Invasive Ageratina adenophora (Asteraceae) in Agroecosystems of Kumaun Himalaya, India: A Threat to Plant Diversity and Sustainable Crop Yield
Abstract
1. Introduction
2. Materials and Method
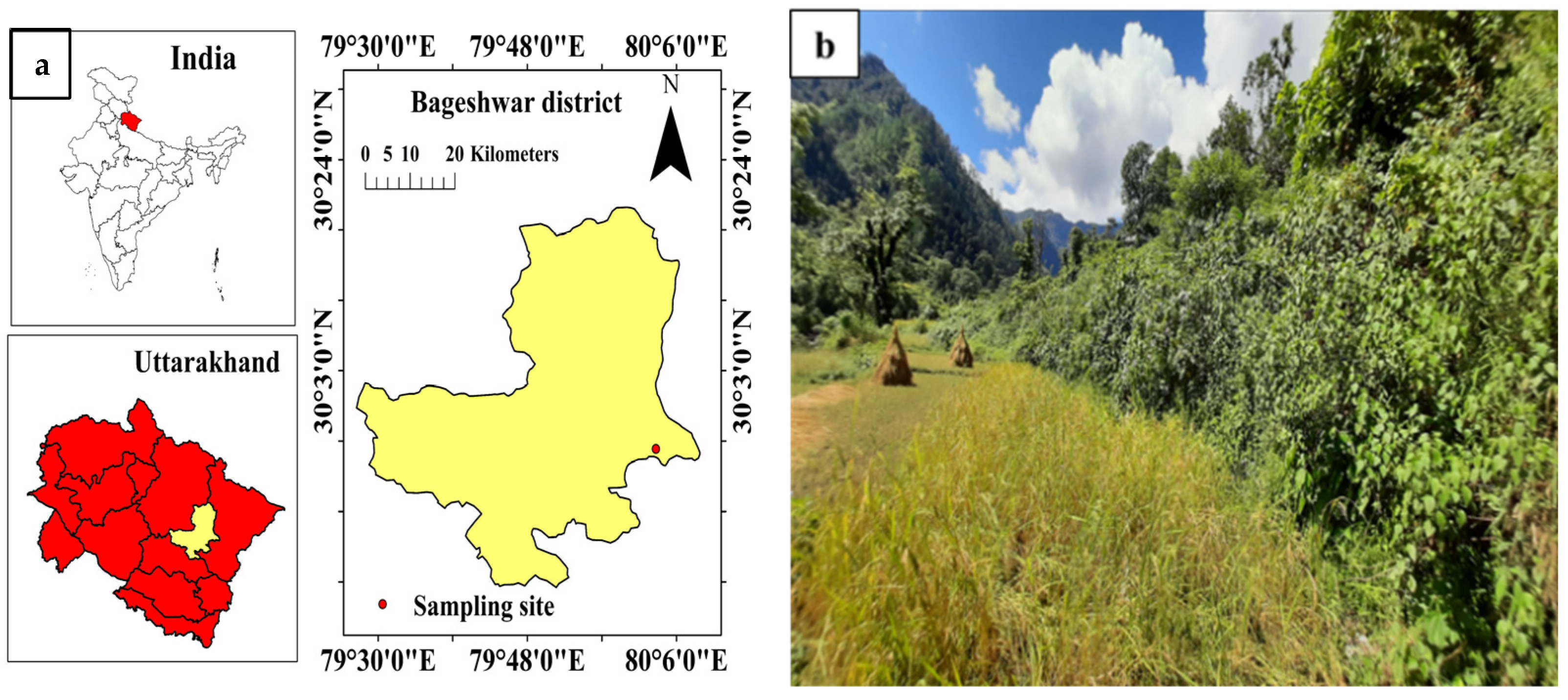
2.1. Description of the Study Site
2.2. Experimental Design
2.2.1. Ridge/Riser Vegetation Sampling
2.2.2. Assessment of Crop Density
2.2.3. Morphological Parameters
2.2.4. Yield and Yield–Related Parameters
2.2.5. Soil Sampling and Analysis
2.3. Data Analysis
3. Results
3.1. Effect of Invasion on Composition, Structure, and Soil Characteristics of Ridge Vegetation
3.2. Effect of Invasion on Light Intensity
3.3. Effect of Invasion on Field Crops
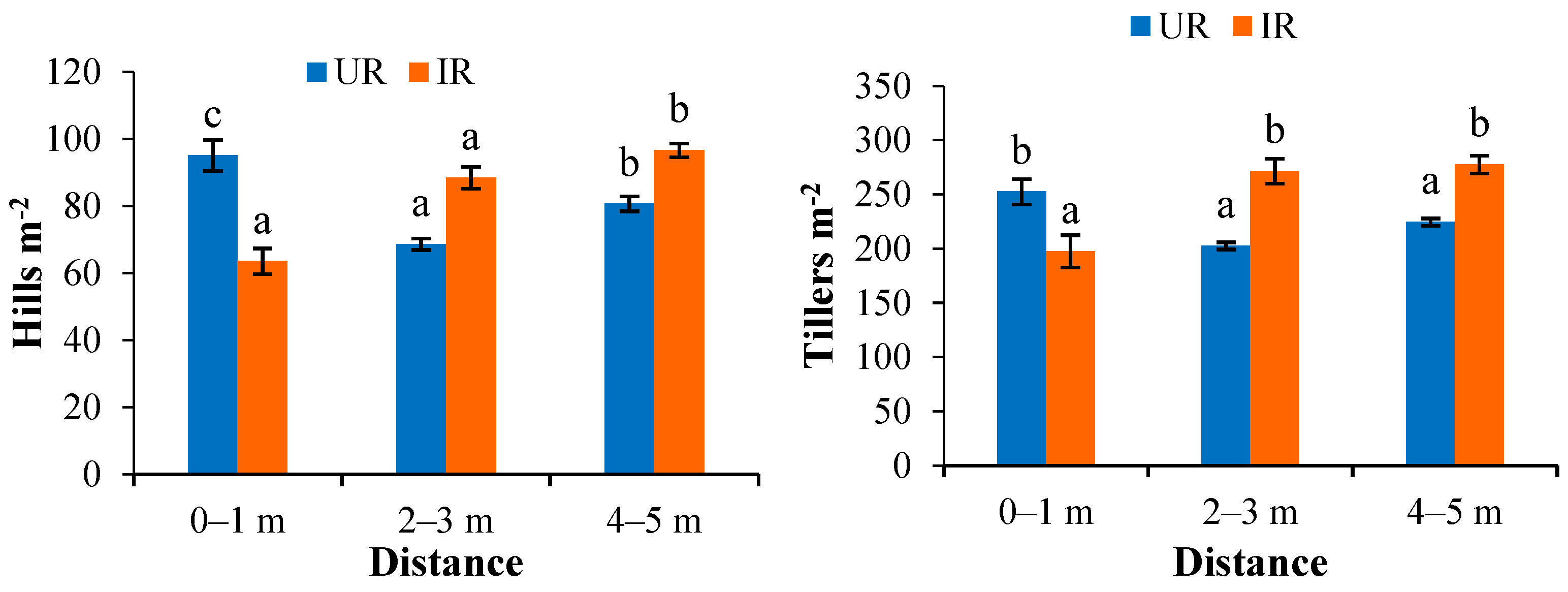
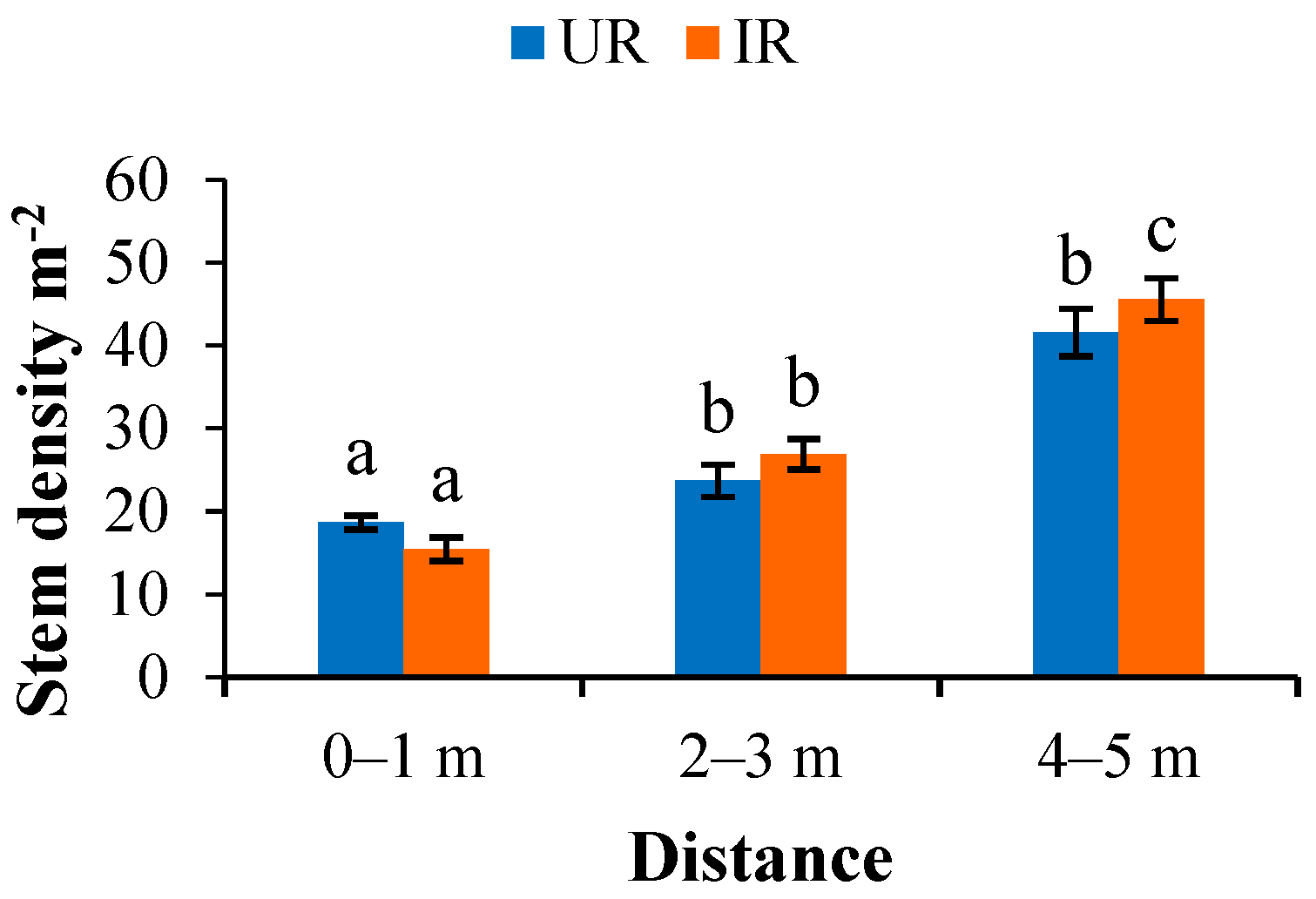
3.3.1. Planting Density
3.3.2. Morphological Parameters
3.3.3. Yield and Yield–Related Parameters
3.4. Effect of Invasion on Soil Characteristics of Crop Fields
4. Discussion
4.1. Effect of Crofton Weed on Ridge Vegetation and Soil Characteristics
4.2. Variation in Soil Characteristics of Crop Fields
4.3. Variation in Crop Performance
4.3.1. With Increasing Distance from Ridge
4.3.2. Near Ridges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palni, L.M.S.; Rawal, R.S. The Himalayan Biodiversity: Richness, Representativeness, Uniqueness and Life–Support Values; G. B. Pant Institute of Himalayan Environment and Development (GBPIHED): Almora, India, 2013; pp. 1–86.
- Mishra, P.K.; Rai, A.; Abdelrahman, K.; Rai, S.C.; Tiwari, A. Analysing challenges and strategies in land productivity in Sikkim Himalaya, India. Sustainability 2021, 13, 11112. [Google Scholar] [CrossRef]
- Pandey, L.; Arunachalam, A.; Joshi, N. Challenges of hill farming due to crop–raiding by wild pigs in the Indian Himalayan region. Curr. Sci. 2019, 116, 1015–1019. [Google Scholar] [CrossRef]
- Bargali, S.S.; Shahi, C.; Bargali, K.; Negi, B.; Khatri, K. Energy and monetary efficiencies at the different altitudinal agroecosystems in central Himalaya, India. Heliyon 2022, 8, e11500. [Google Scholar] [CrossRef]
- Choudhary, M.; Panday, S.C.; Meena, V.S.; Singh, S.; Yadav, R.P.; Mahanta, D.; Mondal, T.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Long–term effects of organic manure and inorganic fertilization on sustainability and chemical soil quality indicators of soybean–wheat cropping system in the Indian mid–Himalayas. Agric. Ecosyst. Environ. 2018, 257, 38–46. [Google Scholar] [CrossRef]
- Bargali, S.S.; Padalia, K.; Bargali, K. Effects of tree fostering on soil health and microbial biomass under different land use systems in the Central Himalayas. Land Deg. Dev. 2019, 30, 1984–1998. [Google Scholar] [CrossRef]
- Rawat, L.S.; Maikhuri, R.K.; Bahuguna, Y.M.; Maletha, A.; Phondani, P.C.; Jha, N.K.; Pharswan, D.S. Interference of Eupatorium adenophorum (Spr.) and its allelopathic effect on growth and yield attributes of traditional food crops in Indian Himalayan Region. Ecol. Res. 2019, 34, 587–599. [Google Scholar] [CrossRef]
- Dobhal, P.K.; Kohli, R.K.; Batish, D.R. Impact of Lantana camara L. invasion on riparian vegetation of Nayar region in Garhwal Himalayas (Uttarakhand, India). J. Ecol. Nat. Environ. 2011, 3, 11–22. [Google Scholar]
- Chapagain, T.; Ghimire, B.; Pudasaini, R.; Gurung, K.; Choi, K.; Rai, L.; Bishnu, B.K.; Raizada, M.N. The underutilized terrace wall can be intensified to improve farmer livelihoods. Agron. Sustain. Dev. 2019, 39, 29. [Google Scholar] [CrossRef]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Spatial variation in allelopathic inhibition by Ageratina adenophora on growth and yield of two traditional millet crops. Vegetos 2022, 35, 663–673. [Google Scholar] [CrossRef]
- Kato–Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Dar, P.A.; Reshi, Z.A. Assessment of plant invasions in agroecosystems of Kashmir Himalaya for better management. Front. Agron. 2022, 3, 116. [Google Scholar] [CrossRef]
- Arun, M.N.; Kumar, R.M.; Sreedevi, B.; Padmavathi, G.; Revathi, P.; Pathak, N.; Srinivas, D.; Venkatanna, B. The Rising Threat of Invasive Alien Plant Species in Agriculture. In Resource Management in Agroecosystems; Ondrasek, G., Zhang, L., Eds.; IntechOpen Limited: London, UK, 2023. [Google Scholar] [CrossRef]
- Radicetti, E.; Mancinelli, R. Sustainable weed control in the agroecosystems. Sustainability 2021, 13, 8639. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Kumar, M.; Garkoti, S.C. Allelopathy effects of invasive alien Ageratina adenophora on native shrub species of chir pine forest in the central Himalaya, India. J. For. Res. 2022, 27, 53–62. [Google Scholar] [CrossRef]
- Datta, A.; Kuhn, I.; Ahmad, M.; Michalski, S.; Auge, H. Processes affecting altitudinal distribution of invasive Ageratina adenophora in western Himalaya: The role of local adaptation and the importance of different life–cycle stages. PLoS ONE 2017, 12, e0187708. [Google Scholar] [CrossRef]
- Poudel, A.S.; Jha, P.K.; Shrestha, B.B.; Muniappan, R. Biology and management of the invasive weed Ageratina adenophora (Asteraceae): Current state of knowledge and future research needs. Weed Res. 2019, 59, 79–92. [Google Scholar] [CrossRef]
- Auld, B.; Martin, P.M. The autecology of Eupatorium adenophorum Spreng. in Australia. Weed Res. 1975, 15, 27–31. [Google Scholar] [CrossRef]
- Darji, T.B.; Adhikari, B.; Pathak, S.; Neupane, S.; Thapa, L.B.; Bhatt, T.D.; Pant, R.R.; Pant, G.; Pal, K.B.; Bishwakarma, K. Phytotoxic effects of invasive Ageratina adenophora on two native subtropical shrubs in Nepal. Sci. Rep. 2021, 11, 13663. [Google Scholar] [CrossRef]
- Thapa, L.B.; Kaewchumnong, K.; Sinkkonen, A.; Sridith, K. “Soaked in rainwater” effect of Ageratina adenophora on seedling growth and development of native tree species in Nepal. Flora 2020, 263, 151554. [Google Scholar] [CrossRef]
- Kumar, M.; Garkoti, S.C. Functional traits, growth patterns, and litter dynamics of invasive alien and co–occurring native shrub species of Chir pine forest in the central Himalaya, India. Plant Ecol. 2021, 222, 723–735. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Sun, Y.; Tong, B.; Guan, R.; Wu, M. Allelopathic effects of aqueous extract of Ageratina adenophora on seven native plant seedlings in growth and chlorophyll. Guangxi Zhiwu/Guihaia 2019, 39, 79–86. [Google Scholar]
- Fu, D.; Wu, X.; Huang, N.; Duan, C. Effects of the invasive herb Ageratina adenophora on understory plant communities and tree seedling growth in Pinus yunnanensis forests in Yunnan, China. J. For. Res. 2018, 23, 112–119. [Google Scholar] [CrossRef]
- Khatri, K.; Bargali, K.; Bargali, S.S.; Negi, B. Effects of leaf residues from Ageratina adenophora on germination, growth and productivity of two rabi crops. Acta Ecol. Sin. 2023, 43, 363–374. [Google Scholar] [CrossRef]
- Broadbent, A.; Stevens, C.J.; Peltzer, D.A.; Ostle, N.J.; Orwin, K.H. Belowground competition drives invasive plant impact on native species regardless of nitrogen availability. Oecologia 2018, 186, 577–587. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 23, 833–840. [Google Scholar] [CrossRef]
- Tripathi, R.S.; Khan, M.L.; Yadav, A.S. Biology of Mikania micrantha HBK: A Review. In Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent; CABI: Cambridge, MA, USA, 2012; pp. 99–107. [Google Scholar]
- Chapagain, T.; Raizada, M.N. Agronomic challenges and opportunities for smallholder terrace agriculture in developing countries. Front. Plant Sci. 2017, 8, 331. [Google Scholar] [CrossRef]
- Sati, V.P. Systems of agriculture farming in the Uttaranchal Himalaya, India. J. Mt. Sci. 2005, 2, 76–85. [Google Scholar] [CrossRef]
- FAO. AQUASTAT: FAO’s Global Information System on Water and Agriculture. 2021. Available online: https://www.fao.org/aquastat/en/geospatial–information/climate–information (accessed on 22 February 2022).
- Curtis, J.T.; McIntosh, R.P. The Interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Misra, R. Ecology Workbook; Oxford and IBH Publishing Company: Calcutta, India, 1968. [Google Scholar]
- Cottom, G.; Curtis, J.T. The use of distance measures in phyto–sociological sampling. Ecology 1956, 35, 451–460. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; pp. 1–35. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Sys. 1958, 3, 36–71. [Google Scholar]
- Pielou, E.C. Species–diversity and pattern–diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Mueller–Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Wiley: Hoboken, NJ, USA, 1974. [Google Scholar] [CrossRef]
- Available online:. Available online: http://www.worldfloraonline.org/ (accessed on 16 June 2023).
- Porker, K.; Straight, M.; Hunt, J.R. Evaluation of G× E× M interactions to increase harvest index and yield of early sown wheat. Front. Plant Sci. 2020, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Asefa, G. The role of harvest index in improving crop productivity. J. Nat. Sci. Res. 2019, 9, 24–28. [Google Scholar]
- Kaur, A.; Singh, A. Effect of weed infestation on root functional traits of wheat (Triticum aestivum L.) under different treatments. Ann. Plant Soil Res. 2020, 22, 269–274. [Google Scholar]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan Forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Walkely, A.; Black, C.A. An experiment of Degtjareff methods for determining soil organic matter and a proposed modification of the chronic acid titration methods. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Englewood Clift, NJ, USA, 1958. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular No. 939; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Black, C.A. Methods of Soil Analysis; Academic Press Inc.: New York, NY, USA, 1965; pp. 1–369. [Google Scholar]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Kumar, S.; Mathur, M. Impact of invasion by Prosopis juliflora on plant communities in arid grazing lands. Trop. Ecol. 2014, 55, 33–46. [Google Scholar]
- Shiferaw, H.; Schaffner, U.; Bewket, W.; Alamirew, T.; Zeleke, G.; Teketay, D.; Eckert, S. Modelling the current fractional cover of an invasive alien plant and drivers of its invasion in a dryland ecosystem. Sci. Rep. 2019, 9, 1576. [Google Scholar] [CrossRef]
- Mahla, N.; Mlambo, D. Influence of two co–occurring invasive plant species on resident woody species and surface soil properties in Chipinge Safari Area, Zimbabwe. Trop. Ecol. 2019, 60, 129–139. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Efects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Ye, X.Q.; Yan, Y.N.; Wu, M.; Yu, F.H. High capacity of nutrient accumulation by invasive Solidago canadensis in a coastal grassland. Front. Plant Sci. 2019, 10, 575. [Google Scholar] [CrossRef]
- Rodgers, V.L.; Wolfe, B.E.; Werden, L.K.; Finzi, A.C. The invasive species Alliaria petiolata (garlic mustard) increases soil nutrient availability in northern hardwood–conifer forests. Oecologia 2008, 157, 459–471. [Google Scholar] [CrossRef]
- Wang, R.; Zou, R.; Liu, J.; Liu, L.; Hu, Y. Spatial distribution of soil nutrients in farmland in a hilly region of the Pearl River Delta in China based on geostatistics and the inverse distance weighting method. Agriculture 2021, 11, 50. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, A.K.; Garkoti, S.C. Lantana camara and Ageratina adenophora invasion alter the understory species composition and diversity of Chir Pine forest in central Himalaya, India. Acta Oecol. 2020, 109, 103642. [Google Scholar] [CrossRef]
- Perkins, L.B.; Nowak, R.S. Soil conditioning and plant–soil feedbacks affect competitive relationships between native and invasive grasses. Plant Ecol. 2012, 213, 1337–1344. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y.; Li, X. Effects of soil phosphorus level on morphological and photosynthetic characteristics of Ageratina adenophora and Chromolaena odorata. Ying Yong Sheng Tai Xue Bao J. App. Ecol. 2006, 17, 602–606. [Google Scholar]
- Zheng, Y.L.; Feng, Y.L.; Liu, W.X.; Liao, Z.Y. Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecol. 2009, 203, 263–271. [Google Scholar] [CrossRef]
- Sadiq, S.; Jan, A. Effect of Graded Application of Potash on Kharif Maize Sown at Different Fertility Levels. Master’s Thesis, Department of Agronomy, The University of Agriculture, Peshawar, Pakistan, 2001. [Google Scholar]
- Hong, S.; Gan, P.; Chen, A. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Ahmed, A.; Aftab, S.; Hussain, S.; Nazir Cheema, H.; Liu, W.; Yang, F.; Yang, W. Nutrient accumulation and distribution assessment in response to potassium application under maize–soybean intercropping system. Agronomy 2020, 10, 725. [Google Scholar] [CrossRef]
- Sun, J.; Li, W.; Li, C.; Chang, W.; Zhang, S.; Zeng, Y.; Zeng, C.; Peng, M. Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front. Plant Sci. 2020, 11, 613760. [Google Scholar] [CrossRef]
- Brown, J.; Caligari, P.D.S.; Campos, H.A. Second Edition of Introduction to Plant Breeding; University of Idaho: Moscow, ID, USA; Monsanto: St. Louis, MO, USA, 2014; pp. 1–295. [Google Scholar]
- Alipour Abookheili, F.; Mobasser, H.R. Effect of planting density on growth characteristics and grain yield increase in successive cultivations of two rice cultivars. Agrosyst. Geosci. Environ. 2021, 4, e20213. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhou, D.W. Root morphological responses to population density vary with soil conditions and growth stages: The complexity of density effects. Ecol. Evol. 2021, 11, 10590–10599. [Google Scholar] [CrossRef]
- Michelan, T.S.; Thomaz, S.M.; Bando, F.M.; Bini, L.M. Competitive Effects Hinder the Recolonization of Native Species in Environments Densely Occupied by One Invasive Exotic Species. Front. Plant Sci. 2018, 9, 126. [Google Scholar] [CrossRef]
- Carvalho, P.; Foulkes, M.J. Roots and Uptake of Water and Nutrients. In Encyclopedia of Sustainability Science and Technology; Meyers, R., Ed.; Springer: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Zhang, D.; Lyu, Y.; Li, H.; Tang, X.; Hu, R.; Rengel, Z.; Zhang, F.; Whalley, W.R.; Davis, W.J.; Cahill, J.F., Jr.; et al. Neighbouring plants modify maize root foraging for phosphorus: Coupling nutrients and neighbours for improved nutrient–use efficiency. New Phytol. 2020, 226, 244–253. [Google Scholar] [CrossRef]
- Köchy, M.; Wilson, S.D. Competitive effects of shrubs and grasses in prairie. Oikos 2000, 91, 385–395. [Google Scholar] [CrossRef]
- Patterson, D.T. Effects of environmental stress on weed/crop interactions. Weed Sci. 1995, 43, 483–490. [Google Scholar] [CrossRef]
- Muniappan, R.; Raman, A.; Reddy, G.V. Ageratina adenophora (Sprengel) King and Robinson (Asteraceae); Cambridge University Press: Cambridge, UK, 2009; pp. 63–73. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, Y.; Yang, X.; Sun, S. Linking trait differences to community dynamics: Evidence from Eupatorium adenophorum and co–occurring native species during a three–year succession. PLoS ONE 2013, 8, e50247. [Google Scholar] [CrossRef] [PubMed]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Phenotypic variation in morphology and associated functional traits in Ageratina adenophora along an altitudinal gradient in Kumaun Himalaya, India. Biologia 2023, 78, 1333–1347. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Wang, J.-F.; Sang, W.-G. Biomass allocation, morphology and photosynthesis of invasive and non–invasive exotic species grown at four irradiance levels. Acta Oecol. 2007, 31, 40–47. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.P.; Zeng, Y.L.; Deng, F.; Ren, W.J. Effect of different shading materials on grain yield and quality of rice. Sci. Rep. 2019, 9, 9992. [Google Scholar] [CrossRef]
- Xu, C.; Tao, H.; Wang, P.; Wang, Z. Slight shading after anthesis increases photosynthetic productivity and grain yield of winter wheat (Triticum aestivum L.) due to the delaying of leaf senescence. J. Integr. Agric. 2016, 15, 63–75. [Google Scholar] [CrossRef]
- Dong, B.; Yang, H.; Liu, H.; Qiao, Y.; Zhang, M.; Wang, Y.; Xie, Z.; Liu, M. Effects of shading stress on grain number, yield, and photosynthesis during early reproductive growth in wheat. Crop Sci. 2019, 59, 363–378. [Google Scholar] [CrossRef]
- Zheng, G.; Luo, S.; Li, S.; Hua, J.; Li, W.; Li, S. Specialized metabolites from Ageratina adenophora and their inhibitory activities against pathogenic fungi. Phytochemistry 2018, 148, 57–62. [Google Scholar] [CrossRef]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Trait variability in co–occurring invasive and native plant species in road side population of Kumaun Himalaya. Braz. J. Bot. 2022, 45, 1099–1110. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Jabran, K.; Farooq, M. Implications of potential allelopathic crops in agricultural systems. In Allelopathy; Cheema, Z.A., Farooq, M., Wahid, A., Eds.; Springer: Berlin, Germany, 2013; pp. 349–385. [Google Scholar] [CrossRef]
- Balke, N.E. Effects of allelochemicals on mineral uptake and associated physiological processes. In The Chemistry of Allelopathy: Biochemical Interactions among Plants; Thompson, A.C., Ed.; American Biochemical Society: Washington, DC, USA, 1985; pp. 161–178. [Google Scholar]
- Zhang, F.; Guo, J.; Chen, F.; Liu, W.; Wan, F. Identification of volatile compounds released by leaves of the invasive plant Crofton weed (Ageratina adenophora, Compositae), and their inhibition of rice seedling growth. Weed Sci. 2012, 60, 205–211. [Google Scholar] [CrossRef]
- Yang, G.Q.; Wan, F.H.; Guo, J.Y.; Liu, W.X. Cellular and ultrastructural changes in the seedling roots of upland rice (Oryza sativa) under the stress of two allelochemicals from Ageratina adenophora. Weed Biol. Manag. 2011, 11, 152–159. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.-X.; Zheng, M.-F.; Xu, Q.-L.; Wan, F.-H.; Wang, J.; Lei, T.; Zhou, Z.-Y.; Tan, J.-W. Bioactive quinic acid derivatives from Ageratina adenophora. Molecules 2013, 18, 14096–14104. [Google Scholar] [CrossRef]
- Ma, J.; Xing, G.; Yang, W.; Ma, L.; Gao, M.; Wang, Y. Inhibitory effects of leachate from Eupatorium adenophorum on germination and growth of Amaranthus retroflexus and Chenopodium glaucum. Acta Ecol. Sin. 2012, 32, 50–56. [Google Scholar] [CrossRef]
- Das, M.B.B.; Acharya, B.D.; Saquib, M.; Chettri, M.K. Effect of aqueous extract and compost of invasive weed Ageratina adenophora on seed germination and seedling growth of some crops and weeds. J. Biodivers. Conserv. Bioresour. Manag. 2018, 4, 11–20. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Yuan, L.; Huang, J. Allelopathy of uncomposted and composted invasive aster (Ageratina adenophora) on ryegrass. J. Hazard. Mater. 2021, 402, 123727. [Google Scholar] [CrossRef]
- Kato–Noguchi, H.; Kato, M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Baličević, R.; Ravlić, M.; Živković, T. Allelopathic effect of invasive species giant goldenrod (Solidago gigantea Ait.) on crops and weeds. Herbologia 2015, 15, 19–29. [Google Scholar] [CrossRef]

| Parameters | Rice | Soybean | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UR | IR | t | d.f | Sig. (2–Tailed) | UR | IR | t | d.f | Sig. (2–Tailed) | |
| Vegetative | ||||||||||
| Density | 138.44 ± 17.37 | 88.56 ± 12.22 | 2.35 | 4 | 0.079 | 234.33 ± 34.51 | 34.93 ± 3.70 | 5.75 | 4 | 0.028 |
| TBA | 8.64 ± 1.71 | 9.65 ± 2.93 | −0.30 | 4 | 0.781 | 6.58 ± 0.95 | 11.77 ± 7.93 | −0.65 | 4 | 0.581 |
| H′ | 1.77 ± 0.15 | 1.04 ± 0.06 | 4.26 | 4 | 0.013 | 1.04 ± 0.17 | 1.06 ± 0.05 | −0.18 | 4 | 0.869 |
| E | 0.74 ± 0.06 | 0.37 ± 0.02 | 5.16 | 4 | 0.007 | 0.27 ± 0.14 | 0.33 ± 0.09 | −0.41 | 4 | 0.703 |
| CD | 0.24 ± 0.04 | 0.01 ± 0.00 | 6.10 | 4 | 0.004 | 0.55 ± 0.08 | 0.004 ± 0.00 | 6.66 | 4 | 0.022 |
| R | 1.66 ± 0.03 | 2.64 ± 0.13 | −7.09 | 4 | 0.002 | 1.42 ± 0.10 | 2.07 ± 0.10 | −4.65 | 4 | 0.010 |
| Soil | ||||||||||
| Gravel (%) | 47.90 ± 1.78 | 56.82 ± 1.68 | −3.65 | 4 | 0.022 | 78.10 ± 0.78 | 63.52 ± 1.72 | 7.73 | 4 | 0.002 |
| Sand (%) | 15.90 ± 0.54 | 14.09 ± 0.44 | 2.61 | 4 | 0.060 | 6.90 ± 0.67 | 11.93 ± 0.58 | −5.65 | 4 | 0.005 |
| Silt (%) | 9.60 ± 0.33 | 7.10 ± 0.37 | 5.07 | 4 | 0.007 | 3.43 ± 0.47 | 7.70 ± 0.99 | −3.88 | 4 | 0.018 |
| Clay (%) | 26.61 ± 0.91 | 21.99 ± 1.02 | 3.38 | 4 | 0.028 | 11.57 ± 0.63 | 16.84 ± 0.99 | −4.49 | 4 | 0.011 |
| ST (° C) | 22.67 ± 0.33 | 22.67 ± 0.33 | 0.00 | 4 | 1.000 | 23.67 ± 0.88 | 22.00 ± 0.58 | 1.58 | 4 | 0.189 |
| SMC | 18.33 ± 1.50 | 21.52 ± 2.22 | −1.19 | 4 | 0.301 | 46.95 ± 2.18 | 42.39 ± 0.68 | 2.00 | 4 | 0.116 |
| BD (g cm−3) | 1.33 ± 0.04 | 1.19 ± 0.04 | 2.60 | 4 | 0.060 | 1.03 ± 0.04 | 0.90 ± 0.00 | 3.77 | 4 | 0.062 |
| Porosity (%) | 48.84 ± 1.71 | 54.30 ± 1.35 | −2.50 | 4 | 0.067 | 60.45 ± 1.41 | 65.56 ± 0.11 | −3.62 | 4 | 0.022 |
| OC (%) | 1.88 ± 0.02 | 1.96 ± 0.04 | −1.91 | 4 | 0.129 | 2.24 ± 0.02 | 2.80 ± 0.01 | −24.29 | 4 | 0.000 |
| pH | 6.20 ± 0.03 | 7.39 ± 0.07 | −15.14 | 4 | 0.000 | 7.52 ± 0.02 | 7.53 ± 0.01 | −0.26 | 4 | 0.811 |
| TN (%) | 0.30 ± 0.01 | 0.33 ± 0.02 | −1.56 | 4 | 0.195 | 0.32 ± 0.01 | 0.39 ± 0.01 | −6.06 | 4 | 0.004 |
| P (%) | 0.0017 ± 0.00 | 0.0041 ± 0.00 | −4.22 | 4 | 0.045 | 0.0051 ± 0.00 | 0.0047 ± 0.00 | 3.05 | 4 | 0.038 |
| K (%) | 0.0036 ± 0.00 | 0.0051 ± 0.00 | −7.89 | 4 | 0.001 | 0.0044 ± 0.00 | 0.0056 ± 0.00 | −5.34 | 4 | 0.006 |
| Parameters | Rice | Parameters | Soybean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Distance | Treatment × Distance | Treatment | Distance | Treatment × Distance | ||||||||
| d.f | F | d.f | F | d.f | F | d.f | F | d.f | F | d.f | F | ||
| SL | 1 | 18.73 *** | 2 | 3.64 * | 2 | 0.37 NS | SL | 1 | 1.13 NS | 2 | 314.03 *** | 1 | 0.08 NS |
| RL | 1 | 0.08 NS | 2 | 2.89 NS | 2 | 1.07 NS | RL | 1 | 4.68 * | 2 | 34.45 *** | 1 | 1.21 NS |
| SD | 1 | 44.45 *** | 2 | 16.29 *** | 2 | 1.30 NS | SD | 1 | 0.13 NS | 2 | 65.44 *** | 1 | 0.14 NS |
| PL | 1 | 25.22 *** | 2 | 8.60 *** | 2 | 0.64 NS | PN | 1 | 0.24 NS | 2 | 199.18 *** | 1 | 4.81 * |
| GN | 1 | 31.19 *** | 2 | 8.19 *** | 2 | 1.90 NS | GN | 1 | 23.45 *** | 2 | 84.33 *** | 1 | 7.11 *** |
| SY | 1 | 45.52 *** | 2 | 12.34 *** | 2 | 1.10 NS | SY | 1 | 0.00 NS | 2 | 127.25 *** | 1 | 0.13 NS |
| RY | 1 | 27.54 *** | 2 | 2.74 NS | 2 | 0.06 NS | RY | 1 | 0.03 NS | 2 | 157.39 *** | 1 | 2.15 NS |
| GY | 1 | 19.85 *** | 2 | 14.12 *** | 2 | 0.44 NS | GY | 1 | 16.39 *** | 2 | 106.17 *** | 1 | 3.97 * |
| SPP | 1 | 22.95 *** | 2 | 10.31 *** | 2 | 5.16 ** | PY | 1 | 9.66 ** | 2 | 5.25 ** | 1 | 2.46 NS |
| SRL | 1 | 38.07 *** | 2 | 3.58 * | 2 | 0.25 NS | SRL | 1 | 0.89 NS | 2 | 139.92 *** | 1 | 2.15 NS |
| HI | 1 | 0.66 NS | 2 | 2.94 NS | 2 | 0.24 NS | HI | 1 | 2.19 NS | 2 | 1.58 NS | 1 | 0.05 NS |
| R:S | 1 | 5.95 * | 2 | 2.41 NS | 2 | 0.30 NS | R:S | 1 | 11.19 *** | 2 | 9.45 *** | 1 | 0.30 NS |
| TD | 1 | 7.75 ** | 2 | 3.55 * | 2 | 23.67 *** | Density | 1 | 0.65 NS | 2 | 89.57 *** | 1 | 1.89 NS |
| HD | 1 | 0.31 NS | 2 | 6.51 ** | 2 | 42.07 *** | |||||||
| TPH | 1 | 5.90 * | 2 | 1.12 NS | 2 | 1.83 NS | |||||||
| Rice | Soybean | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Distance | Treatment × Distance | Treatment | Distance | Treatment × Distance | |||||||
| Parameters | d.f | F | d.f | F | d.f | F | d.f | F | d.f | F | d.f | F |
| Gravel | 1 | 109.39 *** | 2 | 294.03 *** | 2 | 38.28 *** | 1 | 3.36 NS | 2 | 81.47 *** | 2 | 2.41 NS |
| Sand | 1 | 57.31 *** | 2 | 30.14 *** | 2 | 47.21 *** | 1 | 0.001 NS | 2 | 15.84 ** | 2 | 0.21 NS |
| Silt | 1 | 6.57 * | 2 | 1.55 NS | 2 | 7.08 ** | 1 | 2.32 NS | 2 | 4.87 * | 2 | 0.15 NS |
| Clay | 1 | 116.40 *** | 2 | 12.46 *** | 2 | 15.86 *** | 1 | 0.92 NS | 2 | 62.22 *** | 2 | 4.83 NS |
| ST | 1 | 0.17 NS | 2 | 41.23 *** | 2 | 32.88 *** | 1 | 0.06 NS | 2 | 40.39 *** | 2 | 1.06 NS |
| SMC | 1 | 23.60 *** | 2 | 4.67 * | 2 | 0.67 NS | 1 | 4.28 NS | 2 | 4.04 * | 2 | 1.61 NS |
| BD | 1 | 100.71 *** | 2 | 140.00 *** | 2 | 37.77 *** | 1 | 0.08 NS | 2 | 36.62 *** | 2 | 1.19 NS |
| Porosity | 1 | 94.20 *** | 2 | 5.97 * | 2 | 13.40 *** | 1 | 0.10 NS | 2 | 36.51 *** | 2 | 1.19 NS |
| OC | 1 | 810.99 *** | 2 | 28.20 *** | 2 | 83.01 *** | 1 | 1.61 NS | 2 | 304.11 *** | 2 | 28.29 *** |
| pH | 1 | 48.11 *** | 2 | 10.61 ** | 2 | 4.67 * | 1 | 167.92 *** | 2 | 1.50 NS | 2 | 0.95 NS |
| TN | 1 | 7.69 * | 2 | 0.94 NS | 2 | 4.98 * | 1 | 27.84 *** | 2 | 8.86 ** | 2 | 14.70 *** |
| P | 1 | 1075.00 *** | 2 | 14.81 *** | 2 | 15.79 *** | 1 | 984.02 *** | 2 | 10.25 ** | 2 | 8.38 ** |
| K | 1 | 294.03 *** | 2 | 38.28 *** | 2 | 9.78 ** | 1 | 94.25 *** | 2 | 41.15 *** | 2 | 27.65 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, B.; Khatri, K.; Bargali, S.S.; Bargali, K. Invasive Ageratina adenophora (Asteraceae) in Agroecosystems of Kumaun Himalaya, India: A Threat to Plant Diversity and Sustainable Crop Yield. Sustainability 2023, 15, 10748. https://doi.org/10.3390/su151410748
Negi B, Khatri K, Bargali SS, Bargali K. Invasive Ageratina adenophora (Asteraceae) in Agroecosystems of Kumaun Himalaya, India: A Threat to Plant Diversity and Sustainable Crop Yield. Sustainability. 2023; 15(14):10748. https://doi.org/10.3390/su151410748
Chicago/Turabian StyleNegi, Bhawna, Kavita Khatri, Surendra Singh Bargali, and Kiran Bargali. 2023. "Invasive Ageratina adenophora (Asteraceae) in Agroecosystems of Kumaun Himalaya, India: A Threat to Plant Diversity and Sustainable Crop Yield" Sustainability 15, no. 14: 10748. https://doi.org/10.3390/su151410748
APA StyleNegi, B., Khatri, K., Bargali, S. S., & Bargali, K. (2023). Invasive Ageratina adenophora (Asteraceae) in Agroecosystems of Kumaun Himalaya, India: A Threat to Plant Diversity and Sustainable Crop Yield. Sustainability, 15(14), 10748. https://doi.org/10.3390/su151410748







