Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Landfill Leachate Sludge Derived Biochar
2.2. Adsorption of Representative Contaminants
2.3. Characterizations
3. Results and Discussion
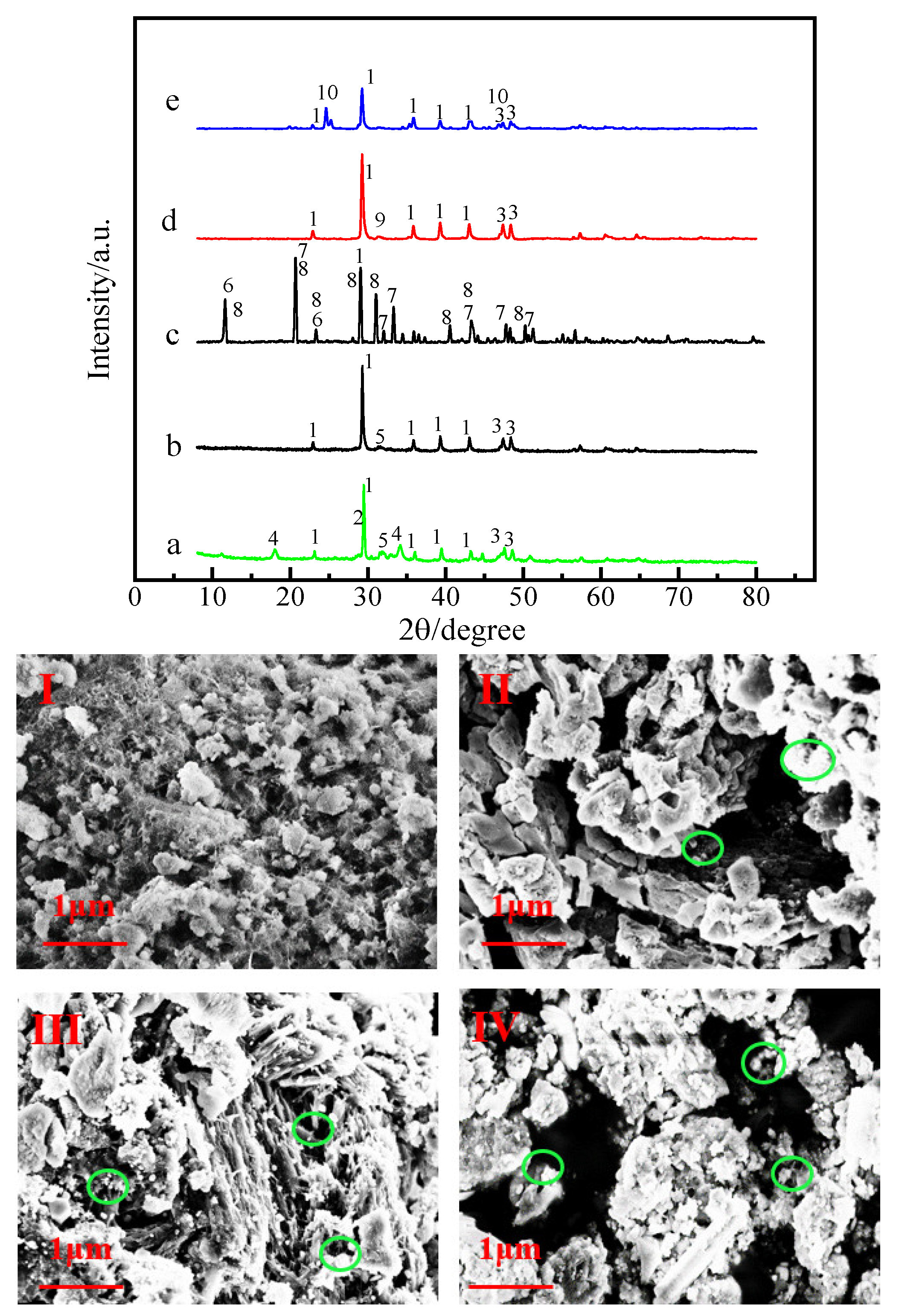
3.1. Physicochemical Properties of LLSDBs
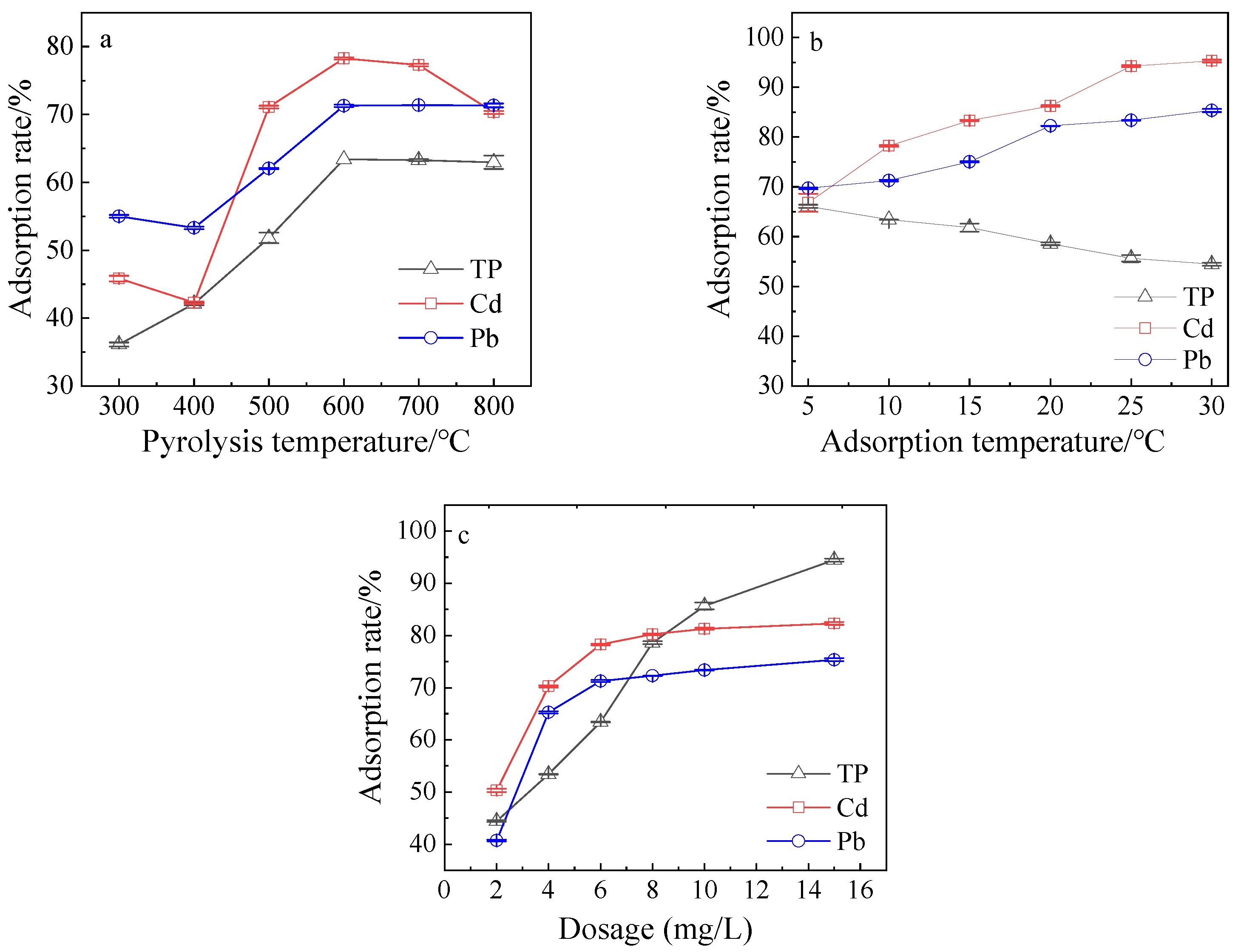
3.2. Adsorption Effect under Different Conditions
3.3. Kinetics and the Thermodynamics for Adsorption
3.3.1. Kinetics for Adsorption
3.3.2. Thermodynamics for Adsorption
3.4. Adsorption Characteristics of LLSDB600
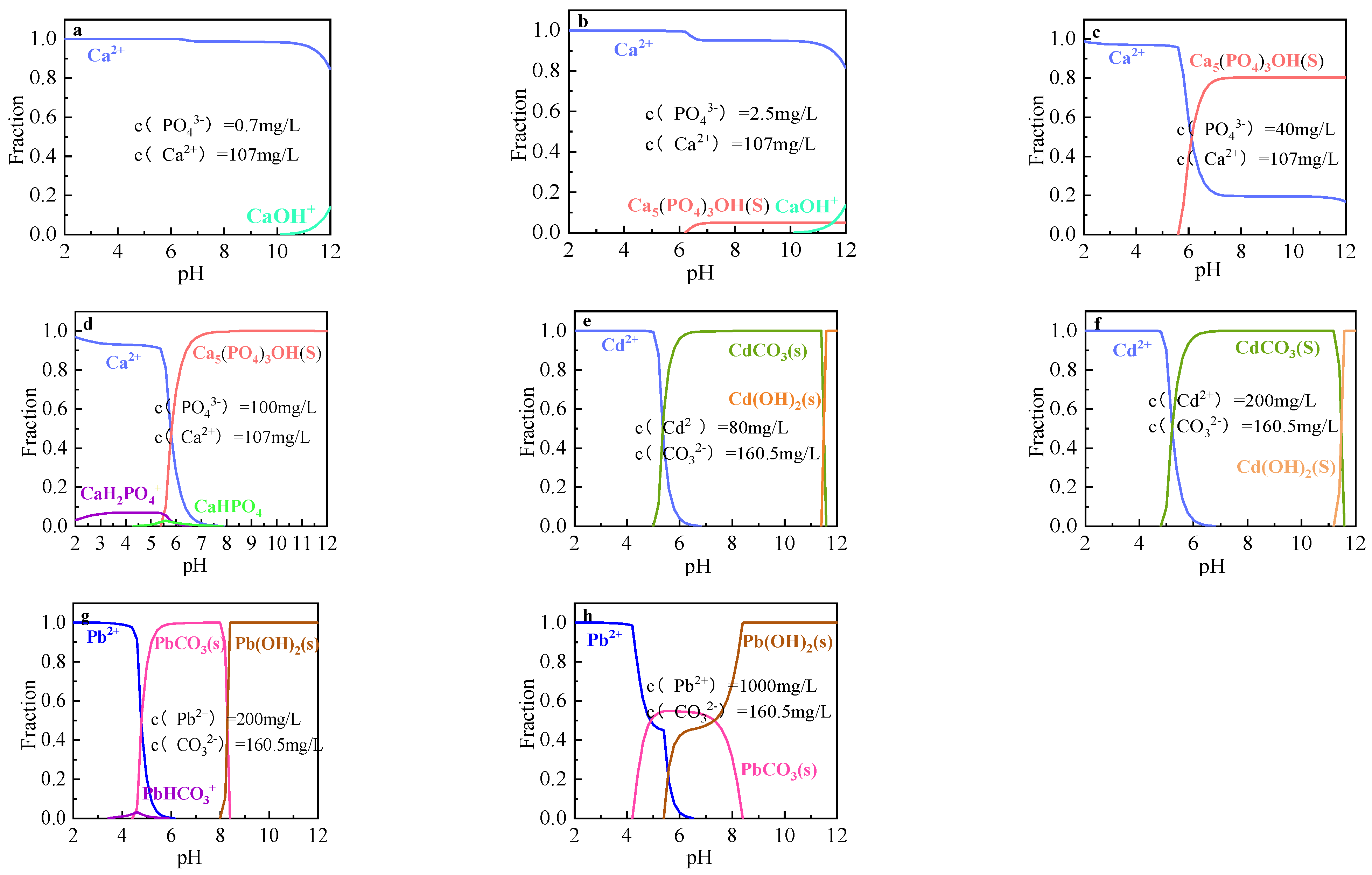
3.5. Proposed Adsorbed Mechanisms
- (1)
- The pyrolysis process and decomposition for LLSDB:
- (2)
- Dissolution for LLSDB and pH increase in the wastewater:
- (3)
- Precipitation for pollutions by LLSDB in the wastewater:
- (4)
- Exchange for pollution by LLSDB in the wastewater:
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Zhu, C.; Gao, J.; Fan, Y.; He, L.; He, C.; Wu, J. Deep-level nutrient removal and denitrifying phosphorus removal (DPR) potential assessment in a continuous two-sludge system treating low-strength wastewater: The transition from nitration to nitritation. Sci. Total Environ. 2020, 744, 140940. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Xiao, R.; Zhou, B.; Delaune, R.D.; Seo, D.C. Effect of pyrolysis temperature on phosphate adsorption characteristics and mechanisms of crawfish char. J. Colloid Interface Sci. 2018, 525, 143. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Mahmood, A.; Shahid, S.A.; Shah, S.S.; Khalid, A.M.; McKay, G. Sorption of lead from aqueous solution by chemically modified carbon adsorbents. J. Hazard. Mater. 2006, 138, 604–613. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Haritha, M.; Rajeshwari, K.; Abilash, V.G. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium) induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef]
- Chen, Z.L.; Zhang, J.Q.; Huang, L.; Yuan, Z.H.; Li, Z.-J.; Liu, M.C. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Int. Agric. 2019, 18, 10. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Zhang, Y.; Pan, W.P. The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: Considering the increased oxygen functional groups by HCl treatment. Bioresour. Technol. 2019, 291, 121859. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- González Rendón, E.S.; Cano, G.G.; Alcaraz-Zubeldia, M.; Garibay-Huarte, T.; Fortoul, T.I. Lead inhalation and hepatic damage: Morphological and functional evaluation in mice. Toxicol. Ind. Health 2018, 34, 128–138. [Google Scholar] [CrossRef]
- Abdulrazzaq, A.M.; Mohd, H.; Wahid, H.A.; Yusof, S.M.; Ali, R.M. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660–671. [Google Scholar]
- Towle, K.M.; Garnick, L.C.; Monnot, A.D. A human health risk assessment of lead (Pb) ingestion among adult wine consumers. Int. J. Food Contam. 2017, 4, 7. [Google Scholar] [CrossRef]
- Yan, J.; Huang, H.; Liu, Z.; Shen, J.; Ni, J.; Han, J.; Wang, R.; Lin, D.; Hu, B.; Jin, L. Hedgehog signaling pathway regulates hexavalent chromium-induced liver fibrosis by activation of hepatic stellate cells. Toxicol. Lett. 2020, 320, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health Risk Assessment of Dietary Cadmium Intake: Do Current Guidelines Indicate How Much is Safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Esposito, G.; Veeken, A.; Weijma, J.; Lens, P. Use of biogenic sulfide for ZnS precipitation. Sep. Purif. Technol. 2006, 51, 31–39. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D. Ion exchange of some heavy metal ions from polar organicsolvents into zeolite. Desalination 2007, 211, 238–248. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Yuan, L.; Zhou, D. Comparison of adsorption behavior studies of Cd2+ by vermicompost biochar and KMnO4-modified vermicompost biochar. J. Environ. Manag. 2020, 256, 109959. [Google Scholar] [CrossRef]
- Machado, M.; Santos, M.; Gouveia, C.; Soares, H.; Soares, E.V. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: The flocculation as a separation process. Bioresour. Technol. 2008, 99, 2107–2115. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2020, 401, 123608. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.; Méndez, A.; Pazferreiro, J.; Fernández, J.M.; Plaza, C.; Gascó, G. Biochar from deinking paper sludge as a peat replacement in growing media preparation. EGU Gen. Assem. Conf. 2015, 17, 201–203. [Google Scholar]
- Sherlala, A.; Raman, A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004. [Google Scholar] [CrossRef]
- Bai, T.; Qu, W.; Yan, Y.; Ma, K.; Xu, Y. Influence of pyrolysis temperature on the properties and environmental safety of heavy metals in chicken manure-derived biochars. J. Environ. Sci. Health Part B 2020, 55, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. Evaluation of sewage sludge and slow pyrolyzed sewage sludge-derived biochar for adsorption of phenanthrene and pyrene. Bioresour. Technol. 2015, 192, 618–626. [Google Scholar] [CrossRef]
- Wesenbeeck, S.V.; Prins, W.; Ronsse, F.; Antal, M.J. Sewage sludge carbonization for biochar applications: The fate of heavy metals. Energy Fuels 2014, 28, 5318–5326. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M. Effect of process parameters for production of microporous magnetic biochar derived from agriculture waste biomass. Microporous Mesoporous Mater. 2017, 253, 29–39. [Google Scholar] [CrossRef]
- Liu, L.; Deng, G.; Shi, X. Adsorption characteristics and mechanism of p-nitrophenol by pine sawdust biochar samples produced at different pyrolysis temperatures. Sci. Rep. 2020, 10, 5149. [Google Scholar] [CrossRef]
- Angin, D.; Sensoz, S. Effect of Pyrolysis Temperature on Chemical and Surface Properties of Biochar of Rapeseed (Brassica napus L.). Int. J. Phytorem. 2014, 16, 684–693. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Du, C.; Liu, W.; Gerson, A.R.; Pi, K. Properties and heavy metal leaching characteristics of leachate sludge-derived biochar. Water Environ. Res. 2021, 93, 3064–3074. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.; Malakahmad, A.; Sapari, N.B.; Yavari, S. Sorption properties optimization of agricultural wastes-derived biochars using response surface methodology. Process Saf. Environ. Prot. 2017, 109, 509–519. [Google Scholar] [CrossRef]
- Mishra, U.; Paul, S.; Bandyopadhaya, M. Removal of zinc ions from wastewater using industrial waste sludge: A novel approach. Environ. Prog. Sustain. Energy 2013, 32, 576–586. [Google Scholar] [CrossRef]
- Xi, X.; Guo, X. Preparation of bio-charcoal from sewage sludge and its performance on removal of Cr (VI) from aqueous solutions. J. Mol. Liq. 2013, 183, 26–30. [Google Scholar] [CrossRef]
- Jing, P.Z.; Yong, S.; Meng, W.; Lian, Z.; Xu, K. Preparation of steam activated carbon from black liquor by flue gas precipitation and its performance in hydrogen sulfide removal: Experimental and simulation works. J. Taiwan Inst. Chem. Eng. 2016, 59, 395–404. [Google Scholar]
- Han, X.; Chen, H.; Liu, Y.; Pan, J. Study on Removal of Gaseous Hydrogen Sulfide Based on Macroalgae Biochars. J. Nat. Gas Sci. Eng. 2019, 73, 103068. [Google Scholar] [CrossRef]
- Tavakoli, H.; Sepehrian, H.; Cheraghali, R. Encapsulation of nanoporous MCM-41 in biopolymeric matrix of calcium alginate and its use as effective adsorbent for lead ions: Equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2013, 44, 343–348. [Google Scholar] [CrossRef]
- Gerente, C.; Lee, V.K.C.; Cloirec, P.L.; Mckay, G. Application of Chitosan for the Removal of Metals From Wastewaters by Adsorption—Mechanisms and Models Review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 41–127. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2009, 156, 2–10. [Google Scholar] [CrossRef]
- Aksu, Z.; Aiikel, Ü. A single-staged bioseparation process for simultaneous removal of copper(II) and chromium(VI) by using C. vulgaris. Process Biochem. 1999, 34, 589–599. [Google Scholar] [CrossRef]
- Webi, T.W.; Chakravort, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar]
- Kousha, M.; Daneshvar, E.; Sohrabi, M.S.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Sun, K.; Kang, M.; Zhang, Z.; Jin, J.; Wang, Z.; Pan, Z.; Xu, D.; Wu, F.; Xing, B. Impact of Deashing Treatment on Biochar Structural Properties and Potential Sorption Mechanisms of Phenanthrene. Environ. Sci. Technol. 2013, 47, 11473–11481. [Google Scholar] [CrossRef] [PubMed]
- Néher-Neumann, E. The Liquid Junction Potential in Potentiometric Titrations. XII. The Calculation of Potentials for Emf Cells with Liquid Junctions, of the Type, AY | AY + A2MoO4+HY, Involving the Formation of Iso-Polymolybdates in the Range −log10[H+] ≤ 7, at [A+] = C mol⋅L−1, Constant, and 25 °C. J. Solution Chem. 2011, 40, 989–1040. [Google Scholar] [CrossRef]
- Ewelina, G.; Marek, M. A DFT study of uranyl hydroxyl complexes: Structure and stability of trimers and tetramers. J. Radioanal. Nucl. Chem. 2017, 313, 126–131. [Google Scholar]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef]
- Akram, M.; Xu, X.; Gao, B.; Wang, S.; Khan, R.; Yue, Q.; Duan, P.; Dan, H.; Pan, J. Highly efficient removal of phosphate from aqueous media by pomegranate peel co-doping with ferric chloride and lanthanum hydroxide nanoparticles. J. Clean. Prod. 2021, 292, 125311. [Google Scholar] [CrossRef]


| LLSDBs | Yield/% | Ash/% | Specific Surface Area (m2/g) | Total Pore (cm3/g) | pH |
|---|---|---|---|---|---|
| LLSDB300 | 85.7 ± 2 | 51.3 ± 2 | 42.1 | 2.2 | 9.1 |
| LLSDB400 | 77.3 ± 2 | 55.8 ± 1 | 42.2 | 15.3 | 9.5 |
| LLSDB500 | 71.2 ± 1 | 62.8 ± 2 | 49.2 | 16.2 | 10.7 |
| LLSDB600 | 69.8 ± 2 | 61.5 ± 1 | 59.4 | 19.1 | 11.3 |
| LLSDB700 | 67.9 ± 1 | 69.4 ± 2 | 55.0 | 19.1 | 12.2 |
| LLSDBs | C | N | H | S | O | Others |
|---|---|---|---|---|---|---|
| LLSDB300 | 17.90 | 1.87 | 1.68 | 0.07 | 23.03 | 55.45 |
| LLSDB400 | 16.30 | 0.98 | 1.02 | 0.15 | 23.55 | 58.00 |
| LLSDB500 | 13.01 | 0.59 | 0.39 | 0.31 | 22.46 | 66.24 |
| LLSDB600 | 13.74 | 0.50 | 0.28 | 0.73 | 22.20 | 62.55 |
| LLSDB700 | 12.65 | 0.28 | 0.24 | 0.63 | 21.61 | 64.94 |
| LLSDB800 | 10.79 | 0.00 | 0.32 | 0.81 | 18.16 | 69.92 |
| Model | Parameter | Adsorption Temperature (°C) and Initial Concentration (mg/L) for TP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 °C | 15 °C | 25 °C | ||||||||
| 0–1.0 | 1.0–20 | 20–120 | 0–1.0 | 1.0–20 | 20–120 | 0–1.0 | 1.0–20 | 20–120 | ||
| Langmuir | qm (mg/g) | 0.02 | 0.88 | 4.55 | 0.04 | 1.16 | 4.76 | 0.02 | 1.75 | 4.35 |
| KL (L/mg) | −2.15 | 0.20 | 0.03 | −1.17 | 0.25 | 0.05 | −1.54 | 0.24 | 0.10 | |
| R2 | 0.77 | 0.83 | 0.70 | 0.29 | 0.80 | 0.81 | 0.28 | 0.51 | 0.94 | |
| Freundlich | KF (L/mg) | 0.44 | 0.15 | 0.60 | 0.42 | 0.22 | 0.98 | 0.04 | 0.33 | 1.35 |
| 1/n | 1.99 | 0.61 | 0.39 | 2.20 | 0.67 | 0.32 | 0.56 | 0.77 | 0.25 | |
| R2 | 0.96 | 0.87 | 0.49 | 0.39 | 0.87 | 0.36 | −0.31 | 0.85 | 0.40 | |
| D-R | β | 1.74 | 2.16 | 8.75 | 2.17 | 1.93 | 3.10 | 1.53 | 1.69 | 9.48 |
| qm (mg/g) | 0.48 | 0.68 | 2.88 | 0.47 | 0.78 | 3.24 | 0.23 | 0.89 | 3.16 | |
| E | 0.51 | 0.49 | 0.31 | 0.49 | 0.50 | 0.44 | 0.53 | 0.52 | 0.30 | |
| Temkin | Kt | 2.44 | 0.64 | 0.33 | 2.41 | 0.70 | 0.64 | 0.77 | 0.75 | 1.34 |
| A | 0.11 | 0.33 | 2.25 | 0.09 | 0.45 | 2.33 | −0.03 | 0.61 | 1.96 | |
| R2 | 0.91 | 0.86 | 0.69 | 0.09 | 0.93 | 0.58 | −0.30 | 0.95 | 0.61 | |
| Model | Parameter | Adsorption temperature (°C) and initial concentration (mg/L) for Cd(II)/Pb(II) | ||||||||
| 10 °C | 20 °C | 30 °C | ||||||||
| 0–100/0–500 | 100–500/500–5000 | 0–100/0–500 | 100–500/500–5000 | 0–100/0–500 | 100–500/500–5000 | |||||
| Langmuir | qm (mg/g) | 2.27/58.78 | 181.82/36.90 | 8.13/85.47 | 33.44/263.16 | 30.67/185.19 | 38.76/263.16 | |||
| KL (L/mg) | 0.001/0.013 | 0.006/0.021 | 1.30/0.007 | 0.091/0.006 | 0.022/0.003 | 0.10/0.017 | ||||
| R2 | 0.02/0.21 | 0.83/0.95 | 0.51/0.71 | 0.97/0.49 | 0.59/0.42 | 0.99/0.77 | ||||
| Freundlich | KF (L/mg) | 0.39/3.68 | 10.30/81.47 | 2.02/0.95 | 21.48/58.88 | 0.93/0.34 | 20.50/104.71 | |||
| 1/n | 0.89/0.48 | 0.20/0.092 | 0.45/0.81 | 0.10/0.18 | 0.79/1.15 | 0.007/0.11 | ||||
| R2 | 0.89/0.68 | 0.69/0.82 | 0.73/0.51 | 0.47/0.98 | 0.76/0.68 | 0.98/0.93 | ||||
| D-R | β | 2.69/4.55 | 66.21/0.001 | 0.12/54.64 | 13.21/0.001 | 4143.81/252,068.6 | 69,636.61/296,844.2 | |||
| qm (mg/g) | 2.35/4.72 | 4.39/1.00 | 2.41/5.14 | 4.47/1.00 | 2.56/5.77 | 4.82/10.28 | ||||
| E | 0.46/0.39 | 0.12/0.71 | 0.69/0.14 | 0.26/0.71 | 0.016/5.77 | 0.004/0.002 | ||||
| Temkin | Kt | 0.57/0.59 | 1.04/5.77 | 1.35/0.36 | 11.67/0.68 | 0.76/0.32 | 37.36/3.82 | |||
| A | 8.94/26.20 | 12.61/32.74 | 5.21/45.63 | 7.79/82.33 | 9.75/70.92 | 5.29/54.18 | ||||
| R2 | 0.69/0.51 | 0.69/0.55 | 0.54/0.43 | 0.44/0.95 | 0.56/0.64 | 0.97/0.89 | ||||
| Concentration (mg/L) | Temperature (°C) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (kJ/mol·K) | |
|---|---|---|---|---|---|
| TP | 0–1 | 5 15 25 | −1.06 −0.98 −0.95 | −237.30 | −0.85 |
| 1–20 | 5 15 25 | −0.11 −0.61 −1.59 | 1986.34 | 7.14 | |
| 20–120 | 5 15 25 | −0.08 −0.75 −0.93 | 1851.31 | 6.66 | |
| Cd(II) | 0–100 | 10 20 30 | −0.28 −0.88 −1.44 | 16.82 | 0.060 |
| 100–500 | 10 20 30 | −2.14 −3.74 −4.44 | 43.18 | 0.16 | |
| Pb(II) | 0–500 | 10 20 30 | −0.015 −0.021 −2.01 | 2.19 | 0.00 |
| 500–5000 | 10 20 30 | −3.02 −4.48 −7.59 | 38.3 | 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lu, K.; Zhang, J.; Ma, C.; Wang, Z.; Tian, X. Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar. Sustainability 2023, 15, 10045. https://doi.org/10.3390/su151310045
Zhang H, Lu K, Zhang J, Ma C, Wang Z, Tian X. Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar. Sustainability. 2023; 15(13):10045. https://doi.org/10.3390/su151310045
Chicago/Turabian StyleZhang, Huiqin, Kexin Lu, Juan Zhang, Chao Ma, Zixian Wang, and Xiaofang Tian. 2023. "Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar" Sustainability 15, no. 13: 10045. https://doi.org/10.3390/su151310045
APA StyleZhang, H., Lu, K., Zhang, J., Ma, C., Wang, Z., & Tian, X. (2023). Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar. Sustainability, 15(13), 10045. https://doi.org/10.3390/su151310045







