Abstract
N,N′-diacetylchitobiose, also known as GlcNAc2, is a chitin oligosaccharide and is reported to possess antimicrobial activity against pathogenic bacteria. In this study, 1% (w/v) GlcNAc2 solution was applied on ready-to-eat (RTE) shrimp and evaluated as an antimicrobial coating against Listeria monocytogenes during storage at 4 °C for 16 days. Texture properties, colour, TBARS values, moisture content and bacterial counts were monitored and analysed every four days. The results indicated that the GlcNAc2 coating retarded the changes in texture properties, TBARS values and moisture content of the RTE shrimp during storage. The presence of GlcNAc2 showed no significant changes in RTE shrimp colour in contrast to the control. However, the growth of L. monocytogenes inoculated on the GlcNAc2-coated RTE shrimp was slower than that of the control sample with the highest log reduction of 0.5 log CFU/mL being observed. This study showed that the GlcNAc2 used as an antimicrobial coating was able to inhibit the growth of L. monocytogenes, while maintaining the quality of the RTE shrimp during refrigerated storage.
1. Introduction
Chitin is the second largest biomass resource after cellulose and the most abundant nitrogen-containing biopolymer on earth. It is a linear polysaccharide formed by repeating units of 2-acetamido-2-deoxy-D-glucopyranose (GlcNAc, or else N-acetylglucosamine) connected by β-(1 → 4)-glycosidic bonds and is a major component of fungal cell walls, crustacean shells and insect exoskeletons. As a natural polymer, chitin and its derivatives, chitin oligosaccharides (CTOSs) and N-acetylglucosamine, are biocompatible, biodegradable and non-toxic. They have also been reported to have anti-bacterial, anti-oxidant, and anti-tumor properties, and are thus widely used in medicine, cosmetics, agriculture and the food sector. Examples of chitin-based commercial products include ChitoFlex® PRO by Tricol Biomedical Inc for wound dressing and bleeding control, BST-Gel® by Piramal Healthcare Canada Inc for bone filling, cartilage repair and invertebral disc restoration, as well as Reaxon® by Medovent for nerve regeneration [1,2].
Similarly to that of high-value carbon based chemicals, the preparation of high-value N-containing chemicals from biomass is of great significance for green and sustainable development [3]. Exploiting chitin, a type of nitrogen-containing biomass, as a resource by developing green and environmentally friendly technologies for deriving CTOSs and GlcNAc will enable the sustainable production of various bio-based products and contribute towards a robust and economic biorefinery supply chain, i.e., one based on crustacean waste valorisation streams [4]. However, it is crucial to investigate the functionalities of such products and identify areas of high-end applications, especially when it comes to CTOSs.
According to previously reported studies, several natural oligosaccharides have shown antimicrobial properties similar to those of chemical preservatives, that are typically used by the food industry. Some of these have also been able to inhibit the growth of food pathogens via synergistic effects, thus becoming promising ingredients for a range of food products for quality assurance [5,6,7]. More specifically, CTOSs have been reported to improve defence against pathogenic infections since molecular recognition and plant defence mechanisms of CTOSs occur in plant species or fruit [8]. Moreover, CTOSs are able to inhibit the growth and multiplication of several filamentous (P. tardum, P. chrizogenum, A. flavus, P. betae and C. herbarum) and yeast-like fungi (C. scotti and R. rubra), most likely due to their interaction with the microbial cell wall [9].
In our previous study, we investigated the inhibitory activity of CTOSs that were obtained via enzymatic digestion, against various bacteria and reported that the minimum inhibitory concentrations for E. coli and L. monocytogenes were 5% and 10% (w/v), respectively [10]. L. monocytogenes is a food pathogen that may cause serious disease in humans and can grow and survive even in chilled environments. Therefore, it is important to understand the shelf life of ready-to-eat products and the efficiency of CTOSs in inhibiting its growth under storage conditions.
Ready-to-eat (RTE) shrimp (cooked) is a street food, is retailed either in chilled or frozen form, and more often than not is unpackaged in tropical countries [11,12]. Consumer demand for this product has steadily grown over the years [11]. RTE shrimp requires minimum thermal processing (normally heated for 2 to 5 min, to temperatures above 63 °C at the centre of the shrimp meat) prior to consumption, which generally inactivates vegetative cells of bacteria that are pathogenic to humans [13,14]. However, the chances of contamination by pathogenic bacteria, such as Listeria monocytogenes, cannot be ruled out, either due to insufficient thermal processing, due to post-processing contamination, caused by using the same area and utensils used for raw shrimp, or due to temperature abuse during storage [13,15]. In order to ensure safety, extend the shelf life and maintain the quality of RTE shrimp, it is essential to introduce a post-processing intervention to inhibit the growth of L. monocytogenes in the product.
Various methods have been proposed to control the post-process contamination of RTE products against L. monocytogenes. The growth of L. monocytogenes on RTE shrimp has been successfully inhibited by employing antimicrobial coatings such as spice and herb extracts, as well as by using films made from chitosan [11,16,17]. Pathogenic growth has also been inhibited by employing thermal and non-thermal treatments or storing the product within modified-atmosphere packaging at an appropriate temperature [18,19,20,21]. Several previous studies reported a significant growth of L. monocytogenes inoculated on RTE shrimp and shrimp in brine stored at temperatures between 4–12 °C, concluding that the possibility of Listeria growth in such products cannot be avoided [22,23]. However, pathogenic growth was significantly inhibited by introducing an antimicrobial coating, such as a solution of chitosan coating in a concentration of 1–2% (w/v), which reduced bacterial counts by approximately 2 log CFU/g compared to those of non-coated samples [18,19]. Chitosan incorporated with spice and herb extracts as well as organic acids was reported to be more effective at inhibiting the growth of L. monocytogenes and aerobic mesophilic bacteria (in terms of total viable count) than was the chitosan coating alone [18,19,24].
Chitin, chitosan and their oligosaccharides are known to possess excellent biological properties, especially antimicrobial activity [25,26]. The use of chitosan as an edible coating on ready-to-eat food for inhibiting L. monocytogenes seems to be receiving more attention recently, compared to chitin [18,19,27]. This is due to the significantly higher solubility of chitosan in organic acids, compared to that of crystalline chitin as the poor solubility of chitin in any solvent limits its potential application as an antimicrobial agent [28]. More recently, water-soluble CTOSs (GlcNAc1–10), composed of one to ten N-acetyl-D-glucosamine covalent β-(1 → 4) linkages, obtained either via acidic or enzymatic hydrolysis have been pursued as simpler alternatives to the use of chitosan [25,29,30,31,32,33].
In this study, the aim was to determine the effectiveness of the water-soluble CTOS N,N′-diacetylchitobiose (GlcNAc2), as an antimicrobial coating on RTE shrimp. In addition to monitoring the inhibition of L. monocytogenes by GlcNAc2, this paper also reports the changes in physicochemical properties occurring during storage at 4 °C for 16 days.
2. Materials and Methods
GlcNAc2 was prepared as described by Abidin et al. [10]. In brief, 1.0% (w/v) of chitin in a 0.05 M sodium acetate buffer at pH 6.0 was enzymatically hydrolysed with 0.1% (w/v) chitinase from Streptomyces griseus (Sigma Aldrich, Gillingham, UK). The reaction mixture was incubated in a shaker water bath at 40 °C. Gram-positive bacterium L. monocytogenes 10403S was cultivated in-house (Department of Food and Nutritional Sciences, University of Reading, Reading, UK).
The acetic acid (≥99.7% ACS reagent, CAS: 64-19-7), hydrochloric acid (37% ACS reagent, CAS: 7647-01-0), thiobarbituric acid (≥98%, CAS: 504-17-6) and trichloroacetic acid (≥99% ACS reagent, CAS: 76-03-9) used in this study were of analytical grade and commercially available.
2.1. Ready-to-Eat (RTE) Shrimp Preparation
Frozen headless and shell-less shrimps (Litopenaeus Vannamei) were purchased from a local retailer (Diamond Foods, Wembley, UK). The shrimps were transported to the Pilot Plant (University of Reading, Reading, UK) immediately after they were purchased and stored at −18 °C. A day prior to the experiments, the shrimps were thawed overnight at 4 °C and cooked in boiling water for 2 min with a thermocouple inserted into the meat ensuring that the meat temperature exceeded 63 °C, as recommended by U.S FDA for seafood [17]. In fact, the actual cooking temperature of the shrimp meat was 92 °C at the end of the cooking process. After cooking, it was drained and cooled to dry [17]. The samples were packed temporarily in the sterile bags prior to inoculation and coating with GlcNAc2 on the same day the shrimps were cooked.
2.2. Inoculum and Inoculation
L. monocytogenes 10403S was grown on Brain Heart Infusion (BHI) agar (Sigma Aldrich, Gillingham, UK) at 37 °C for 24 h. The agar plates were then stored at 4 °C. For the bacterial suspension preparation, 2–3 colonies were picked up from the agar plate using a sterile plastic loop and transferred into sterile Tryptic Soy broth (TSB) under aseptic conditions. The suspension was incubated at 37 °C for 16–18 h with continuous agitation [10]. The suspension was then centrifuged at 7000 rpm for 15 min to separate the supernatant and cell pellet. The supernatant was discarded and the cell pellet was washed and resuspended in sterile 0.85% saline twice [11]. The optical density (OD) of the resuspension was then adjusted to 0.5 McFarland using a UV-Vis spectrophotometer (Orion AquaMate 8000, Thermo Scientific, UK) at 620 nm, which corresponds to approximately 1.5 × 108 CFU/mL. Then, the resuspension was diluted with sterile 0.85% saline to give a final colony count of 1.5 × 105 CFU/mL and used as inoculum.
The RTE shrimp samples were inoculated by immersing them into the 1.5 × 105 CFU/mL L. monocytogenes inoculum at 20 °C for 5 min, with continuous agitation for bacterial attachment. After immersion, the samples were aseptically removed and allowed to drain on a sterile basket at 20 °C for 15 min [11].
2.3. Coating with GlcNAc2
A coating solution of 1% (w/v) GlcNAc2 was prepared by dissolving the compound in sterile deionised water. In this study, a 1% (w/v) GlcNAc2 concentration was chosen, even though the MIC value of GlcNAc2 against L. monocytogenes 10403S has been previously reported to be 10% (w/v) [10]. The inoculated and non-inoculated (control) RTE shrimp samples were dipped in the treatment solution for 5 min. The coated samples were drained with the sterile basket, aseptically transferred into sterile Whirl-Pack® sample bags (Nasco, Fort Atkinson, WI, USA), sealed and stored at 4 °C for 16 days. The control for this experiment included sets of inoculated and non-inoculated RTE shrimps that were dipped in separate sterile deionised water for the same dipping time as that used in the case of GlcNAc2. The coating solutions were separate for the inoculated and non-inoculated RTE shrimp samples in order to avoid contamination with the bacteria strain.
The non-inoculated samples were analysed for texture, colour, lipid oxidation and moisture content, whereas the microbial analysis was conducted for the inoculated samples after 0, 4, 8, 12 and 16 days of storage at 4 °C.
2.4. Physicochemical Analysis
2.4.1. Texture Profile Analysis (TPA)
The texture (hardness, cohesiveness, springiness and chewiness) of RTE shrimp samples was determined via texture profile analysis (TPA) with Texture Analyser CT3 Version 1.2 (Brookfield Engineering Laboratories, Middleboro, MA, USA) [21,34]. TPA was measured using a compression test with the texture analyser fitted with a cylindrical probe (6 mm in diameter) and 25 kg load cell moving at a 1 mm/s test speed until the deformation was 70% of the original height [34]. Two measurements were taken at the second to third segment of the shrimp abdomen for each sample [34]. The texture of three shrimp samples were recorded, and these measurements were replicated thrice for each treatment.
2.4.2. Colour
The colour of the RTE shrimp was determined using a colour spectrophotometer (Minolta CR-400 chroma metre, Konica Minolta Sensing, Inc., Tokyo, Japan). L* represented the brightness on a scale from 0 (dark) to 100 (white), whereas a* and b* were negative to positive scale ranges between greenness to redness and blueness to yellowness, respectively [21]. The measurements of surface colour were taken on both sides of the shrimp abdomen at the second and third segment and three shrimp samples were measured for each treatment. The colourimeter was calibrated with white tile, prior to measurement.
2.4.3. Moisture Content
The moisture content was determined according to the protocols recommended by AOAC (1990) by drying each sample in the oven at 105 °C until the sample weight became constant. The final moisture content was expressed on a wet weight basis.
2.4.4. Lipid Peroxidation
The thiobarbituric acid-reacting substances (TBARS) in the RTE shrimp samples were determined as described by the previous study with some modifications [21]. The shrimp meat (1 g) was mixed with 9 mL of 0.25 N HCl containing 0.375% thiobarbituric acid (TBA) and 15% trichloroacetic acid (TCA). The mixture was heated in a boiling water bath for 10 min, giving a pink colour to the mixture, followed by cooling with running water. The mixture was centrifuged (Centaur 2, MSE, East Grinstead, UK) at 4000 rpm for 10 min and the supernatant was collected for the measurement of absorbance at 532 nm using a UV-Vis spectrophotometer (Cecil CE 7400, Cambridge, UK). The TBARS content was calculated from the standard curve of malonaldehyde (0 to 2 ppm) and expressed as mg malonaldehyde/kg shrimp meat.
2.5. Microbial Analysis
Microbial analysis was performed using the method described in the previous study with some modifications [11]. The shrimp meat (10 g) was blended with 100 mL of 0.85% sterile saline solution in a Stomacher® strainer bag (Seward, Worthing, UK) and homogenised at normal speed for 2 min. Serial decimal dilutions of a bacterial suspension were prepared with 0.85% sterile saline solution and 0.1 mL of each dilution was spread onto PALCAM Listeria Selective Agar Plate (Sigma Aldrich, Gillingham, UK) with PALCAM Listeria Selective Supplement (Sigma Aldrich, Gillingham, UK). The plate was incubated at 37 °C for 48 h, prior to enumeration. The results were expressed in log CFU/g sample. Aerobic plate counts of the non-inoculated shrimp were analysed additionally to evaluate the effects of GlcNAc2 on natural background microflora. Aerobic bacteria were enumerated by plating 0.1 mL on plate count agar (Sigma Aldrich, Gillingham, UK), which was incubated at 37 °C for 48 h.
2.6. Statistical Analysis
Minitab® 18 (State College, PA, USA) statistical software was used for the analysis of the difference between means using Tukey’s multiple range test. The results were determined via one-way analysis of variance (ANOVA) at a significance level of 0.05 (p < 0.05). Three replicates were conducted for each physicochemical analysis, whereas the microbial analysis was run in duplicate for each treatment.
3. Results
3.1. Effect of GlcNAc2 Coating on the Physicochemical Properties of RTE Shrimp
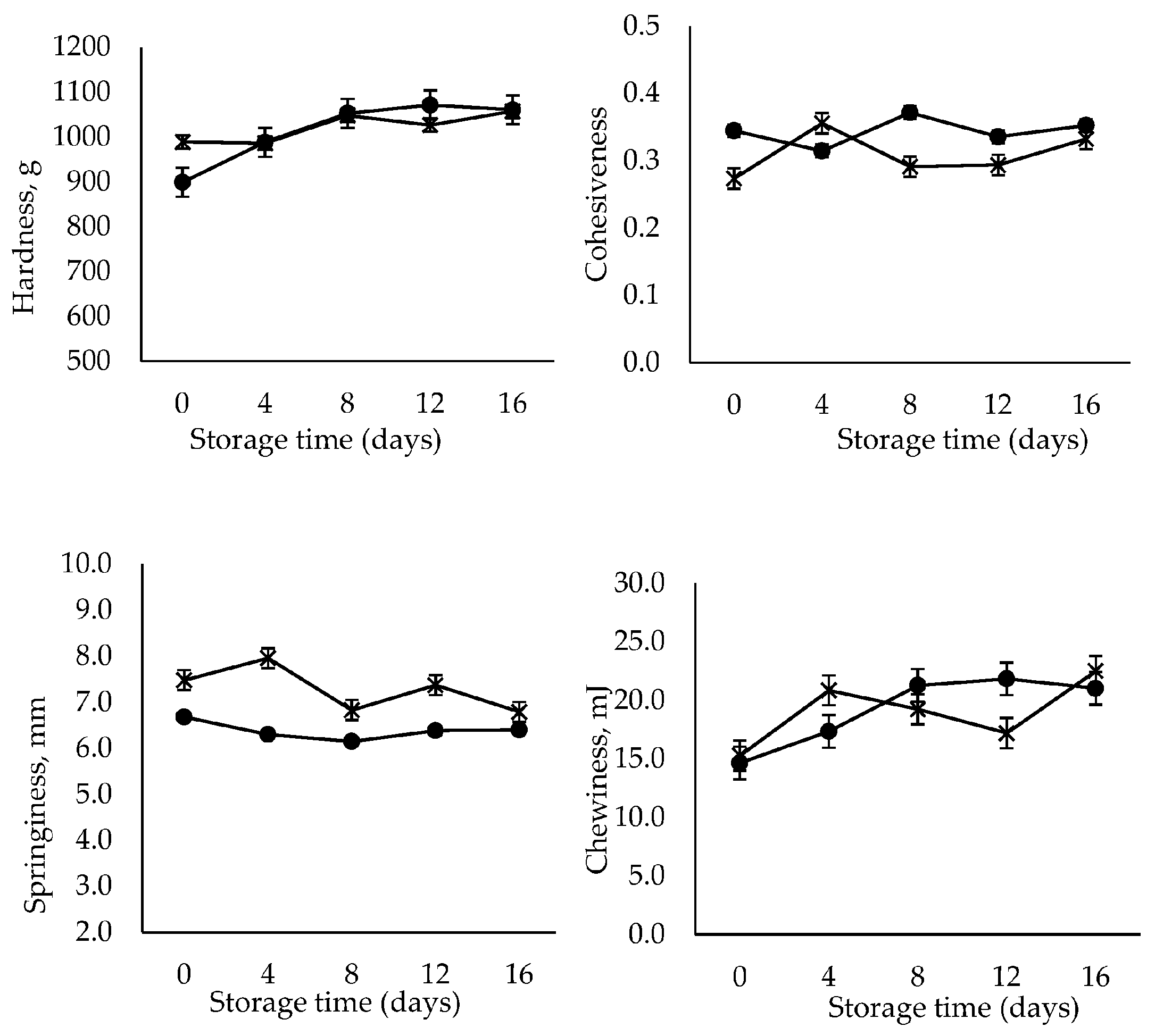
Texture profile analysis (TPA) is an instrumental double compression test to measure the textural properties of food [35]. Texture properties are the main quality attributes that assess the acceptability of RTE shrimp. TPA was carried out to determine hardness, cohesiveness, springiness and chewiness indicating firmness, elasticity, stickiness and tenderness of the RTE shrimp meats, respectively [21]. Based on the TPA shown in Figure 1, the hardness and chewiness of the control sample increased significantly (p < 0.05) with storage time (16 days), whereas no significant changes occurred in the case of the GlcNAc2-coated sample. In addition, the cohesiveness and springiness in all samples were maintained throughout the storage period lasting 16 days. The results obtained here are comparable with those of an earlier publication, which reported that RTE shrimp meat, thermally processed at 85 °C for 30 min, became firmer and tender over 10 days of storage [21]. The shrimp meat exposed to heat treatment above 70 °C underwent hardening caused by protein denaturation and the shrinkage of collagen [36,37]. The thermal shrinkage experienced in the early storage period, i.e., in the first 10 days, resulted in the tightening and stiffening of the meat structure [21].
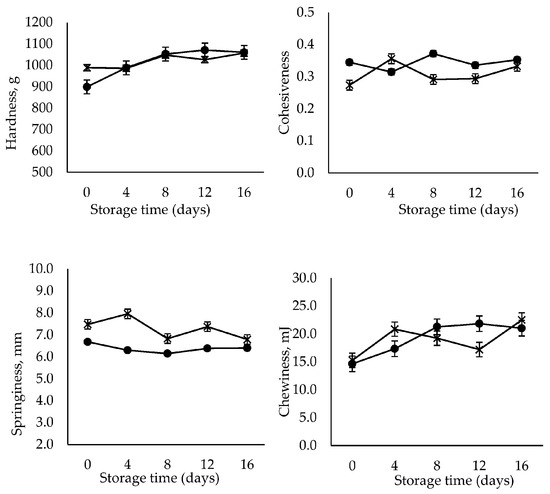
Figure 1.
Changes in the TPA of control (●) and GlcNAc2-coated (×) RTE shrimp occurring during 16-day storage at 4 °C.
Based on the results obtained, it was clarified that the cooking process employed enhanced the firmness and chewiness of the shrimp meat. In contrast, the presence of the GlcNAc2 coating prevented structure deformation. This finding is agreement with that of the previous studies that the chitosan coating was reported to effectively retard the changes in texture parameters in shrimp during storage [38,39]. The bonds between chitosan and myofibrillar proteins could be associated with the improvement of texture in shrimp muscle, with the final structure being formed by both covalent and noncovalent interactions [38]. It is noteworthy that the texture of RTE shrimp maintained its firmness without notable deterioration, regardless of treatment, throughout the storage period.
Colour is one of the main quality attributes of RTE products which critically influences consumer perception and purchasing decision. Brightness (L*), redness (a*) and yellowness (b*) were recorded for the GlcNAc2-coated and control samples (Table 1) showing significant changes occurring over 16 days of storage (p < 0.05). The values of L* of the control and GlcNAc2-coated samples increased (p < 0.05) from 47 to 60 and 46 to 57, respectively. The yellowness of the sample increased as the brightness increased. The value of b* of the control and GlcNAc2-coated samples increased significantly (p < 0.05) from −2 to 5.8 and −0.3 to 7.0, respectively. In contrast, the redness of the samples significantly decreased (p < 0.05) with storage. However, the GlcNAc2-coated sample did not show any significant change compared to the control sample.

Table 1.
Changes in surface colour of control and GlcNAc2-coated samples during 16-day storage at 4 °C.
An increase in the L* and b* of cooked shrimp has been previously discussed, with the increase in protein denaturation being induced during sample heating [21]. The brightness of the cooked shrimp increases due to protein coagulation, thereby changing the shrimp surface properties, increasing light reflection and creating a whitened colour of the meat [21]. As protein denaturation occurs, astaxanthin is released from carotenoproteins changing the colour from orange to red after heating [40]. However, the degradation of astaxanthin continued when the redness values of RTE shrimp decreased throughout the storage period.
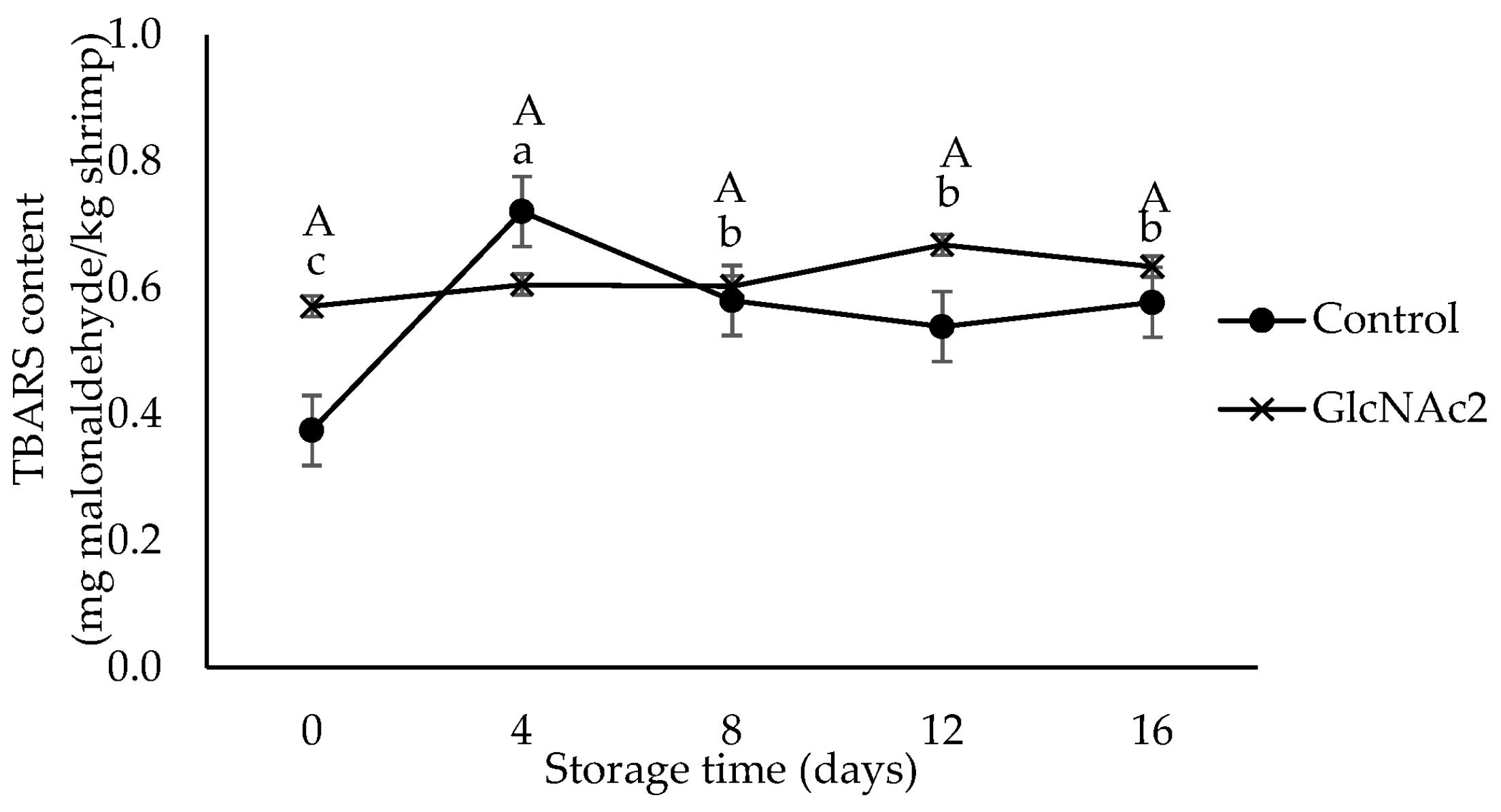
The TBARS test uses the concentration of malonaldehyde as a marker of oxidative rancidity and is widely used for measuring lipid oxidation in meat, which can lead to the production of off-flavours and odours [41,42]. The TBARS values shown in Figure 2 indicate that the lipid oxidation of the RTE shrimp occurred during storage. The TBARS value of GlcNAc2-coated RTE shrimp was higher than that of the control, whereas the TBARS value of the control sample increased sharply from 0.4 to 0.7 mg malonaldehyde/kg shrimp after 4 days of storage, decreased significantly to 0.6 mg malonaldehyde/kg shrimp at day 8 and maintained this value until day 16. In general, the development of lipid oxidation in the RTE shrimp meat is influenced by several factors such as packaging, storage and other processing conditions [43]. Thermal cooking is known to accelerate lipid peroxidation [43]. The presence of oxygen during storage is also likely to accelerate oxidative deterioration [19]. In addition, chitosan is also reported to be a good barrier to oxygen permeation when it is applied on the surface of pink salmon [44]. From the findings here, it is clear that the GlcNAc2 coating was also able to maintain lipid oxidation in RTE shrimp meat, i.e., no significant changes in the TBARS values were observed during storage, probably due to a similar mechanism. According to the previous study, the TBARS value for a high-quality food product must be less than 3 mg malonaldehyde/kg [45]. Based on this criterion, all the RTE shrimp samples studied here, regardless of the presence of GlcNAc2, could be considered “high-quality product” as the TBARS values of the samples were less than 3 mg malonaldehyde/kg even after 16 days of storage.
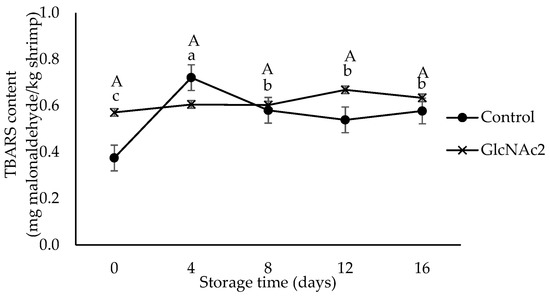
Figure 2.
TBARS values for control and GlcNAc2-coated RTE shrimp during 16-day storage at 4 °C. Small and capital letters indicate significant differences (p < 0.05) between control and GlcNAc2-coated samples during storage, respectively.
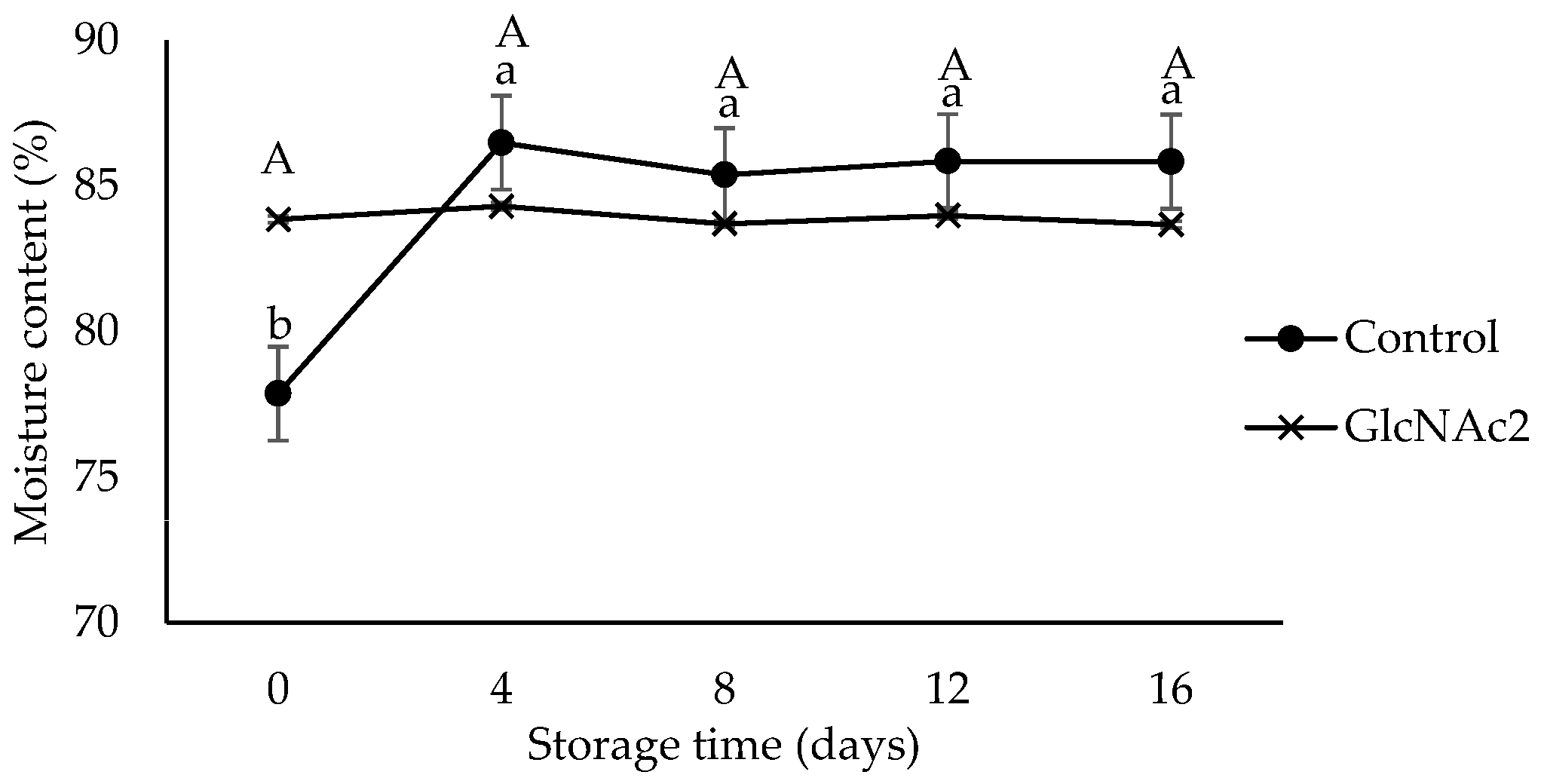
Moisture has been reported to be the most important property of RTE shrimp affecting quality, stability and safety during storage [21]. The moisture content of the control sample shown in Figure 3 increased sharply (p < 0.05) from 78% at the start of storage to 86% on day 4 due to the moisture absorption capability of the sample. The moisture contents of the control sample then remained constant at approximately 86%, until end of the storage period. Employing GlcNAc2, on the other hand, retained the moisture and the moisture content remained constant at 84% throughout the storage period. The stability of the moisture content of the RTE shrimp may have been due to the GlcNAc2 layer acting as a protective barrier against moisture escape. Previously, the chitosan coating also was reported to act as a protective barrier against moisture loss [41].
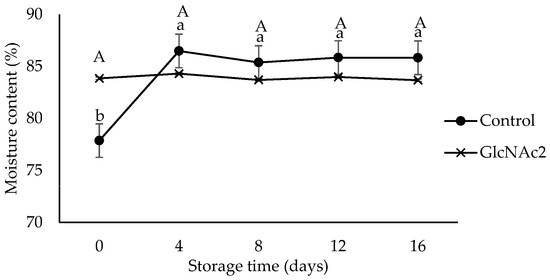
Figure 3.
Moisture content changes in control and GlcNAc2-coated RTE shrimp during 16-day storage at 4 °C. Small and capital letters indicate significant differences (p < 0.05) between control and GlcNAc2-coated samples during storage, respectively.
3.2. Effect of GlcNAc2 Coating on the RTE Shrimp Inoculated by L. monocytogenes
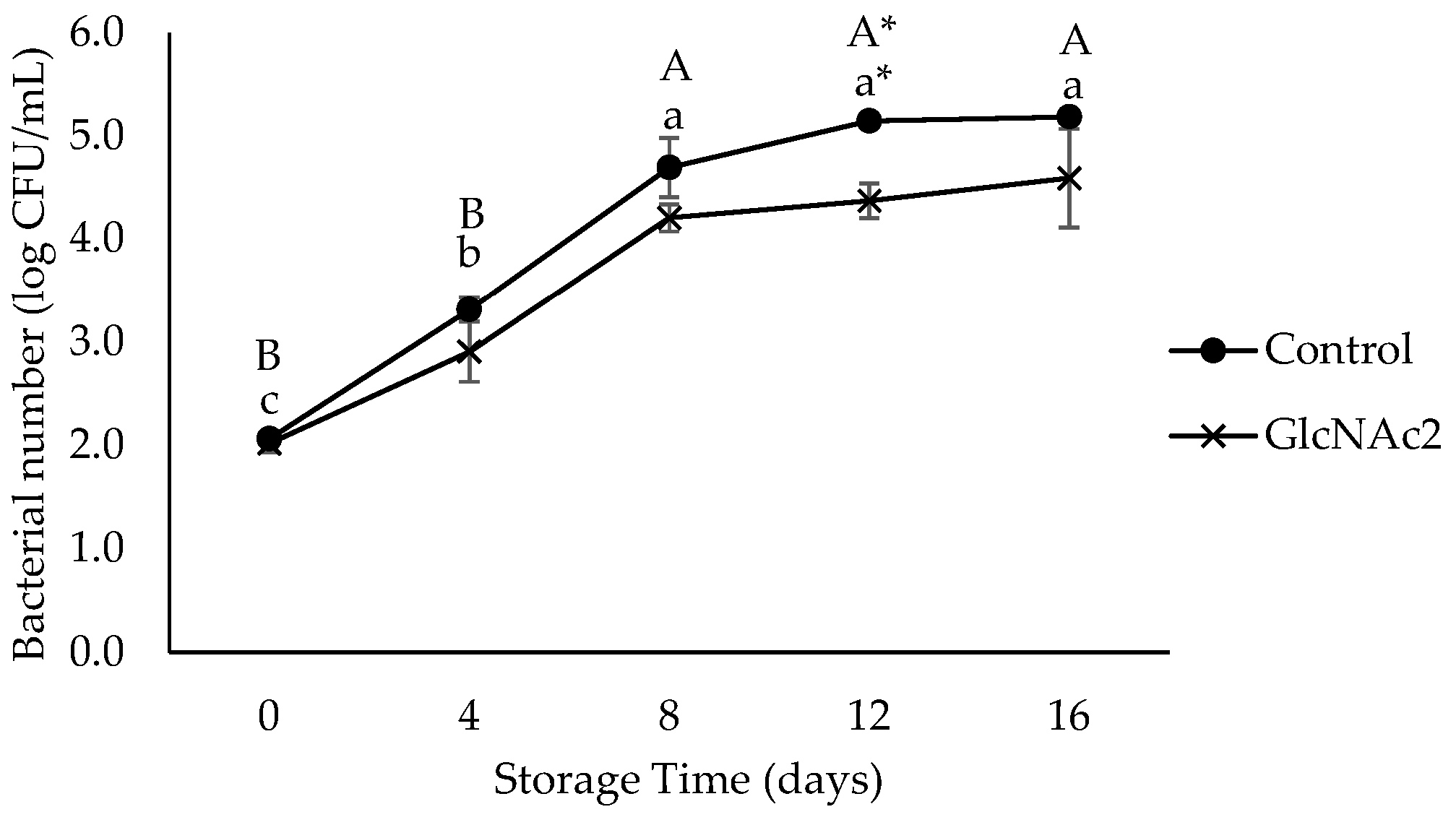
In this study, aerobic bacteria did not grow on the samples during storage (data not shown). L. monocytogenes counts on the inoculated shrimp were initially 2 log CFU/mL. The growth showed no significant differences between samples until day 8 of storage. However, a significantly lower growth (p < 0.05) of L. monocytogenes in GlcNAc2-coated samples was detected on day 12, with the highest log reduction of 0.5 log CFU/mL being observed, as shown in Figure 4. On day 16, the growth on the GlcNAc2-coated sample approached similar colony counts to those of the control.
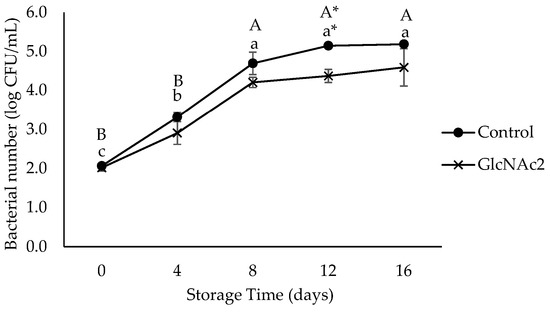
Figure 4.
Growth of L. monocytogenes in control and GlcNAc2-coated RTE shrimp during 16-day of storage at 4 °C. Small and capital letters indicate significant differences (p < 0.05) between control and GlcNAc2-coated samples during storage, respectively. Asterisk (*) indicates significant differences (p < 0.05) between samples over storage time.
The application of chitin and its oligosaccharides as a antimicrobial coating for RTE foods is not widespread due to their low solubility and availability, respectively. Therefore, chitosan, a chitin derivative, is used against L. monocytogenes. Based on earlier research, either acid-soluble or water-soluble chitosan oligosaccharides have been used successfully against the growth of listeria in inoculated samples of raw and RTE shrimp [16,17,43]. According to the previous study, the increase in concentration of water-soluble chitosan from 0.5 to 5% significantly (p < 0.05) increased the antimicrobial activity by lowering counts by 7.43 log CFU/g [43]. In another related study, the count of L. innocua inoculated on RTE shrimp was reduced by 2.29 log CFU/g after treatment with 2% chitosan in combination with 2% organic acid [16]. In a similar work, it was reported that 1% chitosan incorporation in 2% organic acid was the most effective treatment against L. monocytogenes inoculated on RTE shrimp, which caused a 5.38-log CFU/g bacterial reduction [17]. It is most likely that the mechanism of action of GlcNAc2 is not very different from that of chitosan and that a precipitate on the microbial cell surface forms an impervious layer around the cell thereby blocking the channels to transport essential solutes crucial for cell survival [44]. The impervious layer can also destabilise the cell wall beyond repair [44].
4. Conclusions
The results demonstrated that a 1% (w/v) GlcNAc2 coating retarded the changes in texture properties, TBARS values and moisture content of RTE shrimp during 16-day storage at 4 °C. The GlcNAc2 coating showed inhibition against L. monocytogenes only up to day 12 of storage, with a net log reduction of 0.5 log CFU/mL. Furthermore, the absence or presence of GlcNAc2 did not influence colour changes in shrimp meat. This result shows that GlcNAc2 can be applied as an antimicrobial coating in RTE products and might support the growth of L. monocynogenes. Moreover, compared to our previous study where we reported a MIC of 10% (w/v) for L. monocytogenes following broth microdilution anti-bacterial susceptibility testing, it is shown that in chilled conditions a low concentration of 1% (w/v) of a GlcNAc2 coating is enough to inhibit its growth. CTOSs are expected to play a key role in the food industry in the very near future as sustainably produced natural antimicrobial ingredients.
Author Contributions
Conceptualization, M.Z.A., K.N. and K.K.; methodology, M.Z.A. and K.K.; software, M.Z.A.; validation, M.Z.A. and K.K.; formal analysis, M.Z.A.; investigation, M.Z.A.; resources, K.N. and K.K.; data curation, M.Z.A.; writing—original draft preparation, M.Z.A.; writing—review and editing, K.N. and K.K.; visualization, M.Z.A.; supervision, K.N.; project administration, M.Z.A., K.N. and K.K.; funding acquisition, M.Z.A., K.N. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was supported by a scholarship from the Ministry of Higher Education of Malaysia (MOHE) and Universiti Tun Hussein Onn Malaysia (UTHM) which provided a TIER 1 research grant (H964).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food. 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Cao, S.; Liu, Y.; Shi, L.; Zhu, W.; Wang, H. N-Acetylglucosamine as a platform chemical produced from renewable resources: Opportunity, challenge, and future prospects. Green Chem. 2022, 24, 493–509. [Google Scholar] [CrossRef]
- Riofrio, A.; Alcivar, T.; Baykara, H. Environmental and economic viability of chitosan production in Guayas-Ecuador: A robust investment and life cycle analysis. ACS Omega 2021, 6, 23038–23051. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Ma, Q.; Fu, Y.; Zhou, Z.; Zhao, X. Preparation, evaluation and characterization of rutin–chitooligosaccharide complex. Plant Foods Hum. Nutr. 2019, 74, 328–333. [Google Scholar] [CrossRef]
- Li, R.; Zhu, L.; Liu, D.; Wang, W.; Zhang, C.; Jiao, S.; Wei, J.; Ren, L.; Zhang, Y.; Gou, X.; et al. High molecular weight chitosan oligosaccharide exhibited antifungal activity by misleading cell wall organization via targeting PHR transglucosidases. Carbohydr. Polym. 2022, 285, 119253. [Google Scholar] [CrossRef]
- Rao, M.S.; Chander, R.; Sharma, A. Synergistic effect of chitooligosaccharides and lysozyme for meat preservation. LWT Food Sci. Technol. 2008, 41, 1995–2001. [Google Scholar] [CrossRef]
- García, Y.H.; Zamora, O.R.; Troncoso-Rojas, R.; Tiznado-Hernández, M.E.; Báez-Flores, M.E.; Carvajal-Millan, E.; Rascón-Chu, A. Toward understanding the molecular recognition of fungal chitin and activation of the plant defense mechanism in horticultural crops. Molecules 2021, 26, 6513. [Google Scholar] [CrossRef]
- Vasilieva, T.; Lopatin, S.; Varlamov, V.; Miasnikov, V.; Hein, A.M.; Vasiliev, M. Hydrolysis of chitin and chitosan in low temperature electron-beam plasma. Pure Appl. Chem. 2016, 88, 873–879. [Google Scholar] [CrossRef]
- Abidin, M.Z.; Kourmentza, C.; Karatzas, A.K.; Niranjan, K. Enzymatic hydrolysis of thermally pre-treated chitin and antimicrobial activity of N,N′-diacetylchitobiose. J. Chem. Technol. Biotechnol. 2019, 94, 2529–2536. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Caffin, N.; Dykes, G.A.; Turner, M.S. Effect of antimicrobial spice and herb extract combinations on Listeria monocytogenes, Staphylococcus aureus, and spoilage microflora growth on cooked ready-to-eat vacuum-packaged shrimp. J. Food Prot. 2011, 74, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, S.F.; Yang, T.Y.; Hung, W.C.; Chan, M.Y.; Tseng, S.P. Antimicrobial resistance and genetic diversity in ceftazidime non-susceptible bacterial pathogens from ready-to-eat street foods in three Taiwanese cities. Sci. Rep. 2017, 7, 15515. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L. Microbiological criteria for cooked, ready-to-eat shrimp and crabmeat. Food Technol. 1991, 45, 157–160. [Google Scholar]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Rutherford, T.J.; Marshall, D.L.; Andrews, L.S.; Coggins, P.C.; Schilling, M.W.; Gerard, P. Combined effect of packaging atmosphere and storage temperature on growth of Listeria monocytogenes on ready-to-eat shrimp. Food Microbiol. 2007, 24, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jin, T.Z.; Scullen, O.J.; Sommers, C.H. Effects of antimicrobial coatings and cryogenic freezing on survival and growth of Listeria innocua on frozen ready-to-eat shrimp during thawing. J. Food Sci. 2013, 78, M1195–M1200. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Fang, W.; Li, Y. Inhibitory effects of chitosan coating combined with organic acids on Listeria monocytogenes in refrigerated ready-to-eat shrimps. J. Food Prot. 2013, 76, 1377–1383. [Google Scholar] [CrossRef]
- Mejlholm, O.; Bøknæs, N.; Dalgaard, P. Shelf life and safety aspects of chilled cooked and peeled shrimps (Pandalus borealis) in modified atmosphere packaging. J. Appl. Microbiol. 2005, 99, 66–76. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chawla, S.P.; Chander, R.Ã.; Sharma, A. Development of shelf-stable, ready-to-eat (RTE) shrimps (Penaeus indicus) using g-radiation as one of the hurdles. LWT Food Sci. Technol. 2006, 39, 621–626. [Google Scholar] [CrossRef]
- Mahmoud, B.S.M. Effect of X-ray treatments on inoculated Escherichia coli O157: H7, Salmonella enterica, Shigella flexneri and Vibrio parahaemolyticus in ready-to-eat shrimp. Food Microbiol. 2009, 26, 860–864. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, L.; Ding, G.; Hu, X.; Liao, X.; Zhang, Y. High hydrostatic pressure and thermal treatments for ready-to-eat wine-marinated shrimp: An evaluation of microbiological and physicochemical qualities. Innov. Food Sci. Emerg. Technol. 2013, 20, 16–23. [Google Scholar] [CrossRef]
- Mejlholm, O.; Kjeldgaard, J.; Modberg, A.; Vest, M.B.; Bøknæs, N.; Koort, J.; Björkroth, J.; Dalgaard, P. Microbial changes and growth of Listeria monocytogenes during chilled storage of brined shrimp (Pandalus borealis). Int. J. Food Microbiol. 2008, 124, 250–259. [Google Scholar] [CrossRef]
- Tirloni, E.; Nauta, M.; Vasconi, M.; Di Pietro, V.; Bernardi, C.; Stella, S. Growth of Listeria monocytogenes in ready-to-eat “shrimp cocktail”: Risk assessment and possible preventive interventions. Int. J. Food Microbiol. 2020, 334, 108800. [Google Scholar] [CrossRef] [PubMed]
- Carrión-granda, X.; Fernández-pan, I.; Jaime, I.; Rovira, J.; Maté, J.I. Improvement of the microbiological quality of ready-to-eat peeled shrimps (Penaeus vannamei) by the use of chitosan coatings. Int. J. Food Microbiol. 2016, 232, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Osada, M.; Miura, C.; Nakagawa, Y.S.; Kaihara, M.; Nikaido, M.; Totani, K. Effect of sub- and supercritical water pretreatment on enzymatic degradation of chitin. Carbohydr. Polym. 2012, 88, 308–312. [Google Scholar] [CrossRef]
- Abidin, M.Z.; Junqueira-Gonçalves, M.P.; Khutoryanskiy, V.V.; Niranjan, K. Intensifying chitin hydrolysis by adjunct treatments—An overview. J. Chem. Technol. Biotechnol. 2017, 92, 2787–2798. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Wang, L.; Scullen, O.J.; Sommers, C.H. Antimicrobial films and coatings for inactivation of Listeria innocua on ready-to-eat deli turkey meat. Food Control 2014, 40, 64–70. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Roy, I.; Mondal, K.; Gupta, M.N. Accelerating enzymatic hydrolysis of chitin by microwave pretreatment. Biotechnol. Prog. 2003, 19, 1648–1653. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Dziril, M.; Grib, H.; Laribi-Habchi, H.; Drouiche, N.; Abdi, N.; Lounici, H.; Pauss, A.; Mameri, N. Chitin oligomers and monomers production by coupling γ radiation and enzymatic hydrolysis. J. Ind. Eng. Chem. 2015, 26, 396–401. [Google Scholar] [CrossRef]
- Villa-Lerma, G.; González-Márquez, H.; Gimeno, M.; Trombotto, S.; David, L.; Ifuku, S.; Shirai, K. Enzymatic hydrolysis of chitin pretreated by rapid depressurization from supercritical 1,1,1,2-tetrafluoroethane toward highly acetylated oligosaccharides. Bioresour. Technol. 2016, 209, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Noordin, W.N.M.; Shunmugam, N.; Huda, N.; Adzitey, F. The effects of essential oils and organic acids on microbiological and physicochemical properties of whole shrimps at refrigerated storage. Curr. Res. Nutr. Food Sci. 2018, 6, 273–283. [Google Scholar] [CrossRef]
- Chen, L.; Linus, U. Texture measurement approaches in fresh and processed foods—A review. Food Res. Int. 2013, 51, 823–835. [Google Scholar] [CrossRef]
- Mizuta, S.; Yamada, Y.; Miyagi, T.; Yoshinaka, R. Histological changes in collagen related to textural development of prawn meat during heat processing. J. Food Sci. 1999, 64, 991–995. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Kijroongrojana, K.; Sriket, P. Effect of heating on physical properties and microstructure of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Int. J. Food Sci. Technol. 2008, 43, 1066–1072. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Zhou, J.; Ruan, X.; Lin, J.; Fu, L. Effect of chitosan nanoparticle coatings on the quality changes of postharvest whiteleg shrimp, Litopenaeus vannamei, during storage at 4 °C. Food Bioprocess Technol. 2015, 8, 907–915. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Tume, R.K.; Sikes, A.L.; Tabrett, S.; Smith, D.M. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon): Effective method for improvement of cooked colour. Aquaculture 2009, 296, 129–135. [Google Scholar] [CrossRef]
- Butler, B.L.; Vergano, P.J.; Testin, R.F.; Bunn, J.M.; Wiles, J.L. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J. Food Sci. 1996, 61, 953–956. [Google Scholar] [CrossRef]
- Sathivel, S.; Liu, Q.; Huang, J.; Prinyawiwatkul, W. The influence of chitosan glazing on the quality of skinless pink salmon (Oncorhynchus gorbuscha) fillets during frozen storage. J. Food Eng. 2007, 83, 366–373. [Google Scholar] [CrossRef]
- Alfaro, L.; Chotiko, A.; Chouljenko, A.; Janes, M.; King, J.M.; Sathivel, S. Development of water-soluble chitosan powder and its antimicrobial effect against inoculated Listeria innocua NRRL B-33016 on shrimp. Food Control 2018, 85, 453–458. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Cadun, A.; Kıs, D.; Şükran, Ç. Marination of deep-water pink shrimp with rosemary extract and the determination of its shelf-life. Food Chem. 2008, 109, 81–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).