Abstract
The tank goby Glossogobius giuris is a commercially important dominant fish species in the Rabnabad Channel in southern Bangladesh. However, information on the population parameters of this species is not available to support its sustainable management. Therefore, the present study was undertaken to estimate the population parameters to understand both the current status and yield, and to suggest sustainable management measures for this species, using monthly samples collected from September 2021 to August 2022. Our results showed that the size of this species at first sexual maturity was 8.5 cm in total length (TL). The gonadosomatic index indicated a prolonged spawning season, with three peaks in January–February (minor peak), April–May (minor peak), and August–November (major peak). Recruitment occurred at ~8.2 cm TL for an extended period of the year with three pulses in March (major pulse), May–June (minor pulse), and December (minor pulse). The von Bertalanffy growth parameters were TL∞ = 25.0 cm and K = 1.10 year−1. The growth performance index and longevity were 2.84 and 2.7 years, respectively. The estimated fishing, natural, and total mortalities were 0.42, 2.00, and 2.42 year−1, respectively. Therefore, the exploitation rate was 0.17, and the maximum sustainable yield was 0.37, indicating that the stock of G. giuris was not subjected to overexploitation. Hence, management intervention is not needed at this moment. Rather, a substantial amount of fishing pressure could be increased to obtain the maximum benefit.
1. Introduction
The fishing industry supports livelihoods, food security, and human health throughout the world. However, the sustainability of the fishing industry has become a global concern because of the rapid increase in fishing pressure and arbitrary exploitation. As a result, stocks are declining to levels that threaten ecosystems and societies [1]. This situation is worse, especially in developing countries where there is a lack of proper management tools and political will, and where the persistent illegal fishing of juvenile and brood fishes threatens the sustainability of fisheries [2]. Furthermore, the commoditization of fish and the pursuit of economic growth via industrialization and market expansion in developing countries drive overexploitation and may be more important than the weak institutions mentioned above [3,4]. Considering the worst-case scenario of the stocks, it is crucial to formulate effective management tools, focusing on all concerned stakeholders’ participation to promote the conservation and sustainable perpetuity of these fisheries [2,5]. An effective management plan incorporates various management tools, such as effort control, mesh size restriction for gears, allowable catch size, and seasonal closure of the fishery [6,7]. Before setting up these management tools, it is important to address the stock status first, especially the current level of exploitation and stock spawning biomass status.
Glossogobius is one of the largest genera of the Gobiidae family comprising at least 32 described species and several undescribed species [8]. The species of this genus are widely distributed in the tropical Indo-Pacific region, extending from East Africa to the Caroline Islands. Most species are benthic, riverine predators that are restricted to freshwater as adults, although a few species are found in estuaries [8]. The latter assemblage of species is suspected to be amphidromous with a marine larval stage, which accounts for their wide distribution [9]. G. giuris is widely distributed in fresh and brackish waters in the Indo-Pacific regions [10]. Fish have a special consumer preference in the diet of South Asian people because of their unique taste, low fat, and high protein content [11].
In Bangladesh, this species is primarily found in all kinds of fresh and estuarine waters and is of great importance due to consumer demand and high market value [12]. Out of the 18 gobiid species so far recorded in Bangladesh [13], the majority do not constitute an important fishery because of their small size and low consumer demand and market value. But G. giuris, which grows about a foot in length and has consumer demand and high market value, remarkably forms a fishery of some magnitude in the southern part of Bangladesh [14]. Therefore, this fish is caught in large quantities in both subsistence and artisanal fisheries in this area, including Rabnabad Channel. The latest study identified a total of 54 species from this channel [15], where G. giuris was in the fifth position based on abundance.
Numerous studies have been undertaken on this species from its major distribution areas, such as ecology and ecosystems [16,17,18,19,20,21], food and feeding habits [22,23,24,25,26,27,28,29], size relationships and growth [30,31], reproductive traits [14,16,22,24,28,32,33,34,35,36], and stock assessment [37]. However, there is no research on population parameters (e.g., reproduction, recruitment, growth, and mortality) of this species in Bangladeshi waters, which would help to estimate the maximum sustainable yield (MSY) and formulate fisheries management approaches for the resources [38]. Among the population parameters, growth and mortality help to determine the MSY, whereas information about various aspects of reproduction and recruitment helps in formulating management measures. Therefore, the aim of this study was to estimate the population parameters of G. giuris, which may provide a basis for the sustainable utilization and management of this species in the Rabnabad Channel in southern Bangladesh.
2. Materials and Methods
2.1. Study Site and Sampling
The present study was carried out in the Rabnabad Channel (21°52′ N, 90°16′ E), a vital navigational channel in the southern part of Bangladesh (Figure 1). The long channel stretching about 4 km was formed by merging the downstream of two rivers, Galachipa and Tentulia, and flowing into the Bay of Bengal. The channel is rich in aquatic resources, which play an important role in supporting the livelihoods of thousands of fishers [15]. A number of commercially important fish species, including G. giuris, are fished by small-scale artisanal fishers throughout the year. Monthly samples of the population were collected from September 2021 to August 2022 from the fishermen’s catch, who used a set bagnet to catch fish. After collection, the samples were immediately preserved in ice and then fixed with 10% formalin upon arrival at the laboratory. The collection details of G. giuris are shown in Table 1.
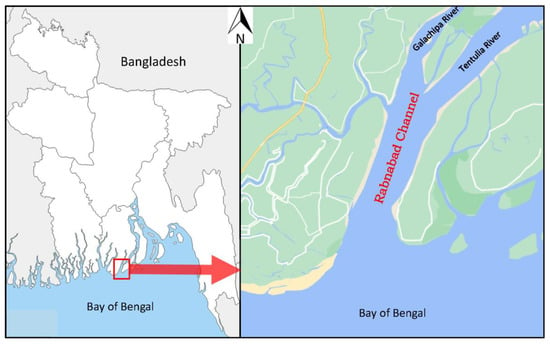
Figure 1.
Map showing the study site, Rabnabad Channel, southern Bangladesh.

Table 1.
Collection record of G. giuris from the Rabnabad Channel, southern Bangladesh.
2.2. Fish Measurement
The fixed specimens were measured for (i) total length (TL) using a measuring scale to the nearest 0.1 cm, and (ii) body weight (BW) using a digital balance (AND, FSH, Republic of Korea) to 0.01 g accuracy. Specimens were sexed by abdominal incision and visual inspection of the gonad. To estimate the parameter reproduction (e.g., length at first sexual maturity and spawning season), only female individuals were used, whereas other parameters were estimated using combined sexes.
2.3. Sexual Maturity and Spawning Season
Whole ovaries were removed from each female, and fat, connective tissue, and blood vessels were carefully removed before weighing to the nearest 0.001 g (OW). The gonadosomatic index (GSI) was calculated as follows:
GSI (%) = 100 × OW/BW
This index assumes that the gonad increases in size with development. Size at first sexual maturity was determined by the relationship between TL and GSI of all females, and the smallest female with an advanced development ovary was considered the minimum size of maturity for the population. The spawning season was estimated based on the monthly variation in GSI, and the results were correlated with monthly air temperature, rainfall, and photoperiod data obtained from World Weather Online (www.worldweatheronline.com/kuakata-weather-averages/bd.aspx, accessed on 15 February 2022) using the Spearman rank correlation test.
2.4. Recruitment Pattern
Length–frequency distribution using TL data pooled from all monthly samples of 1 cm class intervals was constructed. A series of component normal distributions were fitted to the frequency distribution using the program FiSAT II [39] based on the method by Bhattacharya [40]. Each identified normal distribution was assumed to present a distinct age group in the population. The outputs from this analysis include the mean TL and standard deviation explained by each component’s normal distribution. The mean TL of the smallest size group was considered the length at recruitment of G. giuris in the Rabnabad Channel, and individuals with mean + SD TL or smaller sizes were considered recruits in the stock [41]. The yearly recruitment pattern was determined by the occurrence of the monthly percentage of recruits.
2.5. Growth Analysis
Monthly length–frequency data using 1 cm class intervals of TL for combined sexes were inputted into the FiSAT II software [39] to estimate the growth parameters using the following von Bertalanffy [42] equation:
where Lt is the TL (cm) at age t (month), L∞ is the asymptotic TL (cm), K is the growth coefficient (year−1), and t0 is the hypothetical age when TL would be zero. First, we estimated L∞ and Z/K from the Powell–Wetherall plot [43,44] given by a linear regression equation:
where Lm = the mean length of all fish, Lʹ = the cut-off length, a = the intercept, and b = the slope. L∞ and Z/K were computed using the above equation as follows:
Lt = L∞ [1 − exp{−K(t − t0)}]
Lm–L′ = a + bL′
L∞ = a/b
Z/K = −(1 + b)/b
This initial L∞ was used as the seed value to fit the von Bertalanffy growth function (VBGF) to the length–frequency data using the ELEFAN I procedure. The best growth curve was identified based on the index of goodness-of-fit (Rn), along with the final L∞ and K values.
2.6. Growth Performance and Longevity
The growth performance index (Ø’) [45] was calculated using the estimated values of L∞ and K of the VBGF as follows:
Ø’ = log10 K + 2log10L∞
Longevity (tmax) was estimated as tmax ≈ 3/K [46,47].
2.7. Estimation of Mortality and Exploitation Rate
Total mortality (Z) was estimated using the length-converted catch curve method [48] as follows:
where N = the number of individuals of relative age (t) and Δt = time needed for the fish to pass through a length class. The slope b of the curve, with its sign changed, provides an estimate of Z [41]. Natural mortality (M) was estimated using the following formula [47]:
where T = the average annual water temperature (°C) of the habitat. Fishing mortality (F) and exploitation rate (E) were calculated from F = Z − M and E = F/Z, respectively.
ln(Nt/Δt) = a + bt
log10M = −0.006 − 0.279 log10L∞ + 0.654 log10K +0.463 log10T
2.8. Maximum Sustainable Yield
We calculated the relative yield-per-recruit (Y′/R) using the length-based Beverton and Holt [49] methods. The relative biomass per recruit (B′/R) was obtained from B′/R = (Y′/R)/F. Then, the maximum sustainable yield (Emax), the exploitation rate with the minimal increase of 10% of Y′/R (E0.1), and the exploitation rate with the reduction of stock to 50% (E0.5) were estimated using knife-edge selection [49].
3. Results
3.1. Sexual Maturity
Figure 2 illustrates the relationship between TL and GSI of female G. giuris. The lowest and highest GSIs were recorded during the study period as 0.03 and 16.97, respectively. It was found that the GSI value was relatively lower up to a female size of >8.5 cm. However, beyond this size, the GSI value rose sharply. Therefore, the size at first sexual maturity of G. giuris was considered to be 8.5 cm TL at the study site.
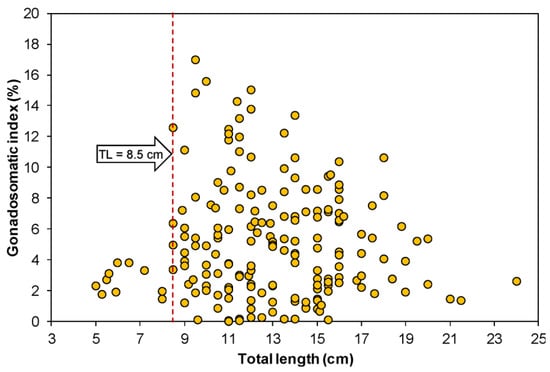
Figure 2.
Relationship between gonadosomatic index and total length (cm) of female Glossogobius giuris in the Rabnabad Channel.
3.2. Spawning Season
Figure 3 shows the monthly changes in the mean GSI of female G. giuris in relation to environmental factors. The mean GSI increased sharply in January, started to decrease afterward and declined significantly in March. The GSI again started to increase in April, remained high till May, and then began to decrease sharply until July. Thereafter, the GSI started to increase from August, remained high from September to October, and then gradually decreased in the subsequent months. Therefore, the GSI of G. giuris indicated a prolonged spawning season with three peaks in January–February (minor peak), April–May (minor peak), and August–November (major peak). There were no correlations between spawning season and monthly air temperature (rs = 0.214, p = 0.502), rainfall (rs = −0.399, p = 0.199), and photoperiod (rs = −0.392, p = 0.210).
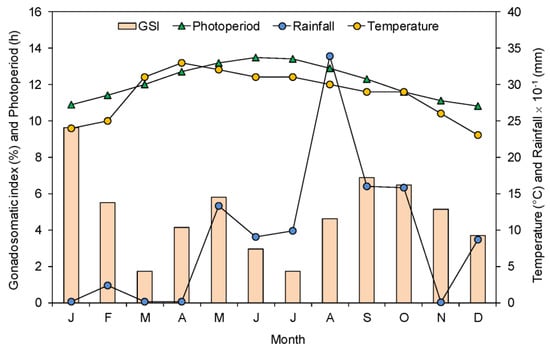
Figure 3.
Monthly changes in the mean GSI of female Glossogobius giuris in the Rabnabad Channel and average monthly variations in air temperature, rainfall, and photoperiod.
3.3. Recruitment Pattern
We detected four distinct normal distributions using the Bhattacharya [40] method, each of which represents a size group with their mean TL (Figure 4A). The calculated mean length of the smallest size group 8.2 cm TL was considered as the length at recruitment of G. giuris in the Rabnabad Channel where fishing occurred. The yearly recruitment indicated an extended pattern with three pulses in March (major pulse), May–June (minor pulse), and December (minor pulse) (Figure 4B).
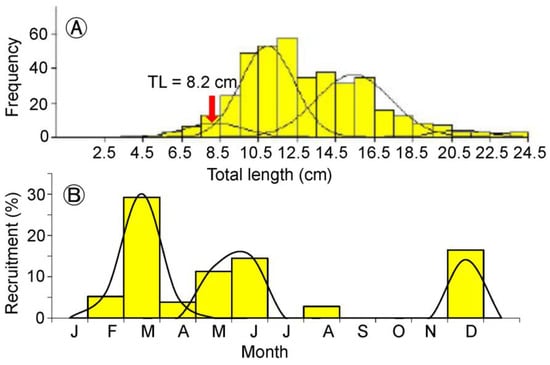
Figure 4.
Recruitment patterns with regard to (A) size and (B) month of Glossogobius giuris in the Rabnabad Channel.
3.4. Growth Analysis
The analysis of the length–frequency data by the Powell–Wetherall plot provided initial estimated values of TL∞ = 24.6 cm and Z/K = 3.71 (Figure 5). Using this initial TL∞ as the seed value, the ELEFAN I analysis yielded an optimized von Bertalanffy growth curve with the following parameters: TL∞ = 25.0 cm and K = 1.10 year−1. The third parameter of the von Bertalanffy growth function t0 was assumed to be zero [50]. The graphical outputs of the growth curve are presented in Figure 6. The estimated growth performance index and the longevity of G. giuris were 2.84 and 2.7 years, respectively.
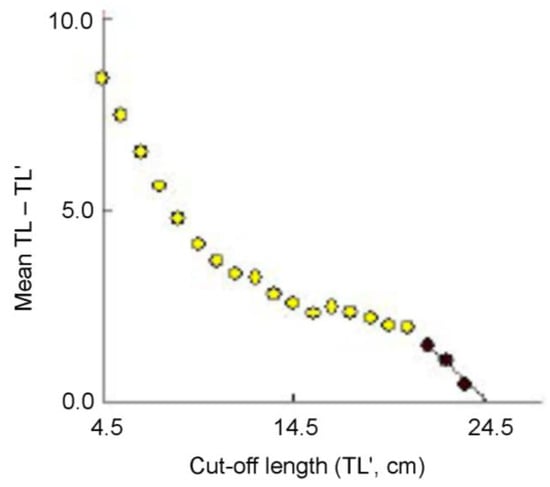
Figure 5.
A Powell–Wetherall plot for Glossogobius giuris. Solid black points are used in the regression, which provides TL∞ = 24.6 cm and Z/K = 3.71.
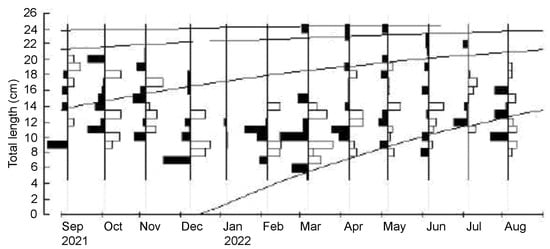
Figure 6.
The von Bertalanffy growth curve (TL∞ = 25.0 cm, K = 1.10 year−1, and Rn = 0.208) of Glossogobius giuris estimated by means of ELEFAN I superimposed on restructured length–frequency data.
3.5. Mortality and Exploitation Rate
We obtained a Z value of 2.24 year−1 from the length-converted catch curve, and the graphical outputs are presented in Figure 7. The average annual water temperature of the Rabnabad Channel is 28 °C. The calculated value of M and F were 2.00 and 0.42 year−1, respectively. Therefore, the calculated value of E for G. giuris was 0.17 in the Rabnabad Channel.
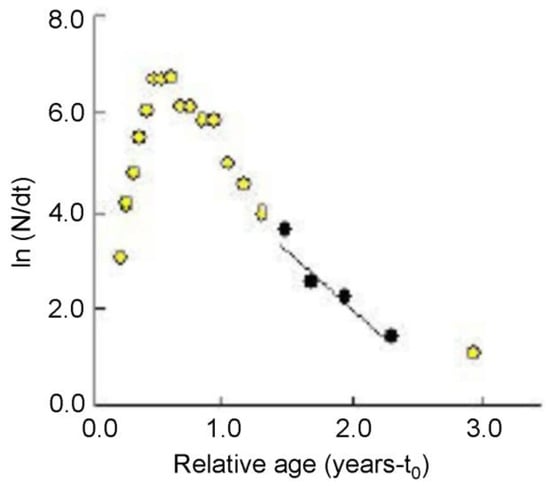
Figure 7.
Length-converted catch curves for Glossogobius giuris in the Rabnabad Channel. Data included in the regression are shown as black solid points.
3.6. Maximum Sustainable Yield
The analyses of relative yield per recruit and relative biomass per recruit showed the maximum sustainable yield Emax = 0.37, the optimum yield E0.1 = 0.31, and the yield at the stock reduction of 50% E0.5 = 0.24 (Figure 8).
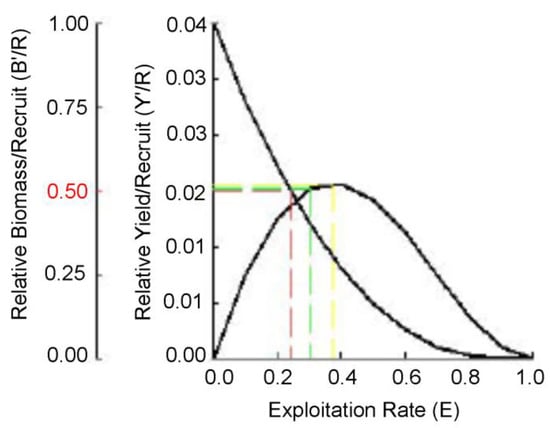
Figure 8.
Relative yield per recruit (Y′) and relative biomass per recruit (B′) of Glossogobius giuris in the Rabnabad Channel.
4. Discussion
The purpose of fisheries management is to ensure that catches from a fish stock are ecologically sustainable in the long term and that benefits to fishers and communities are maximized. To formulate explicit management measures, a fish stock needs information on its parameters, namely, reproduction (e.g., sexual maturity and spawning season), recruitment, growth, and mortality. The first two parameters were used to formulate management measures, while the latter two parameters helped in the stock assessment. Therefore, the present study attempted to provide necessary information for the fisheries management of G. giuris from Bangladeshi waters.
Diversified methods are used for the traditional data-rich and data-poor stock assessments [51]. However, the data required for traditional stock assessments are rarely available for most of the exploited fisheries, especially in developing countries [52,53]. Currently, two types of methods are commonly used in data-poor fisheries: (i) catch-based methods and (ii) length-based methods [54]. The catch-based methods require catch time series and supplementary data, e.g., intrinsic rate of increase, natural mortality, and age at maturity, whereas the length-based methods require length–frequency data. In the present study, we used electronic length frequency analysis (ELEFAN), which is one of the widely used length-based methods.
Determination of sexual maturity (Lm) is imperative for the assessment of the minimum permissible capture size of exploited stocks [55,56]. This information helps in the mesh size selection of the gear to restrict the catching of immature individuals, thus giving them an opportunity to spawn at least once in their life cycle. In our study, the size at first sexual maturity of G. giuris was found to be 8.5 cm TL based on the relationship between TL and GSI. The size at sexual maturity of some gobiid species is summarized in Table 2. We used the Lm/TL ratio to compare Lm between or within species as the total length changes with species. Our result is similar to that of a recent study by Hasan et al. [57] from Gajner Beel, Bangladesh. However, the Lm/TL ratio in the present study was lower than that in the previous study [57], indicating that this species in Rabnabad Channel matured earlier than in Gajner Beel. It can be noted that the Gajner Beel is a freshwater habitat, and the Rabnabad Channel is a coastal habitat. Therefore, the early maturation of G. giuris in the present study compared to the previous study in Gajner Beel is related to salinity as also reported by Dinh et al. [36]. The Lm of G. giuris may vary with location due to the divergent climatic and trophic parameters [58], as this value in the present study was lower than the study in Manchar Lake, Pakistan [35] and higher than the study in Mekong Delta, Vietnam [36]. The Lm/TL ratio indicates that G. giuris matured earlier than other gobies [59,60,61,62,63,64,65,66] and even earlier than its congener, G. sparsipapillus [67].

Table 2.
Size at sexual maturity in some gobiid species.
The GSI showed that G. giuris could release eggs throughout the year with three peaks: two minor peaks in January–February and April–May, and a major peak occurring in August–November. The most vital environmental factors directly or indirectly influencing the reproduction of aquatic animals are temperature, rainfall, and photoperiod [68,69,70,71,72,73]. The seasonality of these factors also influences biological cycles by ensuring larval occurrence during the periods of available food [73,74]. In our study, no significant distinct pattern of temperature, rainfall, or photoperiod was found to explain the seasonality of spawning. But if we could collect samples from at least two different sites with different environmental parameter values, we may detect significant correlations between the spawning season and environmental parameters. In fact, this was not possible in this case, as the present study was based on fishery-dependent data. However, relatively high temperatures (23–33 °C) throughout the year at our study site may cause year-round spawning of this species, as reported by several studies [38,71,72,73,74,75,76,77,78,79,80]. The spawning seasons of some gobiid species are summarized in Table 3. Dinh et al. [36] reported that G. giuris spawn throughout the year, with a peak in the late dry season for those inhabiting freshwater and in the wet season for those inhabiting brackish waters, thus salinity has a significant impact on the spawning season of this species. However, this statement does not concur with other studies on freshwater [33,34,35]. The main spawning season of G. giuris varied with location, but it can be concluded that this goby released eggs mainly from the late dry season (April) to the wet season (May–December). Comparisons among the gobies suggested that peak spawning was species-specific and varied with location.

Table 3.
Spawning season in some gobiid species.
The recruitment pattern of G. giuris showed an extended form with three pulses (a major pulse in March and two minor pulses in May–June and December) associated with three spawning peaks. Dinh et al. [37] also reported a prolonged recruitment pattern of this species with two peaks: a minor peak in March–April and a mean peak occurring in September. It seems that the variations in the recruitment time of this goby could be related to the variations in spawning time and location.
The estimated TL∞ and K values in the present study were compared with the available studies on this and other gobiid species (Table 4). In comparison with other studies of G. giuris, our TL∞ exceeded the value reported by Ahmed and Latifa [31] in Titas River, Bangladesh, and Dinh et al. [37] in Mekong Delta, Vietnam. In contrast to other gobiids, our estimated L∞ value was higher than that of some species [59,81,82,83,84], lower than that of some species [85,86], and comparable with that of some species [87,88,89,90]. These differences in asymptotic length were species-specific and varied with the differences in environmental factors across habitats. The K-value in our study was lower than the value reported by Ahmed and Latifa [31] and higher than the value reported by Dinh et al. [37]. However, compared to other gobiids, our estimated K-value was in the upper range, except for Periophthalmodon schlosseri [85] (Table 4). Generally, L∞ and K are intrinsically negatively correlated [45]. Therefore, it is recommended to use the growth performance index (Ø′) to compare growth between species rather than the comparison of L∞ and K. The growth performance index helps in better comparisons of growth between species and/or habitats [91]. Our estimated Ø′ was higher than that of other gobiid species, except for Periophthalmodon schlosseri [85]. The difference in Ø′ could be due to the variations in K and L∞ among these gobiids. The estimated longevity (tmax) of this fish was lower than that reported in previous studies [31,37], even though it was lower than that of the other gobiid species, except for Periophthalmodon schlosseri [85]. The tmax of fish can be influenced by several intrinsic and extrinsic factors.

Table 4.
Population parameters of some gobiid species.
To develop a sustainable management approach for an exploited fishery, it is imperative to know the current and desired levels of fishing mortality. Although many other matrices may regulate the fishery’s status for operational purposes, these two indices have been widely used to formulate management actions for obtaining the maximum sustainable yield by keeping the fishing pressure at a sustainable level to maintain the productivity of the stock [92]. In our study, fishing mortality (0.42 year−1) was significantly lower than the natural mortality (2.00 year−1), indicating that the survival rate depends more on the likely impact of environmental factors. Similar environmental impacts were also observed in the stock of some goby species living in the Mekong Delta, Vietnam (MDV), such as Boleophthalmus boddarti [81], Stigmatogobius pleurostigma [82], Butis koilomatodon [83], Glossogobius sparsipapillus [84], and Trypauchen vagina [89]. Our computed exploitation rate (E = 0.17) was much lower than our predicted maximum sustainable yield (Emax = 0.37), indicating that the stock of G. giuris was not subjected to overexploitation. Likewise, the stock of other goby species reported from Mekong Delta, such as Boleophthalmus boddarti [81], Stigmatogobius pleurostigma [82], Butis koilomatodon [83], Glossogobius sparsipapillus [84], Pseudapocryptes elongatus [87], and Parapocryptes serperaster [88], were not subjected to overexploitation. On the contrary, the only study [37] on this species and the studies on Glossogobius aureus [86], Trypauchen vagina [89], and Butis butis [90] stocks from Mekong Delta and Periophthalmus barbarus [59] stock from Imo River, Nigeria, were overexploited. The divergence of the fishing status of these gobiids could be related to the variations in fishing preferences.
5. Conclusions
This was a complete and rigorous study of G. giuris from Bangladeshi waters. The main purpose of this study was to assess the population parameters of G. giuris and to show how this information can support fishery managers in designing their management plans and managing the fishery. The results of the study indicate that G. giuris become mature at ~8.5 cm in total length and spawn throughout the year with three peaks with a subsequent occurrence of recruitment in three pulses. Their lifespan is ~2.7 years. The species is underexploited, and the situation does not require management intervention at present. Rather, a substantial amount (20%) of fishing pressure could be increased to obtain the maximum benefit from the fishery.
Author Contributions
Conceptualization, F.A. and Z.F.A.; methodology, F.A.; validation, F.A. and Z.F.A.; formal analysis, F.A.; investigation, M.H.R., D.R. and H.A.; resources, F.A.; data curation, M.H.R., D.R. and H.A.; writing—original draft preparation, F.A.; writing—review and editing, F.A. and Z.F.A.; visualization, F.A.; supervision, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research and Training Centre (RTC) of Patuakhali Science and Technology University (Grant No: fish-114).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated during this study are available from the corresponding authors upon reasonable request.
Acknowledgments
We acknowledge the support of the Department of Fisheries Management, Patuakhali Science and Technology University, for providing laboratory facilities. We would like to thank the local fishers for their help in sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mora, C.; Myers, R.A.; Coll, M.; Libralato, S.; Pitcher, T.J.; Sumaila, R.U.; Zeller, D.; Watson, R.; Gaston, K.J.; Worm, B. Management effectiveness of the World’s marine fisheries. PLoS Biol. 2009, 7, e1000131. [Google Scholar] [CrossRef] [PubMed]
- Jabado, R.W.; Spaet, J.L.Y. Elasmobranch fisheries in the Arabian Seas region: Characteristics, trade and management. Fish Fish. 2017, 18, 1096–1118. [Google Scholar] [CrossRef]
- Mansfield, B. “Modern” industrial fisheries and the crisis of overfishing. In Global Political Ecology; Peet, R., Robbins, P., Watts, M., Eds.; Routledge: London, UK, 2011; pp. 84–99. [Google Scholar]
- Pitcher, T.J.; Lam, M.E. Fish commoditization and the historical origins of catching fish for profit. Marit. Stud. 2015, 14, 2. [Google Scholar] [CrossRef]
- Jabado, R.W.; Al Ghais, S.M.; Hamza, W.; Shivji, M.S.; Henderson, A.C. Shark diversity in the Arabian/Persian Gulf higher than previously thought: Insights based on species composition of shark landings in the United Arab Emirates. Mar. Biodivers. 2015, 45, 719–731. [Google Scholar] [CrossRef]
- Ye, Y.; Cochrane, K.; Bianchi, G.; Willmann, R.; Majkowski, J.; Tandstad, M.; Carocci, F. Rebuilding global fisheries: The World Summit goal, costs and benefits. Fish Fish. 2013, 14, 174–185. [Google Scholar] [CrossRef]
- Bell, J.D.; Watson, R.A.; Ye, Y. Global fishing capacity and fishing effort from 1950 to 2012. Fish Fish. 2017, 18, 489–505. [Google Scholar] [CrossRef]
- Hoese, D.F.; Allen, G.R. Descriptions of three new species of Glossogobius (Teleostei: Gobiidae) from New Guinea. Zootaxa 2015, 3986, 201–216. [Google Scholar] [CrossRef]
- Hoese, D.F.; Allen, G.R. A review of the amphidromous species of the Glossogobius celebius complex, with description of three new species. Cybium 2012, 35, 269–284. [Google Scholar]
- Riede, K. The ‘‘Global register of migratory species”—First results of global GIS analysis. In Biological Resources and Migration; Werner, D., Ed.; Springer: Berlin, Germany, 2004; pp. 211–218. [Google Scholar]
- Islam, M.N.; Joadder, M.A.R. Seasonal variation of the proximate composition of freshwater gobi, Glossogobius giuris (Hamilton) from the river Padma. Pak. J. Biol. Sci. 2005, 8, 532–536. [Google Scholar]
- Islam, M.S.; Tuly, D.M.; Hasnahena, M.; Bahadur, P.; Hassan, M.R. Induced breeding of freshwater Goby, Glossogobius giuris (Hamilton, 1822) in the captivity: A preliminary study. J. Fish. Aquat. Sci. 2014, 9, 24–32. [Google Scholar] [CrossRef]
- Rahman, A.K.A. Freshwater Fishes of Bangladesh; Zoological Society of Bangladesh: Dhaka, Bangladesh, 1989. [Google Scholar]
- Latifa, G.A.; Ahmed, A.T.A.; Ahmed, M.S.; Rahman, M.M.; Asaduzzaman, M.; Obaida, M.A.; Hossain, M.M.; Biswas, A.R. Fishes of Gobiidae family, recorded from the rivers and estuaries of Bangladesh: Some morphometric and meristic studies. Bangladesh J. Zool. 2015, 43, 157–171. [Google Scholar] [CrossRef]
- Rahman, M.B.; Hoque, M.S.; Hasan, M.M. Selectivity of fishing gears and their effects on fisheries diversity of Rabnabad Channel of Patuakhali district in Bangladesh. Acad. Res. Int. 2015, 6, 184–196. [Google Scholar]
- Tandon, K.K. Biology of Channa panctatus (Bloch) and Glossogobius giuris (Hamilton). Res. Bull. Punjab Univ. 1962, 13, 257–262. [Google Scholar]
- Bhowmick, R.M. Studies on some aspects of the biology of Glossogobius giuris (Hamilton) with notes on the fishery in the Hooghly estuary. In Proceedings of the Indo-Pacific Fisheries Council, Kuala Lumpur, Malaysia, 16–31 October 1964; Volume 11, pp. 99–115. [Google Scholar]
- Doha, S. Investigation into the biology of the goby, Glossogobius giuris (Hamilton-Buchanan). Bangladesh J. Zool. 1974, 2, 95–106. [Google Scholar]
- Allen, G.R. Freshwater Fishes of Australia; T.F.H. Publications, Inc.: Neptune City, NJ, USA, 1989. [Google Scholar]
- Islam, M.N. Eco-biology of freshwater gobi, Glossogobius giuris (Hamilton) in relation to its fishery: A review. J. Biol. Sci. 2004, 4, 780–793. [Google Scholar]
- Nguyen, N.T.; Nguyen, V.Q. Biodiversity and Living Resources of the Coral Reef Fishes in Vietnam Marine Waters; Science and Technology Publishing House: Hanoi, Vietnam, 2006. [Google Scholar]
- Hora, S.L. Ecology and bionomics of the gobioid fishes of Gangetic delta. Congr. Int. Zool. 1936, 2, 841–865. [Google Scholar]
- Mookerjee, H.K. On the food of Glossogobius giuris (Hamilton). Sci. Cult. 1947, 13, 162–163. [Google Scholar]
- Alikunhi, K.H.; Rao, G.L.; Jacob, P.K. Bionomics and development of Glossogobius giuris (Hamlton). J. Madras Univ. 1951, 21, 238–248. [Google Scholar]
- Das, S.M.; Moitra, S.K. Studies on the food of some common fishes of Uttar Pradesh, India. Part I. Proc. Natl. Acad. Sci. USA 1955, 25, 1–6. [Google Scholar]
- Karamchandrani, S.J. On the occurrence of associates of carp fry in the fry collection nets and the destructive role played by predatory fish. Indian J. Fish. 1957, 4, 47–61. [Google Scholar]
- Datta Munshi, J.S.; Singh, O.N.; Singh, D.K. Food and feeding relationship of certain aquatic animals in Ganga ecosystem. Trop. Ecol. 1990, 31, 138–144. [Google Scholar]
- Rao, L.M.; Rao, P.S. Food and feeding habits of Glossogobius giuris from Gosthani estuary. Indian J. Fish. 2002, 49, 35–40. [Google Scholar]
- Talde, C.M.; Mamaril, A.C.; Palomares, M.L.D. The diet composition of some economically important fishes in the three floodplain lakes in Agusan Marsh wildlife sanctuary in the Philippines. Sri Lanka J. Aquat. Sci. 2004, 9, 45–56. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Ohtomi, J.; Ahmed, Z.F.; Ibrahim, A.H.M.; Jasmine, S. Length-weight and morphometric relationships of the tank goby Glossogobius giuris (Hamilton, 1822) (Perciformes: Gobiidae) in the Ganges of Northwestern Bangladesh. Asian Fish. Sci. 2009, 22, 961–969. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Latifa, G.A. Determination of the age and growth of Glossogobius giuris (Hamilton-Buchanan, 1822) using sectioned otolith. Bangladesh J. Zool. 2012, 40, 13–19. [Google Scholar] [CrossRef]
- Willey, A. Notes on the freshwater fishing of Ceylon. Spol. Zeyl. 1911, 7, 85–105. [Google Scholar]
- Hossain, M.S. Reproductive characteristics of Bele, Glossogobius giuris from Mithamoin Haor, Kissorgonj, Bangladesh. World J. Fish Mar. Sci. 2014, 6, 537–543. [Google Scholar]
- Roy, A.; Hossain, M.S.; Rahman, M.L.; Salam, M.A.; Ali, M.M. Fecundity and gonadosomatic index of Glossogobius giuris (Hamilton, 1822) from the Payra River, Patuakhali, Bangladesh. J. Fish. 2014, 2, 141–147. [Google Scholar] [CrossRef]
- Qambrani, G.R.; Soomro, A.N.; Palh, Z.A.; Baloch, W.A.; Tabasum, S.; Lashari, K.H.; Qureshi, M.A. Reproductive biology of Glossogobius giuris (Hamilton), in Manchar Lake Sindh, Pakistan. J. Aquacul. Res. Devel. 2016, 7, 392–394. [Google Scholar]
- Dinh, Q.M.; Truong, N.T.; Tran, N.S.; Nguyen, T.H.D. Ovarian and spawning reference, size at first maturity and fecundity of Glossogobius giuris caught along Vietnamese Mekong Delta. Saudi J. Biol. Sci. 2022, 29, 1911–1917. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Phan, Y.N.; Tran, D.D. Population biology of the goby Glossogobius giuris (Hamilton 1822) caught in the Mekong Delta. Asian Fish. Sci. 2017, 30, 26–37. [Google Scholar] [CrossRef]
- Ahamed, F.; Ahmed, Z.F.; Ohtomi, J. Estimation of key population parameters of Penaeus indicus H. Milne Edwards, 1837 (Crustacea: Penaeidae) in the Andharmanik River, southern Bangladesh: Implications for sustainable management. Nauplius 2022, 30, e2022015. [Google Scholar] [CrossRef]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. The FAO-ICLARM Stock Assessment Tools (FiSAT) User’s Guide; Food and Agricultural Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Bhattacharya, C.G. A simple method of resolution of a distribution into Gaussian components. Biometrics 1967, 23, 115–135. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment, and Management; Fishing News Books: London, UK, 1995; 341p. [Google Scholar]
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws, II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Powell, D.G. Estimation of mortality and growth parameters from the length frequency of a catch [model]. Rapp. Proces-Verbaux Des Reun. 1979, 175, 167–169. [Google Scholar]
- Wetherall, J.A. A new method for estimating growth and mortality parameters from length-frequency data. Fishbyte 1986, 4, 12–14. [Google Scholar]
- Pauly, D.; Munro, J.L. Once more on the comparison of growth in fish and invertebrates. Fishbyte 1984, 2, 21. [Google Scholar]
- Taylor, C.C. Cod growth and temperature. J. Mar. Sci. 1958, 23, 366–370. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Pauly, D. Some Simple Methods for Assessment of Tropical Fish Stocks; FAO Fisheries Technical Paper: Roma, Italy, 1983; Volume 234, p. 52. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment: Part II. Tables of Yield Function; FAO Fisheries Biology Technical Paper: Roma, Italy, 1966; Volume 38. [Google Scholar]
- Pauly, D.; David, N. ELEFAN I, Basic programme for the objective extraction of recruitment pattern from length frequency data. Meeresforsch 1981, 27, 201–210. [Google Scholar]
- Maunder, M.N.; Punt, A.E. A review of integrated analysis in fisheries stock assessment. Fish. Res. 2013, 142, 61–74. [Google Scholar] [CrossRef]
- Graaf, G.D.; Bartley, D.; Jorgensen, J.; Marmulla, G. The scale of inland fisheries, can we do better? Alternative approaches for assessment. Fish. Manag. Ecol. 2015, 22, 64–70. [Google Scholar] [CrossRef]
- Prince, J.; Hordyk, A. What to do when you have almost nothing: A simple quantitative prescription for managing extremely data-poor fisheries. Fish Fish. 2019, 20, 224–238. [Google Scholar] [CrossRef]
- Liang, C.; Xian, W.; Liu, S.; Pauly, D. Assessments of 14 exploited fish and Invertebrate stocks in Chinese waters using the LBB method. Front. Mar. Sci. 2020, 7, 314. [Google Scholar] [CrossRef]
- Lucifora, L.O.; Valero, J.L.; Garcia, V.B. Length at maturity of the green-eye spurdog shark, Squalus mitsukuii (Elasmobranchii: Squalidae) from the SW Atlantic, with comparisons with other regions. Mar. Freshw. Res. 1999, 50, 629–632. [Google Scholar] [CrossRef]
- Vitale, F.; Svedang, H.; Cardinale, M. Histological analysis invalidates macroscopically determined maturity ogives of the Kattegat cod (Gadus morhua) and suggests new proxies for estimating maturity status of individual fish. ICES J. Mar. Sci. 2006, 63, 485–492. [Google Scholar] [CrossRef]
- Hasan, M.R.; Hossain, M.D.; Mawa, Z.; Tanjin, S.; Rahman, M.A.; Sarkar, U.K.; Ohtomi, J. Evaluating the size at sexual maturity for 20 fish species (Actinopterygii) in wetland (Gajner Beel) ecosystem, north-western Bangladesh through multi-model approach: A key for sound management. Acta Ichthyol. Piscat. 2015, 51, 29–36. [Google Scholar] [CrossRef]
- Sinovcic, G.; Zorica, B. Reproductive cycle and minimum length at sexual maturity of Engraulis encrasicolus (L.) in the Zarmanja River estuary (Adriatic Sea, Croatia). Estuar. Coast. Shelf Sci. 2006, 69, 439–448. [Google Scholar] [CrossRef]
- Etim, L.; King, R.P.; Udo, M.T. Breeding, growth, mortality and yield of the mudskipper Periophthalmus barbarus (Linneaus 1766) (Teleostei: Gobiidae) in the Imo River estuary, Nigeria. Fish. Res. 2002, 56, 227–238. [Google Scholar] [CrossRef]
- Tran, D.D. Some Aspects of Biology and Population Dynamics of the Goby Pseudapocryptes elongatus (Cuvier, 1816) in the Mekong Delta. Ph.D. Thesis, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia, 2008. [Google Scholar]
- Dinh, Q.M.; Nguyen, T.T.G.; Nguyen, T.K.T. Reproductive biology of the mudskipper Boleophthalmus boddarti in Soc Trang. Tap. Chi. Sinh. Hoc. 2005, 37, 362–369. [Google Scholar]
- Dinh, Q.M.; Qin, J.G.; Dittmann, S.; Tran, D.D. Reproductive biology of the burrow dwelling goby Parapocryptes serperaster. Ichthyol. Res. 2016, 63, 324–332. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Tran, N.T.T. Reproductive biological traits of the goby Stigmatogobius pleurostigma (Bleeker, 1849) from the Mekong Delta, Vietnam. Indian J. Fish. 2018, 65, 20–25. [Google Scholar] [CrossRef]
- Dinh, Q.M. Aspects of reproductive biology of the red goby Trypauchen vagina (Gobiidae) from the Mekong Delta. J. Appl. Ichthyol. 2018, 34, 103–110. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Tran, L.T.; Ngo, N.C.; Pham, T.B.; Nguyen, T.T.K. Reproductive biology of the unique mudskipper Periophthalmodon septemradiatus living from estuary to upstream of the Hau River. Acta Zool. 2020, 101, 206–217. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Lam, T.T.H.; Nguyen, T.H.D.; Nguyen, T.M.; Nguyen, T.T.K.; Nguyen, N.T. First reference on reproductive biology of Butis koilomatodon in Mekong Delta, Vietnam. BMC Zool. 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Nguyen, H.D.T.; Nguyen, T.T.H.; Tran, C.C.; Nguyen, T.N.Y.; Dinh, Q.M. Ovarian development, spawning characteristics, size at first mature and fecundity of Glossogobius sparsipapillus (Gobiiformes: Gobiidae) living along estuarine and coastal regions in the Mekong Delta, Vietnam. Acta Zool. Bul. 2021, 73, 253–260. [Google Scholar]
- Pinheiro, M.A.A.; Hebling, N.J. Biologia de Macrobrachium rosenbergii (De Man, 1879). In Carcinicultura de Água Doce. Tecnologia Para Produção de Camarões; Valenti, W.C., Ed.; IBAMA: Brasilia, Brazil, 1998; pp. 21–46. [Google Scholar]
- Huang, K.-H.; Wu, J.-P.; Wang, S.-Y.; Huang, D.-J.; Chen, H.-C. Ovarian development in the freshwater prawn Macrobrachium asperulum (Decapoda: Palaemonidae). J. Crustac. Biol. 2010, 30, 615–623. [Google Scholar] [CrossRef]
- Ahamed, F.; Saha, N.; Ahmed, Z.F.; Hossain, M.Y.; Ohtomi, J. Reproductive biology of Apocryptes bato (Gobiidae) in the Payra River, southern Bangladesh. J. Appl. Ichthyol. 2018, 34, 1169–1175. [Google Scholar] [CrossRef]
- Ahmed, Z.F.; Ahamed, F.; Rahman, M.M.; Fatema, M.K. Spawning season, recruitment, and growth of the freshwater prawn Macrobrachium lamarrei (H. Milne-Edwards, 1837) in a perennial wetland, northeastern Bangladesh. Nauplius 2021, 29, e2021021. [Google Scholar] [CrossRef]
- Ahamed, F.; Baroi, P.; Ahmed, Z.F.; Ohtomi, J. Reproductive biology of the palaemonid prawn Macrobrachium villosimanus (Tiwari, 1949) (Decapoda: Caridea: Palaemonidae). J. Crust. Biol. 2022, 42, ruac041. [Google Scholar] [CrossRef]
- Lawrence, A.J. Environmental and endocrine control of reproduction in two species of polychaete: Potential bio-indicators for global climate change. J. Mar. Biol. Assoc. UK 1996, 76, 247–250. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Soame, J.M. The effects of climate change on the reproduction of coastal invertebrates. Ibis 2004, 146, 29–39. [Google Scholar] [CrossRef]
- Kikuchi, T. An ecological study on animal communities of the Zostera marina belt in Tomioka Bay, Amakusa, Kyushu. Publ. Amakusa. 1966, 1, 1–106. [Google Scholar]
- Allen, J.A. The dynamics and interrelationships of mixed populations of Caridea found off north-east coast of England. In Some Contemporary Studies of Marine Science; Barnes, H.B., Ed.; Allen and Unwin: London, UK, 1966; p. 4566. [Google Scholar]
- Wear, R.G. Incubation in British decapod Crustacea, and the effects of temperature on the rate and success of embryonic development. J. Mar. Biol. Assoc. UK 1974, 54, 745–762. [Google Scholar] [CrossRef]
- Bauer, R.T. Testing generalization about latitudinal variation in reproduction and recruitment patterns with sicyoniid and caridean shrimp species. Invertebr. Reprod. Dev. 1992, 22, 193–202. [Google Scholar] [CrossRef]
- Oh, C.W.; Suh, H.L.; Park, K.Y.; Ma, C.W.; Lim, H.S. Growth and reproductive biology of the freshwater shrimp Exopalaemon Modestus (Decapoda: Palaemonidae) in a lake of Korea. J. Crust. Biol. 2002, 22, 357–366. [Google Scholar] [CrossRef]
- Ahamed, F.; Ohtomi, J. Reproductive biology of the pandalid shrimp Plesionik izumiae (Decapoda: Caridea). J. Crust. Biol. 2011, 31, 441–449. [Google Scholar] [CrossRef]
- Dinh, Q.M. Population dynamics of Boleophthalmus boddarti in the Mekong Delta, Vietnam. J. Anim. Plant Sci. 2017, 27, 603–610. [Google Scholar]
- Dinh, Q.M.; Nguyen, N.P.D. Population and age structure of the goby Stigmatogobius pleurostigma (Perciformes: Gobiidae) from the Mekong Delta. Int. J. Aquat. Sci. 2018, 9, 23–29. [Google Scholar]
- Dinh, Q.M.; Lam, T.H.T.; Nguyen, T.K.T.; Nguyen, M.T.; Tran, D.D. Population biology of Butis koilomatodon in the Mekong Delta. AACL Bioflux 2020, 13, 3287–3299. [Google Scholar]
- Nguyen, T.T.K.; Dinh, Q.M.; Tran, N.S.; Nguyen, T.H.D. Stock assessment of two populations of Glossogobius sparsipapillus (Osteichthyes, Gobiidae) in the Mekong Delta. Egypt. J. Aquat. Res. 2021, 47, 401–407. [Google Scholar] [CrossRef]
- Mazlan, A.G.; Rohaya, M. Size, growth and reproductive biology of the giant mudskipper, Periophthalmodon schlosseri (Pallas, 1770), Malaysian waters. J. Appl. Ichthyol. 2008, 24, 290–296. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Tran, N.Q.; Tran, D.D. Some biological parameters of Glossogobius aureus population from the Mekong Delta. Iran. J. Fish. Sci. 2021, 20, 84–95. [Google Scholar]
- Dinh, T.D.; Ambak, M.A.; Hassan, A.; Phuong, N.T. Population Biology of the Goby Pseudapocryptes elongatus (Cuvier, 1816) in the Coastal Mud Flat Areas of the Mekong Delta, Vietnam. Asian Fish. Sci. 2007, 20, 165–179. [Google Scholar] [CrossRef]
- Dinh, Q.M.; Qin, J.G.; Tran, D.D. Population and age structure of the goby Parapocryptes serperaster (Richardson, 1864; Gobiidae: Oxudercinae) in the Mekong Delta. Turk. J. Fish. Aquat Sci. 2015, 15, 345–357. [Google Scholar] [CrossRef]
- Dinh, Q.M. Population dynamics of the goby Trypauchen vagina (Gobiidae) at downstream of Hau River, Vietnam. Pak. J. Zool. 2018, 50, 105–110. [Google Scholar] [CrossRef]
- Dinh, Q.M. Biological parameters of Butis butis (Hamilton, 1822) population from the Mekong Delta. In Proceedings Scientific Research Results for Training; Science and Technics Publishing House, Kien Giang University: Kien Giang, Vietnam; pp. 306–314.
- Gabche, C.E.; Hockey, H.-U.P. Growth and mortality of the giant African river prawn Macrobrachium völlenhovenii (Herklots: Crustacea: Palaemonidae) in the Lobe river, Cameroon: A preliminary evaluation. J. Shellfish Res. 1995, 14, 185–190. [Google Scholar]
- Rochet, M.J.; Trenkel, V.M. Why and how could indicators be used in an ecosystem approach to fisheries management? In The Future of Fishery Science in North America; Springer Nature BV: Dordrecht, The Netherlands, 2009; pp. 209–226. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).