Chemical Treatment of Banana Blossom Peels Adsorbent as New Approach for Manganese Removal: Isotherm and Kinetic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Banana Blossom Peels Adsorbent Preparation
2.2. Characterisation of Banana Blossom Peels Powder
Zeta Potential
2.3. Batch Adsorption Experiment
2.4. Adsorption Isotherms Studies
2.5. Adsorption Kinetics Studies
2.6. Desorption Experiment
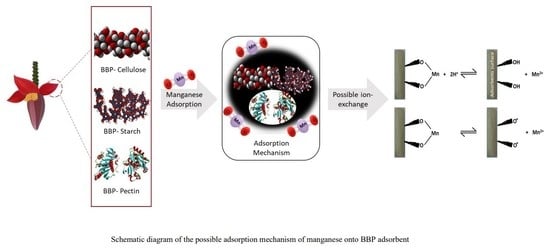
3. Results and Discussion
3.1. Characterisation of BBP Adsorbent
3.1.1. Zeta Potential
3.1.2. Surface Morphology and EDX Analysis
3.1.3. FTIR Analysis
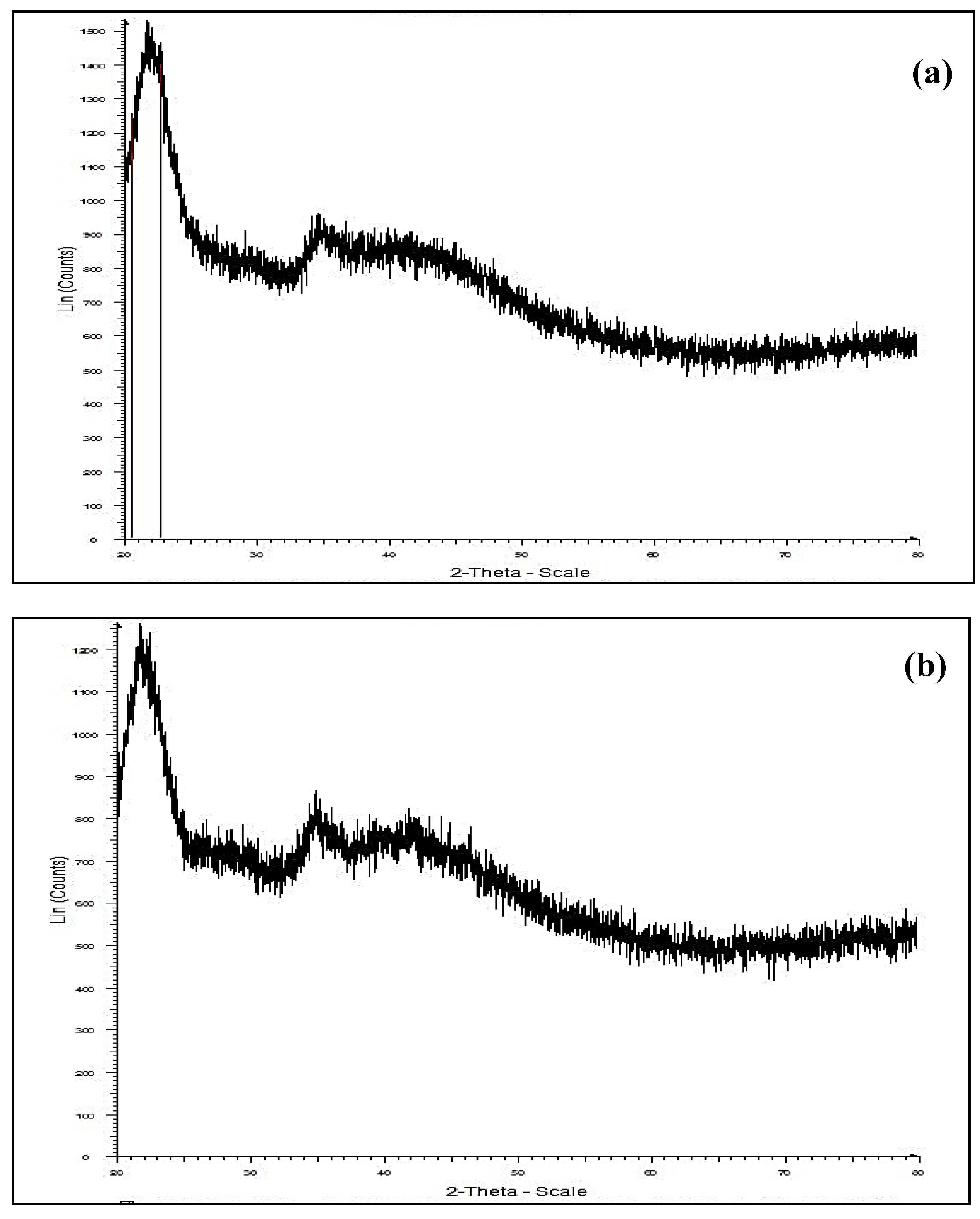
3.1.4. XRD Analysis
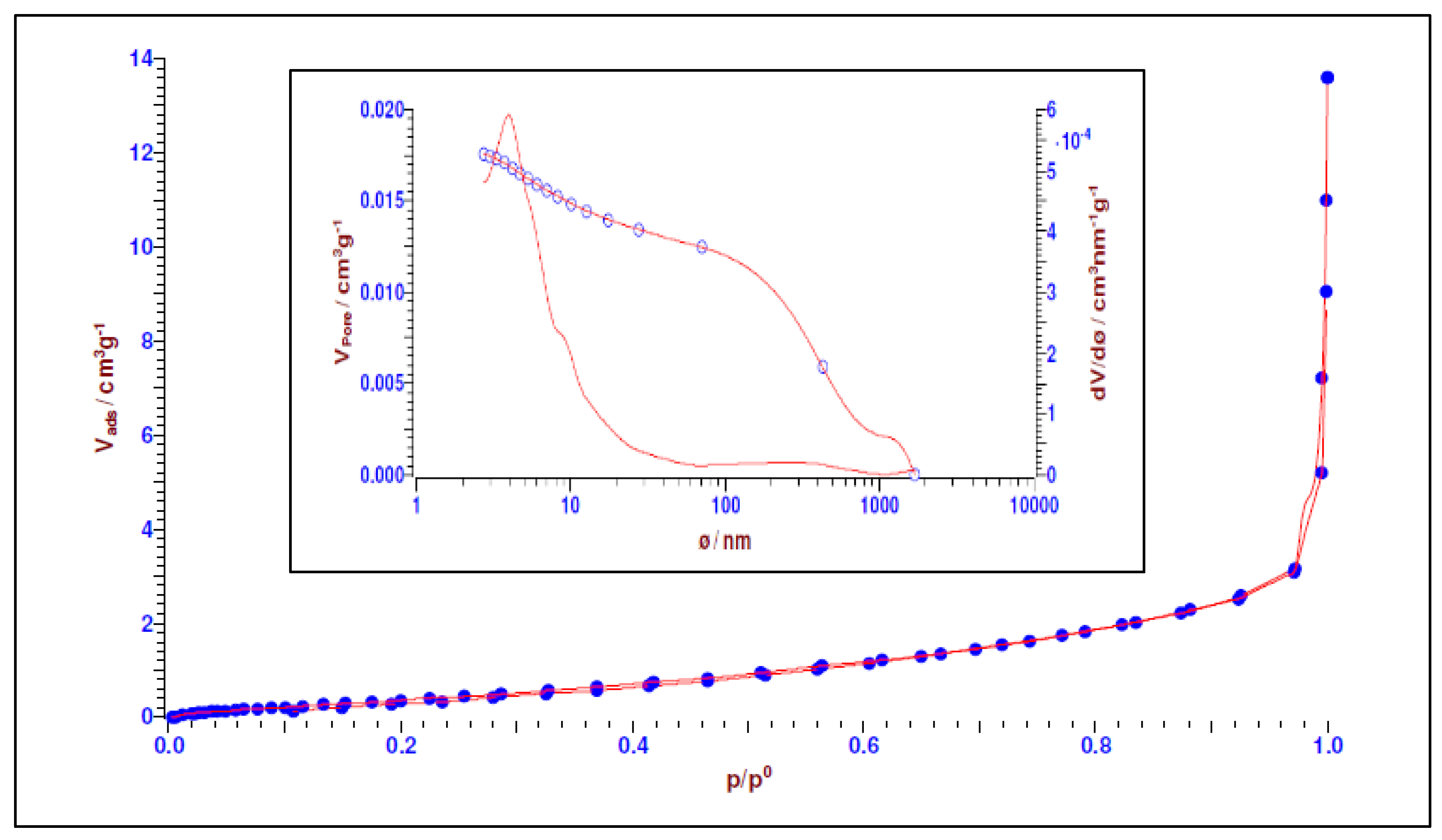
3.1.5. BET Analysis
3.2. Batch Adsorption Studies
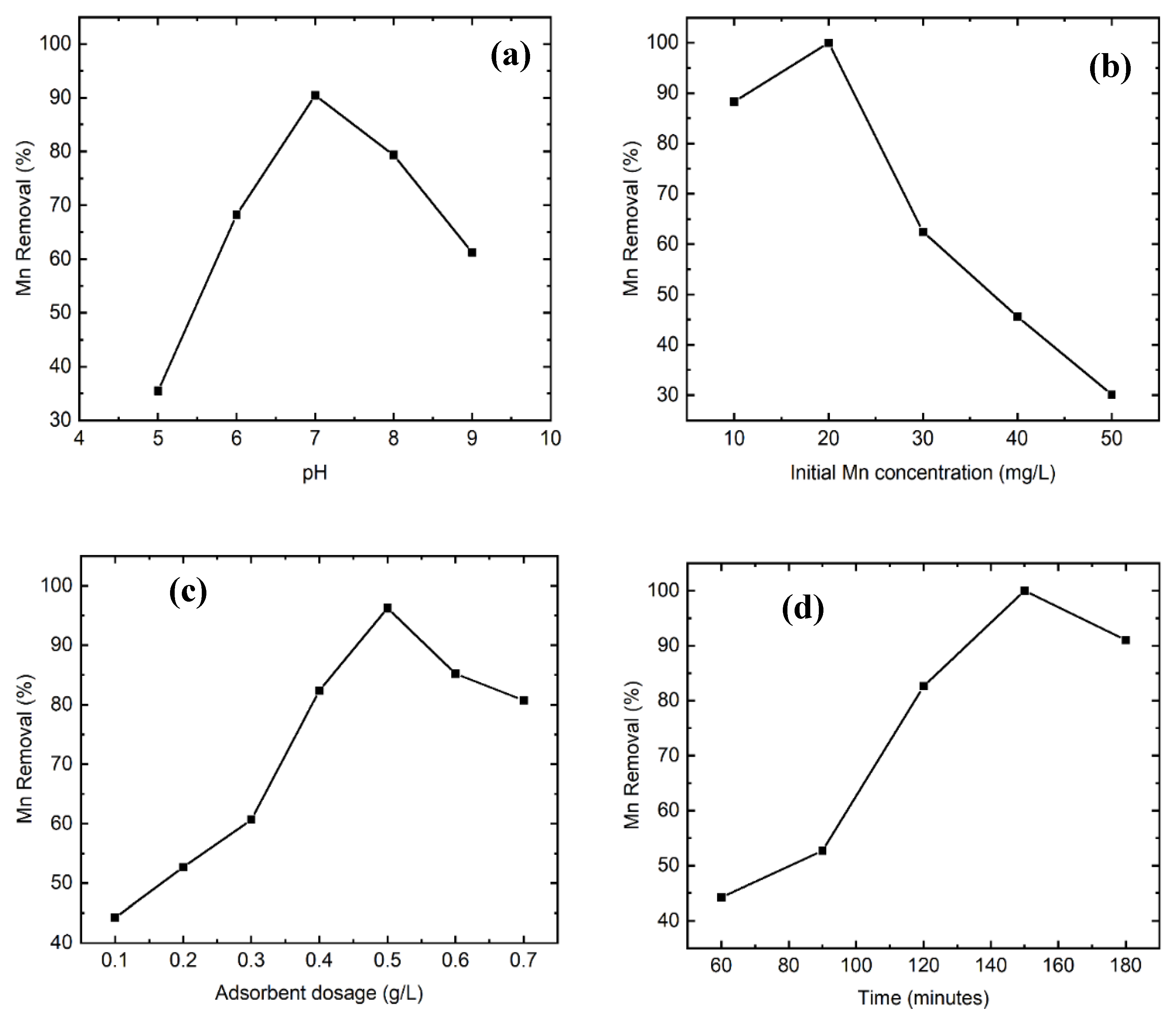
3.2.1. Effect of pH
3.2.2. Effect of Initial Manganese Concentration
3.2.3. Effect of Adsorbent Dosage
3.2.4. Effect of Contact Time
3.3. Comparison with Previous Studies on Mn Removal under Optimum Conditions
3.4. Adsorption Isotherm Studies
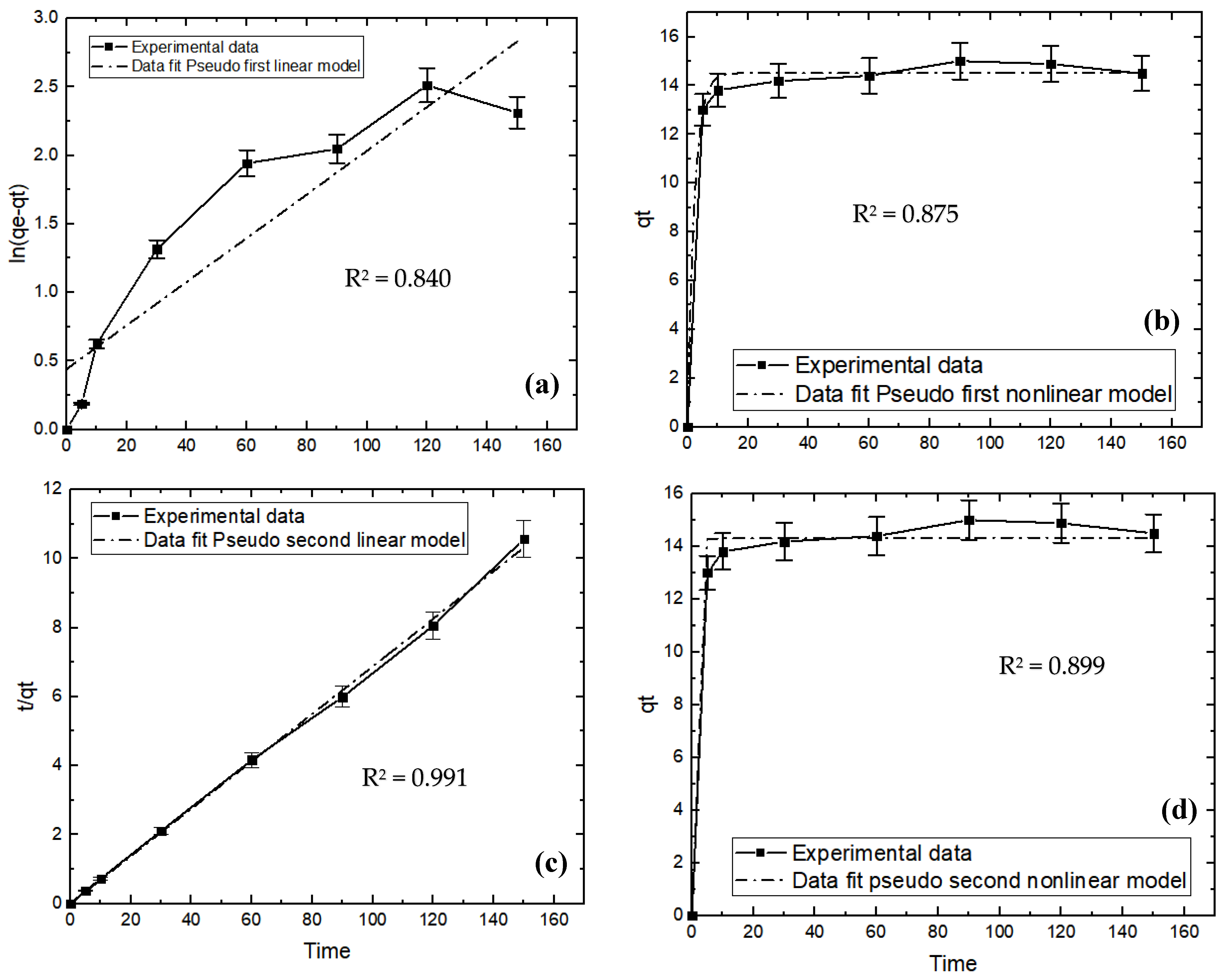
3.5. Adsorption Kinetics
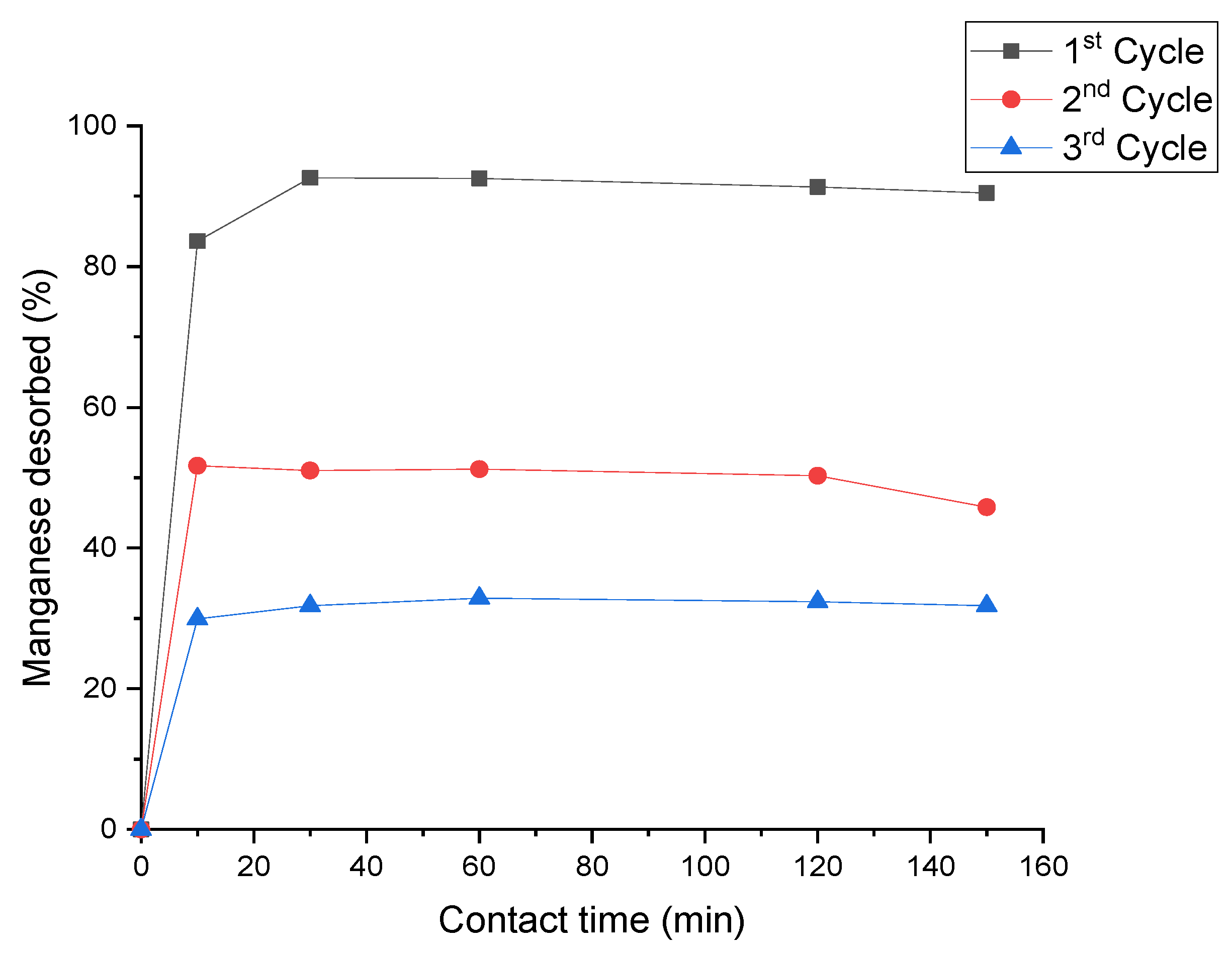
3.6. Desorption Studies
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, F.; Siwar, C.; Begum, R.A. Water resources in Malaysia: Issues and challenges. J. Food Agric. Environ. 2014, 12, 1100–1104. [Google Scholar]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Environmental Technology & Innovation Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar]
- Marsidi, N.; Abu Hasan, H.; Abdullah, S.R.S. A review of biological aerated filters for iron and manganese ions removal in water treatment. J. Water Process Eng. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Milatovic, D.; Gupta, R.C. Manganese. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 445–454. [Google Scholar]
- Tobiason, J.E.; Bazilio, A.; Goodwill, J.; Mai, X.; Nguyen, C. Manganese Removal from Drinking Water Sources. Curr. Pollut. Rep. 2016, 2, 168–177. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Surette, C.; Cormier, P.; Foucher, D. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology 2018, 64, 110–117. [Google Scholar] [CrossRef]
- Ali, A. Removal of Mn(II) from water using chemically modified banana peels as efficient adsorbent. Environ. Nanotechnol. Monit. Manag. 2017, 7, 57–63. [Google Scholar] [CrossRef]
- O’keeffe, L.; Pigat, S. Micronutrient exposure modelling: To build a refined safety assessment for micronutrients. Toxicol. Lett. 2014, 229, S119–S120. [Google Scholar] [CrossRef]
- Gerke, T.L.; Little, B.J.; Maynard, J.B. Manganese deposition in drinking water distribution systems. Sci. Total Environ. 2016, 541, 184–193. [Google Scholar] [CrossRef]
- Miah, M.R.; Ijomone, O.M.; Okoh, C.O.; Ijomone, O.K.; Akingbade, G.T.; Ke, T.; Krum, B.; da Cunha Martins, A., Jr.; Akinyemi, A.; Aranoff, N.; et al. The effects of manganese overexposure on brain health. Neurochem. Int. 2020, 135, 104688. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Sauvé, S.; Barbeau, B.; Legrand, M.; Brodeur, M.; Bouffard, T.; Limoges, E.; Bellinger, D.C.; Mergler, D. Intellectual Impairment in School-Age Children Exposed to Manganese from Drinking Water. Environ. Health Perspect. 2011, 119, 138–143. [Google Scholar] [CrossRef]
- Ozbek, N.; Akm, S. Determination of Trace Metals in Waste Water and Their Removal Processes. In Waste Water—Treatment Technologies and Recent Analytical Developments; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Holmes, S.M. Hierarchically porous zeolite X composites for manganese ion-exchange and solidification: Equilibrium isotherms, kinetic and thermodynamic studies. Chem. Eng. J. 2017, 308, 476–491. [Google Scholar] [CrossRef]
- Du, X.; Zhang, K.; Xie, B.; Zhao, J.; Cheng, X.; Kai, L.; Nie, J.; Wang, Z.; Li, G.; Liang, H. Peroxymonosulfate-assisted electro-oxidation/coagulation coupled with ceramic membrane for manganese and phosphorus removal in surface water. Chem. Eng. J. 2019, 365, 334–343. [Google Scholar] [CrossRef]
- Alvarez-Bastida, C.; Martínez-Miranda, V.; Solache-Ríos, M.; Linares-Hernández, I.; Teutli-Sequeira, A.; Vázquez-Mejía, G. Drinking water characterization and removal of manganese. Removal of manganese from water. J. Environ. Chem. Eng. 2018, 6, 2119–2125. [Google Scholar] [CrossRef]
- Jeirani, Z.; Sadeghi, A.; Soltan, J.; Roshani, B.; Rindall, B. Effectiveness of advanced oxidation processes for the removal of manganese and organic compounds in membrane concentrate. Sep. Purif. Technol. 2015, 149, 110–115. [Google Scholar] [CrossRef]
- Seyedpour, S.F.; Rahimpour, A.; Mohsenian, H.; Taherzadeh, M.J. Low fouling ultrathin nanocomposite membranes for efficient removal of manganese. J. Membr. Sci. 2018, 549, 205–216. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Ahmadi, S.; Ganjidoust, H. Using banana peel waste to synthesize BPAC/ZnO nanocomposite for photocatalytic degradation of Acid Blue 25: Influential parameters, mineralization, biodegradability studies. J. Environ. Chem. Eng. 2021, 9, 106010. [Google Scholar] [CrossRef]
- Jawed, A.; Pandey, L.M. Application of bimetallic Al-doped ZnO nano-assembly for heavy metal removal and decontamination of wastewater. Water Sci. Technol. 2019, 80, 2067–2078. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Shahraki, H.S.; Bushra, R.; Shakeel, N.; Ahmad, A.; Quratulen; Ahmad, M.; Ritzoulis, C. Papaya peel waste carbon dots/reduced graphene oxide nanocomposite: From photocatalytic decomposition of methylene blue to antimicrobial activity. J. Bioresour. Bioprod. 2023, 8, 162–175. [Google Scholar] [CrossRef]
- Liu, L.; Tan, S.; Horikawa, T.; Do, D.; Nicholson, D.; Liu, J. Water adsorption on carbon—A review. Adv. Colloid Interface Sci. 2017, 250, 64–78. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Ihsanullah; Baig, N.; Osman, A.M. Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Sep. Purif. Technol. 2018, 191, 400–423. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Herawati, D.; Santoso, S.D.; Amalina, I. Kondisi Optimum Adsorpsi-Fluidisasi Zat Warna Limbah Tekstil Menggunakan Adsorben Jantung Pisang. J. Sain Health 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, G.; Lin, J. The Physiochemical Properties and Adsorption Characteristics of Processed Pomelo Peel as a Carrier for Epigallocatechin-3-Gallate. Molecules 2020, 25, 4249. [Google Scholar] [CrossRef]
- Surovka, D.; Pertile, E. Sorption of Iron, Manganese, and Copper from Aqueous Solution Using Orange Peel: Optimization, Isothermic, Kinetic, and Thermodynamic Studies. Pol. J. Environ. Stud. 2017, 26, 795–800. [Google Scholar] [CrossRef]
- Zavala-Franco, A.; Hernández-Patlán, D.; Solís-Cruz, B.; López-Arellano, R.; Tellez-Isaias, G.; Vázquez-Durán, A.; Méndez-Albores, A. Assessing the Aflatoxin B1 Adsorption Capacity between Biosorbents Using an In Vitro Multicompartmental Model Simulating the Dynamic Conditions in the Gastrointestinal Tract of Poultry. Toxins 2018, 10, 484. [Google Scholar] [CrossRef]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Temperature dependences of IR spectra of humic substances of brown coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of food waste digestate to ash and biochar composites for high performance adsorption of methylene blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Adekola, F.A.; Hodonou, D.S.S.; Adegoke, H.I. Thermodynamic and kinetic studies of biosorption of iron and manganese from aqueous medium using rice husk ash. Appl. Water Sci. 2016, 6, 319–330. [Google Scholar] [CrossRef]
- Fathi, A.; Gahlan, A.A.; Farghaly, O.A. Decontamination of Copper and Manganese from Aqueous Media by Untreated and Chemically Treated Sugarcane Bagasse: A Comparative Study. Chem. Adv. Mater. 2020, 5, 15–33. [Google Scholar]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Deliver; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Abdeen, Z.; Mohammad, S.; Mahmoud, M. Adsorption of Mn (II) ion on polyvinyl alcohol/chitosan dry blending from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Bediako, J.K.; Sarkar, A.K.; Lin, S.; Zhao, Y.; Song, M.-H.; Choi, J.-W.; Cho, C.-W.; Yun, Y.-S. Characterization of the residual biochemical components of sequentially extracted banana peel biomasses and their environmental remediation applications. Waste Manag. 2019, 89, 141–153. [Google Scholar] [CrossRef]
- Chen, L.; Fang, Y.; Jin, Y.; Chen, Q.; Zhao, Y.; Xiao, Y.; Zhao, H. Biosorption of Cd2+by untreated dried powder of duckweedLemna aequinoctialis. Desalination Water Treat. 2013, 53, 183–194. [Google Scholar] [CrossRef]
- DeRosa, T.F. Advances in Synthetic Organic Chemistry and Methods Reported in US Patents; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Mohamed, R.M.; Hashim, N.; Abdullah, S.; Abdullah, N.; Mohamed, A.; Daud, M.A.A.; Muzakkar, K.F.A. Adsorption of Heavy Metals on Banana Peel Bioadsorbent. J. Phys. Conf. Ser. 2020, 1532, 012014. [Google Scholar] [CrossRef]
- Kim, H.; Ko, R.-A.; Lee, S.; Chon, K. Removal Efficiencies of Manganese and Iron Using Pristine and Phosphoric Acid Pre-Treated Biochars Made from Banana Peels. Water 2020, 12, 1173. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous activated carbons of low-cost agricultural bio-wastes with high adsorption capacity: Preparation and artificial neural network modeling of dye removal from single and multicomponent (binary and ternary) systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Goher, M.E.; Hassan, A.M.; Abdel-Moniem, I.A.; Fahmy, A.H.; Abdo, M.H.; El-Sayed, S.M. Removal of aluminum, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H. Egypt. J. Aquat. Res. 2015, 41, 155–164. [Google Scholar] [CrossRef]
- Maia, L.S.; Duizit, L.D.; Pinhatio, F.R.; Mulinari, D.R. Valuation of banana peel waste for producing activated carbon via NaOH and pyrolysis for methylene blue removal. Carbon Lett. 2021, 31, 749–762. [Google Scholar] [CrossRef]
- García-Mendieta, A.; Olguín, M.T.; Solache-Ríos, M. Biosorption properties of green tomato husk (Physalis philadelphica Lam) for iron, manganese and iron–manganese from aqueous systems. Desalination 2012, 284, 167–174. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Mukhtar, H.; Anwar, F.; Saari, N. RSM Based Optimization of Chemical and Enzymatic Transesterification of Palm Oil: Biodiesel Production and Assessment of Exhaust Emission Levels. Sci. World J. 2014, 2014, 526105. [Google Scholar] [CrossRef]
- Akl, M.A.; Yousef, A.M.; AbdElnasser, S. Removal of Iron and Manganese in Water Samples Using Activated Carbon Derived from Local Agro-Residues. J. Chem. Eng. Process Technol. 2013, 4. [Google Scholar] [CrossRef]
- Esfandiar, N.; Nasernejad, B.; Ebadi, T. Removal of Mn(II) from groundwater by sugarcane bagasse and activated carbon (a comparative study): Application of response surface methodology (RSM). J. Ind. Eng. Chem. 2014, 20, 3726–3736. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Ullah, H.; Hussain, Q.; Al-Wabel, M.I.; Ahmad, M.; Hussain, A.; Riaz, M.; Ok, Y.S.; Kong, J. Adsorption and thermodynamic mechanisms of manganese removal from aqueous media by biowaste-derived biochars. J. Mol. Liq. 2018, 266, 373–380. [Google Scholar] [CrossRef]
- Adeogun, A.I.; Idowu, M.A.; Ofudje, A.E.; Kareem, S.O.; Ahmed, S.A. Comparative biosorption of Mn(II) and Pb(II) ions on raw and oxalic acid modified maize husk: Kinetic, thermodynamic and isothermal studies. Appl. Water Sci. 2013, 3, 167–179. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef]
- Abdić, M.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: Tangerine peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Ahmed, S.M.; Mohammad, S.G.; Elmagd, A.M.A. Evaluation of Egyptian Banana Peel (Musa sp.) as a Green Sorbent for Groundwater Treatment. Int. J. Eng. Technol. 2014, 4, 648–659. [Google Scholar]
- Ahmed, S.A.; El-Roudi, A.M.; Salem, A.A. Removal of Mn(II) from Ground Water by Solid Wastes of Sugar Industry. J. Environ. Sci. Technol. 2015, 8, 338–351. [Google Scholar] [CrossRef]
- Kurniawati, D.; Bahrizal; Sari, T.K.; Adella, F.; Sy, S. Effect of Contact Time Adsorption of Rhodamine B, Methyl Orange and Methylene Blue Colours on Langsat Shell with Batch Methods. J. Phys. Conf. Ser. 2021, 1788, 012008. [Google Scholar] [CrossRef]
- Marques, T.L.; Alves, V.N.; Coelho, L.M.; Coelho, N.M.M. Assessment of the Use of Moringa oleifera Seeds for Removal of Manganese Ions from Aqueous Systems. Bioresources 2013, 8, 2738–2751. [Google Scholar] [CrossRef]
- Hegazy, I.; Ali, M.E.A.; Zaghlool, E.H.; Elsheikh, R. Heavy metals adsorption from contaminated water using moringa seeds/olive pomace byproducts. Appl. Water Sci. 2021, 11, 95. [Google Scholar] [CrossRef]
- Meseldžija, S.; Petrović, J.; Onjia, A.E.; Volkov-Husović, T.; Nešić, A.; Vukelić, N. Removal of Fe2+, Zn2+ and Mn2+ from the mining wastewater by lemon peel waste. J. Serb. Chem. Soc. 2020, 85, 1371–1382. [Google Scholar] [CrossRef]
- Bangaraiah, P. Biosorption of Manganese using Tamarind fruit Shell Powder as a Biosorbent. Res. J. Pharm. Technol. 2018, 11, 4313–4316. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Liu, L.; Yang, J.; Liu, S.; Zhang, T. Preparation, characterization serpentine-loaded hydroxyapatite and its simultaneous removal performance for fluoride, iron and manganese. RSC Adv. 2021, 11, 16201–16215. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: A critical review. Desalination Water Treat. 2014, 57, 518–544. [Google Scholar] [CrossRef]






| Adsorbent | Manganese Removal (%) | pH | Contact Time (min) | Adsorbent Dosage (g) | Mn Conc. (mg/L) | Reference |
|---|---|---|---|---|---|---|
| Moringa seed | 93 | 5 | 120 | 5 | 50 | Hegazy et al. [60] |
| Olive pomace | 91 | 5 | 120 | 5 | 50 | Hegazy et al. [60] |
| Biochar-banana peel | 46 | 7 | 180 | 3 | 10 | Kim et al. [43] |
| Lemon peel | 78.2 | 4 | 15 | 1 | 25 | Meseldzija et al. [61] |
| Beet pulp | 86.4 | 6 | 90 | 1 | 2 | Ahmed et al. [57] |
| Tamarind fruit shell | 74 | 3 | 60 | 1.2 | 100 | Bangaraiah [62] |
| Sugarcane Bagasse | 62.5 | 6 | 150 | 0.15 | 2 | Ahmed et al. [57] |
| BBP | 98 | 7 | 150 | 0.5 | 20 | This study |
| Model | Parameters | Non-Linear | Linear |
|---|---|---|---|
| Langmuir | qmax | 15 ± 1.14 | 15.089 ± 0.80 |
| RL | 2.14 ± 0.06 | 0.02 ± 0.12 | |
| R2 | 0.388 ± 0.07 | 0.984 ± 0.53 | |
| Freundlich | 1/n | 0.2365 ± 0.42 | 0.25 ± 0.06 |
| kf | 8 ± 0.12 | 2.294 ± 1.36 | |
| R2 | 0.516 ± 0.17 | 0.995 ± 0.60 | |
| Pseudo-first order | qe | 14.15 ± 1.39 | 62.783 ± 9.70 |
| K1 | 1.81 ± 1.03 | 2.04 ± 0.70 | |
| R2 | 0.875 ± 1.51 | 0.840 ± 1.24 | |
| Pseudo-second order | qe | 14.33 ± 0.23 | 14.547 ± 2.0 |
| K1 | 1.671 ± 1.53 | 0.881 ± 0.23 | |
| R2 | 0.899 ± 0.05 | 0.991 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudi, N.N.; Mohd Apandi, N.; Muhamad, M.S.; Mohamed Sunar, N.; Mohd Apandi, A.; Te Chuan, L.; Nagarajah, R.; Omar, S. Chemical Treatment of Banana Blossom Peels Adsorbent as New Approach for Manganese Removal: Isotherm and Kinetic Studies. Sustainability 2023, 15, 10223. https://doi.org/10.3390/su151310223
Rudi NN, Mohd Apandi N, Muhamad MS, Mohamed Sunar N, Mohd Apandi A, Te Chuan L, Nagarajah R, Omar S. Chemical Treatment of Banana Blossom Peels Adsorbent as New Approach for Manganese Removal: Isotherm and Kinetic Studies. Sustainability. 2023; 15(13):10223. https://doi.org/10.3390/su151310223
Chicago/Turabian StyleRudi, Nurul Nadia, Najeeha Mohd Apandi, Mimi Suliza Muhamad, Norshuhaila Mohamed Sunar, Affah Mohd Apandi, Lee Te Chuan, Ramathasan Nagarajah, and Suhair Omar. 2023. "Chemical Treatment of Banana Blossom Peels Adsorbent as New Approach for Manganese Removal: Isotherm and Kinetic Studies" Sustainability 15, no. 13: 10223. https://doi.org/10.3390/su151310223
APA StyleRudi, N. N., Mohd Apandi, N., Muhamad, M. S., Mohamed Sunar, N., Mohd Apandi, A., Te Chuan, L., Nagarajah, R., & Omar, S. (2023). Chemical Treatment of Banana Blossom Peels Adsorbent as New Approach for Manganese Removal: Isotherm and Kinetic Studies. Sustainability, 15(13), 10223. https://doi.org/10.3390/su151310223