Abstract
Black soldier fly (BSF) (Hermetia illucens) larvae are currently being developed as bioconversion agents for organic waste treatment. The resulting larvae or prepupae have a high protein and lipid content, primarily used as feed for fish, poultry, and other animals. The lipid content of BSF larvae/prepupae is influenced by the feed for growth and can reach up to 57.8%. BSF lipids mainly consist of medium-chain saturated fatty acids, with lauric acid (LA) being the dominant component. The LA content in BSF larvae/prepupae can be enhanced by incorporating or utilizing substrates containing highly digestible carbohydrates for larval growth. The LA content can reach 76.13% in larvae reared on fruit waste. LA has been reported to exhibit antibacterial, antifungal, antiviral, and anticancer properties. Moreover, it has applications in various fields such as pharmaceuticals, food and beverages, cosmetics, body care, soaps and detergents, plastics, and textiles. This review aims to investigate the LA content in BSF larvae and explore its potential applications, thereby establishing BSF larvae/prepupae as a novel source of LA for diverse fields.
1. Introduction
The black soldier fly (BSF) (Hermetia illucens) is an insect species that is currently gathering significant attention in several countries. Often referred to as maggots, these fly larvae are highly advantageous due to their ability to decompose organic waste and utilize it as a substrate during their larval growth stage. BSF larvae consume various types of organic waste, including vegetable waste, fruit waste, food waste, agro-industrial by-products, chicken manure, chicken feed, dairy manure, biogas digestate, sewage sludge, etc. [1,2,3]. BSF exhibits excellent reproductive capacity and rapid growth, even when feeding on low-grade materials such as organic garbage or waste.
Multiple studies have demonstrated the success of BSF larvae in converting organic waste into nutrient-rich products. Lalander et al. [4] stated that BSF larvae voraciously feed on decaying organic matter, making them a valuable protein source for animal feed. Other researchers have reported that BSF larvae can convert organic waste into nutrient-rich biomass, serving as a sustainable solution for enhancing food production [5,6,7]. BSF larvae can effectively digest organic waste, resulting in a daily reduction of organic matter ranging from 65.5% to 78.9% [8,9]. Approximately 15 thousand BSF larvae can consume around 2 kg of food and organic waste within 24 h [8,9]. Amrul et al. [3] found that BSF larvae can reduce the initial weight of organic waste by approximately 50% in less time compared to conventional composting methods [3]. Utilizing BSF larvae for waste treatment offers an environmentally friendly and cost-effective approach, significantly reducing waste volume and curbing disease transmission [10]. Leveraging BSF larvae for waste treatment presents an alternative solution to address organic waste accumulation from various sources, including the food and beverage industry, markets, households, hotels and restaurants, agricultural practices, livestock, fisheries, and other sources. Furthermore, employing BSF larvae for waste treatment yields larvae suitable for feed production, while the residual biomass serves as fertilizer.
The primary components of BSF larvae are proteins and lipids, the composition of which varies depending on the rearing substrate. Generally, BSF larvae contain approximately 40% protein and 30% lipid on a dry matter basis [11,12,13,14]. Owing to their high protein and lipid content, the larvae can be utilized for feed production in the poultry and aquaculture industries, offering nutritional value comparable to conventional animal feed [5,6,15,16,17]. The utilization of BSF larvae as a food source primarily stems from their high protein content, while their lipid content is often considered a by-product of animal feed production [18]. Consequently, lipids or fatty acids derived from BSF larvae or prepupae are often underutilized, despite research indicating their suitability for biodiesel production [1,12,19,20,21]. Lipids from insect raw materials are broadly extolled because insects have a short life cycle, simple rearing requirements, high body mass gain, and high fat content, and they require less energy, water, and food inputs [22].
Numerous studies have reported that the lipid composition of BSF larvae is influenced by the composition of their growth media [12,13], but lipid content will always be dominated by lauric acid (LA) [7,17,23,24,25]. Meneguz et al. [6] examined the larvae reared in vegetable, fruit, and agroindustry waste. The results showed that BSF larvae reared in all substrates contained higher levels of lauric acid than other fatty acids, which contained 32.4–57.4%. Ewald et al. [7] conducted a study on the effect of 11 substrates based on four different organic waste sources (shellfish, bread, fish, and food waste) on the fatty acid composition of larvae. The results revealed that the fatty acid composition of the organic waste and larvae weight affects the larvae fatty acid profile. The fatty acid composition of larvae mainly consists of lauric acid and can reach 52% [7]. Surendra et al. [25] stated that the rearing of BSF in food waste resulted in a prepupae fatty acid composition which was dominated by lauric acid as well (45%) [25]. Several other studies have also revealed that BSF oil has a high LA content and is of similar quality to coconut oil and palm kernel oil. Many of the beneficial properties of this coconut oil have been attributed to LA [2,18,26]. LA has been reported to have antibacterial [27,28,29,30,31,32], antiviral [33], antifungal [29,34], and anticancer activities [35]. In addition, LA is also widely utilized as a raw material for various surfactants in the food industry and for other materials in the pharmaceutical, cosmetic, soap, and shampoo industries. This review aims to study the content of LA in BSF larvae and the potential application of LA in diverse fields. This paper is expected to provide information to increase the added value of BSF larvae/prepupae as a new source of LA for the industry, such as LA obtained from coconut oil and palm kernel oil, etc.
The extensive literature study on this review conducted in several scientific databases, including Google Scholar, Elsevier, PubMed, MDPI, Scopus, ResearchGate, and many database including from website page, whether in the English or the Indonesian language literature. The keywords for searching suitable literature included “Hermetia illucens”, “black soldier fly (BSF)”, “organic waste”, “extract BSF bioactivity”, and so on. We collected in total 178 source information regarding the applications of BSF in many fields spanning from 1971 to 2023.
2. Black Soldier Fly (Hermetia illucens)
BSF, belonging to the order Diptera and the family Stratiomyidae, is a species of fly within the genus Hermetia [36,37]. Native to the Americas, BSF has spread across a wide geographical range, occupying regions between 45° north latitude and 40° south latitude [36,38]. It has been observed in numerous countries across Europe, Africa, Oceania (Australia and New Zealand), and Asia (Indonesia, Japan, Philippines, and Sri Lanka). The optimal temperature range for the life cycle of BSF larvae is between 20 °C and 30 °C, while for the adult stage (imago), it ranges from 25 to 32 °C [38,39].
The life cycle of BSF (Figure 1) encompasses several stages, commencing with the production of eggs by adult BSF, followed by egg hatching into larvae, larval development into prepupae, and eventually pupae transforming into adult black soldiers. The duration of the BSF life cycle, from egg to adult fly, ranges from 40 to 43 days, influenced by environmental factors such as temperature, humidity, and feeding substrates [40]. Female flies require 20–30 min to lay eggs, with egg production ranging from 546 to 1505 eggs [41]. The eggs are typically laid in close proximity to food sources, facilitating the larvae’s growth on moist decomposing organic matter. Adult BSF does not consume solid food; they solely require water, as nutrient intake is essential only during the larval phase, supporting their reproductive needs [42].
BSF is a tropical fly species with exceptional organic matter decomposition capabilities [43]. The larvae exhibit proficiency in recycling both solid and liquid forms of organic waste, making them suitable candidates for monoculture breeding due to their ease of propagation, safety, and adaptability to various conditions. Additionally, they display resilience against microorganisms and parasites, surviving in challenging environments while effectively cooperating with microorganisms to degrade organic waste [44]. The economic value of the waste processed by BSF larvae remains low, thus promoting the development of BSF biotechnology. Moreover, BSF is not considered a pest and poses a lower disease transmission risk compared to other fly species [44,45].
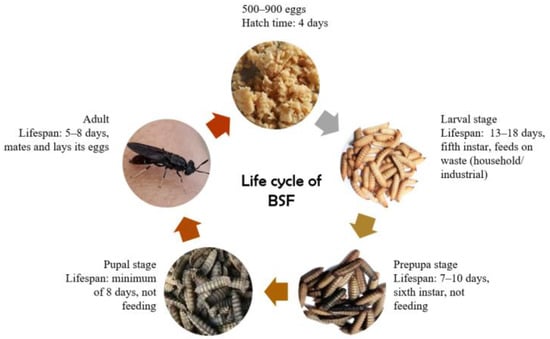
Figure 1.
The life cycle of BSF [46].
The use of BSF larvae offers several advantages, including the ability to degrade organic waste into nutrient-rich materials for growth, convert organic waste into compost with high fertilizer content, and control odor and pests. Additionally, BSF larvae contribute to the reduction of greenhouse gas emissions during the waste decomposition process. For instance, conventional composting methods for agricultural waste and food waste emit methane gas at rates of 10.52 g/kg and 88.1 g/kg, respectively. In contrast, composting with BSF larvae results in significantly lower methane gas emissions of 0.08–0.76 g/kg and 1.0–3.0 g/kg, respectively [47]. Furthermore, the bodies of BSF larvae contain high levels of chitin and protein, making them suitable as animal feed [44]. The high fat content in BSF larvae allows them to be utilized as feedstock for biodiesel production. Overall, the utilization of BSF larvae offers numerous advantages, contributing to the establishment of a circular economy through the bioconversion of various types of waste into biomass rich in protein, lipids, chitin, and other bioactive compounds [21,48].
2.1. The Effectiveness of Organic Waste Treatment by BSF Larvae
The effectiveness of waste treatment with BSF larvae can be seen from various parameters of waste treatment and larval growth, such as substrate reduction (%SR), biomass conversion ratio (BCR), waste reduction index (WRI), the efficiency of conversion of digested food (ECD) or the ingested food (ECI), survival rate, larval biomass, and larval growth rate (GR) [4,6,7].
From Table 1, Lalander’s research [4] shows that the main treatment factors that were found to be influenced by the substrate were waste-to-biomass conversion ratio (% DM), larval development time, and final prepupae weight. The survival rate did not vary greatly between the different substrates. The total volatile solids content and substrate protein content had a major effect on the biomass conversion ratio (BCR) and larval development time, whereas only the total volatile solids content affected the final prepupae weight. The volatile solids feeding rate was found to be the most important parameter, contributing to 60% of the variance in the BCR [4]. The highest waste-to-BCR was achieved with abattoir waste (15% DW) [4]. The BCR for abattoir waste was higher than the mixture of abattoir and fruit and vegetable waste, likely because fruits and vegetables contain materials that are difficult for larvae to degrade. The BCR for fruit and vegetable substrates is much lower than abattoir waste. Rehman et al. in [49] found a significantly lower BCR for dairy manure than chicken manure, even though cow manure had higher total organic carbon due to higher proportions of lignin, cellulose, and hemicellulose. The largest prepupae were those reared on the mixture of abattoir and fruit and vegetable waste, which weighed just over 250 mg/larva on average because the nutrient balance was better in the mixture of abattoir and fruit and vegetable waste, as the carbon added by the fruit and vegetable fraction balanced the high nitrogen content of the abattoir waste, enabling the larvae to utilize the available nutrients to a higher degree [4]. This may also be why in [7], for example, BSF larvae rearing in bread substrates had a higher BCR but lower biomass than fresh mussels. Another factor that affects BSF biomass is the higher survival rate (SR) of larvae reared in fresh mussels (89.3%) than larvae-fed bread (69.8%).

Table 1.
Process efficiency and larval biomass in processing organic waste with BSF larvae.
The capacity of BSFL to degrade waste in a specific time can be evaluated using the waste reduction index (WRI). Higher WRI values indicate good reduction efficiency. A higher WRI value (5.3) was achieved by researchers [6] on brewery by-product waste (BRE), which produced the highest biomass of 11.32 g (total biomass). The ECD value for this waste was also the highest (0.14). The ECD value indicates how efficiently the larvae convert the feed supplied into their biomass. ECD is closely related to mortality, and low mortality values allow for the best performance in terms of ECD. BRE larvae reduce the amount of substrate less than winery by-product larvae; however, the WRI was higher in BRE larvae because they needed less time to reach the prepupal stage, which was also confirmed by the higher larval growth rate values. BRE larvae showed the best ECD combined with the absolute highest total final biomass production and the shortest developmental period [6].
Based on research by Lalander et al. [4], the most suitable substrate for BSFL composting and producing high biomass is a substrate that contains most of the easily available carbon and high enough protein content to support larval development. Abattoir waste, fruit and vegetable and abattoir waste, food waste, and human waste are examples of substrates that provide good conditions for larval growth, and larval rearing would be a good choice. Substrates containing a large proportion of easily available carbon, but low nitrogen content, do not support larval development and thus reduce process efficiency.
2.2. Nutrients Composition of BSF
Extensive research has revealed that BSF larvae are rich in proteins and lipids, making them economically valuable. Surendra et al. [25] reported that the nutritional quality of BSF larvae primarily stems from their lipid and protein content [25]. Barragan-Fonseca et al. found that dried prepupae contain a high percentage of protein (37–63%) and fat (7–39%) [11]. Furthermore, Ebeneezar et al. [50] reported that BSF larvae reared in food waste exhibited a crude protein content of 41.44% and a crude lipid content of 35.69% [50]. Wang et al. conducted research on larvae reared in food waste and found that they contained 43.69% crude protein and 37.24% crude fat, surpassing the levels found in BSF larvae reared in pig manure, chicken manure, and cow manure [51]. Aldi [52], in their study utilizing four types of waste (tofu dregs, chicken blood, oil palm cake, and fish waste), demonstrated that larvae grown in chicken blood media produced a crude protein content of 41.18% with a crude fat content of 43.18%. The highest fat content was obtained from larvae reared in fish waste, with a value of 47.73%, accompanied by a protein content of 31.96% [52]. In general, BSF larvae contain approximately 40% protein and 30% lipid [11,12,13,14]. It is worth noting that the main nutritional composition of BSF larvae, namely, proteins and lipids, varies significantly and is influenced by their rearing substrates.
Liu et al. conducted research to investigate the metabolic changes in the nutritional composition of BSF from eggs to adults (Table 2). The larvae were fed with a mixture of chicken feed and approximately 60% water content. The study revealed a rapid increase in crude fat content from day 4 to day 14 of larval development, reaching a maximum level of 28.4% in dry mass on day 14. Subsequently, from the early prepupa to the late pupa stages, a sharp decrease in crude fat content was observed, with values of 24.2% and 8.2%, respectively. Conversely, protein content was higher in the early stages of growth, exceeding 50%. It then decreased until the age of 12 days but increased again during the late prepupa and pupa stages, with levels reaching 46.2% [53]. This research indicates that the nutritional content of BSF larvae is influenced by the stage of development, emphasizing the importance of considering the larvae’s age at harvest. Moreover, it is possible to modify the specific nutritional composition of BSF larvae to suit the requirements of various applications [54].

Table 2.
Crude protein, crude fat, and ash content in various life phases of BSF [53].
The larvae and prepupae stages are particularly valuable for use as feed, while pupae are less suitable due to a loss of approximately 20% of their lipid content compared to the larval stage [53]. Under identical rearing conditions, larvae contain higher amounts of lipids and nitrogen-free extracts but lower levels of ash and protein compared to prepupae and pupae stages [53,55]. Prepupae also have approximately 20% higher water content than larvae, requiring longer drying times [2,56]. Therefore, the choice of harvest stage depends on the intended use of BSF, with consideration for the nutritional content. Additionally, the nutritional quality of BSF larvae is influenced by their growth substrate and age. Younger larvae harvested at an early age exhibit higher protein content, while fat content increases with the larvae’s age [53,57,58].
2.3. Lipid and Lauric Acid Content in BSF
Lipids are abundantly present in the last instar larvae and prepupae of BSF, obtained through the bioconversion process [48,53]. BSF larvae have been reported to feed on various organic materials, including food waste, fruit waste, sewage sludge, cattle manure, chicken manure, pig manure, palm decanter cake from oil palm mills, rice straw, brown algae (Ascophyllum nodosum), and others [19,23,25,59,60,61]. The lipid content in BSF larvae is influenced by the variation in substrates. For example, the bioconversion of fruit waste, palm decanter cake, and sewage sludge by BSF larvae demonstrated the highest lipid content when fruit waste was used as the substrate, reaching 44.46%. In comparison, palm decanter cake and sewage sludge resulted in lipid contents of 36.51% and 29.85%, respectively [1]. Additionally, Scala et al. reported that larvae fed with an apple diet contained an average lipid content of 36%, whereas larvae fed a mixed grain diet exhibited approximately 22% lipid content [14]. Purnamasari et al. also reported the crude fat content of BSF larvae reared on various wastes, such as cassava peel, fruit waste, tofu pulp, and food waste. The highest fat content was observed in larvae fed with cassava peel, at 28.9%, while the levels in other waste substrates were nearly the same: 22.5% (food waste), 21.2% (fruit waste), and 20.1% (tofu dregs) [59]. Table 3 presents the lipid content in BSF biomass cultivated using different types of wastes. The data indicate that the lipid content in BSF larvae biomass varies depending on the waste material employed for larvae growth, ranging from 11.2% to 57.8%. The highest lipid content was observed in larvae grown on a bread medium (57.8%) due to the high carbohydrate content of bread. This finding aligns with the research conducted by Scala et al., which demonstrated that larvae produced higher lipid content when fed substrates rich in carbohydrates, such as apples and bananas. BSF larvae can synthesize lipids from the carbohydrates present in the substrate [2,14,62].

Table 3.
Lipid content in BSF biomass with different types of wastes.
The [66,67,68,69] major lipid component of BSF larvae is lauric acid (LA), a medium-chain saturated fatty acid, irrespective of the substrate they are grown in [17,23]. Leong et al. [1] reported that LA was the dominant fatty acid across all substrates, with the highest amount produced from larvae fed with fruit waste (approximately 76.13%). Additionally, prepupa BSF had a fat content of approximately 32%, with saturated fatty acids comprising the majority, and LA dominating at 67% [24,25]. Table 4 presents the main fatty acid profile of BSF larvae and prepupae across various types of substrates. Generally, LA is the primary fatty acid in larvae and prepupae fed with different substrates, except for a few exceptions such as mitigation mussels, rapeseed cake, and shrimp waste, where oleic acid is the main fatty acid [53]. This contradicts previous studies that identified saturated fatty acids (SFAs) as the primary fatty acid class in BSF larvae or prepupae, with LA typically being the most dominant. The discrepancy may be explained by differences in the nutritional composition of the substrates, particularly the digestible carbohydrate content [47]. The high carbohydrate content in the substrate can increase the LA content in BSF larvae or prepupae, as BSF can metabolize carbohydrates into LA within its body biomass [7,20,54]. Previous research by Eggink et al. [54] found no LA content in the substrate, indicating that BSF metabolizes carbohydrates through its biosynthetic pathway rather than accumulating LA from the substrate. On the other hand, the higher proportion of oleic acid is primarily obtained from the accumulation of oleic acid from substrates, in addition to its production through the biosynthetic pathway [20,54]. Eggink et al. [54] observed higher levels of oleic acid in mitigation mussels, rapeseed meal, and shrimp waste compared to other substrates. These findings align with the research conducted by Hoc et al. [20], who investigated lipid metabolism in Hermetia illucens, specifically the origin of fatty acids in prepupae. Interestingly, on the same substrate, LA content was higher in the sixth instar (prepupae stage) than in the fifth instar larvae, whereas fat content was higher in the fifth instar than in the sixth instar [21,52,66]. Wong et al. [21] suggested that for industrial-scale production, it is advisable to harvest BSF larvae at the fifth instar as it increases the annual product batch and yield without affecting the fatty acid composition of the BSF larvae biomass and is more time and cost-efficient.

Table 4.
Main fatty acid profile of BSF biomass with various types of substrates.
2.4. Comparison of Lauric Acid Content in BSF and Other Insects
Furthermore, Jayanegara et al. [68] investigated the fatty acid profiles of various insect oils, including BSF larvae (Hermetia illucens), crotos (Oecophylla smaragdina), super worms (Zophobas morio), mealworms (Tenebrio molitor), and crickets (Gryllus bimaculatus) (Table 5). The data in Table 5 reveal distinct fatty acid profiles among the five insect species, with oleic acid being the dominant fatty acid in general, except for BSF larvae and crickets. Among the insects, BSF larvae exhibited the highest content of lauric acid (LA) at 43.1%. In contrast, the LA content in other insect oils was negligible, at less than 1%. These findings are consistent with the research conducted by Lawal et al. [70], which demonstrated that Tenebrio molitor larvae had an LA content of only 0.46%, while Hermetia illucens larvae contained 31% LA. The LA content in other insects was also minimal, with oleic acid being the prevailing fatty acid. This research underscores the potential of BSF larvae as a valuable source of LA.

Table 5.
Lipids, lauric acid, and fatty acid dominant in some insects.
3. Lauric Acid
LA (Figure 2(1)) is a 12-carbon carboxylic acid classified as a medium-chain saturated fatty acid (MCFA). It is known by its IUPAC name, dodecanoic acid, and has the molecular formula C12H24O2. LA possesses a melting point of 44 °C, a boiling point of 296.1 °C at 760 mm Hg, and an acidity (pKa) of 5.3 at 20 °C. At room temperature, LA appears as a white solid with a slight odor reminiscent of bay oil. It readily melts when heated and is considered non-toxic and safe for use in food products. LA exhibits solubility in both polar and non-polar solvents due to the presence of a hydrocarbon (methyl) group at one end and a carboxyl group at the other. Its solubility in water is relatively low, measuring around 55 mg/L at 20 °C, but increases with rising temperatures. While LA is slightly soluble in chloroform, it dissolves in acetone, petroleum ether, toluene, and ethyl acetate. It displays high solubility in methanol, ethanol, and mixed solvents containing benzene. The soap and shampoo industry takes advantage of LA’s solubility in polar and non-polar solvents [75]. Notably, sodium lauryl sulfate (Figure 2(2)) stands as the most commonly used derivative in this industry LA also serves as a precursor for monolaurin (Figure 2(3)), which acts as a potent antimicrobial agent, surpassing the antimicrobial properties of its precursor compounds. Monolaurin exhibits the ability to combat viral and bacterial infections effectively [76]. Moreover, LA has applications in chemical laboratories for determining the molar mass of unknown substances through freezing point depression [77].
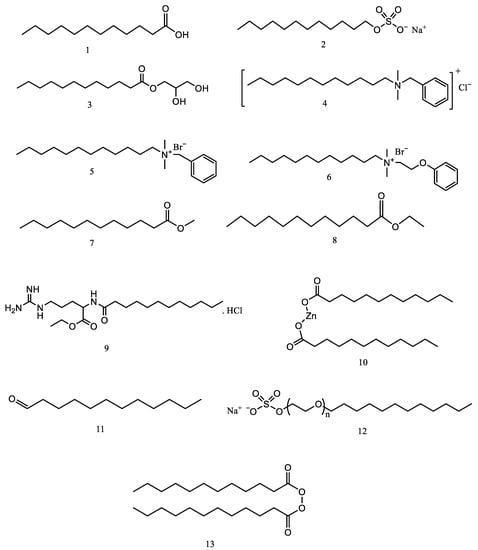
Figure 2.
The structure of LA (1) and some derivative.
3.1. Sources of LA
LA is naturally present in various plant (Table 6) and animal fats and oils. LA can be found in human milk (6.2%), cow’s milk (2.9%), and goat’s milk (3.1%) [78]. While present in small quantities, LA can be found in plants such as wild nutmeg, peach palm seed, betel nut, date palm, macadamia nut, and watermelon seed [78]. The Cuphea genus, consisting of around 240–250 species of herbs and subshrubs, also contains LA in its seed oil. Its content can reach 38.5% in C. sincorana and 76.6% to 85.9% in C. melanium [79,80] Although C. melanium boasts exceptionally high LA content, its agronomic challenges, such as seed shattering and indeterminate growth, have hindered large-scale commercial cultivation [81]. Genetic engineering efforts have been employed to enhance LA content in plants. For instance, through recombinant DNA technology, canola or rapeseed (Brassica napus) underwent genetic modification, resulting in nearly 40 mol% LA content [82]. LauricalTM, a trademarked genetically modified rapeseed oil developed by Calgene (now Monsanto), exemplifies the success of this approach [83].
The most notable sources of LA are coconut and palm kernel oil [84]. Remarkably, research suggests that the high LA content in BSF larval oil is comparable to that of coconut and palm kernel oil. The oil derived from BSF larvae consists of monoglycerides, diglycerides, and triglycerides, displaying a fatty acid profile and quality similar to those of the two plants [2,18,26,85]. Almeida et al. [85] conducted research employing different extraction methods such as decoction, microwaves, maceration, and ultrasound, and results show that despite using different extraction techniques and solvents, similar fatty acid composition profiles were obtained, and found LA is dominant in all the fat extracts ranging from 37% to 62%. Another study by Leong et al. [1] reported the highest LA composition of 76.13% in larvae-fed fruit waste. The oil obtained from BSF larvae is recognized for its high quality and serves as a by-product of animal feed production [85]. BSF larvae present a promising new source of LA, offering an alternative substitute for coconut oil, palm kernel oil, and other oils. Utilizing BSF larvae as a source of LA provides numerous advantages, including shorter harvest times, easy and cost-effective maintenance, relatively easy harvesting, and the use of organic waste as a source of feed. Thus, BSF larvae emerge as a viable and advantageous choice as a new source of LA for many applications.

Table 6.
The LA content in plant oil.
Table 6.
The LA content in plant oil.
| Sources | Amount of LA (%) | Reference |
|---|---|---|
| Palm kernel oil (Elaeis guineensis) | 45.7–48.2 | [86] |
| Coconut oil (Cocos nucifera) | 45–53 | [87] |
| Babasu oil (Attalea speciosa) | 47.4 | [88] |
| Cohune oil (Attalea cohune) | 46.5 | [78] |
| Ucuuba butter (Virola sebifera Aubl.) | 73 | [89] |
| Murumuru butter (Astrocaryum murumuru) | 40 | [89] |
| Ouricury oil (Syagrus coronate) | 43.64 | [90] |
| Tucum oil (Astrocaryum vulgare) | 45.5 | [91] |
| Laurical oil (Brassica napus) | 37.6 | [92] |
3.2. Potential Application of Lauric Acid
3.2.1. Biodiesel Use
Biodiesel has gained significant attention as an alternative to fossil fuels due to its renewable, biodegradable, and environmentally friendly properties. Biodiesel offers several advantages, including comparable calorific value to fossil fuels, low toxic emissions, absence of sulfur and toxins, and high flash point and cetane number [93,94,95,96].
Biodiesel primarily consists of mono-alkyl ester compounds derived from long-chain fatty acids and is used as an alternative fuel for diesel engines. However, medium-chain fatty acid compounds such as lauric acid (LA) also have the potential to serve as precursors for biodiesel production, as demonstrated by several studies. For instance, Zhang et al. [97] produced biodiesel through the esterification of LA catalyzed by ammonium and silver co-doped phosphotungstic acid embedded in a zirconium metal–organic framework nanocomposite. They achieved a 75.6% conversion of LA to methyl ester using a 10% catalyst weight. The optimal conditions for this reaction involved a molar ratio of LA to methanol of 1:15, a reaction temperature of 150 °C, and a reaction time of 3 h [97]. Another study using copper phosphomolybdate (Cu-PMo) as a catalyst achieved a conversion yield of 78.7% in the esterification of LA into biodiesel. The optimal reaction conditions included a temperature of 70 °C, a reaction time of 60 min, a catalyst dose of 3% by weight, and a molar ratio of LA to methanol of 1:6 [98]. Additionally, Zatta et al. [99] achieved even higher conversion results using raw halloysite catalysts, with a 95.02% conversion of LA to biodiesel. However, this required a higher temperature of 160 °C and a longer reaction time of 2 h compared to other methods [100].
Raw materials rich in LA, such as coconut oil (CO), Litsea cubeba kernel oil (LCKO), and palm kernel oil (PKO), have also been successfully converted into biodiesel [101,102,103,104,105,106]. BSF larvae, which contain high levels of lipids, have emerged as a potential source for biodiesel production. Similar to CO, PKO, and LCKO, the oil/lipid composition in BSF larvae or prepupae is typically dominated by LA. Extensive research has been conducted into using BSF larvae to convert organic waste into biodiesel. Biodiesel obtained from larvae grown on various substrates, including manure (chicken, beef, and pork), food waste (restaurants and fruit), soybean residue, and coconut endosperm waste, has shown yields ranging from 90% to 98% [12,19,22,60,107,108,109]. Various biodiesel production processes from BSF larvae have been investigated, such as a two-step process involving esterification with an acid catalyst followed by transesterification with a base catalyst [12,19,20,21,60,108], enzymatic catalysts (biocatalysts) [107,110,111], non-catalytic transesterification using SiO2 porous materials [111], and direct transesterification without lipid extraction [112]. Table 7 provides a summary of research methods for biodiesel production from BSF larvae and the corresponding biodiesel yields obtained.

Table 7.
Methods and yield of biodiesel production from black soldier fly larvae.
Furthermore, several studies have compared the biodiesel yield and characteristics of BSF larvae-derived biodiesel with those of other biodiesel sources. These studies have revealed some distinct differences in the chemical composition and properties of BSF larvae biodiesel compared to biodiesel from sources such as rapeseed oil, soybean oil, palm oil, jatropha oil, and waste cooking oil [12,19,95,114,115,116,117,118]. BSF larvae biodiesel is characterized by a higher proportion of C12 methyl laurate, a saturated fatty acid, whereas other biodiesels are dominated by different fatty acid methyl esters such as methyl oleate (C18:1) in rapeseed biodiesel and methyl linoleate (C18:2) in soybean biodiesel. The iodine value of BSF larvae biodiesel is lower than that of soybean oil and waste cooking oil biodiesels but higher than that of palm oil biodiesel, indicating its higher content of saturated fatty acid methyl esters. The acid value of BSF larvae lipid-derived biodiesel is lower than palm biodiesel and jatropha biodiesel but higher than rapeseed oil, waste cooking oil, and soybean oil biodiesels. However, BSF larvae biodiesel has a higher sulfur content compared to other biodiesels, which may be attributed to the presence of unreacted or degraded free fatty acids, sulfuric acid catalysts, or intrinsic sulfur content in BSF larvae lipids. To meet standards, the sulfur and acid content can be reduced through fractional distillation to obtain purer fatty acid methyl esters [19,95,112,114].
Furthermore, other biodiesel quality parameters were also tested and compared with other sources, including rapeseed oil, soybean oil, palm oil, jatropha oil, and used cooking oil. In general, the quality parameters of biodiesel as density, viscosity, ester content, flash point, cetane number, etc., are comparable to these biodiesel sources, even though the methods for making biodiesel are different [19,110,112,114]. BSF larvae lipid-based biodiesel has a higher percentage of saturated fatty acid methyl esters, giving it higher oxidative stability. However, research by Park et al. [114] showed that the oxidizing properties of biodiesel were lower and did not meet the standards. Although the addition of the antioxidant tert-butylhydroquinone improved the oxidation stability, further research is needed to determine the cause.
Various studies have also shown that the characteristics of biodiesel produced from BSF larvae or prepupae have characteristics of biodiesel that are almost similar to biodiesel from other sources, and it meets the American Society for Test and Materials (ASTM) biodiesel standard D6751, European Standard 14214, and Korea standard KS M 2965 [111,112,113,114]. These results indicate that BSFL grown from organic waste can be a feasible non-food raw material for biodiesel production [12,19,60,107,110,111,112,113,114,119,120,121,122].
The advantage of using BSF insects as raw materials for biodiesel is that they do not compete with food raw materials such as coconut, palm, rapeseed, soybean oil, etc. In addition, the yield productivity of lipids and biodiesel per unit area per year is known to be higher than other raw materials, both edible and non-edible oils. The fat content of 18% soybean, 41% rapeseed, 36% oil palm, 28% jatropha, 50% microalgae, and 30 to 40% BSF larvae yielded oil productivity of 636 L oil/ha/year, 974 L oil/ha/year, 5366 L oil/ha/year, 741 L oil/ha/year, 97,800 L oil/ha/year, and 162,000 L oil/ha/year, respectively. This showed that with less cultivation area, the BSF larvae could produce much higher biodiesel, namely, 145,800 kg/ha/year (based on 40% BSF oil content), compared to soybeans (which could only produce 562 kg/ha/year), rapeseed (862 kg/ha/year), oil palm (4747 kg/ha/year), jatropha (656 kg/ha/year), and microalgae (86,515 kg/ha/year) [114].
From the explanation above, it can be concluded that biodiesel can be produced from a single LA compound and a mixture of fatty acids. However, it seems that the use of single compounds will require more expensive processing costs due to LA separation. The use of fatty acids containing high levels of LA from BSF lipids has the opportunity as a raw material for making biodiesel with quality similar to biodiesel from other sources. However, biodiesel from BSF may be cheaper because it is obtained from very cheap and even worthless materials, namely, organic waste. In addition, another advantage of using BSF lipid as a raw material for biodiesel production is that it can contribute to reducing organic waste, thereby reducing greenhouse gas emissions [48].
3.2.2. Pharmaceutical Use
In the medical field, lauric acid (LA) is known for its powerful antibacterial and antiviral properties. LA is widely used in various medications to treat viral infections, including certain types of influenza, fever blisters, cold sores, bronchitis, yeast infections, gonorrhea, genital herpes caused by the herpes simplex virus (HSV), genital warts caused by human papillomavirus (HPV), HIV/AIDS, intestinal infections caused by the parasite Giardia lamblia, and ringworm. Additionally, LA is used to prevent mother-to-child transmission of HIV [76,123].
LA has been extensively studied for its antibacterial [27,28,29,30,31,32], antiviral [33], antifungal [29,34], and anticancer [35,99] properties. Numerous studies have explored LA’s antimicrobial properties, as shown in Table 8. Both in vitro and in vivo research studies have demonstrated that LA possesses antimicrobial properties 15 times stronger than benzoyl peroxide (BPO), making it a potential treatment option for acne [28]. Among saturated fatty acids, LA is the most potent against Gram-positive bacteria [27,87,124,125,126] and Gram-negative bacteria [127,128,129]. Studies examining the antimicrobial effects of saturated fatty acids with hydrocarbon chains ranging from 6 to 18 carbons have identified LA as the most effective against Gram-positive bacteria such as S. aureus [27], B. megaterium [124], Pneumococci sp., and Corynebacterium sp. [27]. Regarding Gram-negative bacteria, LA has shown significant bactericidal activity against H. pylori [69], C. trachomatis, and N. gonorrhoeae [127,128]. Furthermore, when comparing saturated and unsaturated fatty acids, LA testing of some bacteria exhibits higher Gram-positive antibacterial activity than linolenic acid (C18:3) but lower than linoleic acid (C18:2) [27,124]. In research conducted on human gut microbes, LA exhibits low antimicrobial activity against commensal lactic acid bacteria but high antimicrobial activity against pathogenic Bacteroides and Clostridium, suggesting its potential in modulating gut health [130].
Several researchers have evaluated the antimicrobial properties of BSF extracts to encourage the use of BSF larvae in the pharmaceutical field. These research studies led to the presence of high LA in BSF extract [31,131,132]. In addition, the presence of antimicrobial peptide compounds also plays a role in antimicrobial properties. However, due to the very high amount of LA in BSF larvae or prepupae, this review focuses on the antimicrobial properties of LA. Auza et al. [131] evaluated the antibacterial activity of BSF methanol extract in vitro against the growth of Salmonella typhimurium, Escherichia coli, and Pseudomonas aureginosa and showed that the antibacterial activity of BSF extract increased in line with the increase in the concentration of BSF extract. A BSF extract of 325 mg/mL was an effective concentration to inhibit the growth of S. typhimurium, E. coli, and P. aureginosa bacteria. The average diameter of the inhibition zone for those bacteria was 11.77 ± 0.03 mm, 11.15 ± 0.05 mm, and 11.15 ± 0.23 mm, respectively, which was categorized as a strong inhibition zone [131]. These results were very similar to the research conducted by Harlystiarini et al. [132], which tested two important bacterial strains in poultry, namely, Salmonella sp. and E. coli. The methanol extract was tested on these bacteria at various concentrations using the agar diffusion method. Based on the diameter of the inhibition zone, the BSF larvae extract had strong antibacterial activity (p < 0.05) against Salmonella sp. and E. coli with a concentration of 320 mg/mL. This antibacterial property was associated with the high presence of lauric acid (49.18%).
Another study investigated lyophilized H. illucens larvae, which were homogenized and extracted with acidic methanol. The extract demonstrated broad-spectrum antibacterial activity and showed efficacy against methicillin-resistant Staphylococcus aureus (MRSA), suggesting that H. illucens larvae could serve as a source of novel antibiotic-like compounds for infection control [29]. This finding aligns with the research conducted by Kitahara et al. [133], which tested saturated fatty acids ranging from C8 to C18. The results showed that lauric acid (LA) was the most potent saturated fatty acid against MRSA and methicillin-susceptible Staphylococcus aureus (MSSA) strains, inhibiting their growth at a concentration of 400 μg/mL Furthermore, the LA derivative, namely, monolaurin, has demonstrated significant antimicrobial effects against Gram-positive bacteria, fungi, and several viruses [134]. Comparing C11–C13 fatty acids and their monoglyceride equivalents showed that LA and GML were the most potent antibacterial compounds against Gram-positive bacteria [87]. Other researchers stated that LA, linolenic acid, and GML showed strong antibacterial activity against L. monocytogenes at 10–20 µg/mL [125].
Related to anticancer properties, LA promotes cancer cell destruction and prevention of cancer growth, particularly in breast and colon cancer cells [99]. Research conducted by Lappano et al. [35] revealed that LA induces antiproliferative and pro-apoptotic responses in breast and endometrial cancer cells without affecting the growth of normal breast epithelial cells, suggesting its potential as an anticancer agent.
LA offers several other benefits, including its role as an antioxidant, its ability to increase high-density lipoprotein (HDL) levels, its ability to lower blood pressure and heart rate, and its role in reducing oxidative stress on the heart and kidneys [99]. Alves et al. [135] conducted a study on the effects of acute administration of LA on blood pressure, heart rate, and oxidative stress in rats. An intravenous LA dose of 1–10 mg/kg can reduce blood pressure and heart rate. LA (10−3 M) reduced NADPH-dependent superoxide accumulation in the heart and kidney, which is related to its capability of reducing oxidative stress in the heart and kidneys [135]. Lauric acid has been shown to have antioxidant properties as a compound on its own and in natural sources such as virgin coconut oil and breast milk. Lauric acid is easily absorbed by the intestine, highly metabolized by the liver, and can act as a regulator of metabolism [136]. Research conducted by Anuar et al. [137] showed that lauric acid, especially at 50 mg/kg, has the potential to improve hyperglycemia, insulin sensitivity, hormonal profile, serum oxidative status, and male reproductive organs in STZ-induced diabetic rats. Lauric acid can reduce blood glucose levels, which correlates with decreased insulin resistance and the balance of glucose metabolism and the oxidant–antioxidant system [137].
Furthermore, most ingested LA is transported directly to the liver, where it is metabolized into energy and other metabolites, rather than being stored as fat. These metabolites include ketone bodies, which can be utilized by extrahepatic tissues such as the brain and heart as a direct source of energy [134]. Importantly, LA metabolism by the liver does not harm organs such as the pancreas, liver, and digestive system, making it safe for the body [99].

Table 8.
Several research studies of LA with positive results as an antimicrobial.
Table 8.
Several research studies of LA with positive results as an antimicrobial.
| Antimicrobial Character | Microorganism | Level of LA Bioactivity | Reference |
|---|---|---|---|
| Antibacterial | B. megaterium | 0.15 mM a | [124] |
| Pneumococci Micrococcus sp. Corynebacterium sp. N. asteroides | 0062 µmoles/mL a 0.624 µmoles/mL a 0.124 µmoles/mL a 0.124 µmoles/mL a | [27] | |
| N. asteroides S. aureus Strep. faecalis Strep. pyogenes | 62 µg/mL a 500 µg/mL a 500 µg/mL a 62 µg/mL a | [87] | |
| Helicobacter pylori | 1 mM b | [138] | |
| Chlamydia trachomatis | 5 mM for 10 min c | [127] | |
| S. aureus P. acnes | 0.97 μg/mL a 3.9 μg/mL a 60 μg/mL b (ATCC 6919) 80 μg/mL b (ATCC 11827) | [28] | |
| Methicillin-resistant Staphylococcus aureus (MRSA) Methicillin-susceptible Staphylococcus aureus (MSSA) | 400 μg/mL a 400 μg/mL a | [133] | |
| Salmonella S. aureus E. coli Micrococcus Bacillus stearothermophillus Pseudomonas | 3.13% equivalent to 31.3 mg/mL a 3.13% a 3.13% a 10% a 30% a 50% a | [139] | |
| Neisseria gonorrhoeae | 2.5 mM c | [128] | |
| Antifungal | Candida albicans | 10 mg/mL a | [34] |
| Candida albicans | 2.5 and 5 mM c | [140] | |
| Antivirus | Vesicular stomatitis virus Herpes simplex virus type 1 Visna virus | 2 mg/mL c 2 mg/mL c 2 mg/mL c | [33] |
| Vesicular stomatitis virus | 40 µg/mL c | [141] | |
| HIV | LA as GML 40 μg/mL LA as GML 2.4 g (3 capsules), 3 times daily or 7.2 g daily | [142] | |
| [143] | |||
| Junin virus (JUNV) | 46–188 µM (IC50) | [144] |
a Minimum inhibitory concentration (MIC), b minimum bactericidal concentration (MBC), c reduction of titer, GML: glycerol mono laurate (monolaurin).
3.2.3. Other Applications of Lauric Acid
LA is a versatile oleochemical with applications across the plastics, food, and personal care industries. Additionally, it is used widely in the surfactant industry, and some surfactants derived from LA and dodecanol are antiseptic, such as dodecyl dimethyl benzyl ammonium chloride (geramine) (Figure 2(4)), dodecyl dimethyl benzyl ammonium bromide (bromo-geramine) (Figure 2(5)), and dodecyl dimethyl (2-phenoxyethyl) ammonium bromide (domifene bromide) (Figure 2(6)). In addition to these compounds, many other derivatives also are needed in the industry, such as methyl laurate (Figure 2(7)), ethyl laurate (Figure 2(8)), ethyl lauroyl arginate hydrochloride (Figure 2(9)), zinc laurate (Figure 2(10)), dodecyl aldehyde (Figure 2(11)), sodium lauryl ether sulfate (sodium laureth sulfate) (Figure 2(12)), and others [145].
In the plastics industry, LA functions as a chemical intermediate, namely, as a surfactant, either anionic or nonionic. LA can reduce surface tension between liquids and solids as an anionic and nonionic surfactant. In textile manufacturing applications, LA works well as a lubricant and process agent because it can help water mix with oil and be used as a fragrance, surfactant, and cleaning agent [146,147]. LA is a precursor to dilauroyl peroxide (Figure 2(13)), a common polymerization initiator. This compound is used as a bleaching agent, drying agent (fats, oils, and waxes), burn-out agent for acetate yarns, polymerization catalysts, and preservatives (rubber and resin industries) [147].
Saponified fatty acid (soap) is a common anionic surfactant and is used widely as a detergents and emulsifier. Among fatty-acid soaps, LA soap often is used as a body cleanser, shampoo, dishwashing detergent, and laundry detergent because of its good foaming capacity and strong detergent. Furthermore, LA is neutralized with sodium hydroxide to produce sodium lauric, which is a soap. In the personal care industry, it acts as an emulsifier for facial creams and lotions and as a base ingredient in the production of liquid and transparent soaps. LA can control the level of foam, add conditioning properties, and improve overall cleaning ability [93].
Another use of LA is as a raw material for emulsifiers in various food and beverage additives, especially in the manufacture of vegetable fats. LA is safe for usage in food production because it has non-toxic properties [123]. Moreover, it can also be observed in the animal feed industry, where it is used as a feed additive to improve gut health and digestion, thereby improving food safety and meat quality [99,148,149]. The results of previous research showed that LA supplementation in rats during lactation could improve the lactation function of rats related to increased mammary gland development and turnover of serum lipid metabolites. These findings provide a more theoretical and experimental basis for LA application in mammary gland development and the lactation function of mammals [150].
Furthermore, several researchers have researched surfactants, emulsifiers, and others to support LA applications from BSF oils or fats. Verheyen et al. [151] evaluated the possibility of using fat from three insect species (H. illucens, Locusta migratoria, and A. domesticus) for cosmetic formulations. The results indicated that insects could be an alternative source to provide derivatives of such fatty acids that are suitable for the formulation of shower gel and soaps. Their application depends on the fatty-acid profile. Generally, despite a low amount of palmitoleic acid, which is considered a great ingredient for good skin penetration, locust and cricket lipids are more suitable in cosmetics [76]. Currently, BSF oil is starting to be applied in the cosmetics industry. The plant oil formulation is integrated with BSF oil for skin care to increase skin hydration and cell rejuvenation [152]. The oil extracted from the BSF has a fatty acid profile that is perfect for skin care applications. This product is rich in omega 3s, 6s, and 9s and has LA, another fatty acid that is important for skin health. LA has antimicrobial/bacterial properties, which function to soothe inflamed skin and inhibit acne-causing bacteria [153,154]. On the other hand, since the BSFL fatty acid profile is similar to that of palm kernel and coconut oil, its lipids are suitable for shower gels and soaps.
Verheyen et al. [155] also researched the synthesis of glycine-acyl surfactants from BSF fats, coconut oil, and palm kernel oil through the Schotten–Baumann reaction. Furthermore, surfactant molecules were characterized and compared with commercial surfactants made from Amilite GCS-11® coconut oil. The results showed that glycine-acyl surfactants can be synthesized from BSF fats with performance similar to Amilite GCS-11®. Amilite GCS-11® is a mild and hypoallergenic surfactant recommended for usage in facial washes and soaps. Therefore, BSF oil-based surfactants could have similar applications. It can be concluded that BSF fats are suitable alternatives to coconut or palm kernel oil for the manufacture of glycine-acyl surfactants.
Bio-lubricants come from biological-based raw materials, namely, vegetable oils, both edible oils (palm oil, sunflower oil, rapeseed oil, olive oil, etc.) and non-edible oils (jatropha oil, castor oil, etc.), animal fats (lard, fish oil), waste cooking oil, or other environmentally friendly hydrocarbons [156]. However, the potential of insect-based bio-lubricants, especially from BSF, has not been widely explored by researchers. One of the references found is research conducted by Xiong et al. [157]. They researched the use of BSF lipids as a biodegradable lubricant additive. More than 80% of the fat composition was LA, palmitic acid, oleic acid, and linoleic acid. The BSF oil was then synthesized as the odorless sulfur BSF oil after being purified by degumming, alkaline neutralization, and absorption decolorization. The tribological character behavior has been tested as a biodegradable additive. The results show superior load-carrying capacity and better friction reduction ability compared to sulfur grease [157].
The BSF oil-based bio-lubricant will probably be on par with bio-lubricant from coconut oil or Litsea cubeba kernel oil (LCKO) because the content of medium-chain saturated fatty acids is similar, which is dominated by LA. Coconut oil (copra oil) has a high saturated fat content that allows it to oxidize slowly, and it can be used as a lubricant and transformer oil due to its high viscosity [158]. Coconut oil has even better bio-lubricant qualities than mineral oil [159,160]. Bio-lubricant (TMPE) obtained from LCKO has lubricating properties equivalent to ISO VG 20 standards. LCKO has high saturated fatty acids, and especially LA and capric acid contribute 66% of the total oil, so they are not easily oxidized. Therefore, the bio-lubricant from LCKO was known to have good oxidative properties [161]. The BSF oil, which has a fatty acid composition similar to coconut oil and LCKO, may become a candidate raw material source for bio-lubricant with equivalent performance. However, to achieve this, BSF oil-based bio-lubricant research must be continued intensively.
Fats and oils are commonly used as ingredients of various emulsion systems that have many industrial applications. An emulsion is a dispersed system that contains two immiscible liquids stabilized by an emulsifier. Research of lipid applications from BSF larvae as a carrier for drugs or bioactive compounds in the form of oil-in-water (O/W) nano-emulsions is still limited to date. A nano-emulsion is formulated by adding an emulsifier to a mixture of oil and water with the help of physical homogenization. The emulsifier is adsorbed onto the oil–water interface to reduce the interfacial tension and form dispersed droplets [162]. A nano-emulsion is a thermodynamically unstable system that requires an emulsifier to maintain stability during preparation and storage. Chou et al. [163] conducted research to develop a new nano-emulsion using BSFL oil as a lipophilic compound combined with nonionic d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and amphoteric hydrogenated lecithin (HL) as emulsifiers. Their research showed that BSF larvae oil combined with mixing TPGS and HL could form almost spherical nano-emulsions, reducing the nano-emulsion size and increasing its long-lasting storage stability by the homogenization–ultrasonication method. From that research, useful information was obtained to design BSF larva oil-based nano-emulsions that could be applied to drug, food, and cosmetic delivery systems.
Xu et al. [164] conducted a study to develop an efficient strategy for the production of monoacylglycerol (MAG) enriched with LA via enzymatic glycerolysis using BSF larvae oil. The results showed that MAG enriched with LA could be obtained by enzymatic glycerolysis from BSFL oil with high purity. The final product contained 97.7% MAG with 50.2% LA and had a lower acid value and better oxidation stability than the starting BSFL oil. This research can open opportunities to increase the added value of BSFL oil through its use for MAG enriched with LA production. MAG and its derivatives are used widely in the food, cosmetic, pharmaceutical, plastic, and textile industries.
In agriculture, BSF oil emulsion was also studied by Ruban et al. [165]. He researched the effect of BSF larvae fat emulsion on the germination power and energy of pea seeds (Pisum sativum L.). The result showed that using 0.3% weight of xanthan gum as a stabilizer for the fat emulsion of BSF larvae significantly increased the number of germinated seeds and the energy of seed sprouting. Fat of BSF larvae can be used as a pesticide carrier and for increasing seed resistance to contamination with fungi and insects during storage and sprouting [165].
4. Opportunities and Challenges
LA can be obtained from BSF lipids with a composition comparable to palm kernel oil, coconut oil, and other sources of lauric oil. The content of LA in BSF lipids can be increased by modifying the larval growth substrate. Substrates with a high carbohydrate content will increase the amount of lipids and LA in BSF larvae [1,7,14]. Additionally, the varying oil content in the substrates will determine the fatty acid composition. For example, adding fish oil to the substrate significantly increases the content of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the larvae, while adding coconut oil will increase the LA content of BSF larvae [166].
Although the LA content in BSF larvae is high, it has not been utilized as much as LA from other sources. LA has numerous applications (Figure 3), including its use as a surfactant, bio-lubricant, emulsifier, and antimicrobial agent. The abundant LA content in BSF larvae opens up possibilities for exploring it as a raw material, similar to LA derived from other sources, particularly coconut oil and palm kernel oil.
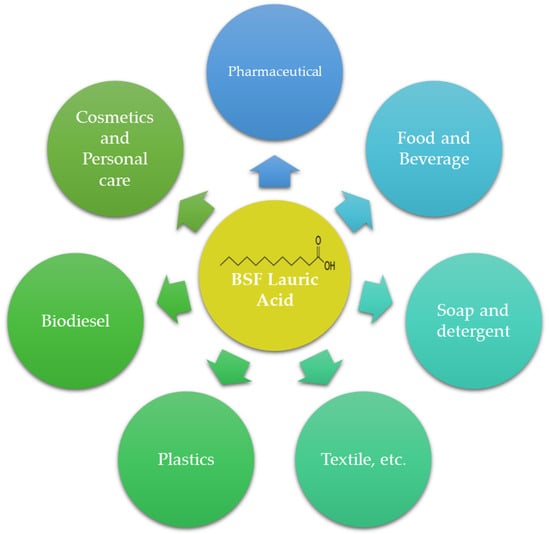
Figure 3.
Potential application of lauric acid from BSF oil.
LA can be used in combination with other fatty acids, such as in the manufacture of biodiesel, bio-lubricants, surfactants, and emulsifiers. However, for certain applications, such as those in the pharmaceutical industry, separation may be required. This is because different fatty acids possess varying antimicrobial properties, necessitating the use of pure LA.
A challenge in the utilization of LA from BSF larvae is the variability in LA content depending on the substrate they feed on. The type of organic waste, substrate composition, or substrate proximate composition determines the amount of lipid and LA obtained. Therefore, a homogeneous substrate with a consistent chemical composition may be preferable to ensure stable LA yields during production.
BSF cultivation has been implemented on large scale in Asia, America, Europe, and Africa. The challenge lies in finding an efficient and effective production process that can compete with other sources of LA. Techniques for lipid extraction, LA separation, and formulation into desired products still require exploration, particularly on a large scale. The utilization of LA from BSF larvae remains limited compared to other sources such as coconut oil and palm kernel oil. Research on their applications, such as for bio-lubricants, surfactants, emulsifiers, and others, is still relatively limited. Therefore, there are opportunities for further research on the applications of LA from BSF larvae across various fields.
Furthermore, utilizing BSF larvae as a raw material for LA offers numerous advantages. Harvest time is faster, and productivity per unit area and per year is higher compared to raw materials from vegetable oil. The feed source is derived from unused organic waste, which is continuously available so that BSF oil will be very sustainable compared to palm kernel oil. Palm kernel oil production depends on climate (e.g., rainfall and temperature), soil quality, pests, diseases, etc. Therefore, the sustainability of lauric oil from palm kernels will depend on these conditions. In addition, increasing the productivity of palm oil through the opening of new plantations has the potential to cause environmental problems, especially if the plantations originate from natural forests. Therefore, BSF larvae as a source of LA will be more sustainable and environmentally friendly. BSF cultivation also supports the circular economy and will be able to create jobs and empower the community more broadly.
Although organic waste poses challenges in every country, in regions abundant with natural resources, the production of lipids and LA from BSF larvae may receive less attention. However, for countries with limited natural resources and restricted land availability, this approach provides a solution for sustainable procurement of raw materials due to organic waste being generated consistently worldwide. Sources of organic waste are diverse and can come from household activities to industrial activities such as the food and beverage industry, the agricultural industry, animal husbandry and fisheries, hotels and restaurants, and traditional and modern markets. Thus, the availability of substrates for the growth of BSF larvae indicates enormous potential that can encourage the increase of the BSF industry.
Furthermore, several things limit the success of the BSF industry. Considering that the cultivation of BSFL is greatly influenced by abiotic factors such as light, temperature, and salinity, the development of culture technology is essential to economic efficiency. Cultivating BSFL in Asian countries with adequate light and a cool climate will allow small businesses to generate high profits without high investment [17]. However, in cold climates countries, more energy is required to adapt the culture area to optimal larval growth conditions. In addition, effective post-harvest technology and cultivation locations must approach the source of the substrate to reduce production costs so that it is feasible to cultivate on a large scale.
In light of this economic feasibility, SkyQuest published a market analysis of black soldier flies in 2022. This study investigated the potential of the BSF as a sustainable and efficient source of protein for animal feed. The study analyzed data from 20 commercial BSF farms in six countries (Brazil, Cambodia, China, Ghana, Kenya, and Vietnam) to assess the economic feasibility of large-scale production of BSF in the global BSF market. The study found that BSF can be produced profitably on a large scale at an estimated cost of USD 0.60–0.70 per kg of dry matter (DM). It makes BSF one of the most cost-effective protein sources available for animal feed [167].
The main driver of the BSF industry is the increasing global demand for protein and the need for sustainable and environmentally friendly animal feeds. Several challenges need to be overcome to realize this potential, including developing efficient and sustainable mass production methods, ensuring regulatory compliance, and building consumer confidence in product quality. Newer and more efficient technologies are being developed in the global BSF market to cultivate and process BSF. These advances will help increase production and reduce costs, making BSF products more accessible to consumers [167].
Furthermore, a search of FAOLEX, the United Nations’ Food and Agriculture Organization’s publicly available database on food regulations worldwide, yielded a single entry that specifically mentions black soldier fly (BSF). Dating to May 2017, the regulation [168] identifies seven insect species “currently reared in the Union”, including Hermetia illucens, that fulfill the safety conditions for insect production for farmed and pet animal feed, namely, “These should not be pathogenic or have other adverse effects on plant, animal or human health; they should not be recognized as vectors of human, animal or plant pathogens and they should not be protected or defined as invasive alien species”. They also place restrictions on the substrates fed to BSFL or these other species. The substrates must contain “products of non-animal origin” or a limited set of animal products that include fishmeal; rendered fats, blood, and gelatin from non-ruminants; milk; eggs; honey; etc. Flesh is not listed, and manure, “catering waste” (human food waste), and “other waste” are explicitly excluded [168]. These restrictions eliminate the risk of prion contamination of the BSFL but greatly limit its usage to close nutrient loops.
In the United States of America, animal feed is considered “food” and should be regulated by the Food and Drug Administration (FDA); however, the FDA has an official Memorandum of Understanding with the Association of American Feed Control Officers (AAFCO) for all regulations regarding animal feed [169]. The FDA and AAFCO regulate BSFL production, packaging, labeling, distribution, sale, import, and export for direct human and animal consumption, respectively. In August 2016, AAFCO approved the dried larvae of Hermetia illucens “that has been raised on a feedstock composed exclusively of feed grade materials (and which) must contain not less than 34% crude protein and 32% fat on an as-fed basis” for use in feeding salmonid fishes [170]. At this time, therefore, BSFL cannot be reared on non-feed grade substrates or fed to non-salmonids. Rearing BSFL on chicken manure and feeding them to fish or chickens or humans is thus not allowed in the USA at this time. Requiring feed-grade substrates for BSFL greatly reduces their environmental benefit; and the protein and fat floors, which were stipulated to ensure a consistent product, further limit the types of feed suitable for the larvae and, therefore, their environmental benefit [169].
In the EU, the legal status (allowed or not allowed) of the use of insect feed sources has been regulated via several regulations. In the EU, insects reared for food or feed fall under the definition of "farmed animal", which has certain consequences for the permission to use a feed (organic resource or substrate) for a farmed animal. General rules for all feed in the EU, including that for insects, are that it has to be (a) safe and (b) it does not have a direct adverse effect on the environment or animal welfare. In addition, there are requirements for feed hygiene and the maximum contents of certain undesirable substances in animal feed [171]. Therefore, more research is needed in particular to evaluate the resources of this waste to assess its potential safety risks. The relaxation of EU laws would allow these resources to be utilized optimally for BSF larvae feed.
Currently, the cosmetics industry has been using more natural and sustainable raw materials because consumers are increasingly concerned about the safety of cosmetic formulations, compositions, and origins of the raw materials that make up these products. Raw materials derived from insects have the potential to be used in cosmetic formulations because they contain bioactive compounds from their biomass, such as antimicrobial peptides, chitin, chitosan, and fatty acids. For some consumers, the idea of insect-based cosmetic products will be disgusting, but considering that bioactive compounds for cosmetics are obtained by extraction from these insects and then used as cosmetic ingredients can increase consumer acceptance.
Recently, a skincare product produced using purified BSF larval fat was patented and internationally marketed [172]. This product shows several beneficial characteristics that improve skin conditions, including smoothing, revitalizing, moisturizing, and tightening the skin. In May 2020, Insect Beauty LLC collaborated with Sibu Sea Berry Therapy, an American natural skincare brand, to launch an entovegan skincare product made from black soldier fly larvae oil [173]. This product, named Point68, is a face and neck oil combining BSF oil with organic sea buckthorn and other plant oils. It was claimed by the founder of Insect Beauty, Josh Galt, to be the first luxury skin oil product derived from insects that is commercially available on the market. The product contains 20% BSF oil. The other plant oils present in Point68 include argan, rose hips, patchouli, frankincense, lavender, and sunflower oils with functions ranging from anti-inflammatory to soothing properties, while sea buckthorn is a rich source of omega-7, a fatty acid vital to collagen production and healthy skin, hair, and nails. Galt also added that BSF insects are one of the world’s most sustainable and nutrient-rich insects because they can turn organic plant waste into nutrient-rich protein and lipids, which have one of the highest concentrations of lauric acid in the world other than coconut oil. Lauric acid is a medium-chain fatty acid that benefits skin health and healing through its powerful antimicrobial, antibacterial, and antiviral properties. With the official launching of this cosmetic product to the market, it can be proven that the government and consumers have started to well accept cosmetics derived from BSF oils.
5. Conclusions
- BSF larvae can serve as a bioconversion agent for converting organic waste into larval products rich in nutrients, including proteins and lipids.
- Numerous studies have demonstrated that the lipid and fatty acid content of BSF larvae are influenced by the growth substrate and the developmental stage of the larvae.
- Generally, the fatty acid composition of BSF larvae/prepupae is predominantly composed of LA, which is comparable in quality to the LA content found in coconut and palm kernel oil.
- Various research studies have shown that LA exhibits bioactivity as an antibacterial, antifungal, antiviral, and anticancer agent.
- LA offers numerous benefits and has found widespread applications in diverse fields, including pharmaceuticals, cosmetics, personal care, food and beverages, soap and detergent, plastics, textiles, and others.
- Potential applications can be applied as a single compound or a mixture with other fatty acids.
- Given the high LA content present in BSF larvae, which is similar to that of coconut and palm kernel oil, there is significant potential for utilizing it as a novel source for raw materials typically obtained from coconut or palm kernel oil.
Author Contributions
Conceptualization, T.S. and E.J.; methodology, T.S, E.J. and A.T.H.; validation, E.J., A.T.H., K.F. and H.A.; formal analysis, T.S. and E.J.; resources, E.J., K.F. and A.T.H.; data curation, T.S. and H.A.; writing—original draft preparation, T.S.; writing—review and editing, T.S., E.J., H.A. and K.F.; visualization, T.S.; supervision, E.J. and A.T.H.; project administration, E.J., A.T.H. and K.F.; funding acquisition, E.J. and A.T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Universitas Padjadjaran from Academic Leadership Grant No. 1549/UN6.3.1/PT.00/2023 by Ace Tatang Hidayat and Euis Julaeha. In addition, the Article Processing Charge (APC) was funded by Universitas Padjadjaran.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This research does not report any data.
Acknowledgments
The authors are grateful to the Academic Leadership Grant (ALG), Universitas Padjadjaran, and Research Center for Environmental and Clean Technology, National Research and Innovation Agency, for all research facilities.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Leong, S.Y.; Kutty, S.R.M.; Tan, C.K.; Tey, L.H. Comparative study on the effect of organic waste on lauric acid produced by Hermetia Illucens larva via bioconversion. J. Eng. Sci. Technol. 2015, 8, 52–63. [Google Scholar]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; Smet, S.D. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Amrul, N.F.; Ahmad, I.K.; Basri, N.E.A.; Suja, F.; Jalil, N.A.A.; Azman, N.A. A Review of organic waste treatment using black soldier fly (Hermetia illucens). Sustainability 2022, 14, 4565. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Bondari, K.; Sheppard, D.C. Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac. Res. 1987, 18, 209–220. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Solano, N.M.; Gutierrez, F.R.; Zurbrugg, C.T. Biological treatment of municipal organic waste using black soldier fly Maggot. Waste Biomass Valor. 2011, 2, 357–363. [Google Scholar] [CrossRef]
- Wahyuni; Dewi, R.K.; Ardiansyah, F.; Fadhlil, R.C. Maggot BSF Kualitas Fisik dan Kimianya; Litbang Pemas Unisla: Lamongan, Indonesia, 2021; pp. 9–10. [Google Scholar]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458, 312–318. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; Loon, J.J.A.V. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed. 2017, 1, 105–120. [Google Scholar] [CrossRef]
- Ishak, S.; Kamari, A. Biodiesel from black soldier fly larvae grown on restaurant kitchen waste. Environ. Chem. Lett. 2019, 17, 1143–1150. [Google Scholar] [CrossRef]
- Fajar, A. Penggunaan Eceng Gondok dan Limbah buah Terfermentasi Sebagai Media Tumbuh BSF (Black Soldier Fly) Terhadap Kualitas Tepung Maggot BSF; Skripsi, Fakultas Peternakan Universitas Islam Lamongan: Lamongan, Indonesia, 2020. [Google Scholar]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef] [PubMed]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Czekała, W. Concept of IN-OIL project based on bioconversion of by-products from food processing industry. J. Ecol. Eng. 2017, 18, 180–185. [Google Scholar] [CrossRef]
- Kim, C.-H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.-Y.; Park, K.Y.; Chung, H. Use of Black Soldier Fly Larvae for Food Waste Treatment and Energy Production in Asian Countries: A Review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Muller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch. 2017, 72, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.L.; Lognay, G.; Francis, F.; Megid, R.C. About lipid metabolism in Hermetia illucens (L. 1758): On the origin of fatty acids in prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Wong, C.-Y.; Rosli, S.-S.; Uemura, Y.; Ho, Y.C.; Leejeerajumnean, A.; Kiatkittipong, W.; Cheng, C.-K.; Lam, M.-K.; Lim, J.-W. Potential Protein and Biodiesel Sources from Black Soldier Fly Larvae: Insights of Larval Harvesting Instar and Fermented Feeding Medium. Energies 2019, 12, 1570. [Google Scholar] [CrossRef]
- Wong, H.Y.; Lim, J.W.; Lam, M.K.; Uemura, Y.; Chong, F.K.; Chew, T.L.; Mohamad, M.; Hasan, H.A. Impact of limited feed medium and different lipid extraction solvents in dealing with black soldier fly larvae. In Proceedings of the AIP Conference Proceedings of International Symposium on Green and Sustainable Technology (ISGST2019), Perak, Malaysia, 23–26 April 2019. [Google Scholar]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Gonzalez-Fernandez, M.J.; Sanchez-Muros-Lozano, M.J.; Barroso, F.G.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Kao, M.C.; Fang, J.Y.; Zouboulis, C.C.; Zang, L.; Gallo, R.L.; Huang, C.M. Antimicrobial Property of LA Against Propionibacterium acnes: Its Therapeutic Potential for Inflammatory Acne Vulgaris. J. Investig. Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect derived LA as promising alternative strategy to antibiotics in the antimicrobial resistance scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
- Herdiyati, Y.; Astrid, Y.; Shadrina, A.A.; Wiani, I.; Satari, M.H.; Kurnia, D. Potential fatty acid as antibacterial agent against oral bacteria of Streptococcus mutans and Streptococcus sanguinis from basil (Ocimum americanum): In vitro and in silico Studies. Curr. Drug Discov. Technol. 2021, 18, 532–541. [Google Scholar] [CrossRef]
- Thormar, H.; Isaacs, C.E.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef]
- Akula, S.T.; Nagaraja, A.; Ravikanth, M.; Kumar, N.G.R.; Kalyan, Y.; Divya, D. Antifungal efficacy of LA and caprylic acid—Derivatives of virgin coconut oil against Candida albicans. Biomed. Biotechnol. Res. J. 2021, 5, 229–234. [Google Scholar] [CrossRef]
- Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D.C.; Galli, G.R.; Curcio, R.; Malaguarnera, R.; Belfiore, A.; Cappello, A.R.; Maggiolini, M. The LA-activated signaling prompts apoptosis in cancer cells. Cell Death Discov. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Diener, S. Valorisation of Organic Solid Waste Using the Black Soldier Fly, Hermetia illucens, in Low and Middle-Income Countries. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2010. [Google Scholar]
- Puspita, H.; Prasetya, A.; Mulyadi, A.D. Guide to Household Organic Waste Management by Black Soldier Fly Biocon-Version; Ministry of Environment and Forestry: Jakarta, Indonesia, 2020.
- Caruso, D.; Devic, E.; Subamia, I.W.; Talamond, P.; Baras, E. Technical Handbook of Domestication and Production of Diptera Black Soldier Fly (BSF) Hermetia illucens, Stratiomyidae; PT Penerbit IPB Press: Bogor, Indonesia, 2013; pp. 2–7. [Google Scholar]
- Dortmans, B.M.A.; Diener, S.; Verstappen, B.M.; Zurbrügg, C. Black Soldier Fly Biowaste Processing—A Step-by-Step Guide; Eawag: Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2017; pp. 6–8. [Google Scholar]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected life history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Ann. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Handayani, D.; Naldi, A.; Larasati, R.R.N.P.; Khaerunnisa, N.; Budiatmaka, D.D. Management of increasing economic value of organic waste with Maggot cultivation. IOP Conf. Ser. Earth Environ. Sci. 2021, 716, 012026. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Vanlaerhoven, S.L.; Tomberlin, J.K. Relative humidity effects on the life history of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2012, 41, 971–978. [Google Scholar] [CrossRef]
- Popa, R.; Green, T. Biology and Ecology of the Black Soldier Fly; DipTerra LCCe-Book: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bullock, N.; Chapin, E.; Elder, B.; Evans, A.; Givens, M.; Jeffay, N.; Pierce, B.; Robinson, W.; Mattox, J. Implementation of Black Soldier Fly Breeding and Chicken Feed Production at Pickards Mountain Eco-Institute. Available online: https://ie.unc.edu/wp-content/uploads/sites/277/2016/03/bsfl_presentation.pdf (accessed on 6 August 2022).
- Maglangit, F.; Alosbanos, R.S. Black Soldier Fly. Encyclopedia. Available online: https://encyclopedia.pub/entry/7597 (accessed on 21 December 2022).
- Purnamasari, L.; Khasanah, H. Black Soldier Fly (Hermetia illucens) as a Potential Agent of Organic Waste Bioconversion. ASEAN J. Sci. Technol. Dev. 2022, 39, 69–83. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Ur Rehman, K.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Ebeneezar, S.; Prabu, D.L.; Tejpal, C.S.; Jeena, N.S.; Summaya, R.; Chandrasekar, S.; Sayooj, P.; Vijayagopal, P. Nutritional evaluation, bioconversion performance and phylogenetic assessment of black soldier fly (Hermetia illucens, Linn. 1758) larvae valorized from food waste. Environ. Technol. Innov. 2021, 23, 101783. [Google Scholar] [CrossRef]
- Wang, S.; Wu, L.; Li, B.; Zhang, D. Reproductive potential and nutritional composition of Hermetia illucens (Diptera: Stratiomyidae) prepupae reared on different organic wastes. J. Econ. Entomol. 2020, 113, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Aldi, M. Pengaruh berbagai media tumbuh terhadap kandungan air, protein dan lemak maggot yang dihasilkan sebagai pakan. J. Res. Innov. Anim. 2018, 2, 14–20. [Google Scholar]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Rehman, U.K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and by-products as rearing substrates for black soldier fly (Hermetia illucens) larvae: Effects on larval body composition and performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef]
- Barroso, F.G.; Haro, C.D.; Sánchez-Muros, M.J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Oonincx, D.G.; van Broekhoven, S.; van Huis, A.; Loon, J.J.V. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Rachmawati; Buchor, D.; Hidayat, P.; Hem, S.; Fahmi, M.R. Perkembangan Dan Kandungan Nutrisi Larva Hermetia illucens (Linnaeus) (Diptera: Startiomyidae) Pada Bungkil Kelapa Sawit. J. Entomol. Indon. 2010, 7, 28–41. [Google Scholar] [CrossRef]
- Wardhana, A.H. Black Soldier Fly (Hermetia illucens) sebagai Sumber Protein Alternatif untuk Pakan Ternak. Wartazoa 2016, 26, 69–78. [Google Scholar] [CrossRef]
- Purnamasari, L.; Sucipto, I.; Muhlison, W.; Pratiwi, N. Komposisi Nutrien Larva Black Soldier Fly (Hermetia illucens) dengan Media Tumbuh, Suhu dan Waktu Pengeringan yang Berbeda. In Proceedings of the Prosiding Seminar Nasional Teknologi Peternakan dan Veteriner, Bogor, Indonesia, December 2019; pp. 675–680. [Google Scholar]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 675–680. [Google Scholar] [CrossRef]
- Pliantiangtam, N.; Chundang, P.; Kovitvadhi, A. Growth performance, waste reduction efficiency and nutritional composition of black soldier fly (Hermetia illucens) larvae and prepupae reared on coconut endosperm and soybean curd residue with or without supplementation. Insects 2021, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Moula, N.; Scippo, M.L.; Douny, C.; Degand, G.; Dawans, E.; Cabaraux, J.F.; Hornick, J.L.; Medigo, R.C.; Leroy, P.; Francis, F.; et al. Performances of local poultry breed fed black soldier fly larvae reared on horse manure. Anim. Nutr. 2018, 4, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Lim, J.W.; Uemura, Y.; Chong, F.K.; Yeong, Y.F.; Mohamad, M.; Hermansyah, H. Insect-based lipid for biodiesel production. In AIP Conference Proceedings; AIP Publishing LLC.: New York, NY, USA, 2018; p. 020150. [Google Scholar]
- Alifian, M.D.; Sholikin, M.M.; Evvyernie, D.; Nahrowi. Potential Fatty Acid Composition of Hermetia illucens Oil Reared on Different Substrates. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 062002. [Google Scholar] [CrossRef]
- Giannetto, A.; Olivaa, S.; Lanes, C.F.C.; Pedron, F.D.A.; Savastano, D.; Baviera, C.; Parrino, V.; Paro, G.L.; Spano, N.C.; Cappelo, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef]
- El-Dakar, M.A.; Ramzy, R.R.; Ji, H. Influence of substrate inclusion of quail manure on the growth performance, body composition, fatty acid and amino acid profiles of black soldier fly larvae (Hermetia illucens). Sci. Total Environ. 2021, 772, 145528. [Google Scholar] [CrossRef]
- Jayanegara, A.; Gustanti, R.; Ridwan, R.; Widyastuti, Y. Fatty acid profiles of some insect oils and their effects on in vitro bovine rumen fermentation and methanogenesis. Ital. J. Anim. Sci. 2020, 19, 1310–1317. [Google Scholar] [CrossRef]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Lipid nutritional indices, regioisomeric distribution, and thermal properties of Tenebrio molitor and Hermetia illucens larvae fat. J. Asia Pac. Entomol. 2022, 25, 101951. [Google Scholar] [CrossRef]
- Yoon, C.H.; Jeon, S.H.; Ha, Y.J.; Kim, S.W.; Bang, W.Y.; Bang, K.H.; Gal, S.W.; Kim, I.S.; Cho, Y.S. Functional chemical components in Protaetia brevitarsis larvae: Impact of supplementary feeds. Food Sci. Anim. Resour. 2020, 40, 461–473. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional potential of selected insect species reared on the Island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; Loon, J.J.A.V. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 3, 500–509. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Lauric Acid. Available online: https://en.wikipedia.org/wiki/Lauric_acid (accessed on 22 May 2022).
- Levy, J. Fight Acne and Infections with Lauric Acid. Available online: https://draxe.com/nutrition/lauric-acid/ (accessed on 25 July 2022).
- Lauric Acid. Available online: https://thechemco.com/chemical/lauric-acid/ (accessed on 30 October 2022).
- Sandhya, S.; Talukdar, J.; Bhaishya, D. Chemical and Biological Properties of LA: A Review. Int. J. Adv. Res. 2016, 4, 1123–1128. [Google Scholar] [CrossRef]
- Graham, S.A.; Jose, G.P.C.; Murad, A.M.; Rech, E.L.; Cavalcanti, T.B.; Inglis, P.W. Patterns of fatty acid composition in seed oils of Cuphea, with new records from Brazil and Mexico. Ind. Crop. Prod. 2016, 87, 379–391. [Google Scholar] [CrossRef]
- Graham, S.A.; Kleiman, R. Composition of seed oils in some Latin American Cuphea (Lythraceae). Ind. Crop. Prod. 1992, 1, 31–34. [Google Scholar] [CrossRef]
- Kenar, J.A.; Moser, B.; List, G.R. Naturally occurring fatty acids: Source, chemistry, and uses. In Fatty Acids: Chemistry, Synthesis, and Applications; Ahmad, M.U., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 23–82. [Google Scholar]
- Voelker, T.A.; Hayes, T.R.; Cranmer, A.M.; Turner, J.C.; Davies, H.M. Genetic engineering a quantitative trait: Metabolic and genetic parameters influencing the accumulation of laurate in rapeseed. Plant J. 1996, 9, 229–241. [Google Scholar] [CrossRef]
- Murphy, D.J. The Status of Industrial Vegetable Oils from Genetically Modified Plants. European Chemicals Agency. Available online: https://echa.europa.eu/documents/10162/2174224/the_status_of_industrial_vegetable_oils_from_genetically_modified_plants_expert_report_en.pdf/e6b0de1b-c9d5–4e07–8fc6–8f4dae318c55 (accessed on 19 December 2022).
- Organic Lauric Acid. Available online: https://www.aryanint.com/organic-lauric-acid/ (accessed on 31 October 2022).
- Almeida, C.; Murta, D.; Nunes, R.; Baby, A.R.; Fernandes, A.; Barros, L.; Rijo, P.; Rosado, C. Characterization of lipid extracts from the Hermetia illucens larvae and their bioactivities for potential use as pharmaceutical and cosmetic ingredients. Heliyon 2022, 8, e09455. [Google Scholar] [CrossRef]
- Sujadi; Hasibuan, H.A.; Rahmadi, H.Y.; Purba, A.R. Komposisi asam lemak dan bilangan iod minyak dari sembilan varietas kelapa kelapa sawit DxP komersial di PPKS. J. Penelit. Kelapa Sawit. 2016, 24, 1–12. [Google Scholar] [CrossRef]
- Kabara, J.; Vrable, R.; Jie, M.L.K. Antimicrobial lipids: Natural and synthetic fatty acids and monoglycerides. Lipids 1977, 12, 753–759. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First Study on the Oxidative Stability and Elemental Analysis of Babassu (Attalea speciosa) Edible Oil Produced in Brazil Using a Domestic Extraction Machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef]
- Feitosa, J.M.; Silva, T.S.A.; Fonseca, A.E.X.; Rodrigues, W.C.S.; da Silva, A.C.E.; Corrêa, C.V.P.; da Aguiar, F.S.; Mourão, R.H.V.; da Oliveira, E.G.; Nunes, K.M. Evaluation of the quality of Amazonian butters as sustainable raw materials for applications in bioproducts. Rev. Ciênc. Farm. Básica Apl. 2021, 42, e708. [Google Scholar] [CrossRef]
- Dos Santos Souza, T.G.; da Silva, M.M.; Feitoza, G.S.; de Melo Alcântara, L.F.; da Silva, M.A.; de Oliveira, A.M.; de Oliveira Farias de Aguiar, J.C.R.; do Amaral Ferraz Navarro, D.M.; de Aguiar Júnior, F.C.A.; da Silva, M.V.; et al. Biological safety of Syagrus coronata (Mart.) Becc. Fixed oil: Cytotoxicity, acute oral toxicity, and genotoxicity studies. J. Ethnopharmacol. 2021, 272, 113941. [Google Scholar] [CrossRef] [PubMed]
- Oboh, F.O.; Oderinde, R.A. Fatty acid and glyceride composition of Astrocaryum vulgare kernel fat. J. Sci. Food Agric. 1989, 48, 29–36. [Google Scholar] [CrossRef]
- Hamam, F.; Daun, J.; Shahidi, F. Lipase-assisted acidolysis of high-laurate canola oil with eicosapentaenoic acid. J. Am. Oil Chem. Soc. 2005, 82, 875–879. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Zulkarnain, N.; Rosid, S.J.M.; Azid, A.; Endut, A.; Toemen, S.; Ismail, S.; Abdullah, W.N.W.; Aziz, S.M.; Yusoff, N.M.; et al. Catalytic Transesterification of Coconut Oil in Biodiesel Production: A Review. Catal. Surv. Asia 2022, 26, 129–143. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sust. Energ. Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sust. Energ. Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Mohan, K.; Sathishkumar, P.; Rajan, D.K.; Rajarajeswaran, J.; Ganesan, A.R. Black soldier fly (Hermetia illucens) larvae as potential feedstock for the biodiesel production: Recent advances and challenges. Sci. Total Environ. 2023, 859, 160235. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Lei, D.; Wang, J.; Zhang, Y. Efficient Production of Biodiesel from Esterification of LA Catalyzed by Ammonium and Silver Co-Doped Phosphotungstic Acid Embedded in a Zirconium Metal–Organic Framework Nanocomposite. ACS Omega 2020, 5, 12760–12767. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Q.; Wei, F.; Huang, J.; Feng, Y.; Ma, H.; Zhang, Y. Preparation of Copper (II) Containing Phosphomolybdic Acid Salt as Catalyst for the Synthesis of Biodiesel by Esterification. J. Oleo Sci. 2018, 67, 427–432. [Google Scholar] [CrossRef]
- Lama, S.C. 7 Benefits of Lauric Acid for Your Body. Available online: https://www.livestrong.com/article/141282-bad-effects-using-coconut-oil/ (accessed on 5 August 2022).
- Zatta, L.; Gardolinski, J.E.F.C.; Wypych, F. Raw halloysite as reusable heterogeneous catalyst for esterification of Lauric acid. Appl. Clay Sci. 2011, 51, 165–169. [Google Scholar] [CrossRef]
- Nakpong, P.; Wootthikanokkhan, S. High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renew. Energy 2010, 35, 1682–1687. [Google Scholar] [CrossRef]
- Herliana; Ilim; Simanjuntak, W.; Pandiangan, K.D. Transesterification of coconut oil (Cocos nucifera L.) into biodiesel using zeolite-A catalyst based on rice husk silica and aluminum foil. J. Phys. Conf. Ser. 2021, 1751, 012091. [Google Scholar] [CrossRef]
- Lai, P.; He, Y.; Li, J.; Xiao, Z.; Zhang, A. Optimization of Process Conditions for Extracting Litsea cubeba Kernel Oil by Microwave-Assisted Water Method. IOP Conf. Ser. Earth Environ. Sci. 2019, 332, 022051. [Google Scholar] [CrossRef]
- Zhong, S.; Cai, H.Q.; Ai, H.D. Preparation of Biodiesel from High-Acid Value Litsea cubeba Kernel Oil. Available online: https://www.researchgate.net/publication/287165652_Preparation_of_biodiesel_fromhighacid_value_litsea_cubeba_kernel_oil/citations (accessed on 7 March 2023).
- Li, D.; Feng, W.; Chen, C.; Chen, S.; Fan, G.; Liao, S.; Wu, G.; Wang, Z. Transesterification of Litsea cubeba kernel oil to biodiesel over zinc supported on zirconia heterogeneous catalysts. Renew. Energy 2021, 177, 13–22. [Google Scholar] [CrossRef]
- Bello, E.I.; Oguntuase, B.; Osasona, A.; Mohammed, T.I. Characterization and Engine Testing of Palm Kernel Oil. Eur. J. Eng. Technol. 2015, 3, 2015. [Google Scholar]
- Nguyen, H.N.; Liang, S.-H.; Doan, T.T.; Su, C.-H.; Yang, P.-Y. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Elsayed, M.; Ran, Y.; Ai, P.; Azab, M.; Mansour, A.; Jin, K.; Zhang, Y.; Abomohra, A.E. Innovative integrated approach of biofuel production from agricultural wastes by anaerobic digestion and black soldier fly larvae. J. Clean. Prod. 2020, 263, 121495. [Google Scholar] [CrossRef]
- Ishak, S.; Kamari, A.; Yusof, S.N.M.; Halim, A.L.A. Optimization of biodiesel production of Black Soldier Fly larvae rearing on restaurant kitchen waste. J. Phys. Conf. Ser. 2018, 1097, 012052. [Google Scholar] [CrossRef]
- He, S.; Lian, W.; Liu, X.; Xu, w.; Wan, W.; Qi, S. Transesterification synthesis of high-yield biodiesel from black soldier fly larvae by using the combination of Lipase Eversa Transform 2.0 and Lipase SMG1. Food Sci. Technol. 2022, 42, e103221. [Google Scholar] [CrossRef]
- Jung, S.; Jung, J.M.; Yiu Fai Tsang, Y.F.; Bhatnagar, A.; Chen, W.-H.; Lin, K.-Y.A.; Kwon, E.E. Biodiesel production from black soldier fly larvae derived from food waste by non-catalytic transesterification. Energy 2022, 238, 121700. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Li, S.Y.; Su, C.H.; Chien, C.C.; Chen, Y.J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 65–169. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Bashir, M.J.K.; Li, Q. A Circular Economy Framework based on Organic Wastes Upcycling for Biodiesel Production from Hermetia illucens. Eng. J. 2021, 25, 223–234. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jung, S.; Na, Y.-G.; Jeon, C.-H.; Cheon, H.-Y.; Yun, E.-Y.; Lee, S.-H.; Kwon, E.E.; Kim, J.-K. Biodiesel production from the black soldier fly larvae grown on food waste and its fuel property characterization as a potential transportation fuel. Environ. Eng. Res. 2022, 27, 20070. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sust. Energ Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Kim, J.K.; Yim, E.S.; Jeon, C.H.; Jung, C.S.; Han, B.H. Cold performance of various biodiesel fuel blends at low temperature. Int. J. Automot. Technol. 2012, 13, 293–300. [Google Scholar] [CrossRef]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animal feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Verified Market Research. Top 10 Black Soldier Fly Companies Feeding Nutrient-Rich Feed to Livestock. Available online: https://www.verifiedmarketresearch.com/blog/top-black-soldier-fly-companies/ (accessed on 17 April 2023).
- Joly, G.; Nikiema, J. Global Experiences on Waste Processing with Black Soldier Fly (Hermetia illucens): From Technology to Business. Resource Recovery and Reuse Series 16, CGIAR Research Program on Water, Land and Ecosystems (WLE); International Water Management Institute (IWMI): Battaramulla, Sri Lanka, 2019; ISSN 2478-0529. [Google Scholar]
- Cheng, J.Y.K.; Chiu, S.L.H.; Lo, I.M.C. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef]
- Larouche, J.; Deschamps, M.H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of Killing Methods on Lipid Oxidation, Colour and Microbial Load of Black Soldier Fly (Hermetia illucens) Larvae. Animals 2019, 9, 182. [Google Scholar] [CrossRef]
- Lauric Acid. Available online: https://www.acmehardesty.com/product/lauric-acid-c12/ (accessed on 22 May 2022).
- Galbraith, H.; Miller, T.; Paton, A.; Thompson, J. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J. Appl. Microbiol. 1971, 34, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. GRAS antimicrobial agents for cosmetic products. J. Soc. Cosmet. Chem. 1980, 31, 1–10. [Google Scholar]
- Kanetsuna, F. Bactericidal effect of fatty acids on mycobacteria, with particular reference to the suggested mechanism of intracellular killing. Microbiol. Immunol. 1985, 29, 127–141. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Karlsson, S.M.; Steingrímsson, Ó.; Thormar, H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998, 42, 2290–2294. [Google Scholar] [CrossRef]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1999, 43, 2790–2792. [Google Scholar] [CrossRef]
- Bergsson, G.; Steingrímsson, Ó.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Matsue, M.; Mori, Y.; Nagase, S.; Sugiyama, Y.; Hirano, R.; Ogai, K.; Ogura, K.; Kurihara, S.; Okamoto, S. Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transplant. 2019, 28, 1528–1541. [Google Scholar] [CrossRef]
- Auza, F.A.; Purwanti, S.; Syamsu, J.A.; Natsir, A. Antibacterial activities of black soldier flies (Hermetia illucens) extract towards the growth of Salmonella typhimurium, E. coli and Pseudomonas aeruginosa. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012024. [Google Scholar] [CrossRef]
- Harlystiarini; Mutia, R.; Wibawan, I.W.T.; Astuti, D.A. In vitro antibacterial activity of black soldier fly (Hermetia illucens) larva extracts against Gram-Negative bacteria. Bul. Peternak. 2019, 43, 125–129. [Google Scholar] [CrossRef]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef]
- Dayrit, F.M. Lauric acid is a Medium-Chain Fatty Acid, Coconut Oil is a Medium-Chain Triglyceride. Philipp. J. Sci. 2014, 143, 157–164. [Google Scholar]
- Alves, N.F.B.; de Queiroz, T.M.; Travassos, R.d.A.; Magnani, M.; Braga, V.d.A. Acute Treatment with Lauric Acid Reduces Blood Pressure and Oxidative Stress in Spontaneously Hypertensive Rats. Basic Clin. Pharmacol. Toxicol. 2017, 120, 348–353. [Google Scholar] [CrossRef]
- Gregor, A.; Auernigg-Haselmaier, S.; Trajanoski, S.; König, J.; Duszka, K. Colonic Medium-Chain Fatty Acids Act as a Source of Energy and for Colon Maintenance but Are Not Utilized to Acylate Ghrelin. Nutrients 2021, 13, 3807. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.S.; Shafie, S.A.; Faris, M.A.; Maznan, N.A.F.; Zin, N.S.N.M.; Azmi, N.A.S.; Raoof, R.A.; Myrzakozha, D.; Samsulrizal, N. Lauric acid improves hormonal profiles, antioxidant properties, sperm quality and histomorphometric changes in testis and epididymis of streptozotocin-induced diabetic infertility rats. Toxicol. Appl. Pharmacol. 2023, 470, 116558. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.W.; Batema, R.P.; Ford, L.L. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob Agents Chemother. 1996, 40, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Su’i, M.; Sumaryati, E.; Prasetyo, R.; Eric, D.P. Anti Bacteria Activities of Lauric Acid from Coconut Endosperm (Hydolysed Using Lipase Endogeneus. Adv. Environ. Biol. 2015, 9, 45–49. [Google Scholar]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Hornung, B.; Amtmann, E.; Sauer, G. LA inhibits the maturation of vesicular stomatitis virus. J. Gen. Virol. 1994, 75, 353–361. [Google Scholar] [CrossRef]
- Welch, J.L.; Xiang, J.; Okeoma, C.M.; Schlievert, P.M.; Stapleton, J.T. Glycerol Monolaurate, an Analogue to a Factor Secreted by Lactobacillus, Is Virucidal against Enveloped Viruses, Including HIV -1. mBio 2020, 11, e00686-20. [Google Scholar] [CrossRef]
- Conrado, S.D. Coconut oil in health and disease: Its and monolaurin’s potential as cure for HIV/Aids. In Proceedings of the XXXVII Cocotech Meeting, Chennai, India, 24–28 July 2002. [Google Scholar]
- Bartolotta, S.; Garcia, C.C.; Candurra, N.A.; Damonte, E.B. Effect of fatty acids on arenavirus replication: Inhibition of virus production by lauric acid. Arch. Virol. 2001, 146, 777–790. [Google Scholar] [CrossRef]
- Lauric Acid. Available online: https://www.chemicalbook.com/ChemicalProductPropertyEN_CB0357278.htm (accessed on 31 October 2022).
- Lauric Acid. Available online: https://acmechem.com/lauric-acid-98/ (accessed on 22 May 2022).
- Lauric Acid. Available online: https://haz-map.com/Agents/5046 (accessed on 30 October 2022).
- Lauric Acid Market—Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2016–2024. Available online: https://www.transparencymarketresearch.com/lauric-acid-market.html (accessed on 22 May 2022).
- Zeiger, K.; Popp, J.; Becker, A.; Hankel, J.; Visscher, C.; Klein, G.; Meemken, D. Lauric acid as feed additive—An approach to reducing Campylobacter spp. in broiler meat. PLoS ONE 2017, 12, e0175693. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, Q.; Li, F.; Yi, W.; Liu, F.; Wang, S.; Jiang, Q. Effects of Dietary Supplementation of lauric acid on Lactation Function, Mammary Gland Development, and Serum Lipid Metabolites in Lactating Mice. Animals 2020, 10, 529. [Google Scholar] [CrossRef]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Miert, S.V.; Meersman, F. Insects as an Alternative Source for the Production of Fats for Cosmetics. J. Cosmet. Sci. 2018, 69, 187–202. [Google Scholar]
- SIBU and Point68. The World’s First Insect Oil Extract in a Luxury Face Oil Launched by SIBU and Point68. Available online: https://www.prweb.com/releases/the_worlds_first_insect_oil_extract_in_a_luury_face_oil_launched_by_sibu_and_point68/prweb17530282.htm (accessed on 4 March 2023).
- Insekt. Available online: https://www.altrene.ca/whyinsects (accessed on 7 March 2023).
- Westendorp, J. Beauty Care by Bugs: Kemptville Man Scores $10K Grant to Launch Insect Oil Skin Care Business. 2020. Available online: https://www.therecord.com/local-kemptville/business/2022/09/15/beauty-care-by-bugs-kemptville-man-scores-10k-grant-to-launch-insect-oil-skin-care-business.html?itm_source=parsely-api (accessed on 7 March 2023).
- Verheyen, G.R.; Theunis, M.; Vreysen, S.; Naessens, T.; Noyen, I.; Ooms, T.; Goossens, S.; Pieters, L.; Foubert, K.; Miert, S.V. Glycine-acyl Surfactants Prepared from Black Soldier Fly Fat, Coconut Oil and Palm Kernel Oil. Curr. Green Chem. 2020, 7, 239–248. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Bio-lubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Xiong, J.; Mao, J.; Wang, T.; Feng, W.; Wang, W.; Yang, C.; Miao, X. Refining and Sulphurization of Oil from Black Soldier Fly and Its Application as Biodegradable Lubricant Additive. J. Am. Oil Chem. Soc. 2020, 97, 1243–1251. [Google Scholar] [CrossRef]
- Negi, P.; Singh, Y.; Tiwari, K. A review on the production and characterization methods of bio-based lubricants. Mater. Today Proc. 2021, 46, 10503–10506. [Google Scholar] [CrossRef]
- Mannekote, J.K.; Kailas, S.V.; Venkatesh, K.; Kathyayini, N. Environmentally friendly functional fluids from renewable and sustainable sources-a review. Renew. Sustain. Energy Rev. 2018, 81, 1787–1801. [Google Scholar] [CrossRef]
- Syahrullail, S.; Kamitani, S.; Shakirin, A. Performance of vegetable oil as lubricant in extreme pressure condition. Procedia Eng. 2013, 68, 172–177. [Google Scholar] [CrossRef]
- Cai, Z.; Zhuang, X.; Yang, X.; Huang, F.; Wang, Y.; Li, Y. Litsea cubeba kernel oil as a promising new medium-chain saturated fatty acid feedstock for bio-lubricant base oil synthesis. Ind. Crop. Prod. 2021, 167, 113564. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid. Interf. Sci. 2018, 251, 24. [Google Scholar] [CrossRef]
- Chou, T.-Z.; Nugroho, D.S.; Cheng, Y.-S.; Chang, J.-Y. Development and Characterization of Nano-emulsions Based on Oil Extracted from Black Soldier Fly Larvae. Appl. Biochem. Biotechnol. 2020, 191, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, W.; Liu, X.; He, S.; Ji, Y.; Wang, W.; Wang, F. An Effective Strategy for the Production of LA–Enriched Monoacylglycerol via Enzymatic Glycerolysis from Black Soldier Fly (Hermetia illucens) Larvae (BSFL) Oil. Appl. Biochem. Biotechnol. 2021, 193, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.A.; Novikova, M.V.; Loskutov, S.I.; Kostin, A.A. An effect of fat emulsions of black soldier fly (Hermetia illucens) larvae on the germination capacity and energy of sprouting of pea (Pisum sativum L.) seeds. Food Syst. 2021, 4, 308–314. [Google Scholar] [CrossRef]
- Hasnol, S.; Lim, J.W.; Wong, C.Y.; Lam, M.K.; Ntwampe, S.K.O. Liminal presence of exo-microbes inoculating coconut en-dosperm waste to enhance black soldier fly larval protein and lipid. Environ. Sci. Pollut. Res. 2020, 27, 24574–24581. [Google Scholar] [CrossRef]
- SkyQuest Technology Consulting Pvt. Ltd. Global Black Soldier Fly Market to Hit Sales of $1437.56 Million by 2028. Black Soldier Fly is Being Looked at as Sustainable Source of Livestock Feed Protein. 2022. Available online: https://finance.yahoo.com/news/global-black-soldier-fly-market-130500021.html?guccounter=1&guce_referer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvLmlkLw&guce_referrer_sig=AQAAAHV6ShxCFi-AmLzjglAyLiXrbbtJ6bp_y0s_0bbj1sl4Qf1TezlmSOvXJ343RrO4IpFjKzZgxVp1LShirtx1doLAamgvN5rqFK9rEY7uLfc7pOBPDQANhx9cTgC4CM3BjHCrnLcmJyKPubf3c9b-QrdnTHPzfWbDIi3RzAQySi8J#:~:text=The%20study%20found%20that%20BSF,sources%20available%20for%20livestock%20feed.2022 (accessed on 25 June 2023).
- European Commission. Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. 2017. Available online: http://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=uriserv:OJ.L_.2017.138.01.0092.01.ENG&toc=OJ:L:2017:138:TOC (accessed on 22 June 2022).
- Klonick, A. Bug Ideas: Assessing the Market Potential and Regulation of Insects. Master’s Thesis, Duke University, Durham, NC, USA, 2017. [Google Scholar]
- Association of American Feed Control Officials. Lab Methods and Services Committee Draft Agenda. In Proceedings of the AAFCO Annual Meeting Agenda and Committee Reports, Pittsburgh, PA, USA, 31 July–3 August 2016; AAFCO: Pittsburgh, PA, USA, 2016; p. 112. [Google Scholar]
- Bosch, G.; van Zanten, H.H.E.; Zamprogna, A.; Veenenbos, M.; Meijer, N.P.; van der Fels-Klerx, H.J.; van Loon, J.J.A. Conversion of organic resources by black soldier fly larvae: Legislation, efficiency and environmental impact. J. Clean. Prod. 2019, 222, 355–363. [Google Scholar] [CrossRef]
- Sangduan, C. Skincare Product Containing Hermetia illucens Extract. U.S. Patent No 15/981,689, 13 September 2018. [Google Scholar]
- Lim, G.Y. Skin-Sect Innovation: Sibu and Insect Beauty to Launch First Luxury Skin Oil Containing Insect Extract. 2020. Available online: https://Www.Cosmeticsdesign-Asia.Com/Article/2020/05/20/Skin-Sect-Innovation-Sibu-And-Insect-Beauty-To-Launch-First-Luxury-Skin-Oil-Containing-Insect-Extract (accessed on 22 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).