South Korea’s atmosphere reports a trend of increasing chemical concentrations such as POPs. They found the major pollutants to be PAHs, PCBs, brominated flame retardants (BFRs) and PBDEs, the combination of which had significantly polluted the atmosphere of South Korea. Based on their findings, South Korea is considered a hotspot for POP pollution, while the level of TBBPA is lower than expected [10]. BFRs, in the manner of other POPs, can accumulate in food chains and have even been found in human milk [11]. As most of the review papers only focus on the environmental aspects of POP pollution, there is a need to evaluate the health impact of POPs on human health. To date, there is no comprehensive review regarding the effect of different types of POPs on human health in South Korea. Therefore, we reviewed the potential POP contamination in food and how it can affect the health of South Koreans by referring to relevant published studies over the last decade. The impacts of different types of POPs, including OCPs, PCBs, PFAS, PBDEs and HBCDs on the health of infants, adults and elderly people have been investigated. Based on our findings, the dominant diseases can be classified as endocrine disruption, cancer and cardiovascular and metabolic problems.
1.1. POPs in Food
Overall, the primary factor that determines the presence of environmental pollutants in food, irrespective of whether they are organically or conventionally produced, is the proximity of anthropogenic pollution sources [12]. Due to their lipophilic nature and ability to bioaccumulate within the food chain, POPs have the potential to accumulate in the adipose tissues of humans, thereby causing detrimental effects on human health [13].
Upon release into the atmosphere, POPs settle onto vegetation, soil and sediments. They subsequently bioaccumulate in aquatic fish and farm animals through the ingestion of contaminated feed, plants and sediment. As marine and freshwater organisms exhibit higher concentrations of POPs compared to their surrounding aquatic environment, they serve as valuable bioindicators. For the majority of individuals not occupationally exposed to POPs, the primary route of exposure (>90% of POP intake) stems from the consumption of animal products and seafood. Additionally, exposure can occur through the consumption of fruits and vegetables treated with pesticides, which serve as an additional source of exposure [4,14]. Figure 1 presents some of the reported POPs in foods in previous studies.
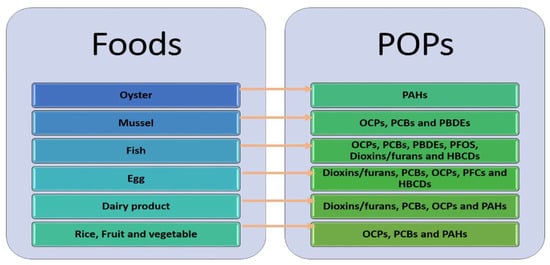
Figure 1.
The reported POPs in food (adopted from [4]).
1.2. POPs in the Human Body
The protection of human health is the ultimate objective of the Stockholm Convention, with humans being the final link in the exposure chain [15]. To monitor the presence of POPs in humans, both invasive and non-invasive techniques are employed for biological monitoring. These techniques involve the analysis of breast milk, blood/serum, hair, saliva, semen, fingernails and urine. These biological samples provide insights into the accumulation of POPs in the body resulting from exposure and the potential transfer of POPs through the placenta and breast milk from mother to child, as well as the excretion of POPs and their metabolites through various bodily fluids. Research indicates that the primary route of exposure for the bioaccumulation of POPs in human fluids is through the intake of contaminated food. A secondary route of exposure includes inhalation of pollutants from e-waste sites and contaminated farms [14].
The high toxicity of POPs is attributed to their property of bioaccumulation. This ability to accumulate in living organisms for extended periods is facilitated by the high-fat solubility of hydrophobic POPs. This characteristic enables them to readily accumulate and persist in fatty tissues [7]. The primary routes through which POPs undergo bioaccumulation are outlined and presented in Table 1.

Table 1.
The main routes of bioaccumulation of POPs.
1.3. Health Effects of POPs
Despite the low level of POPs in humans, various health problems have been associated with this type of pollution. This is due to their metabolic and carcinogenic effects, which lead to human chronic diseases through the mechanism of DNA methylation deregulation [4]. In the current context of environmental pollution, virtually everyone carries traces of POPs in their bodies. Interestingly, even fetuses and embryos have been found to harbor POPs. These pollutants are detected in individuals across all age groups, with higher levels observed in older populations. Exposure to these contaminants poses significant health risks, including cardiovascular diseases, obesity, hormone disruption, reproductive and neurological disorders, cancer, endocrine disturbances, diabetes and learning disabilities [7]. Moreover, health problems such as dizziness, diarrhea, rashes, skin irritation and headaches can be the result of POP exposure. Thus, POPs have demonstrated the ability to induce a range of detrimental effects on human health, including compromising the immune system and rendering the body susceptible to microbial infections [3].
Upon entering the human body, POPs persist throughout an individual’s lifetime. Even small quantities of these substances can contribute to the development of diseases. For instance, certain chlorinated hydrocarbon pesticides such as aldrin and dieldrin have been associated with numerous cases of severe acute poisonings. They can result in gastrointestinal disorders and kidney and nervous system damage, and have the potential to impact immune response systems. Additionally, the combination of aldrin and dieldrin may elevate the risks of liver and biliary cancer [6]. Therefore, if these chemicals surpass their acceptable thresholds, POPs can have harmful effects on the human body. Some of the possible diseases caused by POPs in the human body are presented in Table 2.

Table 2.
Health Problems and their links to POPs.
Based on Table 2, OCP is considered one of the most problematic types of POPs, one which causes several different diseases. A recent study investigated the OCP levels for 12 years in the people of Seoul in South Korea. The level of OCPs showed a decreasing trend; it was higher in females than in males due to metabolism, dietary habits and body mass, while it showed an increasing level with increased age and higher BMI [28].
2. The Availability of POPs in South Korean Food
In response to the globalization and diversification of the food industry, as well as the increasing consumer focus on health, South Korea has implemented food safety policies that are evidence-based. The Total Diet Study (TDS) serves as a common tool for risk assessment, allowing the evaluation of exposure to hazardous elements. International organizations such as the World Health Organization (WHO), the Food and Agriculture Organization (FAO), and the European Food Safety Authority have made efforts to standardize TDS methodologies. In South Korea, periodic TDSs have been conducted to assess dietary intakes of various substances, including pesticides, heavy metals, persistent organic pollutants, mycotoxins and processing contaminants [29]. Notably, 74% of mothers in South Korea receive postpartum care services for one month following delivery, during which they either stay in the hospital or at home [30]. These care services include dietary restrictions that limit the consumption of high-fat foods and fast foods, which often contain elevated levels of per- and poly-fluoroalkyl substances (PFAS) [31].
The extensive pollution of short carbon-chain perfluorocarboxylic acids (PFCAs) in the environment can result in their presence in food products. Among meats, vegetables and fruits, PFHxA was the most commonly detected PFAS [32]. Furthermore, among the 16 PFAS analyzed in the dietary samples from South Korea, PFPeA, and PFHxA were found to be the most commonly detected compounds [33]. Another study compared different pathways of POP exposure in South Korea. PBDEs were ingested via dietary intake and by other pathways. When considering various routes of exposure, it was found that dietary intake played the most significant role in the overall exposure to PBDEs among South Korean adults, accounting for approximately 71% of the total intake.
One study conducted a risk assessment of dioxins in 257 food items in South Korea. They reported that although the levels of POPs in the air have been considerably reduced, their levels in human serum have not reduced, indicating that humans may also be unintentionally subjected to these compounds, primarily through food ingestion [34].
In another study conducted in South Korea, the concentrations of PFAAs were measured in 397 food samples. The findings indicated that long-chain perfluorocarboxylic acids (PFCAs) and Perfluorooctane sulfonate (PFOS) were the predominant PFAAs detected in fish, shellfish and processed foods. On the other hand, perfluorooctanoic acid (PFOA) and short-chain PFCAs were more prevalent in dairy products and beverages. Fish consumption emerged as a significant contributor to PFOS exposure, while dairy foods played a major role in PFOA exposure. Notably, tap water intake emerged as a primary source of PFOA exposure when it served as the main source of drinking water [32].
Another study in South Korea analyzed a total of 521 food samples which were sampled and analyzed for their 1,2,5,6,9,10-hexabromocyclododecane HBCD content. The highest amount of HBCD was found in fish and shellfish, and this was attributed to natural exposure to polluted marine environments [35]. A study was conducted to evaluate the dietary exposure and risk associated with PCBs for the general population in South Korea, focusing on 28 different food items. The findings indicated that the overall dietary exposure to PCBs through food intake among the South Korean population remained below the recommended tolerable daily intake (TDI) levels [36].
Milk serves as an important medium for monitoring the contamination of persistent organic pollutants (POPs). In a South Korean study, the concentrations of POPs in raw bovine milk were determined. The results revealed that the residual levels of PBDEs, HCB, PCDD/Fs and DL-PCBs in raw bovine milk fell within acceptable safety limits [37].
Seafood consumption represents a significant pathway for exposure to legacy persistent organic pollutants (POPs), including PCDD/Fs, PCBs and OCPs, as well as PAHs and mercury, among South Korean populations [38,39]. Despite the increasing demand for polybrominated diphenyl ethers (PBDEs) in parallel with the rapid growth of the electronics market in South Korea, there are no specific regulations in place for PBDEs. The concentrations of PBDEs in commonly consumed seafood were analyzed. The results revealed that the contribution of seafood consumption to PBDE intake in South Korea was the highest among the reported estimated daily intakes (EDIs) of several other countries. For South Korean adults, both seafood consumption and dust ingestion played an equal role in total PBDE intake, whereas dust ingestion was the primary contributor for toddlers [40]. Another study conducted on South Korean seafood found no statistically significant difference in PCB levels between raw samples and various cooking methods. However, there was a noticeable increase in PCB concentrations after cooking raw seafood [41]. Additionally, it was observed that individuals in South Korea who consumed more vegetables, potatoes, fish/shellfish, or popcorn tended to have higher concentrations of various perfluorinated compounds (PFCs) in their serum [42].
On the other hand, exposure to POPs during early-life stages can disrupt the development of the immune and respiratory systems, potentially leading to a diminished ability to combat infections and an elevated susceptibility to allergic manifestations in later life. Existing epidemiological findings indicate that early-life exposure to POPs can have negative impacts on the development of immune and respiratory systems. Moreover, prenatal exposure to POPs poses health risks, not only to expectant mothers, but also to newborns [43]. Table 3 represents the effect of POPs during prenatal and post-natal periods in South Korean people.

Table 3.
The available POPs in samples during the prenatal and post-natal periods in South Korea.
3. The Availability of POPS in Mother Milk and Baby Food
Breastfeeding stands as the benchmark for nourishing newborns, yet breast milk is a biological fluid that may harbor environmental contaminants. These pollutants have the potential to impact the immune system and, in turn, the functioning of different bodily organs [50].
Breast milk consumption constitutes more than 90% of the daily intake of substances following birth. Research conducted in South Korea identified ingestion of house dust and breast milk, inhalation of indoor air, and dermal contact with house dust as the primary pathways and sources of POPs for infants below six months of age. Considering the exposure scenario for children over one year old, the contribution of dietary intake in terms of exposure significantly decreased, from 90% to 30% [51].
In South Korea, measurements were taken to evaluate the levels of PBDEs in synthetic musks, via both fetal and maternal exposure. These measurements aimed to assess the prenatal and postnatal exposures experienced by infants. Comparing the obtained data from other countries and previous data from South Korea, South Korean breast milk showed relatively higher concentrations of PBDEs. Furthermore, these PBDE concentrations were found to gradually increase over time [52].
It was documented that, over 12 years, the average level of perfluorooctanoic acid (PFOA) in South Korean breast milk rose by approximately threefold (278%). The consumption of fish appears to be a prominent dietary factor associated with the concentration of perfluorooctane sulfonate (PFOS). Out of the 14 per- and poly-fluoroalkyl substances (PFAS) analyzed, twelve were detectable in breast milk samples [53]. However, a study conducted in South Korea did not establish any significant connections between phthalate metabolites in breast milk and population characteristics such as age, parity, or pre-pregnancy BMI [54]. Table 4 presents the availability of different types of POPs in tested mothers’ bodies in South Korea.

Table 4.
POPs concentration in the bodies of mothers in South Korea.
Besides breastmilk, baby food also contains POPs, as reported by South Korean research. For instance, one research effort examined the residue levels of PBDEs in homemade baby food. The concentrations of total PBDEs were higher than those found in commercial formulae from the United States. They concluded that baby food is a significant exposure pathway of PBDEs for over-24-month-old infants [61].
It has been reported that as infants grew older, there was an observed increase in the contribution of DDTs to the overall concentrations of organochlorine compounds (OCs). The contribution rose from 30% in 6-month-old infants to 67% in 15-month-old infants. Conversely, the concentrations of PCBs, HCHs, and CHLs showed a gradual decline as infants aged, indicating that addressing the risk associated with DDTs should be given the highest priority amongst reduction efforts [62].
Another research effort aimed to identify the dietary patterns associated with blood levels of persistent organic pollutants (POPs) in South Korean children. The analysis revealed that the dietary pattern related to polychlorinated biphenyls (PCBs) exhibited strong associations with the consumption levels of cheese, nuts, salted seafood and seeds. Similar results were observed for the overall intake of PCBs. The dioxin-like PCB pattern, on the other hand, was characterized by higher consumption of yogurt, beverages and fruit, along with a lower intake of grains, seaweed and processed meat. Moreover, the dietary pattern associated with total organochlorine pesticides (OCPs) showed positive factor-loading values for beverages and shrimp, while seaweeds and processed meat had negative factor-loading values [63].
4. Type of Associated Diseases with POPs in South Korea
A variety of types of diseases have been reported to be caused by POPs. The associated diseases are classified into two categories, (1) endocrine disruption and cancers, and (2) cardiovascular and metabolic diseases [4]. In addition, another classification includes additive and synergistic effects, reproductive problems, endocrine disruption and cardiovascular problems [7]. The reported diseases in South Korea that have been associated with POPs are classified and explained in the following discussion. Figure 2 demonstrates the reported diseases in South Korea that have been linked with POPs.
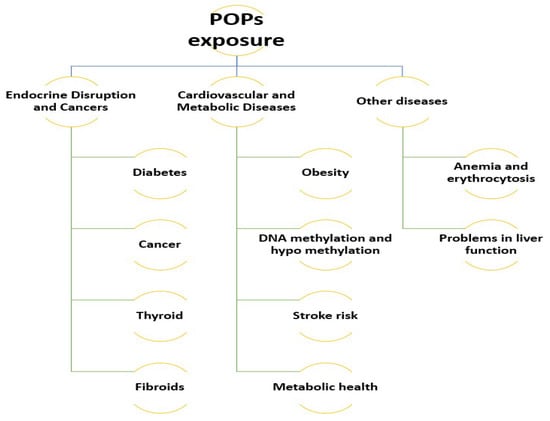
Figure 2.
The diseases in South Korea that have been reported to be due to POP exposure.
4.1. Endocrine Disruption and Cancers
4.1.1. Diabetes
The estimated prevalence of diabetes in South Korea is 12.4%, which corresponds to approximately 4.01 million individuals. Type 2 diabetes comprises over 95% of the diabetes cases in the country [64]. In South Korea, there is a growing interest in POPs due to their potential as endocrine disruptors and their suggested association with diabetes as a possible risk factor [65].
The rapid growth of agriculture and industry in Asia over the last five decades has resulted in elevated levels of potentially harmful chemicals, such as POPs and non-persistent pesticides. Among the countries in the region, China accounts for more than half of the pesticides used. However, when considering use per hectare of agricultural land, South Korea surpasses others by utilizing over 50 kg of formulated products per hectare [66]. In South Korea, a wide range of pesticides containing multiple chemical ingredients have been extensively employed, raising concerns about the potential impact of pesticide exposure on the development of diabetes [67].
The association between POPs and diabetes may be influenced by confounding factors. One potentially confounding factor is the storage of POPs in adipose tissue, which could affect the observed association in cross-sectional studies [68]. However, a study indicating that weight loss results in elevated serum concentrations of POPs, while weight gain can lower them, suggests that adipose tissue acts as a reservoir for POPs, thereby reducing their circulating levels. Furthermore, it has been proposed that the diabetic state itself could alter the metabolism of POPs, leading to variations in their distribution or concentration [69]. A recent study showed that there is an association between dioxin and the risk of type 2 diabetes mellitus (T2DM) and thyroid cancer in South Korean elderly people. The sample study was made up of 48% men and 52% women, with a median age of 54.06 and a BMI median of 24.28 kg/m2. According to the findings, women show a positive association between dioxin and the risk of T2DM but men do not [70]. Table 5 shows the studies related to POPs and diabetes in South Korea.

Table 5.
Association of POPs with Diabetes in South Korea.
4.1.2. Cancer
Two group studies have reported a link between POP exposure and cancers in South Korea. In a prospective study conducted in South Korea, the researchers examined the links between serum concentrations of POPs and the risk of prostate cancer. The study included 110 individuals diagnosed with prostate cancer and a comparison group of 256 participants without prostate cancer. Measurements were taken for serum concentrations of 32 polychlorinated biphenyl (PCB) congeners and 19 organochlorine pesticides (OCPs). To assess the associations between POPs and prostate cancer risk, hazard ratios (HRs) and 95% confidence intervals (95% CIs) were estimated using a weighted Cox regression model. The dose–response curves demonstrated that the cumulative sum of PCBs (∑PCBs) was linked to an increased risk of prostate cancer. These findings suggest a potential role of POPs in the development of prostate cancer [75].
In another group, a study conducted in South Korea investigated the impact of exposure to POPs on lung cancer. Pre-diagnostic serum concentrations of POPs were found to be associated with an increased risk of lung cancer. The study included 118 individuals diagnosed with lung cancer and a control group of 252 participants. Notably, serum concentrations of chlordane and PCBs were linked to a risk of lung cancer in the general population, even several decades after their production and use had been banned. The findings suggest that even low-level environmental exposure to POPs raises the risk of lung cancer, with consistent associations observed between lung cancer risk and chlordane and PCBs across all models. These significant findings were reinforced by comprehensive adjustments for various potential confounding factors, including smoking, alcohol consumption, obesity and exposure to other POPs [76].
4.1.3. Thyroid
The thyroid hormone has a critical role in biological processes in the body such as metabolism and growth. Lack of thyroid hormone affecting fetal development can be caused by pollution of POPs in placenta or breastmilk. Small changes in this hormone cause neurological and cardiovascular problems if they happen in the early stages of pregnancy [77]. In addition, potential endocrine disruptions associated with POPs, especially those of thyroid status associated with PCBs, have been repeatedly reported. A study in South Korea found that PCBs, PBDEs and OCPs cause potential endocrine disruption, especially of the thyroid hormone. They studied the blood samples of one hundred-five pregnant women, and found that several PCBs, such as PCB28, 52, and 118, have negative associations with T3 or T4. In addition, there were a significant associations between BDE47 and total PBDEs with T3 or T4. Concerning OCPs, the presence of DDTs and HCB generally showed associations with decreased levels of thyroid hormones T3 or T4. It is important to note that the thyroid hormone levels of all subjects fell within the reference range. Nonetheless, the exposure to specific targeted POPs raised concerns about the potential disruption of thyroid hormone balance among pregnant women, even at the current level of exposure [78].
Another study in South Korea found an association between thyroid hormones, thyroxine-binding globulin and peripheral deiodinase activity with POPs. They evaluated the modulating effects of sex, menopausal status and age on 1250 participants. TBG and deiodinase activity may mediate the thyroid-disrupting effects of POPs. BDE-47 and β-HCH were related to thyroid autoantibodies. The study found that the thyroid-disrupting influences of POPs may differ by sex-hormonal status, age and sex, and may be mediated by TBG and GD [79].
4.1.4. Fibroids
A study conducted in South Korea revealed associations between various newly introduced consumer chemicals such as APs (DEHTP, DPrHpP and DINCH) and OPEs (TDCIPP and TBOEP) and the occurrence of uterine fibroids among women of reproductive age. The study involved 32 cases and a matched control group (n = 79) comprising premenopausal adult women in South Korea. The results indicated that urinary levels of BDCIPP, BBOEP and BBOEHEP were linked to an increased risk of fibroids. Metabolites of DPrHpP, DEHTP and DINCH demonstrated higher odds of uterine fibroids. Among the phthalates, metabolites of BBzP and DEHP were associated with fibroids. Factor analysis revealed a significant association between a factor predominantly loaded with DPrHpP and DEHP and uterine fibroids, supporting the findings observed in the single chemical regression model. In addition, they found that several metabolites of APs and OPEs are associated with an elevated risk of uterine fibroids among premenopausal women [80].
4.2. Cardiovascular and Metabolic Diseases
4.2.1. Obesity
The accumulation of POPs has been linked to obesity [7]. This association arises from the potential obesogenic properties of POPs, which can impact the development and functioning of adipose tissue, thereby contributing to obesity [81]. Existing literature indicates that there are significant interactions between fat mass and POPs in predicting overall mortality. Among individuals with low POP concentrations, no obesity paradox was observed, as mortality rates increased with higher fat mass. However, in alignment with the obesity paradox, these patterns completely disappeared in individuals with high POP concentrations [82]. The recent studies on the link between POPs and obesity in South Korea are listed in Table 6.

Table 6.
Association of POPs with obesity in South Korea.
According to the following studies in South Korea, adipose tissue can cause additional accumulation of POPs. In a particular study, the presence of Methanobacteriales was identified in 32.5% (27 out of 83) of women. Among these women, both BMI and waist circumference were notably higher compared to those without Methanobacteriales. Additionally, there were positive associations between Methanobacteriales levels in feces and both BMI and waist circumference [87]. Moreover, significant correlations were observed between fecal Methanobacteriales levels and serum concentrations of various OCPs, such as cis-nonachlor, oxychlordane and trans-nonachlor. Moreover, when considering two individuals exposed to equivalent levels of environmental POPs, the one with higher adipose tissue may have an advantage due to the storage of POPs in adipose tissue, which helps alleviate the burden on other vital organs. Consequently, adipose tissue can serve a protective function against the detrimental effects of POPs. However, two scenarios can increase the release of POPs from adipose tissue into the bloodstream, thereby raising the risk of their reaching critical organs: (i) weight loss and (ii) insulin resistance. On the contrary, weight gain diminishes this likelihood. Consequently, avoiding the adverse health consequences of POPs may often contradict conventional assumptions about obesity and changes in body weight [88].
4.2.2. DNA Methylation and Hypomethylation
Exposure to POPs during pregnancy is associated with a disruption in the thyroid hormone balance. Therefore, the POP concentrations in DNA methylation of thyroid hormone-related genes in the placenta and maternal serum of 106 South Korean mothers have been investigated. Based on the findings, seven compounds, including four OCPs, one PBDE and two PCBs were detected in >75% of maternal serum samples. In addition, there was a positive association between POPs in maternal serum and DNA methylation changes of key thyroid-regulating genes in the placenta [89].
To investigate the potential association between low-dose exposure to POPs and global DNA hypomethylation in South Koreans, a study was conducted. Among the study’s subjects, CDH1 methylation was observed in 78.3% of individuals. Interestingly, serum concentrations of OCP or PCB compounds were found to be higher in subjects with CDH1 methylation compared to those without methylation [90]. An additional cross-sectional study was carried out utilizing data from 444 South Korean individuals. Serum measurements were taken for sixteen distinct POPs, comprising six OCPs and ten PCBs. With the exception of PCB101, p,p′-DDE and PCBs displayed positive associations with the LINE-1 assay in women. On the other hand, p,p′-DDE, PCB153 and PCB180 exhibited positive associations with the LINE-1 assay in men [91].
In another study, an inverse association was reported between global DNA methylation levels and blood concentrations of POPs, which are xenobiotics that accumulate in adipose tissue. The study aimed to estimate the extent of global DNA hypomethylation among 86 healthy South Korean individuals. Measurements were taken for various POPs, including OC pesticides, PCBs and PBDEs. The findings revealed that several POPs were linked to global DNA hypomethylation for men, while global DNA hypermethylation was observed for women [92].
4.2.3. Stroke Risk
A study has reported an association of POPs with stroke risk in South Korea. A total of 526 sub-cohort members and 111 stroke incidence cases were identified. Upon accounting for potential confounding variables, participants in the highest tertile of serum concentration of p,p′-DDE displayed an elevated risk of stroke compared to those in the lowest tertile. A similar association was estimated for PCB118, PCB156 and PCB138. Concerning TEQ, individuals in the highest tertile had a threefold-increased likelihood of experiencing a stroke compared to those in the lowest tertile. PCBs exhibited a positive association with ischemic stroke, while no significant association was found with hemorrhagic stroke [93].
4.2.4. Metabolic Health
POP exposure has been linked with problems in metabolic health in previous studies [4]. Three studies in South Korea have reported problems in metabolic health as the outcome of POP exposure. One study considered concentrations of marker PCBs and their link with metabolic health among 214 children aged 7–9. They evaluated the changes in metabolic components after a 1-year follow-up among 158 children. The study’s findings indicated a significant association between concentrations of PCBs and increased changes in diastolic blood pressure (BP) and triglyceride levels over a 1-year follow-up period. Among the metabolic components assessed, the change in diastolic BP exhibited a notable association with specific PCBs, whereas no association was observed with organochlorine pesticides. The researchers concluded that even low-dose exposures to PCBs among children could have a detrimental impact on metabolic health, particularly concerning diastolic BP [94].
In another study examining metabolic diseases such as obesity and diabetes mellitus (DM) and their connection with PAHs and volatile organic compounds (VOCs), spot urine and blood samples were collected from 3787 adult individuals. The study revealed significant associations between exposure to various PAHs and VOCs and increased risks of obesity and DM. Specifically, the benzene metabolites t,t-MA and PAH metabolite 2-OHFlu were found to be linked to an elevated risk of DM. Furthermore, urinary biomarkers for PAHs and VOCs demonstrated positive associations with BMI in the adult population of South Korea [95].
Additionally, another study conducted in South Korea indicated that the concentrations of most PCBs and certain OCPs, including hexachlorobenzene, heptachlor epoxide, β-hexachlorocyclohexane and oxychlordane were predictive of the risk for metabolic syndrome (MetS) and its associated components. The study followed 64 patients newly diagnosed with MetS over 4 years and included 182 control participants. Their findings demonstrated that prolonged exposure to a combination of PCBs and OCPs could increase the risk of developing MetS, even within the low-dose range of POPs [96].
5. Other Reported Diseases
Apart from the above-mentioned categories, POPs have been reported to be associated in South Korea with anemia and erythrocytosis, as well as problems in liver function. One study found that per-fluoroalkyl and poly-fluoroalkyl substances (PFAS) are associated with anemia and erythrocytosis and cause toxicity in the hematologic system. In their study involving 1295 men and 1644 women, the researchers made an intriguing observation regarding the effects of per- and poly-fluoroalkyl on the hematologic system. They found that PFAS exhibited specific effects on red blood cells (RBCs) in both sexes, leading to clinical manifestations such as anemia or erythrocytosis in the general population. These findings suggest that PFAS are associated with alterations in RBC metabolism, independent of bone marrow function, and can impact clinical status even at environmentally relevant levels of exposure [97].
Another research effort examined 1404 South Korean adults and linked problems in liver function with PFOA, PFOS, PFHxS, PFDA and PFNA. Among all participants, all five PFAS were detected. Elevated levels of serum PFOA, PFOS, PFHxS, PFDA and PFNA were found to be associated with increased concentrations of liver enzymes in the bloodstream. The nature of these associations varied depending on factors such as sex and obesity. When considering the combined exposure to multiple PFAS, a positive relationship was observed with liver enzyme levels [98]. Recently, it was found that PAH and VOC increased non-alcoholic fatty liver disease (NAFLD) among South Korean adolescents. A total of 798 adolescent participants were tested, 381 male and 417 female, and the results showed that 73 (9.1%) of the participants had been diagnosed with NAFLD [99].
6. Conclusion and Future Perspectives
This review highlighted for the first time the status of POP contamination in food in South Korea and the health impact of POPs in South Korea. It should be noted that the authors have encountered several limitations in finalizing this review, such as finding the related studies which connect POP pollution and health impacts among only South Korean people, selection of a suitable classification of diseases based on the existing literature and difficulty in searching for keywords due to wide range of journals in the field of environmental pollution and health (environment, health and medical journals). In sum, as this was not a systematic review, by searching in Scopus, Web of Science, and Google Scholar, 99 studies out of 311 have been selected focusing on the keywords (POPs pollution, South Korea, diseases, and health impacts). According to the findings, the following conclusions can be drawn:
- Even though OCPs, PCBs and PFAS are banned in South Korea, there has been a slight increase in environmental POPs levels over time due to urbanization and modernization in the last decade. The studies show that there are considerable amounts of POPs in the human mothers’ milk that is transferred to South Korean infants.
- The concentrations of PBDEs in seafood commonly consumed by South Koreans were analyzed. This analysis revealed that the contribution of seafood consumption to PBDE intake was the highest among the reported estimated daily intake in neighboring countries.
- There is a positive association between age, sex and BMI, and POP exposure and diabetes. Increased age and BMI increase the risk of diabetes among South Korean people. The most-reported diseases in South Korea are disruptions in the endocrine system, diabetes, obesity, cancer and thyroid illnesses. In addition, some of the other diseases, such as DNA methylation and hypomethylation, anemia, and problems in liver functions, have been reported, albeit rarely.
Although research on emerging POPs such as PBDEs and HBCDs in various environmental samples remains limited, the absence of established environmental quality guidelines for POPs in order to safeguard coastal ecosystems is a concern shared by both China and South Korea.
In addition, there is limited research in Asian countries such as China, Japan and Korea regarding the association between PBDE exposure and female reproductive functions affected by the thyroid system. Therefore, it is crucial to investigate the levels of PBDEs in various environmental sources, individuals and food samples to determine the exposure pathways of these POPs in South Koreans.
Author Contributions
Conceptualization, S.R. and L.R.; methodology, S.R. and L.R.; software, M.R. (Mehdi Rezaei); formal analysis, S.R. and L.R.; formal analysis, L.R.; investigation, S.R, and L.R.; resources, S.R.; data curation, S.R.; writing—original draft preparation, S.R. and L.R.; writing—review and editing, S.R., L.R., A.A.M., M.R. (Mahdi Rafieizonooz) and E.K.; visualization, M.R. (Mehdi Rezaei); supervision, S.R.; funding acquisition, L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the KU Research Program of Konkuk University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- POPs. USCOPOP Texts and Annexes. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 29 May 2023).
- Guillotin, S.; Delcourt, N. Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14271. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Bilal, M.; Qamar, S.A.; Imran, H.M.; Riasat, A.; Jahangeer, M.; Ghafoor, M.; Ali, N.; Iqbal, H.M. Nano-remediation technologies for the sustainable mitigation of persistent organic pollutants. Environ. Res. 2022, 211, 113060. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent organic pollutants in food: Contamination sources, health effects and detection methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef] [PubMed]
- Lallas, P.L. The Stockholm Convention on persistent organic pollutants. Am. J. Int. Law 2001, 95, 692–708. [Google Scholar] [CrossRef]
- Islam, R.; Kumar, S.; Karmoker, J.; Kamruzzaman, M.; Rahman, M.A.; Biswas, N.; Tran, T.K.A.; Rahman, M.M. Bioaccumulation and adverse effects of persistent organic pollutants (POPs) on ecosystems and human exposure: A review study on Bangladesh perspectives. Environ. Technol. Innov. 2018, 12, 115–131. [Google Scholar] [CrossRef]
- Alharbi, O.M.; Khattab, R.A.; Ali, I. Health and environmental effects of persistent organic pollutants. J. Mol. Liq. 2018, 263, 442–453. [Google Scholar] [CrossRef]
- Kogevinas, M. Human health effects of dioxins: Cancer, reproductive and endocrine system effects. Apmis 2001, 109, S223–S232. [Google Scholar] [CrossRef]
- Li, Q.Q.; Loganath, A.; Chong, Y.S.; Tan, J.; Obbard, J.P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A 2006, 69, 1987–2005. [Google Scholar]
- Rezania, S.; Talaeikhozani, A.; Oryani, B.; Cho, J.; Barghi, M.; Rupani, P.F.; Kamali, M. Occurrence of persistent organic pollutants (POPs) in the atmosphere of South Korea: A review. Environ. Pollut. 2022, 307, 119586. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, L.; Li, J.; Wu, Y. Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 2018, 198, 522–536. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Occurrence of environmental pollutants in foodstuffs: A review of organic vs. conventional food. Food Chem. Toxicol. 2019, 125, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hong, Y.S.; Ha, E.-H.; Park, H. Serum concentrations of PCBs and OCPs among prepubertal Korean children. Environ. Sci. Pollut. Res. 2016, 23, 3536–3547. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Vanderpuije, P.; Megson, D.; Reiner, E.J.; Bradley, L.; Adu-Kumi, S.; Gardella, J.A., Jr. The state of POPs in Ghana-A review on persistent organic pollutants: Environmental and human exposure. Environ. Pollut. 2019, 245, 331–342. [Google Scholar] [CrossRef]
- Fiedler, H.; Li, X.; Zhang, J. Persistent organic pollutants in human milk from primiparae–correlations, global, regional, and national time-trends. Chemosphere 2023, 313, 137484. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. Concentrations of environmental organic contaminants in meat and meat products and human dietary exposure: A review. Food Chem. Toxicol. 2017, 107, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Tsai, P.-C.; Yang, C.-Y.; Leon Guo, Y. Increased risk of diabetes and polychlorinated biphenyls and dioxins: A 24-year follow-up study of the Yucheng cohort. Diabetes Care 2008, 31, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Umulisa, V.; Kalisa, D.; Skutlarek, D.; Reichert, B. First evaluation of DDT (dichlorodiphenyltrichloroethane) residues and other Persistence Organic Pollutants in soils of Rwanda: Nyabarongo urban versus rural wetlands. Ecotoxicol. Environ. Saf. 2020, 197, 110574. [Google Scholar] [CrossRef]
- Mutiyar, P.; Mittal, A. Status of organochlorine pesticides in Ganga river basin: Anthropogenic or glacial? Drink. Water Eng. Sci. 2013, 6, 69–80. [Google Scholar] [CrossRef]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef]
- Dorsey, A. Toxicological Profile for Alpha-, Beta-, Gamma, and Delta-Hexachlorocyclohexane; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- Kim, Y.A.; Park, J.B.; Woo, M.S.; Lee, S.Y.; Kim, H.Y.; Yoo, Y.H. Persistent organic pollutant-mediated insulin resistance. Int. J. Environ. Res. Public Health 2019, 16, 448. [Google Scholar] [CrossRef]
- Mustafa, M.; Pathak, R.; Tripathi, A.; Ahmed, R.S.; Guleria, K.; Banerjee, B. Maternal and cord blood levels of aldrin and dieldrin in Delhi population. Environ. Monit. Assess. 2010, 171, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gągol, M.; Cako, E.; Fedorov, K.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Hydrodynamic cavitation based advanced oxidation processes: Studies on specific effects of inorganic acids on the degradation effectiveness of organic pollutants. J. Mol. Liq. 2020, 307, 113002. [Google Scholar] [CrossRef]
- Jorgenson, J.L. Aldrin and dieldrin: A review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ. Health Perspect. 2001, 109, 113–139. [Google Scholar] [PubMed]
- González, N.; Domingo, J.L. Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in food and human dietary intake: An update of the scientific literature. Food Chem. Toxicol. 2021, 157, 112585. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, Z.; Wang, L.; Liu, G.; Sun, Y.; Wang, Y.; Wang, T.; Liang, Y. Screening of potential PFOS alternatives to decrease liver bioaccumulation: Experimental and computational approaches. Environ. Sci. Technol. 2019, 53, 2811–2819. [Google Scholar] [CrossRef]
- Seo, S.-H.; Choi, S.-D.; Batterman, S.; Chang, Y.-S. Health risk assessment of exposure to organochlorine pesticides in the general population in Seoul, Korea over 12 years: A cross-sectional epidemiological study. J. Hazard. Mater. 2022, 424, 127381. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, S.-H.; Kim, H.-J.; Yoon, H.-J. Total diet studies as a tool for ensuring food safety. Toxicol. Res. 2015, 31, 221–226. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, M. A study on the change of postpartum care in Korea. Asia Cult. Stud. 2012, 26, 217–240. [Google Scholar]
- Domingo, J.L.; Nadal, M. Per-and polyfluoroalkyl substances (PFASs) in food and human dietary intake: A review of the recent scientific literature. J. Agric. Food Chem. 2017, 65, 533–543. [Google Scholar] [CrossRef]
- Heo, J.-J.; Lee, J.-W.; Kim, S.-K.; Oh, J.-E. Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. J. Hazard. Mater. 2014, 279, 402–409. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, S.-K.; Kang, D.-M.; Hwang, Y.-S.; Oh, J.-E. The relationships between sixteen perfluorinated compound concentrations in blood serum and food, and other parameters, in the general population of South Korea with proportionate stratified sampling method. Sci. Total Environ. 2014, 470, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-S.; Park, M.-K.; Kim, G.; Barghi, M.; Choi, S.-D.; Yang, J.; Chang, Y.-S. Dietary exposure and potential human health risk of dioxins in South Korea: Application of deterministic and probabilistic methods. Chemosphere 2022, 291, 133018. [Google Scholar] [CrossRef] [PubMed]
- Barghi, M.; Shin, E.-S.; Son, M.-H.; Choi, S.-D.; Pyo, H.; Chang, Y.-S. Hexabromocyclododecane (HBCD) in the Korean food basket and estimation of dietary exposure. Environ. Pollut. 2016, 213, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Paek, O.; Suh, J.; Park, H.; Oh, K.; Hong, S.; Lee, H.; Kim, M. Risk Assessment of Polychrorinated Biphenyls (PCBs) through Food Intake for the Korean Population. Korean J. Food Sci. Technol. 2013, 45, 364–369. [Google Scholar] [CrossRef]
- Kim, D.-G.; Kim, M.; Jang, J.-H.; Bong, Y.H.; Kim, J.-H. Monitoring of environmental contaminants in raw bovine milk and estimates of dietary intakes of children in South Korea. Chemosphere 2013, 93, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-B.; Kim, H.-S.; Choi, M.; Choi, H.-G. Intake and potential health risk of polycyclic aromatic hydrocarbons associated with seafood consumption in Korea from 2005 to 2007. Arch. Environ. Contam. Toxicol. 2010, 58, 214–221. [Google Scholar] [CrossRef]
- Moon, H.-B.; Kim, S.-J.; Park, H.; Jung, Y.S.; Lee, S.; Kim, Y.-H.; Choi, M. Exposure assessment for methyl and total mercury from seafood consumption in Korea, 2005 to 2008. J. Environ. Monit. 2011, 13, 2400–2405. [Google Scholar] [CrossRef]
- Lee, S.; Kannan, K.; Moon, H.-B. Assessment of exposure to polybrominated diphenyl ethers (PBDEs) via seafood consumption and dust ingestion in Korea. Sci. Total Environ. 2013, 443, 24–30. [Google Scholar] [CrossRef]
- Moon, H.; Kim, D.-H.; Oh, J.-E. Dietary exposure to PCBs by seafood cooking method: A Korean study. Chemosphere 2019, 215, 775–782. [Google Scholar] [CrossRef]
- Ji, K.; Kim, S.; Kho, Y.; Paek, D.; Sakong, J.; Ha, J.; Kim, S.; Choi, K. Serum concentrations of major perfluorinated compounds among the general population in Korea: Dietary sources and potential impact on thyroid hormones. Environ. Int. 2012, 45, 78–85. [Google Scholar] [CrossRef]
- Gascon, M.; Morales, E.; Sunyer, J.; Vrijheid, M. Effects of persistent organic pollutants on the developing respiratory and immune systems: A systematic review. Environ. Int. 2013, 52, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, S.; Kim, S.; Park, J.; Kim, H.-J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Kim, S. Placental transfer of persistent organic pollutants and feasibility using the placenta as a non-invasive biomonitoring matrix. Sci. Total Environ. 2018, 612, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-T.; Son, M.-H.; Lee, D.-H.; Seong, W.J.; Han, S.; Chang, Y.-S. Partitioning behavior of heavy metals and persistent organic pollutants among feto–maternal bloods and tissues. Environ. Sci. Technol. 2015, 49, 7411–7422. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H.-J.; Kim, S.; Choi, G.; Kim, S.; Park, J.; Shim, S.-S.; Lee, I.; Kim, S.; Moon, H.-B. Current status of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) exposure among mothers and their babies of Korea-CHECK cohort study. Sci. Total Environ. 2018, 618, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Kim, S.; Kim, S.; Kim, S.; Choi, Y.; Kim, H.-J.; Lee, J.J.; Kim, S.Y.; Lee, S.; Moon, H.-B. Occurrences of major polybrominated diphenyl ethers (PBDEs) in maternal and fetal cord blood sera in Korea. Sci. Total Environ. 2014, 491, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-Y.; Lee, S.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Park, J.; Moon, H.-B. Polybrominated diphenyl ethers in maternal serum, breast milk, umbilical cord serum, and house dust in a South Korean birth panel of mother-neonate pairs. Int. J. Environ. Res. Public Health 2016, 13, 767. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.-B.; Kim, S. Association between several persistent organic pollutants and thyroid hormone levels in cord blood serum and bloodspot of the newborn infants of Korea. PLoS ONE 2015, 10, e0125213. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Sinkiewicz-Darol, E.; Gadzała-Kopciuch, R. The impact of environmental pollution on the quality of mother’s milk. Environ. Sci. Pollut. Res. 2019, 26, 7405–7427. [Google Scholar] [CrossRef]
- Shin, M.-Y.; Kim, S.; Lee, S.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Park, J. Prenatal contribution of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) to total body burden in young children. Sci. Total Environ. 2018, 616, 510–516. [Google Scholar] [CrossRef]
- Kang, C.S.; Lee, J.-H.; Kim, S.-K.; Lee, K.-T.; Lee, J.S.; Park, P.S.; Yun, S.H.; Kannan, K.; Yoo, Y.W.; Ha, J.Y. Polybrominated diphenyl ethers and synthetic musks in umbilical cord serum, maternal serum, and breast milk from Seoul, South Korea. Chemosphere 2010, 80, 116–122. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, N.; Lee, J.-W.; Mehdi, Q.; Yun, M.-H.; Moon, H.-B. Time-course trend and influencing factors for per-and polyfluoroalkyl substances in the breast milk of Korean mothers. Chemosphere 2023, 310, 136688. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Park, J.; Kim, H.-J.; Cho, G.; Kim, G.-H.; Eun, S.-H.; Lee, J.J.; Choi, G.; Suh, E. Concentrations of phthalate metabolites in breast milk in Korea: Estimating exposure to phthalates and potential risks among breast-fed infants. Sci. Total Environ. 2015, 508, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ryu, H.-Y.; Lee, J.H.; Lee, Y.J.; Kim, H.-K.; Jang, D.D.; Kim, H.S.; Yoon, H.-S. Organochlorine pesticides and polychlorinated biphenyls in Korean human milk: Contamination levels and infant risk assessment. J. Environ. Sci. Health Part B 2013, 48, 243–250. [Google Scholar] [CrossRef]
- Kang, H.; Choi, K.; Lee, H.-S.; Kim, D.-H.; Park, N.-Y.; Kim, S.; Kho, Y. Elevated levels of short carbon-chain PFCAs in breast milk among Korean women: Current status and potential challenges. Environ. Res. 2016, 148, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.; Park, J.; Kim, H.-J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Kim, S.; Choi, K. Perfluoroalkyl substances (PFASs) in breast milk from Korea: Time-course trends, influencing factors, and infant exposure. Sci. Total Environ. 2018, 612, 286–292. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Lee, D.H.; Moon, H.-B. Association between several persistent organic pollutants in serum and adipokine levels in breast milk among lactating women of Korea. Environ. Sci. Technol. 2015, 49, 8033–8040. [Google Scholar] [CrossRef]
- Kim, S.; Eom, S.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Cho, G.; Kim, Y.D. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age-CHECK cohort study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Choi, K. Synthetic musk compounds and benzotriazole ultraviolet stabilizers in breast milk: Occurrence, time–course variation and infant health risk. Environ. Res. 2015, 140, 466–473. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Kim, S.; Choi, S.-D.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S. Infant exposure to polybrominated diphenyl ethers (PBDEs) via consumption of homemade baby food in Korea. Environ. Res. 2014, 134, 396–401. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Kim, S.; Choi, S.-D.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S. Occurrence and exposure assessment of polychlorinated biphenyls and organochlorine pesticides from homemade baby food in Korea. Sci. Total Environ. 2014, 470, 1370–1375. [Google Scholar] [CrossRef]
- Lee, H.A.; Hwang, H.J.; Oh, S.Y.; Ha, E.H.; Park, H. Dietary patterns related to exposure to persistent organic pollutants based on the Ewha Birth and Growth Cohort. Environ. Pollut. 2018, 243, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Ha, K.H.; Kim, D.J. New risk factors for obesity and diabetes: Environmental chemicals. J. Diabetes Investig. 2015, 6, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ha, E.; Hong, Y.S.; Park, H. Serum Levels of Persistent organic pollutants and insulin secretion among children Age 7–9 Years: A Prospective Cohort Study. Environ. Health Perspect. 2016, 124, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Staimez, L.R. Association of persistent organic pollutants and non-persistent pesticides with diabetes and diabetes-related health outcomes in Asia: A systematic review. Environ. Int. 2015, 76, 57–70. [Google Scholar] [CrossRef]
- Cha, E.S.; Jeong, M.; Lee, W.J. Agricultural pesticide usage and prioritization in South Korea. J. Agromed. 2014, 19, 281–293. [Google Scholar] [CrossRef]
- Magliano, D.; Loh, V.; Harding, J.; Botton, J.; Shaw, J. Persistent organic pollutants and diabetes: A review of the epidemiological evidence. Diabetes Metab. 2014, 40, 1–14. [Google Scholar] [CrossRef]
- Lim, J.; Son, H.; Park, S.; Jacobs, D.; Lee, D. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int. J. Obes. 2011, 35, 744–747. [Google Scholar] [CrossRef]
- Lee, S.; Lim, Y.; Kang, Y.; Jung, K.; Jee, S. The Association between Blood Concentrations of PCDD/DFs, DL-PCBs and the Risk of Type 2 Diabetes Mellitus and Thyroid Cancer in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 8745. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.-K.; Kim, J.-Y.; Lee, K.; Choi, J.R.; Chang, S.-J.; Chung, C.H.; Park, K.-S.; Oh, S.-S.; Koh, S.-B. Exposure to pesticides and the prevalence of diabetes in a rural population in Korea. Neurotoxicology 2019, 70, 12–18. [Google Scholar] [CrossRef]
- Lim, J.-E.; Jee, S.H. Association between serum levels of adiponectin and polychlorinated biphenyls in Korean men and women. Endocrine 2015, 48, 211–217. [Google Scholar] [CrossRef]
- Son, H.-K.; Kim, S.-A.; Kang, J.-H.; Chang, Y.-S.; Park, S.-K.; Lee, S.-K.; Jacobs, D., Jr.; Lee, D.-H. Strong associations between low-dose organochlorine pesticides and type 2 diabetes in Korea. Environ. Int. 2010, 36, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.; Kim, S.; Lee, Y.; Kim, D.; Lee, D. Can persistent organic pollutants distinguish between two opposite metabolic phenotypes in lean Koreans? Diabetes Metab. 2018, 44, 168–171. [Google Scholar] [CrossRef]
- Lim, J.-E.; Nam, C.; Yang, J.; Rha, K.H.; Lim, K.-M.; Jee, S.H. Serum persistent organic pollutants (POPs) and prostate cancer risk: A case-cohort study. Int. J. Hyg. Environ. Health 2017, 220, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Park, E.; Kim, J.; Oh, J.-K.; Kim, B.; Hong, Y.-C.; Lim, M.K. Impact of environmental exposure to persistent organic pollutants on lung cancer risk. Environ. Int. 2020, 143, 105925. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Schramm, K.-W. Perinatal effects of persistent organic pollutants on thyroid hormone concentration in placenta and breastmilk. Mol. Asp. Med. 2022, 87, 100988. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.-B.; Kim, S. Association between several persistent organic pollutants and thyroid hormone levels in serum among the pregnant women of Korea. Environ. Int. 2013, 59, 442–448. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, S.; Kim, S.; Lee, I.; Moon, M.K.; Choi, K.; Park, J.; Cho, Y.H.; Kwon, Y.M.; Yoo, J. Sex, menopause, and age differences in the associations of persistent organic pollutants with thyroid hormones, thyroxine-binding globulin, and peripheral deiodinase activity: A cross-sectional study of the general Korean adult population. Environ. Res. 2022, 212, 113143. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Bastiaensen, M.; Malarvannan, G.; Poma, G.; Casero, N.C.; Gys, C.; Covaci, A.; Lee, S.; Lim, J.-E. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ. Res. 2020, 189, 109874. [Google Scholar] [CrossRef]
- Aaseth, J.; Javorac, D.; Djordjevic, A.B.; Bulat, Z.; Skalny, A.V.; Zaitseva, I.P.; Aschner, M.; Tinkov, A.A. The role of persistent organic pollutants in obesity: A review of laboratory and epidemiological studies. Toxics 2022, 10, 65. [Google Scholar] [CrossRef]
- Hong, N.; Kim, K.; Lee, I.; Lind, P.; Lind, L.; Jacobs, D.; Lee, D. The association between obesity and mortality in the elderly differs by serum concentrations of persistent organic pollutants: A possible explanation for the obesity paradox. Int. J. Obes. 2012, 36, 1170–1175. [Google Scholar] [CrossRef]
- Moon, H.-B.; Lee, D.-H.; Lee, Y.S.; Choi, M.; Choi, H.-G.; Kannan, K. Polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in adipose tissues of Korean women. Arch. Environ. Contam. Toxicol. 2012, 62, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lim, H.J.; Won, A.J.; Ahn, M.Y.; Patra, N.; Chung, K.K.; Kwack, S.J.; Park, K.L.; Han, S.Y.; Choi, W.S. Comparisons of polybrominated diphenyl ethers levels in paired South Korean cord blood, maternal blood, and breast milk samples. Chemosphere 2012, 87, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, J.-H.; Park, H.; Baek, S.-Y.; Kim, Y.-H.; Chang, Y.-S. Assessment of polybrominated diphenyl ethers (PBDEs) in serum from the Korean general population. Environ. Pollut. 2012, 164, 46–52. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, S.; Kim, S.; Choi, S.-D.; Park, J.; Kim, H.-J.; Lee, J.J.; Choi, G.; Choi, S.; Kim, S. Occurrence and prenatal exposure to persistent organic pollutants using meconium in Korea: Feasibility of meconium as a non-invasive human matrix. Environ. Res. 2016, 147, 8–15. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, J.-C.; Lee, I.-K.; Moon, H.-B.; Chang, Y.-S.; Jacobs, D.R., Jr.; Lee, D.-H. Associations among organochlorine pesticides, Methanobacteriales, and obesity in Korean women. PLoS ONE 2011, 6, e27773. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, K.S.; Jacobs, D., Jr.; Lee, D.H. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes. Rev. 2017, 18, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, Y.H.; Won, S.; Ku, J.-L.; Moon, H.-B.; Park, J.; Choi, G.; Kim, S.; Choi, K. Maternal exposures to persistent organic pollutants are associated with DNA methylation of thyroid hormone-related genes in placenta differently by infant sex. Environ. Int. 2019, 130, 104956. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, K.M.; Lee, D.H.; Kim, D.S. Association of low-dose exposure to persistent organic pollutants with E-cadherin promoter methylation in healthy Koreans. Biomarkers 2018, 23, 293–298. [Google Scholar] [CrossRef]
- Lee, M.H.; Cho, E.R.; Lim, J.-E.; Jee, S.H. Association between serum persistent organic pollutants and DNA methylation in Korean adults. Environ. Res. 2017, 158, 333–341. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Kim, D.-S.; Lee, S.-K.; Lee, I.-K.; Kang, J.-H.; Chang, Y.-S.; Jacobs, D.R., Jr.; Steffes, M.; Lee, D.-H. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ. Health Perspect. 2010, 118, 370–374. [Google Scholar] [CrossRef]
- Lim, J.-E.; Lee, S.; Lee, S.; Jee, S.H. Serum persistent organic pollutants levels and stroke risk. Environ. Pollut. 2018, 233, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, S.H.; Hong, Y.S.; Ha, E.H.; Park, H. The effect of exposure to persistent organic pollutants on metabolic health among Korean children during a 1-year follow-up. Int. J. Environ. Res. Public Health 2016, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Park, H.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds is associated with a risk of obesity and diabetes mellitus among Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int. J. Hyg. Environ. Health 2022, 240, 113886. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Kim, K.-S.; Kim, S.-A.; Hong, N.-S.; Lee, S.-J.; Lee, D.-H. Prospective associations between persistent organic pollutants and metabolic syndrome: A nested case–control study. Sci. Total Environ. 2014, 496, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Sakong, J.; Park, C. Association of serum polyfluoroalkyl substances (PFAS) with anemia and erythrocytosis in Korean adults: Data from Korean National Environmental Health Survey cycle 4 (2018–2020). Int. J. Hyg. Environ. Health 2023, 249, 114136. [Google Scholar] [CrossRef]
- Kim, O.-J.; Kim, S.; Park, E.Y.; Oh, J.K.; Jung, S.K.; Park, S.; Hong, S.; Jeon, H.L.; Kim, H.-J.; Park, B. Exposure to serum perfluoroalkyl substances and biomarkers of liver function: The Korean national environmental health survey 2015–2017. Chemosphere 2023, 322, 138208. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Lee, J.-Y.; Moon, K.W. Exposure to volatile organic compounds and polycyclic aromatic hydrocarbons is associated with the risk of non-alcoholic fatty liver disease in Korean adolescents: Korea National Environmental Health Survey (KoNEHS) 2015–2017. Ecotoxicol. Environ. Saf. 2023, 251, 114508. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).