Abstract
The share of molecular hydrogen as a source of “green energy” is currently significantly increasing. It is proposed to use existing underground natural gas storage facilities to store large volumes of hydrogen. In Russia, depleted oil and gas fields (DOGFs) and deep aquifers are used for natural gas storage. The purpose of this work was to determine microbial diversity in DOGF and deep aquifers by cultural and 16S rRNA gene-based approaches and the effect of H2 on the growth of microorganisms from the underground gas storage (UGS) horizons. The composition of the microbial community inhabiting the formation water of the Peschano–Umetskoe depleted oil and gas reservoir was typical for microbial communities of oil reservoirs and included bacteria of the phyla Bacillota (Dethiosulfatibacter, Defluviitalea, Acetobacterium, Syntrophobotulus), Actinobacteriota (Dietzia, Rhodococcus), Spirochaetota (Sphaerochaeta), Pseudomonadota (Shewanella), and Bacteroidota (Petrimonas), together with methanogenic archaea of the phylum Euryarchaeota (Methanobacterium). In some formation water samples, the share of methanogens of the genus Methanobacterium reached 61.6% of the total community; these hydrogen-utilizing organisms may contribute to the formation of methane in the reservoirs used for the storage of molecular hydrogen. Microbial communities of UGSs located in aquifers were less diverse and abundant. Cultivable hydrogenotrophic sulfate-reducing, homoacetogenic, and methanogenic prokaryotes were retrieved from the studied aquifers and from the DOGF used for gas storage. Microorganisms present in the condensation and reservoir waters of the UGS facilities can influence the composition of the water and gas phase, and affect the host rocks and borehole equipment.
1. Introduction
Hydrogen energetics is presently a topic of ever-increasing interest [1,2]. Many industrially developed states are implementing programs on the development of hydrogen technologies [3,4]. Establishment of environmentally friendly power engineering, one of the major components of the new mode of technology, implies expansion in the applications of hydrogen as an energy reservoir, energy carrier, and a chemical reagent. Determination of the optimal conditions for underground storage of hydrogen together with methane is therefore an urgent task. Assessment of the risks associated with activity of the microbial communities competing for hydrogen in the underground storage facilities for methane–hydrogen mixtures is of special importance [5,6]. The major complicating factors are (1) corrosion of the surface and submerged equipment used for storage of the hydrogen–methane mixtures; (2) formation of sulfur deposits on the equipment; (3) plugging of the near-bottom zone of wells by the products of chemical interaction between sedimentary and condensation waters; and (4) decrease in the stored gas volume due to diffusion into the caprock and to hydrogen dissolution in the formation water [7].
For underground gas storage (UGS), depleted oil and gas fields (DOGFs), deep aquifers, and salt caverns are widely applied [1,8]. Abundant information is available concerning the presence of microorganisms capable of H2 utilization in deep oligotrophic environments [9,10] and in oil fields [11,12]. Sulfate-reducing, acetogenic, and methanogenic microorganisms able to utilize H2 have been isolated from subterranean environments [13]. Quantitative assessment of the rates of sulfate reduction, acetogenesis, and methanogenesis in oil fields was carried out [14,15] and stimulation of these processes by exogenous hydrogen was shown [16,17]. Phylogenetically and metabolically diverse microbial communities were detected in groundwater samples and aquifer sediments adjacent to the Colorado River, located near Rifle, CO, USA [18]. Using 2540 draft-quality microbial genomes analysis, it was predicted that microorganisms could conduct multiple sequential redox transformations and participate in the biogeochemical cycles of carbon, nitrogen, sulfur, and hydrogen.
Microorganisms inhabiting UGSs located in deep aquifers [1,19,20,21], oil and gas reservoirs [22,23], and those colonizing natural gas pipelines [24] have been scarcely studied. Molecular hydrogen arriving in the deep environments (aquifers or oil and gas reservoirs) may be expected to stimulate the activity of the deep microbial communities. Šmigáň et al. [25] have reported that autochthonous methanogenic and sulfate-reducing populations inhabited an aquifer used for storage of natural gas and town gas containing molecular hydrogen and carbon dioxide, in Lobodice, former Czechoslovakia (now Czech Republic). These microorganisms utilized H2 with production of methane and hydrogen sulfide, which resulted in losses of up to 17% molecular hydrogen [26]. The results of Thaysen et al. [27] seem to contradict this value. These authors evaluated the risk for microbial growth in 42 depleted oil and gas fields and found that microbial hydrogen-consuming processes could be sustained in only 6 of 42 fields. The potential hydrogen consumption by actively growing homoacetogenic, methanogenic, and sulfur species-reducing microorganisms (SSRM) was <0.1–3.2%, <0.01–1.8%, and <0.01–1.3%, respectively, of the H2 in the aquifer in the Frigg reservoir, and <0.1–2.0%, <0.01–2.1%, and <0.01–0.5% of the H2 in the Hamilton reservoir. These rates were reached in these two low-temperature and low-salinity DOGFs after 0.2–342 days, when microorganisms grew to their maximum cell counts, based on the dissolved nutrient concentrations.
Ranchou-Peyruse et al. [20] using the dsrB and mcrA genes as phylogenetic markers, studied the phylogenetic composition of sulfate-reducing and methanogenic microorganisms in six aquifers used for natural gas storage in France. It was shown that sulfate-reducing bacteria of the genus Desulfotomaculum, uncultured members of the Peptococcaceae, and Candidatus Desulforudis audaxviator predominated in five of the six studied aquifers; distant from the UGS, members of the families Desulfovibrionaceae, Desulfomicrobiaceae, Desulfobulbaceae, and Syntrophaceae also occurred, together with uncultured Deltaproteobacteria. Methanogenic archaea belonging to the families Methanosarcinaceae, Methanobacteriaceae, Methanomicrobiaceae, Methanopyraceae, and Methanosaetaceae, and to the new uncultured group close to the Methanobacteriaceae family, predominated in only one aquifer containing a mixture of natural gas and about 50% H2. It was suggested that the injection of gases into aquifers leads to a change in the composition of underground microbial populations and that affects the production of methane from hydrogen that may be realized in these environments through time [20].
In Russia, 17 UGSs located in depleted oil and gas reservoirs and 8 UGSs located in deep aquifers are in operation. In water samples collected from subsurface horizons in the North Stavropol UGS depleted gas reservoir, cultivable fermenting, acetogenic, methanogenic, iron- and sulfate-reducing prokaryotes were found [22,28,29]. High rates of sulfate reduction and methanogenesis were registered in the water samples collected from surface facilities in the process of gas injection [22]. In water samples from the aquifers used for natural gas storage at the Shchelkovo, Kasimov, and Kaluga UGSs, acetic and other lower fatty acids, methanol, and dissolved gases were detected; these may serve as substrates for microorganisms [30]. Cultivated aerobic organotrophic bacteria and anaerobic fermenting, sulfate-reducing, and methanogenic microorganisms were not abundant in these samples. By high-throughput sequencing of the V4 fragment of the 16S rRNA genes, bacteria of the phyla Bacillota, Bacteroidota, Pseudomonadota, Actinomycetota, Desulfobacterota, Verrucomicrobiota, and Planctomycetota were revealed in the studied water samples. The share of archaeal sequences in the studied libraries did not exceed 1.5%.
The data on the composition of microbial populations in the UGS horizons and of their possible H2 consumption are essential for the prediction of H2 preservation in UGSs, and for assessing the effect of hydrogen on the functional activity of microbial populations and the subsequent effect of microorganisms on the composition of the gas, rocks, and infrastructure of UGS facilities [4,6,31].
The objective of this work was to describe the microbial diversity in subsurface horizons used for UGS in Russia using cultural and 16S rRNA gene metabarcoding approaches and to predict the functional activity of microbial communities. In this study, we report on the microbial diversity in water samples collected from deep aquifers at the Shchelkovo, Kasimov, and Kaluga UGSs and from the Peschano–Umetskoe depleted oil and gas field used for underground gas storage. It was shown that the microorganisms inhabiting the condensation and formation waters of the UGS facilities were able to grow on hydrogen and to affect the composition both of the water and gas phases and of the borehole equipment.
2. Materials and Methods
2.1. Site Description and Water Sample Collection
The study was conducted using water samples from underground gas storages located in Russia. Formation water samples were collected during October–November 2021 from 6 observation wells at the Peschano–Umetskoe, Shchelkovo, Kasimov, and Kaluga UGSs. Four samples of the liquid delivered into the stratum with injected gas were collected from the separators at the gas collection points (GCP). Liquid samples were dispensed to capacity into sterile plastic bottles, sealed hermetically, and transported to the laboratory for physicochemical and microbiological analysis. The water samples for determining the composition of the microbial communities were fixed with 96% ethanol (1:1, v/v) immediately after the collection.
Characteristics of the UGS horizons used for water sampling are listed in Table S1. The Peschano–Umetskoe UGS (Saratov region) is a depleted gas and oil reservoir, which has been used as a UGS since 1967. Geologically, this UGS is located in the deposits of the Devonian and Carboniferous systems (Paleozoic group), Jurassic and Cretaceous systems (Mesozoic group), and Paleogene and Quaternary systems (Cenozoic group). The Bobrikov horizon is represented by micaceous and argillaceous sandstone. The Tula horizon is composed of sandstone with calcareous cement, interlayered with clays, aleurolites, and dolomitic limestone.
The Shchelkovo UGS (Moscow region), Kasimov UGS (Ryazan region), and Kaluga UGS (Kaluga region) were established in aquifers. The collector for gas storage at the Shchelkovo UGS is the Upper Devonian Shchigrovsky horizon, represented mainly by weakly cemented sandstones and aleurolites. At the Kasimov UGS, gas is stored at the aquifer located at the Nizhne Shchigrovsky horizon of the Fran stage, represented by overlapping clay, siltstone, and sand rocks and characterized by significant facies variability. At the Kaluga UGS, the aquifer located at the Gdov horizon of Upper Proterozoic sandstones is exploited for gas storage. The natural water in the subsurface horizons of the Peschano–Umetskoe, Kasimov, and Kaluga UGSs is characterized by high total salinity, belongs to chloride–calcium or chloride–sodium type, and contains about 2.0, 5.5, and 1.1 g of sulfate/L, respectively [30].
2.2. Media Composition and Cultivation Conditions
The water samples were analyzed for the presence of cultivable aerobic organotrophs and methylotrophs, along with anaerobic fermenting, acetogenic, sulfate-reducing, and methanogenic prokaryotes, as described previously [30]. Salinity of the media corresponded to the salinity of the studied water samples and was 10 or 60 g NaCl/L. The TEG medium used for aerobic organotrophs contained (per liter distilled water) 5.0 g bacto-tryptone, 2.5 g yeast extract, and 1.0 g glucose; pH of the medium was 7.0. The medium for aerobic methylotrophic bacteria contained (per liter distilled water) 1.2 g K2HPO4, 0.6 g KH2PO4, 0.05 g CaCl2·6H2O, 0.2 g MgSO4·7H2O, and 2 mL/L methanol; pH of the medium was 7.0. Anaerobic fermentative bacteria were cultivated in the medium with peptone (4 g/L) and glucose (10 g/L) [32] supplemented with 0.1 g/L Na2S·9H2O; pH 6.8–7.0. The medium for sulfate-reducing bacteria contained (per liter distilled water) 0.2 g K2HPO4, 0.5 g KCl, 0.25 g NH4Cl, 0.15 g CaCl2·6H2O, 3.0 g MgSO4·7H2O, 2.0 g NaHCO3, 4.0 g Na2SO4, 4.0 g sodium lactate, 0.5 g yeast extract, and 0.2 g Na2S·9H2O; pH of the medium was 7.0–7.2. For detection of autotrophic sulfate reducers, the medium without lactate was used, and H2 in the headspace acted as the electron donor, while NaHCO3 was the carbon source. Methanogens were cultivated using three media that contained methanol (2 mL/L), acetate (2.5 g/L), and H2/CO2 (4:1, v/v); the mineral base of the medium contained (per liter distilled water) 0.2 g K2HPO4, 0.5 g KCl, 0.25 g NH4Cl, 0.15 g CaCl2·6H2O, 0.3 g MgSO4·7H2O, 2.5 g NaHCO3, and 0.5 g Na2S·9H2O; pH of the medium was 7.0–7.2. The medium for lithoautotrophic acetogens had the same composition as the medium for hydrogenotrophic methanogens, while containing a lower amount of the reducing agent: 0.28 g/L Na2S·9H2O; pH 6.8. All media for anaerobic microorganisms were supplemented with 1.0 mg/L resazurin, 1 mL/L of trace elements solution according to Pfennig and Lippert [33], and 1 mL/L of vitamin solution according to [34]. All media were prepared using Hungate’s anaerobic technique. The gas phase was air for aerobic bacteria, argon for most anaerobic bacteria, and H2/CO2 mixture (4:1, v/v) for hydrogenotrophic sulfate-reducing, methanogenic, and acetogenic microorganisms. If not stated otherwise, the cultures were incubated for 14–28 days at 23–25 °C under stationary conditions.
The potential contribution of microorganisms to corrosion of the steel UGS equipment was determined by cultivation of the Kal_SE sulfate-reducing enrichment in the presence of a steel piece (FSK-89 borehole filter) (Ural Steel Structures Plant, Yekaterinburg, Russia) in liquid anaerobic medium with sodium lactate; pH of the medium was 7.0–7.2. A borehole filter was used to prevent the degradation of the near-bottom zone of weakly cemented collectors and to release sand and other mechanical admixtures during the exploitation of gas wells. To assess the chemical corrosion of the steel filter, it was compared to the uninoculated controls incubated under the same conditions.
2.3. Enumeration of Microorganisms and Determination of Microbial Growth
Cell numbers of microorganisms of such main physiological groups as aerobic organotrophs and methylotrophs, and anaerobic fermenting, acetogenic, sulfate-reducing, and methanogenic prokaryotes, were estimated by the most probable number (MPN) method with two replicate tenfold dilutions of water samples in tubes with selective liquid nutrient media prepared by the authors. Growth of aerobic bacteria was determined by measuring optical density at 600 nm. Growth of sulfate-reducing bacteria was detected by sulfide production in the medium, which was quantified with dimethyl-p-phenylenediamine by a colorimetric method [35]. Growth of methanogenic and fermentative prokaryotes was checked by gas chromatographic measurement of methane and hydrogen concentrations in the gas phase. Content of short-chain low fatty acids, low alcohols, and ketones in the water samples and in the cultures of fermentative and acetogenic bacteria was determined using a Shimadzu GC 2010 Plus gas chromatograph («Shimadzu Corporation», Kyoto, Japan) with a Zebron ZB-FFAP column, as described previously [36]. Each dilution of inoculated culture medium used for MPN counts was examined by phase-contrast microscopy using an Axio Imager.D1 epifluorescence microscope (Carl Zeiss, Oberkochen, Germany).
2.4. Illumina MiSeq Sequencing and Analysis of 16S rRNA Genes of Prokaryotes from Water Samples and Sulfidogenic Enrichment
Water samples (1 L) fixed with 96% ethanol (1:1, v/v) and the Kal_SE sulfate-reducing enrichment (5 mL) were filtered through 0.22 μm membranes (MerckMillipore, Burlington, MA, USA). The biomass was washed off the filters with a lysing solution containing 0.15 M NaCl and 0.1 M Na2EDTA (pH 8.0) and further processed as described previously [30]. Total DNA was isolated from the biomass using the PowerSoil DNA Isolation Kit (MoBio, Carlsbad, CA, USA) according to the manufacturer’s recommendations. The 16S rRNA gene libraries were obtained by high-throughput sequencing on the Illumina MiSeq according to Gohl et al. [37]. The purified DNA preparation was used as a template for PCR amplification of the V4 region of the 16S rRNA gene using two primers. The forward primer (5′-CAAGCAGAAGACGGCAT ACGAGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT XXXXXXXXXXXX ZZZZGTGBCAGCMGCCG CGGTAA-3′) consisted of 5′ Illumina Linker Sequence, Index 1, Heterogeneity Spacer [38], and the 515F primer sequence [39]; the reverse primer (5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCG ATCT XXXXXXXXXXXX ZZZZ GACTACNVGGGTMTCTAATCC-3′) included 3′ Illumina Linker Sequence, Index 2, Heterogeneity Spacer, and the Pro-mod-805R primer sequence [40]. The read length was 200–250 nt. The quality of the paired-end reads was controlled with UPARSE [41]. The reads were grouped into operational taxonomic units (OTUs) at 97% similarity using USEARCH [42]. OTUs were identified using the QIIME (Quantitative Insights into Microbial Ecology) database [43] and the SILVA online resource [44]. The ClustVis online resource [45] was used to construct heatmaps of the microbial communities at the genus level. Statistical calculations were carried out using Microsoft Excel. Diversity indices were calculated with EstimateS [46]. The Venn diagrams were constructed using the Venny 2.1.0 [47] and InteractiVenn [48] online services.
2.5. Functional Annotation
OTUs were used for predicting the functional characteristics of microbial communities with the iVikodak software package [49]. The Global Mapper module of the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [50] was used to obtain the functional profiles, and Local Mapper was used to predict the individual profiles of the enzymes involved in sulfur and methane metabolic pathways and benzoate and polycyclic aromatic hydrocarbon (PAH) degradation. Heatmaps of the functional profiles and enzymes predicted for the communities were constructed using the ClustVis resource [45]. The contribution of various groups of prokaryotes to the metabolic pathways was also evaluated using the KEGG database [50].
Datasets of 16S rRNA gene metabarcoding of microbial communities from several UGSs located in Russia [30] were also downloaded and used for comparison in this study (Bioproject PRJNA724815, SRA 14189839–14189843).
2.6. Scanning Electron Microscopy (SEM) and Elemental Analysis
Steel filter fragments with biofilms were dehydrated with increasing ethanol concentrations (from 30 to 100%, v/v). The samples were then washed twice with 100% acetone and air-dried for 24 h. In the first experimental variant, the samples were examined under a scanning electron microscope (Quattro S, Thermo Fisher Scientific, Waltham, MA, USA) at an accelerating voltage of 10–15 in the “natural medium” ESEM mode. The elemental composition of the samples was also determined in the ESEM mode (at an accelerating voltage of 20 kV, pressure of 500 Pa, and working distance (WD) of 10 mm) using an energy dispersive X-ray spectroscopy detector (EDS X-ray detector), which resulted in the maps of element distribution on the surface. In another experimental variant, dried samples were mounted on special tables, and plated with a thin layer of metal (gold/palladium) on a Hitachi HUS-5 GB (Hitachi Ltd., Tokyo, Japan) system to create a conductive coating. The samples were examined under high vacuum at an accelerating voltage of 20 kV.
2.7. Nucleotide Sequence Accession Number
The libraries for the 16S rRNA gene fragments of microorganisms from water samples and from sulfidogenic enrichment culture were deposited in NCBI SRA, BioProject PRJNA913484.
3. Results and Discussion
3.1. Physicochemical Properties of the Water Samples and Characterization of the Cultivable Prokaryotes
Formation water sampled from the Peschano–Umetskoe depleted gas–oil reservoir and from Shchelkovo, Kasimov, and Kaluga deep aquifers used for underground gas storage belonged to chloride–calcium or chloride–sodium type, characterized by a wide spectrum of salinity (from 2.0 to 163.86 g/L), high sulfate content (from 1.1 to 5.5 g/L), and pH from 5.1 to 10.9 (Tables S1 and S2). The dissolved gases in the Peschano–Umetskoe UGS were mainly represented by hydrocarbons (87.5%) and also contained N2 (7.9%), H2 (3.2%), CO2 (0.5%), and O2 (0.9%). In other UGSs the composition of dissolved gases varied from methane and methane–nitrogen to nitrogen–methane and nitrogen. Reservoir temperature in the Tula horizon of the Peschano–Umetskoe UGS was 33 °C and varied from 17 to 24 °C in other UGSs [30]. Formation water samples from the Peschano–Umetskoe gas–oil reservoir contained from 61.9 to 267.3 mg of acetate/L; in the samples from aquifers of other UGSs, acetate concentrations were lower and varied within the range 34.3–72.5 mg/L. Concentration of C3–C5 volatile fatty acids (VFA) in the studied water samples did not exceed 41.4 mg/L (Table S2). Methanol at concentrations from 2.87 to 10.29 g/L was detected in the samples from the Kaluga UGS. Methanol is usually applied during the period of gas extraction as an inhibitor of formation of gas hydrate plugs.
Most studied liquid samples contained cultivable fermenting bacteria (up to 104 cells/mL; Figure S1). The fermentative enrichment from well 33 of the Peschano–Umetskoe UGS produced about 1040 mg C2–C5 volatile fatty acids, mainly represented by acetic acid, and about 120 mg of ethanol per liter. Aerobic bacteria growing on media with methanol (10–102 cells/mL) or on bacto-tryptone, yeast extract, and glucose (102–105 cells/mL) were present mainly in the samples from the Peschano–Umetskoe UGS, which contained oxygen in a dissolved gas (Table S1). Methanogens growing in media with methanol and acetate were revealed in three water samples from the Kaluga UGS (10 cells/mL) and in two samples from gas collection points at the Kasimov UGS. Methanogens using H2/CO2 were revealed in four samples (161, 33, 13, and GCP-6). Although sulfate was present in all studied water samples, cultivable sulfate-reducing bacteria were only detected in two out of ten samples inoculated into the media both with lactate + SO42−, and with H2/CO2 + SO42−. Lithoautotrophic acetogens were detected (by acetate formation in the medium with H2/CO2) in the samples from wells 6, 33, and 43 at the Peschano–Umetskoe UGS. Thus, formation water samples from the Peschano–Umetskoe gas–oil reservoir exhibited higher abundance of cultivated microorganisms than the samples from UGSs localized in aquifers. Depleted gas and oil reservoirs contain significant amounts of residual oil in the rocks, often exceeding 50% of the original oil reserve in the reservoir [51]. The presence of oil in collector rocks may be responsible for high numbers of microorganisms in the Peschano–Umetskoe UGS compared to the samples from aquifers. Hydrocarbons may act as substrates for both aerobic and anaerobic microorganisms [52]. The results of culture-based studies of the water samples from UGSs located in aquifers correlated with our earlier data [30]. Low concentrations of usable nutrient substrates, phosphorus and nitrogen, typical of aquifers [20,53], probably suppressed development of both organotrophic and autotrophic prokaryotes.
3.2. Composition of Microbial Communities in Water Samples from UGSs
Phylogenetic diversity of prokaryotes was determined for two samples (PU_6 and PU_33) from the Peschano–Umetskoe UGS by high-throughput sequencing of amplicons of the V4 region of the 16S rRNA gene. Previously obtained data on microbial diversity in the samples from other UGSs located in aquifers [30] were used for comparison. The 16S rRNA gene sequences grouped into operational taxonomic units (OTUs) at similarity level of ≥97% were used to characterize the richness and diversity of the microbial communities (Table S3). The coverage for the PU_6 and PU_33 libraries was high (98 and 99%, respectively), indicating that the libraries were highly representative, making it possible to determine quantitatively all the dominant microbial taxa. The Good’s coverage of the Kal_121 library from the Kaluga UGS aquifer [30] did not, however, exceed 91%, although over 9500 reads were analyzed. The values of the Shannon–Wiener and Simpson diversity indices were low for all the studied libraries, while those of the Berger–Parker dominance index were, on the contrary, relatively high (Table S3). The compositions of microbial communities from various UGSs were compared by principal component analysis (PCA) using the relative abundance of the operational taxonomic units. The isolated position of microbial communities in the PU_33 and Kal_121 samples, resulting from their low diversity level and predominance of members of a small number of taxa, is shown in Figure 1. The differences in the composition of the studied communities, with predominance of different phylogenetic groups, should be noted.
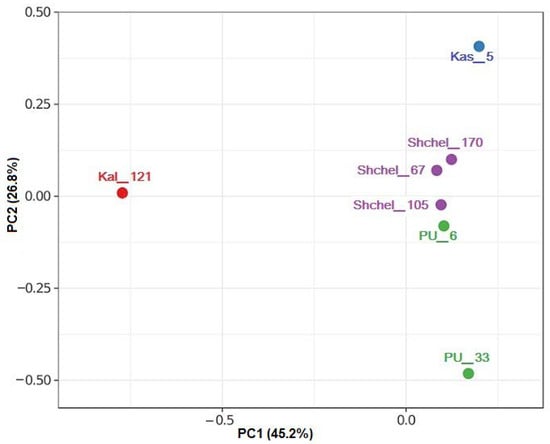
Figure 1.
Principal component analysis (PCA) of 16S rRNA gene sequences (≥97% similarity) of microorganisms from the samples of formation water from the UGSs. Library designations correspond to the well number used for water sampling from the Shchelkovo (Shchel), Kasimov (Kas), Peschano–Umetskoe (PU), and Kaluga (Kal) UGSs.
To reveal the differences between the studied libraries in the number of OTUs, Venn diagrams were constructed. The libraries PU_6 and PU_33 from the Peschano–Umetskoe UGS had significantly more shared phylotypes (25.8%), than between both of them and the Kaluga UGS library (Kal_121) (3.9% each) (Figure S2a). Microbial communities of the Shchelkovo, Kasimov, and Kaluga UGSs located in aquifers with high-salinity water were less abundant and diverse than those of the Peschano–Umetskoe gas–oil reservoir. All studied libraries shared only 2.5% of the phylotypes (Figure S2b).
The distribution of the 16S rRNA gene fragments in the libraries from the UGS formation water at the domain level (Bacteria and Archaea), at the phylum/class level, and at the genus level is shown on Figure S3, Figure 2a,b, respectively.
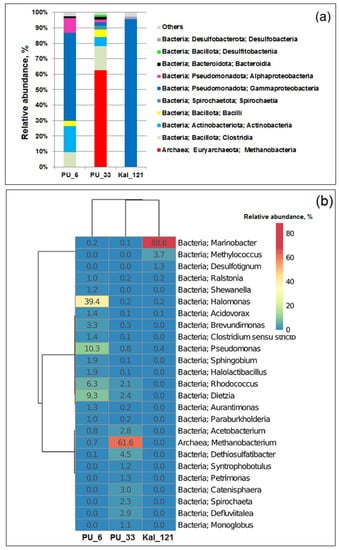
Figure 2.
Taxonomic classification of 16S rRNA gene fragments of Bacteria and Archaea at the phylum/class level (a) and at the genus level (b) retrieved from the UGS formation water. The sequences accounting for more 1% in at least one library are listed. The numerals indicate the share (%) of the total number of sequences in each library. The double hierarchical dendrogram shows the microbial distribution in the three samples.
Bacteria predominated in most of the studied UGS samples, and only in the PU_33 sample was Archaea, represented by hydrogen-utilizing methanogens of the genus Methanobacterium (the phylum Euryarchaeota), constituted by 61.6%. In the PU_6 sample, the predominant phyla were Pseudomonadota (genera Halomonas and Pseudomonas), Actinobacteriota (Dietzia and Rhodococcus), and Bacillota (Halolactibacillus). In the PU_33 sample, bacteria belonged mainly to the phyla Bacillota (Dethiosulfatibacter, Defluviitalea, Acetobacterium, and Catenisphaera), Actinobacteriota (Dietzia and Rhodococcus), Spirochaetota (Sphaerochaeta), and Bacteroidota (Petrimonas).
Predominance of halophilic bacteria of the genera Halomonas and Marinobacter in the studied samples was probably due to high salinity of formation water in the sampled horizons. These bacteria are usual inhabitants of oil-contaminated ecosystems and oilfields with highly saline formation water [54,55,56]. The presence of members of the genera Pseudomonas, Dietzia, and Rhodococcus, which are known to utilize a broad spectrum of hydrocarbons [57,58,59], could be explained by the presence of hydrocarbons and of oxygen in low amounts among the water-soluble gaseous compounds in the Peschano–Umetskoe formation water (Table S1).
Members of the genera Dethiosulfatibacter, Defluviitalea, Petrimonas, and Sphaerochaeta, which were revealed in the studied samples, possess fermentative metabolism. Some members of these genera are also capable of using external electron acceptors, reducing sulfur or thiosulfate to sulfide [36,60,61,62]. Members of the genus Acetobacterium are known as producers of acetic acid from molecular hydrogen and carbon dioxide [63,64]. Both molecular hydrogen and CO2 were detected in the water-soluble gas in the Peschano–Umetskoe UGS, thus providing for growth of these bacteria and production of acetic acid. Acetate can be utilized by other anaerobic members of the microbial community including sulfur- and sulfate-reducing bacteria and methanogenic archaea. Sulfate-reducing bacteria of the genera Desulfotignum, Desulfomicrobium, Desulfosporosinus, and Desulfosalsimonas were detected in samples from the Peschano–Umetskoe and Kaluga UGSs as minor populations.
3.3. Functional Potential of Microbial Communities from UGSs
The functional profiles of subsurface microbial communities predicted using the KEGG database are presented in Figure 3. Microbial communities inhabiting deep subsurface horizons of the Peschano–Umetskoe UGS were able to participate in the major pathways of carbohydrate and energy metabolism, and in the degradation of toluene, benzoate, polycyclic aromatic hydrocarbons, and chlorinated organic compounds. The relative impact of key microorganisms in the sulfur and methane metabolism pathways and benzoate and PAH degradation is presented in Table S4. The presence of the enzymes of the “Methane metabolism”, probably including those of hydrogenotrophic methanogenesis, was predicted for prokaryotes from the observation wells PU_6 and PU_33. Members of both communities possessed the key enzymes of this pathway, formylmethanofuran dehydrogenase [EC: 1.2.7.12], tetrahydromethanopterin S-methyltransferase [EC: 2.1.1.86], and methyl-coenzyme M reductase [EC: 2.8.4.1], responsible for CO2 catabolism to formylmethanofuran, methyl-CoM, and methane, respectively (Figure S4a), although representation of these pathways was considerably higher in the PU_33 community as compared to PU_6. These enzymes probably belonged to methanogenic archaea of the genus Methanobacterium, which were predominant in the PU_33 community and were a minor component in PU_6 (Table S4). Moreover, prokaryotes of both communities tentatively possessed formate dehydrogenase [EC: 1.17.1.9], the enzyme oxidizing formate to CO2, which was probably associated with the ability of some Methanobacterium to carry out methanogenesis on formate. Representation of anaerobic carbon monoxide dehydrogenase [EC: 1.2.7.4] in prokaryotes of the PU_33 community indicated the ability of its archaeal components to carry out methanogenesis on CO (Figure S4a). In prokaryotes of the PU_6 and PU_33 communities, the enzymes of methane oxidation were not reliably represented. Bacteria of the PU_6 community possessed methanol dehydrogenase (cytochrome c) [EC: 1.1.2.7], the enzyme oxidizing methanol to formaldehyde, which was probably indicative of participation of the facultatively methylotrophic members of the genera Halomonas, Pseudomonas, and Rhodococcus in this process.
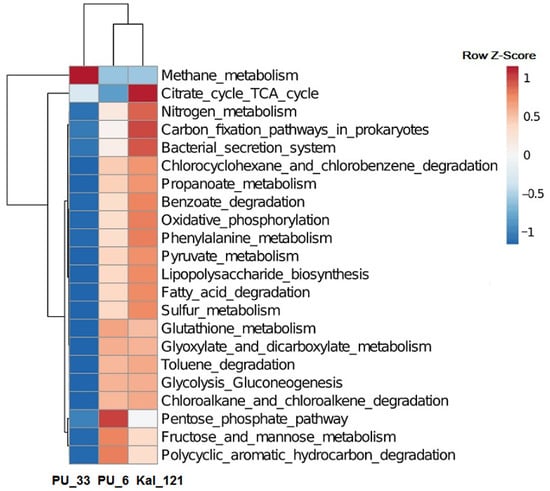
Figure 3.
Heatmap showing the relative percentage of iVikodak-derived microbial community functional profiles.
The functional profiles of subsurface microbial communities predicted using the KEGG database are presented inFigure 3. Microbial communities inhabiting deep subsurface horizons of the Peschano–Umetskoe UGS were able to participate in the major pathways of carbohydrate and energy metabolism, and in the degradation of toluene, benzoate, polycyclic aromatic hydrocarbons, and chlorinated organic compounds. The relative impact of key microorganisms in the sulfur and methane metabolism pathways and benzoate and PAH degradation is presented in Table S4. The presence of the enzymes of the “Methane metabolism”, probably including those of hydrogenotrophic methanogenesis, was predicted for prokaryotes from the observation wells PU_6 and PU_33. Members of both communities possessed the key enzymes of this pathway, formylmethanofuran dehydrogenase [EC: 1.2.7.12], tetrahydromethanopterin S-methyltransferase [EC: 2.1.1.86], and methyl-coenzyme M reductase [EC: 2.8.4.1], responsible for CO2 catabolism to formylmethanofuran, methyl-CoM, and methane, respectively (Figure S4a), although representation of these pathways was considerably higher in the PU_33 community as compared to PU_6. These enzymes probably belonged to methanogenic archaea of the genus Methanobacterium, which were predominant in the PU_33 community and were a minor component in PU_6 (Table S4). Moreover, prokaryotes of both communities tentatively possessed formate dehydrogenase [EC: 1.17.1.9], the enzyme oxidizing formate to CO2, which was probably associated with the ability of some Methanobacterium to carry out methanogenesis on formate. Representation of anaerobic carbon monoxide dehydrogenase [EC: 1.2.7.4] in prokaryotes of the PU_33 community indicated the ability of its archaeal components to carry out methanogenesis on CO (Figure S4a). In prokaryotes of the PU_6 and PU_33 communities, the enzymes of methane oxidation were not reliably represented. Bacteria of the PU_6 community possessed methanol dehydrogenase (cytochrome c) [EC: 1.1.2.7], the enzyme oxidizing methanol to formaldehyde, which was probably indicative of participation of the facultatively methylotrophic members of the genera Halomonas, Pseudomonas, and Rhodococcus in this process.
Significant differences in the pathways of methane metabolism between the communities PU_6 and PU_33 from DOGF and the previously studied deep aquifer community Kal_121, in which complete methanogenesis pathways were absent, while the fragments of methanotrophy were represented, were revealed (Figure S4b). In this process, methane is oxidized to methanol by methane monooxygenase (soluble) [EC: 1.14.13.25] and methane monooxygenase (particulate) [EC: 1.14.18.3], and then to formaldehyde by methanol dehydrogenase (cytochrome c) [EC: 1.1.2.7]. These differences probably resulted from the absence of methanotrophic archaea in the aquifer community Kal_121 and with the presence of obligate methanotrophs of the genus Methylococcus.
For communities PU_6 and PU_33 from the Peschano–Umetskoe UGS, the key enzyme of sulfate reduction, sulfate adenylyltransferase [EC: 2.7.7.4], catalyzing sulfate reduction to adenylyl sulfate, was predicted. Although members of the genus Methanobacterium may be responsible for the major contribution to sulfur metabolism in the PU_33 community (Table S4), according to the BV-BRC 3.30.5h database [65], the genes encoding this enzyme were found only in few genomes of the strains of this genus, while the genes of other sulfate reduction enzymes were absent. However, for members of both communities the existence of adenylylsulfate kinase [EC: 2.7.1.25] and phosphoadenylyl-sulfate reductase (thioredoxin) [EC:1.8.4.8] was predicted, reducing sulfate to sulfide in the assimilatory process (Figure S5a,b), probably due to the presence of members of the genera Halomonas, Pseudomonas, and Rhodococcus. The enzymes of dissimilatory sulfate reduction were not reliably represented in either community. Members of the genus Marinobacter, which were predominant in the Kal_121 sample, were probably responsible for assimilatory sulfate reduction in this community. Moreover, dissimilatory sulfate reduction in the Kal_121 community was possible due to the presence of a small population of sulfate-reducing bacteria (genera Desulfotignum and Desufomicrobium), which possess adenylylsulfate reductase [EC: 1.8.99.2] (Figure S5c).
In prokaryotes of the PU_6 and PU_33 communities, the “Degradation of polycyclic aromatic hydrocarbons (PAH) pathway” was found to contain the enzymes of phthalate degradation, beginning with phthalate 4,5-dioxygenase [EC: 1.14.12.7] (Figure S6). The products of phthalate degradation were then catabolized via the “Benzoate degradation” pathway, incorporating the catechol ortho-cleavage module; its enzymes were reliably represented in both communities. This pathway also comprised two key enzymes of the aerobic benzoate degradation module (“Benzoate degradation I”), benzoate 1,2-dioxygenase (EC: 1.14.12.10) and dihydroxycyclohexadiene carboxylate dehydrogenase (EC: 1.3.1.25), catabolizing catechol formation from benzoate, and the enzymes of the Catechol meta-cleavage module (Figure S7). Benzoate-CoA ligase (EC: 6.2.1.25); the enzyme catalyzing benzoate conversion to benzoyl-CoA, an intermediate in the degradation of many aromatic compounds, was present only in bacteria of the PU_6 community, although the enzymes of the full module for anaerobic benzoate degradation were not reliably revealed. The enzymes of this metabolic pathway were more significantly represented in the PU_6 community, probably due to predomination of bacteria of the genera Halomonas, Pseudomonas, and Rhodococcus, which were present in the PU_33 community only as minor components. The set of enzymes for PAH and benzoate degradation in the Kal_121 community was similar to that of PU_6, although in the latter, members of the dominant genus Marinobacter were the major phthalate and benzoate degraders (Table S4).
While members of the genera capable of hydrogen consumption (oxidation) according to the BRENDA database [66] were revealed in both the PU_6 and PU_33 microbial communities, the composition of these genera differed significantly (Table S5). In PU_33, the main hydrogen consumers were probably archaea of the genus Methanobacterium, predominant in that community, which use H2 for methanogenesis involving the enzymes hydrogen dehydrogenase [EC: 1.12.1.2] and hydrogen dehydrogenase (NADP+) [EC: 1.12.1.3]. In the PU_6 community, methanogens were a minor component, while predominant bacteria of the genera Pseudomonas and Rhodococcus are known to oxidize hydrogen using hydrogenase (acceptor) [EC: 1.12.99.6]. The potential for hydrogen consumption by the microorganisms in the Peschano–Umetskoe gas–oil reservoir was probably significantly higher than in the case for the deep aquifer Kal_121 community, since the share of genera capable of hydrogen oxidation was relatively low in the latter. Analysis of the composition of microbial communities from the Shchelkovo, Kasimov, and Kaluga UGSs located in aquifers [30] using the BRENDA database revealed that the potential H2 consumers in these deep aquifers included bacteria of the genera Hydrogenophaga, Hydrogenophilus, Pelomonas, Cupriavidus, sulfate-reducing bacteria of the genera Desulfotignum, Desulfovibrio, Desufomicrobium, and Desulfobulbus, along with methanogenic archaea of the genera Methanosphaera and Methanobrevibacter.
3.4. Sulfide Production and Steel Corrosion by Anaerobic Enrichments from UGS
Bacterial corrosion caused by sulfide produced by sulfidogenic bacteria and archaea is one of the factors causing the degradation of UGS steel structures [67]. Fermenting bacteria, which produce CO2 and volatile fatty acids in geological formations, may also cause steel corrosion (carbonate corrosion). Corrosive activity of microorganisms is associated with formation of microbial biofilms on metal and rock surfaces and with bioelectrochemical processes caused by “electrogenic” microorganisms [68].
In the present work, microbial communities capable of causing corrosion of the UGS steel equipment were investigated. Enrichment cultures of sulfate-reducing bacteria growing on a number of organic substrates (lactate, pyruvate, fumarate, acetate, methanol, and ethanol), and autotrophically on H2/CO2, were obtained from the UGS samples (Figure S8). Addition of H2 to the media with lactate or methanol enhanced sulfide production. A sulfidogenic enrichment (Kal_SE) growing in the medium with lactate and sulfate or thiosulfate with production of sulfide was obtained from formation water samples Kal_81 and Kal_106. Analysis of the 16S rRNA gene V4 fragment was used to determine the prokaryotic phylogenetic diversity in this enrichment, which comprised sulfate-reducing bacteria of the genus Desulfosporosinus (33.3% of the sequence number in the library), several phylotypes of the genus Bacillus (61.2%, 3.0%, and 0.7%), and Paenibacillus (1.8%). The genus Desulfosporosinus comprises anaerobic mesophilic spore-forming bacteria, which have been isolated from soil, marine and river sediments, permafrost, groundwater contaminated with motor fuel, and acid mining drainage sediments [69,70]. This genus comprises metal-resistant bacteria capable of growth by reduction of sulfate, sulfite, thiosulfate, elemental sulfur, and DMSO as electron acceptors and with organic substrates or molecular hydrogen as electron donors. Lactate is incompletely oxidized to acetate. Desulfosporosinus nitroreducens is also capable of nitrate reduction to nitrite [71]. Members of the genus Desulfosporosinus are found in environments with low pH. It should be noted that some UGS water samples had pH 5–6.
To assess the potential contribution of bacteria to corrosion of steel equipment, the Kal_SE sulfate-reducing enrichment was incubated in a liquid anaerobic medium with sodium lactate (pH of the medium 7.0–7.2) in the presence of a steel filter (Figure 4). Although the known Desulfosporosinus representatives are able to use H2 for sulfate reduction, lactate was used in this experiment to obtain better growth of sulfidogens during the incubation period. After 210 days of incubation at 23–25 °C, sulfide concentrations in two replicate experiments were 60 and 120 mg/L, indicating activity of sulfidogenic microorganisms, while no sulfide was produced in the uninoculated control.

Figure 4.
Fragments of a steel borehole filter exposed in the medium with the Kal_SE sulfidogenic enrichment for 210 days at 23–25 °C.
Biofilms formed on the surface of wet specimens of steel filter were observed using a Quattro S scanning electron microscope in “natural environment” mode (ESEM) (Figure 5). The microbial biofilm on the metal surface consisted of agglomeration of small spherical cells 0.2–0.4 µm in diameter (indicated by a white arrow) and rod-shaped (up to 0.5 µm long) cells (indicated by a red arrow). Analysis of the elemental composition of the filter surface (Figure S9a) showed the presence of the following chemical elements: C, Ca, O, Cr, Fe, Ni, Na, Si, P, S, Pb, and Cl (Figure S9b). The use of an energy dispersive analyzer made it possible to create element maps by gradually rastering the electron beam point by point over the surface of the sample [72,73]. In such maps, brighter areas indicate a higher concentration of elements. The distribution of elements on the metal surface was not uniform (Figure S9c–n). The areas on the image occupied by microbial cells corresponded to the bright areas in the distribution maps of a biogenic component containing such elements as carbon, oxygen, and phosphorus. The maps showing the spatial distribution of the elements indicated the presence of NaCl crystals on the surface of the steel sample, together with elongated crystal formations, which include sulfur and lead.
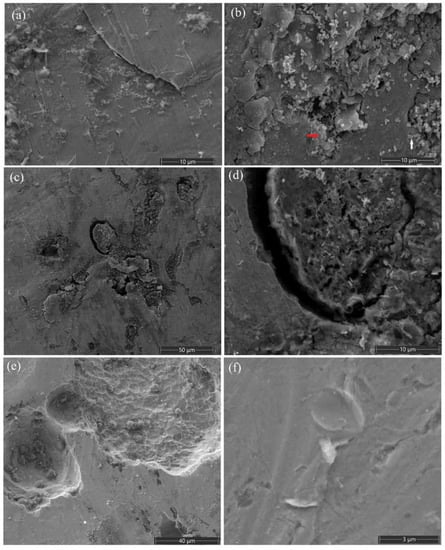
Figure 5.
SEM images of biofilms on the surface of steel borehole filter exposed for 210 days in the medium with sulfidogenic enrichment (a–e) and in the uninoculated control medium (f). Small spherical cells and rod-shaped cells are indicated by white and red arrows, respectively. Scanning electron microscope Quattro S, ESEM mode (Thermo Fisher Scientific, Waltham, MA, USA).
Results of the scanning electron microscopy indicated small changes in surface morphology of the steel filter exposed to the sulfidogenic enrichment. The rate of bacterial corrosion of fragments of this steel filter was estimated in a parallel study by Safarova et al. [74] as 60.3–94.3 g/(m2·year).
While studies on the microorganisms of underground gas storage facilities are relatively few [1,4,6], increased interest in hydrogen energetics necessitates investigation of these habitats. This is the first work in which the Russian underground gas repositories located in DOGFs were investigated using a combination of metabarcoding of the 16S rRNA genes and culture-based techniques. The studied water samples from the Peschano–Umetskoe DOGF were found to contain cultured fermentative, acetogenic, sulfate-reducing, and methanogenic microorganisms, although their abundance was low. Growth of methanogens, acetogens, and sulfate-reducing bacteria on hydrogen follows the reaction Equations (1)–(3):
4H2 + HCO3− + H+ → CH4 + 2H2O
4H2 + 2HCO3− + H+ → CH3COO− + 2H2O
4H2 + SO42− + H+ → HS− + 4H2O
Sulfate concentration in the studied water samples was high and could not limit sulfate reduction. The absence of easily assimilated substrates and nitrogen and phosphorus sources was probably responsible for the low abundance of sulfidogens. Samples from the Peschano–Umetskoe DOGF contained acetate and other volatile fatty acids, with their total concentration in different samples varying from 68 to 291 mg/L. While VFA concentration in the samples from UGSs located in aquifers did not exceed 83 mg/L, the concentration of methanol injected in the subterranean horizons varied widely, reaching 10.3 g/L. Enrichment cultures of sulfate-reducing bacteria obtained from groundwater samples were able to use a broad range of substrates, including VFA and methanol or to grow autotrophically in the presence of H2. Thus, delivery of molecular hydrogen into deep horizons may stimulate the growth of the subterranean microbiota. H2 is known to play a significant part in metabolism of deep microbial communities [75]. High sulfate concentration in the groundwater of the Peschano–Umetskoe UGS did not prevent development of hydrogenotrophic methanogenic archaea of the genus Methanobacterium, whose abundance in some samples reached 61.6% according to the 16S rRNA metabarcoding data. It was shown previously that H2 consumption by homoacetogenic and sulfate- and sulfur-reducing microorganisms did not change within the pH2 range of 0.1–3.5 MPa [76,77]; however, the growth of methanogens was inhibited at high pH2 [78].
Radiotracer studies revealed extremely high rates of methanogenesis (up to 2.8 mL CH4/(L day) and sulfate reduction (up to 0.5 mg S2−/(L day) in the samples from the North Stavropol UGS facilities, which is also a depleted gas reservoir [23]. The gas injected into the North Stavropol UGS also contains, apart from hydrocarbons, up to 0.6% (v/v) CO2 and up to 0.5% (v/v) H2. Methanol, usually injected into production wells as an inhibitor of formation of gas hydrate plugs during gas extraction at the UGSs, may also cause local microbial growth. Anaerobic microorganisms involved in methanol transformation were isolated from water samples from the North Stavropol UGS facilities [28]. Pure cultures of acetogenic (Eubacterium limosum AG12, Sporomusa sphaeroides AG8-2), methanogenic (Methanosarcina barkeri MGZ3), and sulfate-reducing prokaryotes (Desulfovibrio desulfuricans SR12) were adapted to the conditions of the habitat and grew either on methanol or on hydrogen + CO2; the methanogenic strain Methanobacterium formicicum MG134 grew on hydrogen + CO2 and on formate. The authors suggested [28] that organic acids (acetate, butyrate) found in high concentrations in the water samples may be produced by the acetogens inhabiting subsurface horizons of the UGS.
H2 injection into an underground methane storage facility similar to the Peschano–Umetskoe UGS, located in depleted gas–oil reservoir, may result in changed chemical equilibrium between formation water, dissolved gases, and rock minerals. Hydrogen may act as a substrate for the population of methanogenic, acetogenic, and sulfate-reducing microorganisms; their growth will then result in respective formation of methane, CO2 (promoting carbonate corrosion), acetic acid (causing the dissolution of carbonates in the country rocks), and hydrogen sulfide (impairing the gas quality and causing formation of metal sulfides and corrosion of steel equipment). The presence of molecular hydrogen may also cause increased brittleness of metals. The biogeochemical reactions provoked by H2 may also result in hydrogen loss, and in the dissolution/precipitation of minerals causing a higher or lower reservoir capacity and other negative technogenic changes in the geological environment. The scale of these processes and the key microbial agent should, however, be specified for specific UGSs. This will result in more accurate prediction of hydrogen losses in the underground gas storage facilities, which, according to available publications, may vary from 2 to 81% [79,80,81,82].
4. Conclusions
Diversity of the microbial community in the depleted gas and oil reservoir used for underground gas storage was determined in the present work using metabarcoding of the 16S rRNA genes and the culture-based technique. Formation water from the Peschano–Umetskoe DOGF was inhabited by microorganisms typical for petroleum reservoirs, which included bacteria of the phyla Bacillota (genera Dethiosulfatibacter, Defluviitalea, Acetobacterium, Catenisphaera, Syntrophobotulus), Actinobacteriota (Dietzia, Rhodococcus), Spirochaetota (Sphaerochaeta), Pseudomonadota (Shewanella), and Bacteroidota (Petrimonas), together with methanogenic archaea of the phylum Euryarchaeota (Methanobacterium). Enrichment cultures obtained from the Peschano–Umetskoe reservoir were able to grow autotrophically with molecular hydrogen and various electron acceptors, producing methane, sulfide, or/and acetic acid. Bioinformatic analysis revealed that the DOGF underground microbial communities were potentially capable of utilizing the residual oil components (toluene, benzoate, polycyclic aromatic hydrocarbons, and chlorinated organic compounds). Based on the 16S rRNA gene metabarcoding, hydrogenotrophic methanogens of the genus Methanobacterium constituted up to 61.6% of the total formation water community from this reservoir, which may indicate the possibility to apply hydrogen injection biotechnology for biotic in situ methanation in this environment. The microbial communities inhabiting deep aquifers with high-salinity water at the Shchelkovo, Kasimov, and Kaluga UGSs were less abundant and diverse than that in the Peschano–Umetskoe gas and oil field. Sulfidogenic enrichments obtained from UGS samples produced hydrogen sulfide and caused corrosion of a steel sample under laboratory conditions. They likely carry out corrosion of steel equipment at UGS facilities in the case of H2 delivery into the deep horizons. Assessment of the functional activity of prokaryotes in these habitats requires metagenomic research and radiotracer studies of the rates of sulfate reduction, acetogenesis, and methanogenesis. These data will provide for more accurate long-term prediction of the biogeochemical processes and of hydrogen preservation in underground gas storage facilities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15139945/s1, Figure S1: Abundance of microorganisms of the main metabolic groups in water samples from the underground gas storage horizons and from the gas collection point (GCP) at the Peschano–Umetskoe, Shchelkovo, Kasimov, and Kaluga underground gas storages. Designations: AOB, aerobic organotrophic bacteria; AMB, aerobic methylotrophic bacteria; FERM, fermenting bacteria; SRB, sulfate-reducing bacteria grown in the medium with lactate; SRB_H2, sulfate-reducing bacteria grown in the medium with H2; MET, methanogens grown in the medium with methanol; MET_H2, methanogens grown on H2/CO2. Figure S2: Venn diagrams showing the number and proportion of shared and unique OTUs between the three (a) and five (b) libraries of 16S rRNA genes prokaryotes from UGSs. Figure S3: The relative proportion of the 16S rRNA gene fragment sequences of Bacteria and Archaea in the libraries from the UGS water samples. Figure S4: Predicted profile of enzymes for part of the methane metabolism pathway in prokaryotes from water samples PU_33 (a) and Kal_121 (b) according to the KEGG database. The intensity of the color is due to the supposed representation of enzymes. The dominant modules of the methane metabolism pathway are highlighted in red. Figure S5: Predicted profile of enzymes of the sulfur metabolism pathway in water samples PU_6 (a), PU_33 (b), and Kal_121 (c) according to the KEGG database. The intensity of the color is due to the supposed representation of enzymes. The dominant modules of the sulfur metabolism pathway are highlighted in red. Figure S6: Predicted profile of enzymes of the phthalate metabolism (part of the PAH degradation pathway) in prokaryotes from the PU_6 water sample according to the KEGG database. The dominant modules of the phthalate metabolism pathway are highlighted in red. Figure S7: Predicted profile of enzymes of the benzoate degradation pathway in prokaryotes from the PU_6 water sample according to the KEGG database. The dominant modules of the benzoate degradation pathway are highlighted in red. Figure S8: Sulfide production by Kal_SE sulfidogenic enrichment on media with various organic acids, alcohols, or/and H2/CO2 mixture incubated at 25 °C for 85 days. Figure S9: Image of a steel filter fragment with an adhered microbial biofilm (a); elemental composition (b); and maps of spatial distribution of the chemical elements: O (c); C (d); Fe (e); S (f); Na (g); and Cl (h) on the sample surface. Table S1: Characteristics of the UGS horizons used for water sampling. Table S2: Physicochemical characteristics of the water sampled in 2021 from aquifers and gas collection points (GCP) at the Peschano–Umetskoe, Shchelkovo, Kasimov, and Kaluga underground gas storages. Table S3: Diversity indices of the 16S rRNA gene sequences of prokaryotes in the libraries from UGS samples. Table S4: Potential contribution of microorganisms from UGS water samples in the pathways of nitrogen, sulfur, and methane metabolism and in benzoate and polycyclic aromatic hydrocarbon (PAH) degradation according to the KEGG database. Table S5: Potential contribution of microorganisms from UGS water samples to hydrogen metabolism according to the BRENDA database.
Author Contributions
Conceptualization, T.N.N. and L.A.A.; formal analysis, T.P.T. and T.N.N.; funding acquisition, T.N.N. and L.A.A.; investigation, T.P.T., T.L.B., S.K.B., N.G.L., D.S.F. and E.A.S.; project administration, T.N.N. and L.A.A.; software, T.P.T.; validation, T.P.T., T.L.B., S.K.B. and N.G.L.; visualization, T.P.T., T.L.B., S.K.B., N.G.L. and T.N.N.; writing—original draft, T.N.N. and L.A.A.; writing—review and editing, T.N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Science and Higher Education of the Russian Federation. Work of L.A.A., D.S.F., and E.A.S. was performed under the research project FMME-2022-0007 (1220228000276-2). Other researchers, T.N.N., T.P.T., T.L.B., S.K.B. and N.G.L., were funded by grant № 122040800164-6.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The libraries of 16S rRNA gene fragment sequences of prokaryotes from water samples and from sulfidogenic enrichment were deposited in NCBI SRA, BioProject PRJNA913484.
Acknowledgments
SEM studies were carried out at the Shared Research Facility “Electron microscopy in life sciences” at the Moscow State University (Unique Equipment “Three-dimensional electron microscopy and spectroscopy”) and using the equipment purchased under the Moscow State University Development Program. The authors are grateful to the staff of the Laboratory of Physicochemical Research, Gazprom VNIIGAZ branch in Ukhta for providing analyses of the gas dissolved in water from aquifers of the UGSs. The authors are grateful to BioSpark LLC for high-throughput sequencing of 16S rRNA genes of prokaryotes in two water samples.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Molíková, A.; Vítězová, M.; Vítěz, T.; Buriánková, I.; Huber, H.; Dengler, L.; Hanišáková, N.; Onderka, V.; Urbanová, I. Underground gas storage as a promising natural methane bioreactor and reservoir? J. Energy Storage 2021, 47, 103631. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrog. Energy 2020, 46, 23436–23462. [Google Scholar] [CrossRef]
- Agarwal, R. Transition to a hydrogen-based economy: Possibilities and challenges. Sustainability 2022, 14, 15975. [Google Scholar] [CrossRef]
- Reitenbach, V.; Ganzer, L.; Albrecht, D.; Hagemann, B. Influence of added hydrogen on underground gas storage: A review of key issues. Environ. Earth Sci. 2015, 73, 6927–6937. [Google Scholar] [CrossRef]
- Gregory, S.P.; Barnett, M.J.; Field, L.P.; Milodowski, A.E. Subsurface microbial hydrogen cycling: Natural occurrence and implications for industry. Microorganisms 2019, 7, 53. [Google Scholar] [CrossRef]
- Dopffel, N.; Jansen, S.; Gerritse, J. Microbial side effects of underground hydrogen storage—Knowledge gaps, risks and opportunities for successful implementation. Int. J. Hydrog. Energy 2021, 46, 8594–8606. [Google Scholar] [CrossRef]
- Carden, P.; Paterson, L. Physical, chemical and energy aspects of underground hydrogen storage. Int. J. Hydrog. Energy 1979, 4, 559–569. [Google Scholar] [CrossRef]
- Barsuk, N.E.; Khaidina, M.P.; Khan, S.A. “Green gas” in the European gas transport system. Gazov. Promyshlennost 2018, 10, 104–109. (In Russian) [Google Scholar]
- Chapelle, F.H.; O’Neill, K.; Bradley, P.M.; Methé, B.A.; Ciufo, S.A.; Knobel, L.L.; Lovley, D.R. A hydrogen-based subsurface microbial community dominated by methanogens. Nature 2002, 415, 312–315. [Google Scholar] [CrossRef]
- Takai, K.; Mormille, M.R.; McKinley, J.P.; Brockman, F.J.; Holben, W.E.; Kovacik, J.K., Jr.; Fredrickson, J.K. Shifts in archaeal communities associated with lithological and geochemical variations in subsurface Cretaceous rock. Environ. Microbiol. 2003, 5, 309–320. [Google Scholar] [CrossRef]
- Harris, S.; Smith, R.; Suflita, J. In situ hydrogen consumption kinetics as an indicator of subsurface microbial activity. FEMS Microbiol. Ecol. 2007, 60, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, K.; Wang, L.Y.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Different diversity and distribution of archaeal community in the aqueous and oil phases of production fluid from high-temperature petroleum reservoirs. Front. Microbiol. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Magot, M.; Ollivier, B.; Patel, B.K.C. Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 2000, 77, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Gieg, L.M.; Davidova, I.A.; Duncan, K.E.; Suflita, J.M. Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ. Microbiol. 2010, 12, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, S.S.; Borzenkov, I.A. Microbiological transformation of low-molecular-weight carbon compounds in the deep subsurface. In Biogeochemistry of Global Change; Oremland, R.S., Ed.; Chapman & Hall: New York, NY, USA, 1993; pp. 825–838. [Google Scholar] [CrossRef]
- Nazina, T.N.; Shestakova, N.M.; Ivoilov, V.S.; Kostrukova, N.K.; Belyaev, S.S.; Ivanov, M.V. Radiotracer assay of microbial processes in petroleum reservoirs. Adv. Biotechnol. Microbiol. 2017, 2, 555591. [Google Scholar] [CrossRef]
- Bonch-Osmolovskaya, E.A.; Miroshnichenko, M.L.; Lebedinsky, A.V.; Chernyh, N.A.; Nazina, T.N.; Ivoilov, V.S.; Belyaev, S.S.; Boulygina, E.S.; Lysov, Y.P.; Perov, A.N.; et al. Radioisotopic, culture-based, and oligonucleotide microchip analyses of thermophilic microbial communities in a continental high-temperature petroleum reservoir. Appl. Environ. Microbiol. 2003, 69, 6143–6151. [Google Scholar] [CrossRef]
- Anantharaman, K.; Brown, C.T.; Hug, L.A.; Sharon, I.; Castelle, C.J.; Probst, A.J.; Thomas, B.C.; Singh, A.; Wilkins, M.J.; Karaoz, U.; et al. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat. Commun. 2016, 24, 13219. [Google Scholar] [CrossRef]
- Basso, O.; Lascourreges, J.F.; Le Borgne, F.; Le Goff, C.; Magot, M. Characterization by culture and molecular analysis of the microbial diversity of a deep subsurface gas storage aquifer. Res. Microbiol. 2009, 160, 107–116. [Google Scholar] [CrossRef]
- Ranchou-Peyruse, M.; Auguet, J.C.; Mazière, C.; Restrepo-Ortiz, C.X.; Guignard, M.; Dequidt, D.; Chiquet, P.; Cézac, P.; Ranchou-Peyruse, A. Geological gas-storage shapes deep life. Environ. Microbiol. 2019, 21, 3953–3964. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Steliga, T.; Kluk, D.; Gminski, Z. Biomonitoring studies and preventing the formation of biogenic H2S in the Wierzchowice underground gas storage facility. Energies 2021, 14, 5463. [Google Scholar] [CrossRef]
- Ivanova, A.E.; Borzenkov, I.A.; Tarasov, A.L.; Milekhina, E.I.; Belyaev, S.S. A microbiological study of an underground gas storage in the process of gas injection. Microbiology 2007, 76, 453–460. [Google Scholar] [CrossRef]
- Ivanova, A.E.; Borzenkov, I.A.; Tarasov, A.L.; Milekhina, E.I.; Belyaev, S.S. A microbiological study of an underground gas storage in the process of gas extraction. Microbiology 2007, 76, 461–468. [Google Scholar] [CrossRef]
- Staniszewska, A.; Kunicka-Styczyńska, A.; Otlewska, A.; Gawor, J.; Gromadka, R.; Żuchniewicz, K.; Ziemiński, K. High-throughput sequencing approach in analysis of microbial communities colonizing natural gas pipelines. Microbiol. Open 2019, 8, e00806. [Google Scholar] [CrossRef]
- Šmigáň, P.; Greksák, M.; Kozánkova, J.; Buzek, F.; Onderka, V.; Wolf, I. Methanogenic bacteria as a key factor involved in changes of town gas stored in an underground reservoir. FEMS Microbiol. Lett. 1990, 73, 221–224. [Google Scholar] [CrossRef]
- Buzek, F.; Onderka, V.; Vančura, P.; Wolf, I. Carbon isotope study of methane production in a town gas storage reservoir. Fuel 1994, 73, 747–752. [Google Scholar] [CrossRef]
- Thaysen, E.M.; McMahon, S.; Strobel, G.J.; Butler, I.B.; Ngwenya, B.T.; Heinemann, N.; Wilkinson, M.; Hassanpouryouzband, A.; McDermott, C.I.; Edlmann, K. Estimating microbial growth and hydrogen consumption in hydrogen storage in porous media. Renew. Sustain. Energy Rev. 2021, 151, 111481. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Borzenkov, I.A.; Chernykh, N.A.; Belyayev, S.S. Isolation and investigation of anaerobic microorganisms involved in methanol transformation in an underground gas storage facility. Microbiology 2011, 80, 172–179. [Google Scholar] [CrossRef]
- Tarasov, A.L.; Borzenkov, I.A.; Belyayev, S.S. Investigation of the trophic relations between anaerobic microorganisms from an underground gas repository during methanol utilization. Microbiology 2011, 80, 180–187. [Google Scholar] [CrossRef]
- Nazina, T.N.; Abukova, L.A.; Tourova, T.P.; Babich, T.L.; Bidzhieva, S.K.; Filippova, D.S.; Safarova, E.A. Diversity and possible activity of microorganisms in underground gas storage aquifers. Microbiology 2021, 90, 619–629. [Google Scholar] [CrossRef]
- Panfilov, M. Underground and pipeline hydrogen storage. Compendium of Hydrogen Energy, Volume 2: Hydrogen Storage. Transp. Infrastruct. 2016, 91–115. [Google Scholar] [CrossRef]
- Postgate, J.R. The Sulfate-Reducing Bacteria, 2nd ed.; Cambridge University Press: Cambridge, UK, 1984; p. 151. [Google Scholar]
- Pfennig, N.; Lippert, K.D. Über das vitamin B12—Bedürfnis phototropher Schweferelbakterien. Arch. Microbiol. 1966, 55, 245–256. [Google Scholar]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of methane by bacterial extracts. J. Biol. Chem. 1963, 238, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Trüper, H.G.; Schlegel, H.G. Sulfur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Van Leeuwenhoek 1964, 30, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Bidzhieva, S.K.; Sokolova, D.S.; Tourova, T.P.; Nazina, T.N. Bacteria of the genus Sphaerochaeta from low-temperature heavy oil reservoirs (Russia). Microbiology 2018, 87, 757–765. [Google Scholar] [CrossRef]
- Gohl, D.M.; MacLean, A.; Hauge, A.; Becker, A.; Walek, D.; Beckman, K.B. An optimized protocol for high-throughput amplicon-based microbiome profiling. Protoc. Exch. 2016. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Hugerth, L.W.; Muller, E.E.L.; Hu, Y.O.O.; Lebrun, L.A.M.; Roume, H.; Lundin, D.; Wilmes, P.; Andersson, A.F. Systematic design of 18S rRNA gene primers for determining eukaryotic diversity in microbial consortia. PLoS ONE 2014, 9, e95567. [Google Scholar] [CrossRef]
- Merkel, A.Y.; Tarnovetskii, I.Y.; Podosokorskaya, O.A.; Toshchakov, S.V. Analysis of 16S rRNA primer systems for profiling of thermophilic microbial communities. Microbiology 2019, 88, 671–680. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- SILVAngs. Version: 1.9.10/1.4.9; SILVA: r138.1. Available online: https://www.arb-silva.de/ngs/ (accessed on 14 March 2023).
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9. User’s Guide and Application. 2013. Available online: http://purl.oclc.org/estimates (accessed on 9 May 2019).
- Oliveros, J.C. (2007–2015) Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html.
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Nagpal, S.; Haque, M.M.; Singh, R.; Mande, S.S. iVikodak—A platform and standard workflow for inferring, analyzing, comparing, and visualizing the functional potential of microbial communities. Front. Microbiol. 2019, 9, 3336. [Google Scholar] [CrossRef]
- KEGG PATHWAY Database. Available online: https://www.genome.jp/kegg/pathway.html (accessed on 30 March 2023).
- Youssef, N.; Elshahed, M.S.; McInerney, M.J. Microbial processes in oil fields: Culprits, problems and opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [CrossRef]
- Widdel, F.; Musat, F.; Knittel, K.; Galushko, A. Anaerobic degradation of hydrocarbons with sulphate as electron donor. In Sulphate-Reducing Bacteria. Environmental and Engineered Systems; Barton, L.L., Hamilton, W.A., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 265–303. [Google Scholar]
- Kieft, T.L. Microbiology of the deep continental biosphere. In Their World: A Diversity of Microbial Environments Advances in Environmental Microbiology; Hurst, C.J., Ed.; Springer: Cham, Switzerland, 2016; pp. 225–249. [Google Scholar] [CrossRef]
- Kaye, J.Z.; Sylvan, J.B.; Edwards, K.J.; Baross, J.A. Halomonas and Marinobacter ecotypes from hydrothermal vent, subseafloor and deep-sea environments. FEMS Microbiol. Ecol. 2010, 75, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Fathepure, B.Z. Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front. Microbiol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Tourova, T.P.; Sokolova, D.S.; Semenova, E.M.; Ershov, A.P.; Grouzdev, D.S.; Nazina, T.N. Genomic and physiological characterization of halophilic bacteria of the genera Halomonas and Marinobacter from petroleum reservoirs. Microbiology 2022, 91, 235–248. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Ward, O.P. Physical and metabolic interactions of Pseudomonas sp. strain JA5-B45 and Rhodococcus sp. strain F9-D79 during growth on crude oil and effect of a chemical surfactant on them. Appl. Environ. Microbiol. 2001, 67, 4874–4879. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B. Application of Rhodococcus in bioremediation of contaminated environments. In Biology of Rhodococcus; Microbiology, Monographs; Alvarez, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 16, pp. 231–262. [Google Scholar] [CrossRef]
- Nazina, T.N.; Shumkova, E.S.; Sokolova, D.S.; Babich, T.L.; Zhurina, M.V.; Xue, Y.-F.; Osipov, G.A.; Poltaraus, A.B.; Tourova, T.P. Identification of hydrocarbon-oxidizing Dietzia bacteria from petroleum reservoirs based on phenotypic properties and analysis of the 16S rRNA and gyrB genes. Microbiology 2015, 84, 377–388. [Google Scholar] [CrossRef]
- Takii, S.; Hanada, S.; Tamaki, H.; Ueno, Y.; Sekiguchi, Y.; Ibe, A.; Matsuura, K. Dethiosulfatibacter aminovorans gen. nov., sp. nov., a novel thiosulfate-reducing bacterium isolated from a sulfate-reducing mixed culture enriched with Casamino acids from coastal marine sediment. Int. J. Syst. Evol. Microbiol. 2007, 57, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Jabari, L.; Gannoun, H.; Cayol, J.-L.; Hamdi, M.; Fauque, G.; Ollivier, B.; Fardeau, M.-L. Characterization of Defluviitalea saccharophila gen. nov., sp. nov., a thermophilic bacterium isolated from an upflow anaerobic filter treating abattoir wastewaters, and proposal of Defluviitaleaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, A.; Tindall, B.J.; Bardin, V.; Blanchet, D.; Jeanthon, C. Petrimonas sulfuriphila gen. nov., sp. nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int. J. Syst. Evol. Microbiol. 2005, 55, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Balch, W.E.; Schoberth, S.; Tanner, R.S.; Wolfe, R.S. Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 1977, 27, 355–361. [Google Scholar] [CrossRef]
- Braun, M.; Gottschalk, G. Acetobacterium wieringae sp. nov., a new species producing acetic acid from molecular hydrogen and carbon dioxide. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. 1982, 3, 368–376. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2022, 51, D678–D689. [Google Scholar] [CrossRef]
- Schomburg, I.; Chang, A.; Ebeling, C.; Gremse, M.; Heldt, C.; Huhn, G.; Schomburg, D. Brenda, the enzyme database: Updates and major new developments. Nucleic Acids Res. 2004, 32, 431–433. [Google Scholar] [CrossRef]
- Gieg, L.M.; Jack, T.R.; Foght, J.M. Biological souring and mitigation in oil reservoirs. Appl. Microbiol. Biotechnol. 2011, 92, 263–282. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Chambers, B.; Lomans, B.P.; Head, I.M.; Tsesmetzis, N. Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl. Environ. Microbiol. 2016, 82, 2545–2554. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Sproer, C.; Rainey, F.A.; Burghardt, J.; Pauker, O.; Hippe, H. Phylogenetic analysis of the genus Desulfotomaculum: Evidence for the misclassification of Desulfotomaculum guttoideum and description of Desulfotomaculum orientis as Desulfosporosinus orientis gen. nov., comb. nov. Int. J. Syst. Bacteriol. 1997, 47, 1134–1139. [Google Scholar] [CrossRef]
- Panova, I.A.; Ikkert, O.; Avakyan, M.R.; Kopitsyn, D.S.; Mardanov, A.V.; Pimenov, N.V.; Shcherbakova, V.A.; Ravin, N.V.; Karnachuk, O.V. Desulfosporosinus metallidurans sp. nov., an acidophilic, metal-resistant sulfate-reducing bacterium from acid mine drainage. Int. J. Syst. Evol. Microbiol. 2021, 71, 004876. [Google Scholar] [CrossRef]
- Vandieken, V.; Niemann, H.; Engelen, B.; Cypionka, H. Marinisporobacter balticus gen. nov., sp. nov., Desulfosporosinus nitroreducens sp. nov. and Desulfosporosinus fructosivorans sp. nov., new spore-forming bacteria isolated from subsurface sediments of the Baltic Sea. Int. J. Syst. Evol. Microbiol. 2017, 67, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Cheng, C.L.; Huang, P.J.; Lin, S.Y. Application of scanning electron microscopy and X-ray microanalysis: FE-SEM, ESEM-EDS, and EDS mapping for studying the characteristics of topographical microstructure and elemental mapping of human cardiac calcified deposition. Anal. Bioanal. Chem. 2014, 406, 359–366. [Google Scholar] [CrossRef]
- Costa-Bauzá, A.; Grases, F.; Julià, F. The power of desktop scanning electron microscopy with elemental analysis for analyzing urinary stones. Urolithiasis 2023, 51, 50. [Google Scholar] [CrossRef]
- Safarova, E.A.; Filippova, D.S.; Stolyarov, V.E. Features of operation of underground gas storage facilities in joint storage of methane and hydrogen. Sci. J. Russian Gas Soc. 2021, 3, 58–62. (In Russian) [Google Scholar]
- Colman, D.R.; Poudel, S.; Stamps, B.W.; Boyd, E.S.; Spear, J.R. The deep, hot biosphere: Twenty-five years of retrospection. Proc. Natl. Acad. Sci. USA 2017, 114, 6895–6903. [Google Scholar] [CrossRef] [PubMed]
- Berta, M.; Dethlefsen, F.; Ebert, M.; Schäfer, D.; Dahmke, A. Geochemical effects of millimolar hydrogen concentrations in groundwater: An experimental study in the context of subsurface hydrogen storage. Environ. Sci. Technol. 2018, 52, 4937–4949. [Google Scholar] [CrossRef]
- Schieche, D.; Murty, M.V.S.; Kermode, R.I.; Bhattacharyya, D. Biohydrogenation of fumarate using Desulfovibrio desulfuricans: Experimental results and kinetic rate modelling. J. Chem. Technol. Biotechnol. 1997, 70, 316–322. [Google Scholar] [CrossRef]
- Miller, J.F.; Shah, N.N.; Nelson, C.M.; Ludlow, J.M.; Clark, D.S. Pressure and temperature effects on growth and methane production of the extreme thermophile Methanococcus jannaschi. Appl. Environ. Microbiol. 1988, 54, 3039–3042. [Google Scholar] [CrossRef]
- Laban, M. Hydrogen storage in salt caverns: Chemical modelling and analysis of large-scale hydrogen storage in underground salt caverns. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2020. [Google Scholar]
- Bo, Z.; Zeng, L.; Chen, Y.; Xie, Q. Geochemical reactions-induced hydrogen loss during underground hydrogen storage in sandstone reservoirs. Int. J. Hydrog. Energy 2021, 46, 19998–20009. [Google Scholar] [CrossRef]
- Zeng, L.; Keshavarz, A.; Xie, Q.; Iglauer, S. Hydrogen storage in Majiagou carbonate reservoir in China: Geochemical modelling on carbonate dissolution and hydrogen loss. Int. J. Hydrog. Energy 2022, 47, 24861–24870. [Google Scholar] [CrossRef]
- Saeed, M.; Jadhawar, P.; Bagala, S. Geochemical effects on storage gases and reservoir rock during underground hydrogen storage: A depleted North Sea oil reservoir case study. Hydrogen 2023, 4, 323–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).