Remediation of Acid Mine Drainage in the Haizhou Open-Pit Mine through Coal-Gangue-Loaded SRB Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Methods
2.2.1. Completely Randomized Design
2.2.2. Response Surface Methodology (RSM)
2.2.3. Tests to Reveal the Mechanism
2.3. Test Apparatus
3. Results and Discussion
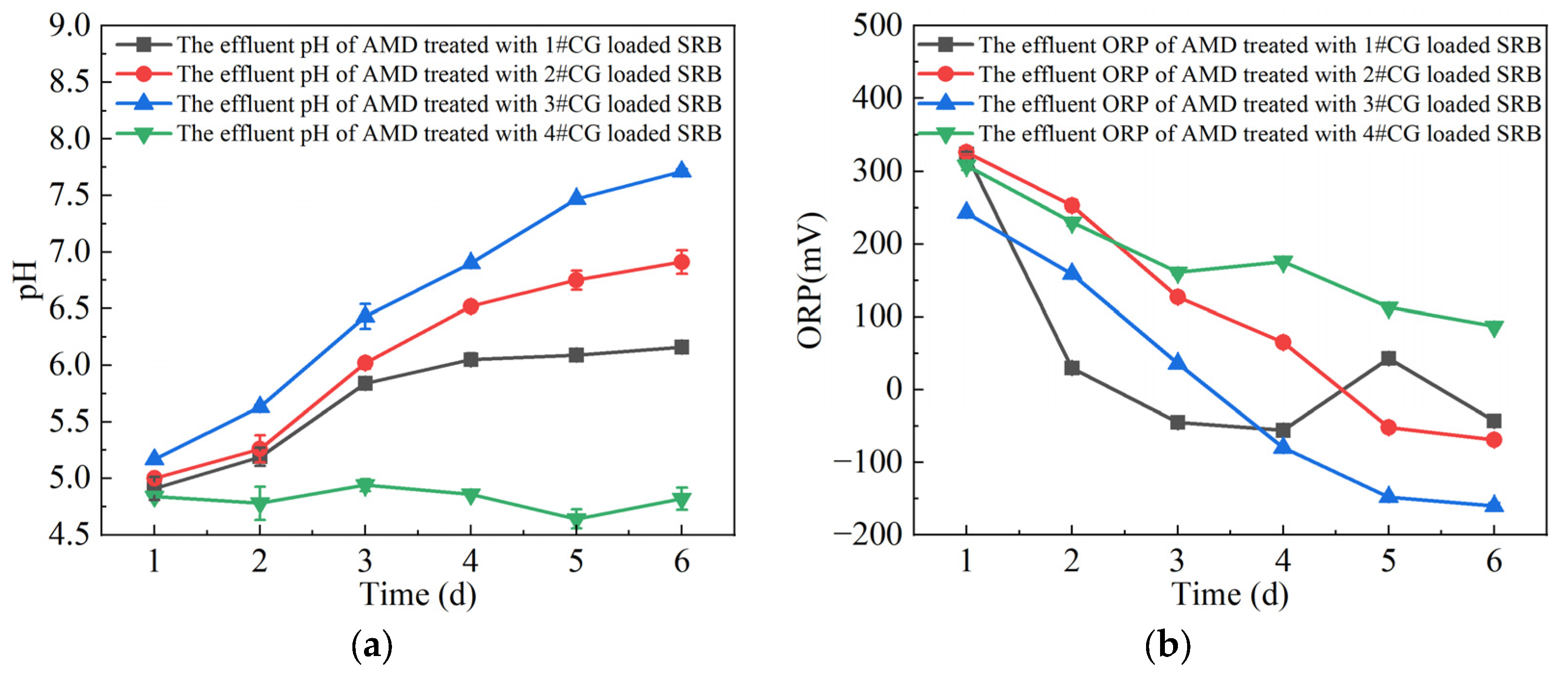
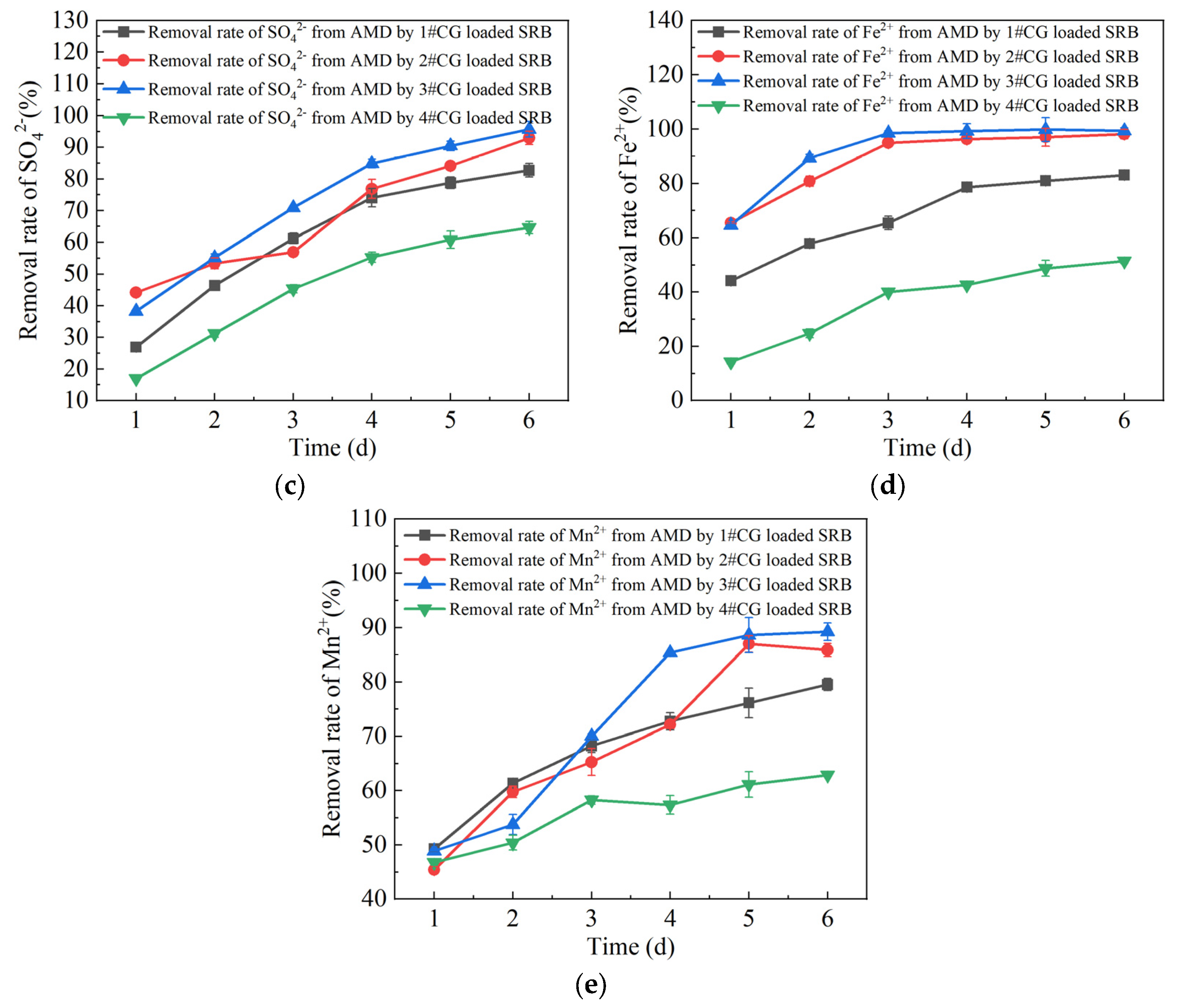
3.1. Analysis of Single-Factor Test Results
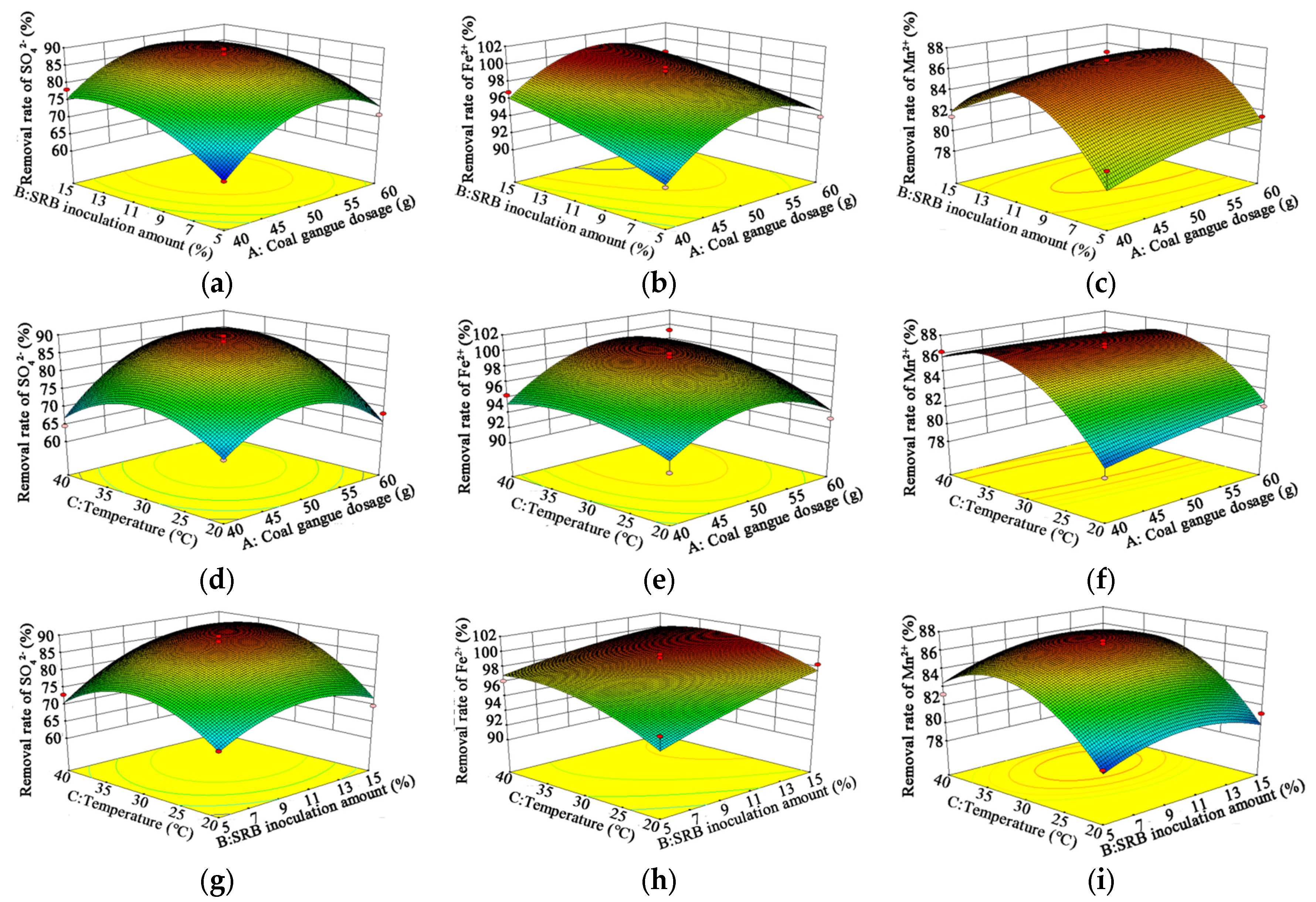
3.2. Analysis of the RSM Results
4.07 × A × C + 2.66 × B × C − 9.35 × A2 − 5.76 × B2 − 8.37 × C2, R2 = 0.9730.
0.87 × A × C − 0.59 × B × C − 3.42 × A2 − 0.45 × B2 − 1.72 × C2, R2 = 0.8982.
2.62 × A × C + 0.64 × B × C − 0.66 × A2 − 4.86 × B2 − 10.53 × C2, R2 = 0.9565.
3.3. Analysis of AMD Characteristic Test Results of Coal-Gangue-Loaded SRB Treatment in the Haizhou Open-Pit Mine
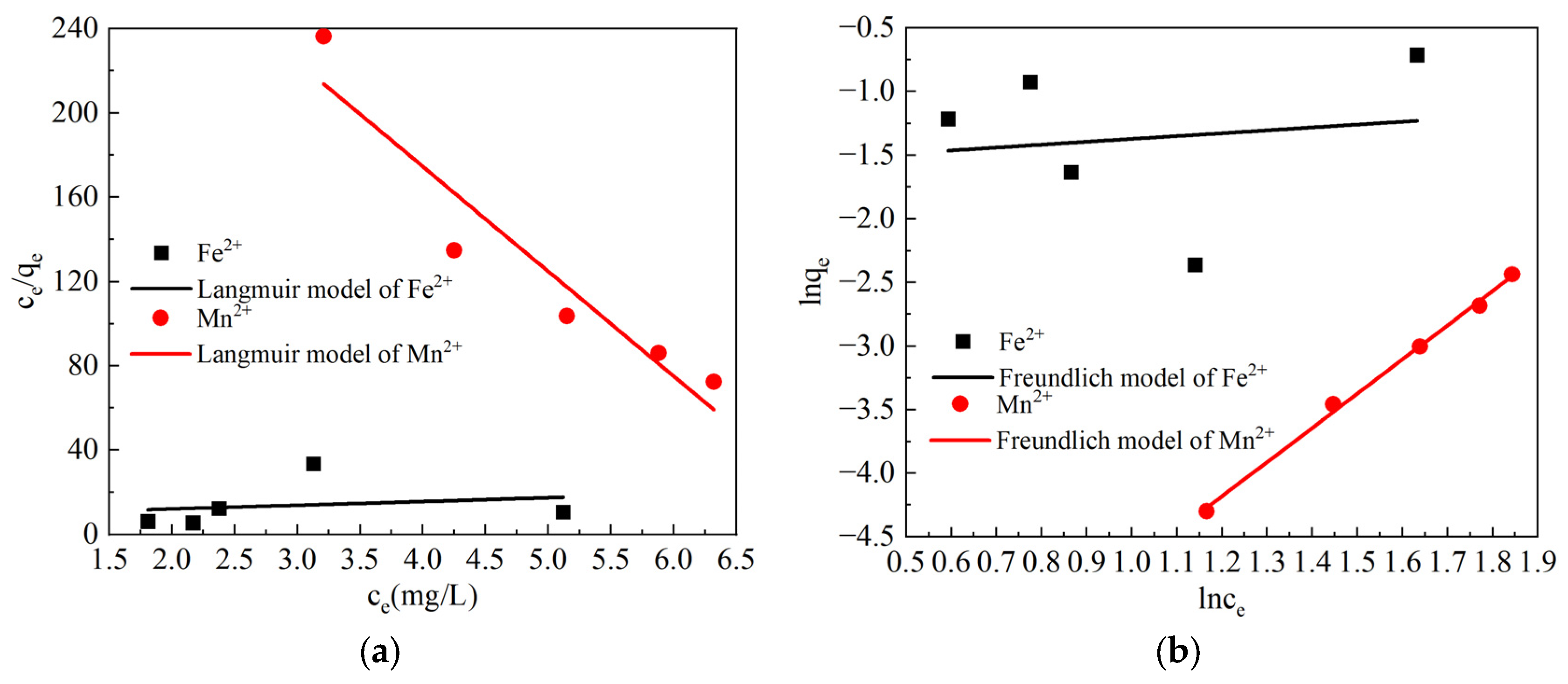
3.3.1. Analysis of the Fe2+ and Mn2+ Adsorption Isotherm Test Results
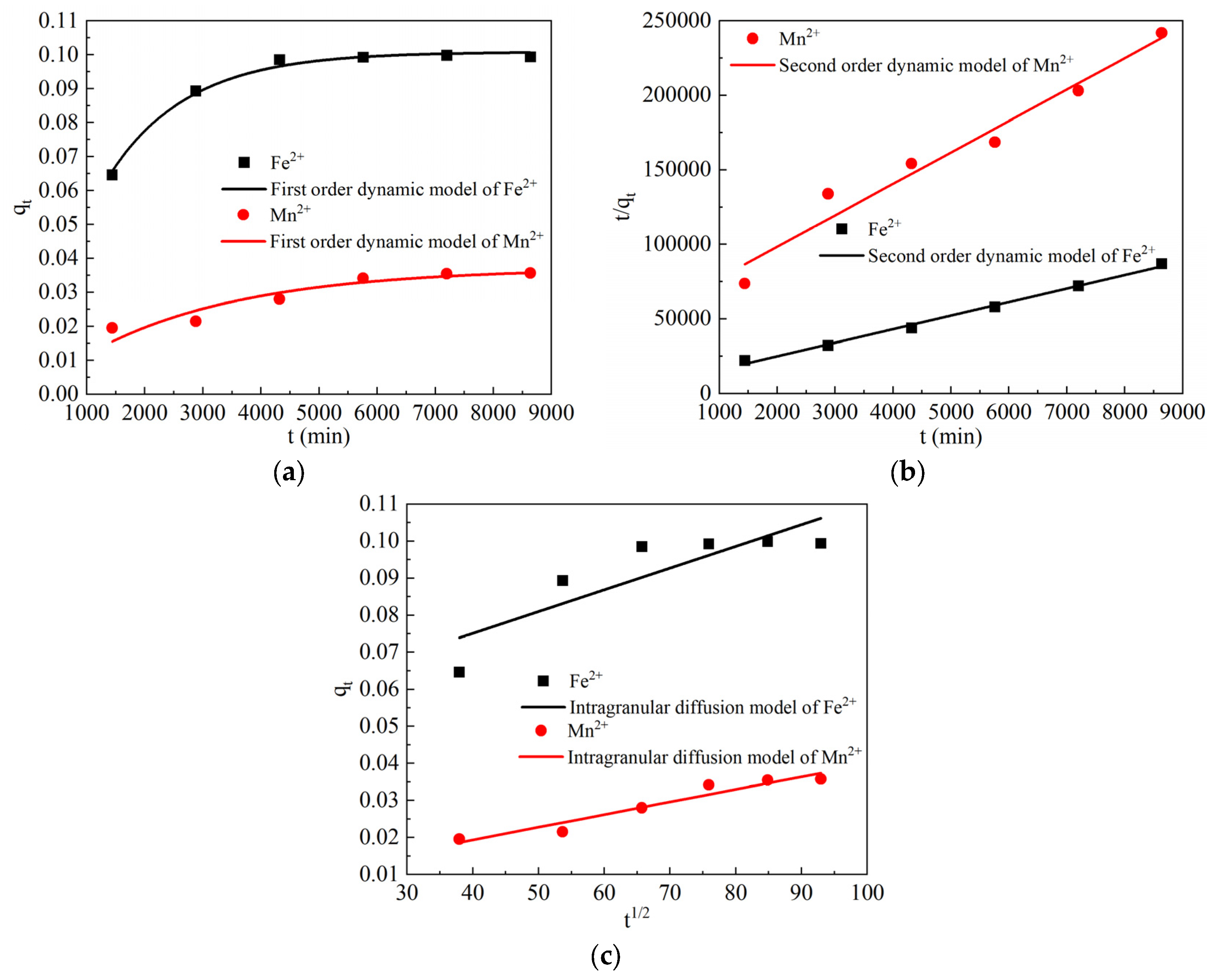
3.3.2. Analysis of the Results of the Fe2+ and Mn2+ Adsorption Kinetics Test
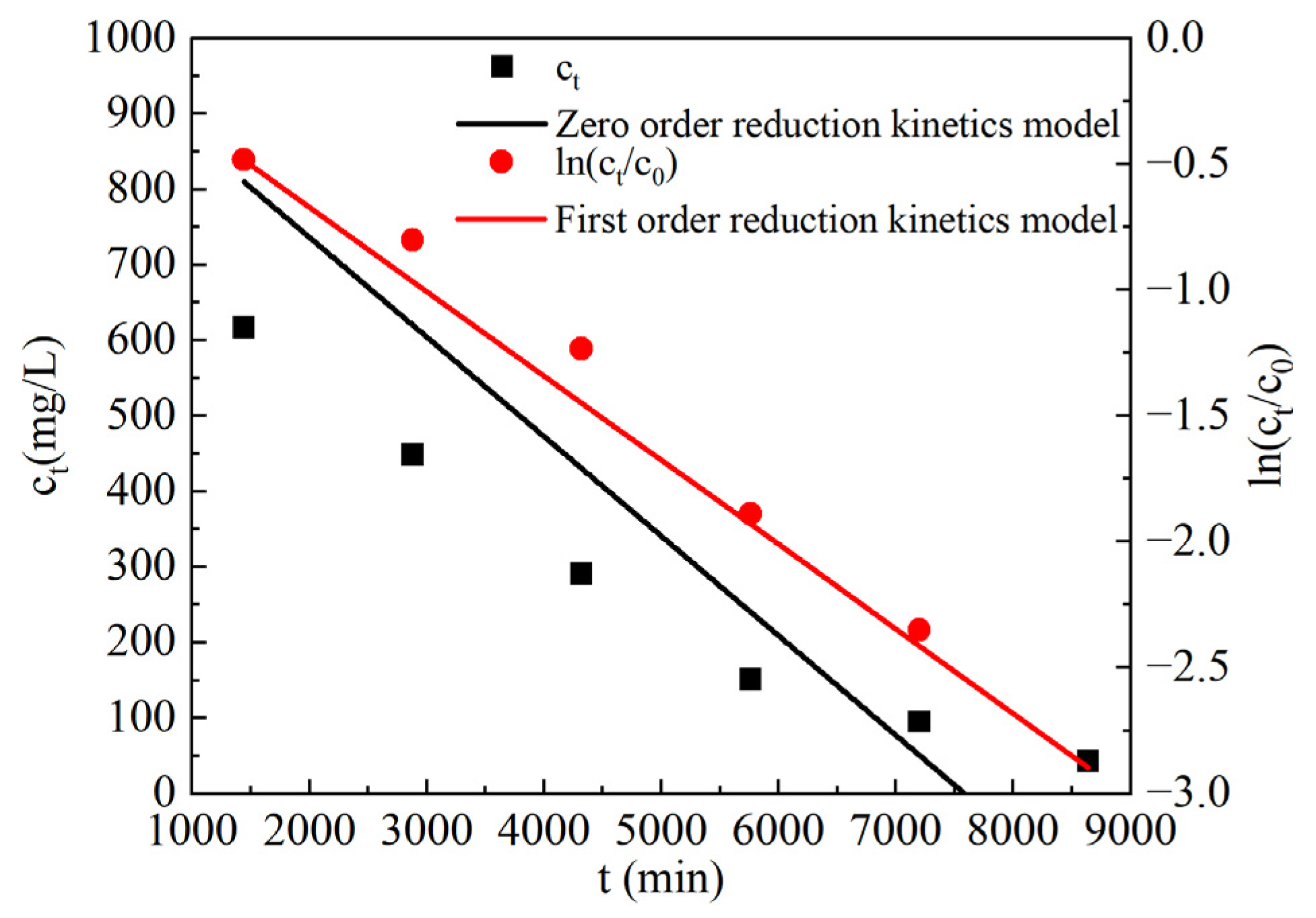
3.3.3. Analysis of Reduction Kinetics Test Results of SO42−
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masindi, V.; Akinwekomi, V.; Maree, J.P.; Muedi, K.L. Comparison of mine water neutralisation efficiencies of different alkaline generating agents. J. Environ. Chem. Eng. 2017, 5, 3903–3913. [Google Scholar] [CrossRef]
- Crafton, E.; Pritchard, C.; Guo, L.; Senko, J.M.; Cutright, T.J. Dynamics of Mn removal in an acid mine drainage treatment system over 13 years after installation. Environ. Earth Sci. 2019, 78, 10. [Google Scholar] [CrossRef]
- Hwang, S.K.; Jho, E.H. Heavy metal and sulfate removal from sulfate-rich synthetic mine drainages using sulfate reducing bacteria. Sci. Total Environ. 2018, 635, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J. Factors affecting the lower limit of the safe mud weight window for drilling operation in hydrate-bearing sediments in the Northern South China Sea. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 82. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, D.; Yin, J.; Zhou, X.; Li, Y.; Chi, P.; Han, Y.; Ansari, U.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Evolution and Mechanism. Nat. Resour. Res. 2023, 1–26. [Google Scholar] [CrossRef]
- Lazar, M.; Apostu, I.-M.; Faur, F.; Rotunjanu, I. Factors influencing the flooding process of former coal open-pits. Min. Miner. Depos. 2021, 15, 124–133. [Google Scholar] [CrossRef]
- Asif, Q.; Yu, J.; Christian, M.; Björn, Ö. Potential of fly ash for neutralisation of acid mine drainage. Environ. Sci. Pollut. Res. Int. 2016, 23, 17083–17094. [Google Scholar]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Wang, B.; Wu, D.; Ekama, G.A.; Tsui, T.-H.; Jiang, F.; Chen, G.-H. Characterization of a new continuous gas-mixing sulfidogenic anaerobic bioreactor: Hydrodynamics and sludge granulation. Water Res. 2018, 135, 251–261. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, H.; Zhu, S. Mercury emission from spontaneously ignited coal gangue hill in Wuda coalfield, Inner Mongolia, China. Fuel 2016, 182, 525–530. [Google Scholar] [CrossRef]
- Wu, H.; Wen, Q.; Hu, L.; Gong, M.; Tang, Z. Feasibility study on the application of coal gangue as landfill liner material. Waste Manag. 2017, 63, 161–171. [Google Scholar] [CrossRef]
- Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers 2022, 14, 3861. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Wu, S.; Lam, P.K.S. The environmental characteristics of usage of coal gangue in bricking-making: A case study at Huainan, China. Chemosphere 2014, 95, 274–280. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Li, Q.; Gai, A.; Yang, T.; Li, R. Study on the Properties and Heavy Metal Solidification Characteristics of Sintered Ceramsites Composed of Magnesite Tailings, Sewage Sludge, and Coal Gangue. Int. J. Environ. Res. Public Health 2022, 19, 11128. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Wu, J.; Gao, M. Calcined Coal Gangue Fines as the Substitute for Slag in the Production of Alkali-Activated Cements and Its Mechanism. Processes 2022, 10, 1557. [Google Scholar] [CrossRef]
- Li, X.; Qiao, Y.; Shao, J.; Bai, C.; Li, H.; Lu, S.; Zhang, X.; Yang, K.; Colombo, P. Sodium-based alkali-activated foams from self-ignition coal gangue by facile microwave foaming route. Ceram. Int. 2022, 48, 33914–33925. [Google Scholar] [CrossRef]
- Wang, C.Q.; Duan, D.Y.; Huang, D.M.; Chen, Q.; Tu, M.J.; Wu, K.; Wang, D. Lightweight ceramsite made of recycled waste coal gangue & municipal sludge: Particular heavy metals, physical performance and human health. J. Clean. Prod. 2022, 376, 134309. [Google Scholar]
- Zhou, C.; Liu, G.; Dun, W.; Ting, F.; Ruwei, W.; Xiang, F. Mobility behavior and environmental implications of trace elements associated with coal gangue: A case study at the Huainan Coalfield in China. Chemosphere 2014, 95, 193–199. [Google Scholar]
- Wang, J.; Lin, C.; Li, Y. Synthesis of gangue-supported Fe/FeO x nanoparticles with application for adsorption of cadmium. Acta Mater. Compos. Sin. 2022, 39, 3317–3329. [Google Scholar] [CrossRef]
- Jabłońska, B.; Kityk, A.V.; Busch, M.; Huber, P. The structural and surface properties of natural and modified coal gangue. J. Environ. Manag. 2017, 190, 80–90. [Google Scholar] [CrossRef]
- Deng, J.; Ma, Z.; Qin, Q.; Liu, W.; Wang, B. A residue reutilization strategy on fluorine-containing water purification. J. Clean. Prod. 2022, 371, 133613. [Google Scholar] [CrossRef]
- Ruifang, Q.; Fangqin, C. Modification of waste coal gangue and its application in the removal of Mn(2+) from aqueous solution. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2016, 74, 524–534. [Google Scholar]
- Li, C.; Wang, S.; Zhang, X.; Wu, J.; Zheng, S.; Sun, Z. In-situ preparation of coal gangue-based catalytic material for efficient peroxymonosulfate activation and phenol degradation. J. Clean. Prod. 2022, 374, 133926. [Google Scholar] [CrossRef]
- Guo, X.; Fu, S.; Di, J.; Dong, Y.; Jiang, G. Study on the Treatment of Acid Mine Drainage Containing Fe2+ and Mn2+ Using Modified Spontaneous Combustion Gangue. J. Renew. Mater. 2021, 9, 541–555. [Google Scholar] [CrossRef]
- Guo, X.; Dong, Y.; Di, J.; Li, Y.; Dong, Y. Experimental Treatment of Acid Mine Drainage by the Synergistic Reaction with SRB and Spontaneous Combustion Coal Gangue. Non-Met. Mines 2016, 39, 28–31. [Google Scholar]
- Wu, J.; Niu, Q.; Li, L.; Hu, Y.; Mribet, C.; Hojo, T.; Li, Y.-Y. A gradual change between methanogenesis and sulfidogenesis during a long-term UASB treatment of sulfate-rich chemical wastewater. Sci. Total Environ. 2018, 636, 168–176. [Google Scholar] [CrossRef]
- Wang, X.; Di, J.; Liang, B.; Yang, Y.; Dong, Y.; Wang, M. Study on treatment of acid mine drainage by nano zero-valent iron synergistic with SRB immobilized particles. Environ. Eng. Res. 2021, 26, 200333. [Google Scholar] [CrossRef]
- Di, J.; Ma, Y.; Wang, M.; Gao, Z.; Xu, X.; Dong, Y.; Fu, S.; Li, H. Dynamic experiments of acid mine drainage with Rhodopseudomonas spheroides activated lignite immobilized sulfate-reducing bacteria particles treatment. Sci. Rep. 2022, 12, 8783. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H. Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Miner. Eng. 2016, 92, 63–71. [Google Scholar] [CrossRef]
- GB 6920-86; Water Quality-Determination of pH Value-Glass Electrode Method. State Bureau of Environment Protection: Washington, DC, USA, 1986.
- SL 94-1994; Determination of Oxidation-Reduction Potential (Electrometric Method). Ministry of Water Resources of China: Bejing, China, 1995.
- HJ/T 342-2007; Water Quality—Determination of Sulfate—Barium Chromate Spectrophotometry. The State Environmental Protection Administration: Bejing, China, 2007.
- HJ/T 345-2007; Water Quality—Determination of Iron—Phenanthroline Spectrophotometry. The State Environmental Protection Administration: Bejing, China, 2007.
- GB 11906-1989; Water Quality—Determination of Manganese—Potassium Periodate Spectrophotometric Method. State Bureau of Environment Protection: Washington, DC, USA, 1989.
- Sun, H.; Sheng, S.; Yang, Z.; Liu, H.; Jiao, X.; Wan, A. Analysis of Black-odor Factors of Lake Sediment and Elimination Strategies of Key Factor. Environ. Sci. Surv. 2019, 38, 12–20. [Google Scholar] [CrossRef]
- Hao, T.-W.; Xiang, P.-Y.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.-K.; van Loosdrecht, M.C.M.; Chen, G.-H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Neufeld, R.D.; Ropelewski, L.; Acheson, M. Sewage as a Mixed Organic Substrate for Desulfurization Bacteria. In Proceedings of the Water Environment Federation, New Orleans, LA, USA, 29 September–3 October 2012; Volume 17. [Google Scholar]
- Taewoon, H.; Mihaela, N.C.; Jong-In, H. Biosulfides precipitation in weathered tailings amended with food waste-based compost and zeolite. J. Environ. Qual. 2012, 41, 1857–1864. [Google Scholar]
- Dong, Y.; Di, J.; Yang, Z.; Wang, X.; Zhang, Y.; Guo, X.; Li, Z.; Zhang, J. Repairing effects of sulfate-reducing bacteria on the dissolved pollutant of coal gangue based on leaching experiments. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–14. [Google Scholar] [CrossRef]
- Yoo, K.; Sasaki, K.; Hiroyoshi, N.; Tsunekawa, M.; Hirajima, T. The Effect of Mn2+ Concentration on Mn Removal by a Sulfate Reducing Bacteria Bioreactor. Mater. Trans. 2004, 45, 2429–2434. [Google Scholar] [CrossRef]
- Jamaledin, M.S.; Fatemeh, A. Adsorption Behavior of Fe(II) and Fe(III) Ions on Polyaniline Coated Sawdust: Batch and Fixed–Bed Studies. Acta Chim. Slov. 2020, 67, 36–46. [Google Scholar]
- Miao, Z.Y.; He, H.; Tan, T.; Zhang, T.; Tang, J.L.; Yang, Y.C.; Shi, K.Y.; Tang, J. Biotreatment of Mn2+ and Pb2+ with Sulfate-Reducing Bacterium Desulfuromonas alkenivorans S-7. J. Environ. Eng. 2018, 144, 04017116. [Google Scholar] [CrossRef]
- Wang, L.; Chen, T.; Chang, D.; Liu, H.; Chen, D. Cd2+ adsorption from aqueous solutions by desulfurization products of manganese nodule leached residue. Acta Petrol. Mineral. 2011, 30, 1001–1006. [Google Scholar]
- Liu, F.; Jia, M.; Men, J.; Wang, X. Selective Adsorption of Mn2+ and Co2+ in Dilute Solution by Cobalt/Manganese Imprinted Cysteine-chitosan. At. Energy Sci. Technol. 2015, 49, 984–991. [Google Scholar]
- Zhang, R.; Yang, S.; Dong, C.; Qiao, Y.; Zhang, J.; Guo, Y. Synthesized akhtenskites remove ammonium and manganese from aqueous solution: Removal mechanism and the effect of structural cations. RSC Adv. 2021, 11, 33798–33808. [Google Scholar] [CrossRef] [PubMed]
- Boughanmi, R.; Borchert, K.B.; Steinbach, C.; Mayer, M.; Schwarz, S.; Svirepa, A.; Schwarz, J.; Mertig, M.; Schwarz, D. Native and Oxidized Starch for Adsorption of Nickel, Iron, and Manganese Ions from Water. Polysaccharides 2022, 3, 556–573. [Google Scholar] [CrossRef]
- Mohammadi, R.; Azadmehr, A.; Maghsoudi, A. Fabrication of the alginate-combusted coal gangue composite for simultaneous and effective adsorption of Zn(II) and Mn(II). J. Environ. Chem. Eng. 2019, 7, 103494. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Qu, P.; Wang, Q.; Miao, Y. Preparation of heavy metal adsorption functional ceramsite by coal gangue loaded chitosan and its adsorption performance. Build. Mater. Technol. Appl. 2021, 11–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Zhu, S.; Chen, P.; Wang, C.; Li, Z. Study on adsorption of heavy metal ions in mineral processing wastewater after chelating modification of coal gangue. Inorg. Chem. Ind. 2023, 55, 97–103. [Google Scholar]
- Qi, L.; Liang, L.; Xudong, Z.; Yong, W.; Yongzhen, W. Cost-effective microwave-assisted hydrothermal rapid synthesis of analcime-activated carbon composite from coal gangue used for Pb2+ adsorption. Environ. Sci. Pollut. Res. Int. 2022, 29, 77788–77799. [Google Scholar]
- Qizheng, Q.; Jiushuai, D.; Huanhuan, G.; Zhongyi, B.; Xiahui, G.; Zhitao, M.; Zhenyong, M. An exploratory study on strategic metal recovery of coal gangue and sustainable utilization potential of recovery residue. J. Clean. Prod. 2022, 340, 130765. [Google Scholar]
- Wang, C.; Feng, K.; Wang, L.; Yu, Q.; Du, F.; Guo, X. Characterization of coal gangue and coal gangue-based sodalite and their adsorption properties for Cd2+ ion and methylene blue from aqueous solution. J. Mater. Cycles Waste Manag. 2023, 25, 1622–1634. [Google Scholar] [CrossRef]
- Liping, Z.; Aolei, C.; Hongbin, Q.; Shouqiang, X.; Xue, Z.; Xuwen, H. Fe and Mn removal from mining drainage using goaf filling materials obtained from coal mining process. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2015, 72, 1940–1947. [Google Scholar]
- Tahir, S.S.; Rauf, N. Removal of Fe(II) from the wastewater of a galvanized pipe manufacturing industry by adsorption onto bentonite clay. J. Environ. Manag. 2004, 73, 285–292. [Google Scholar] [CrossRef]
- Doula, M.K. Removal of Mn2+ ions from drinking water by using Clinoptilolite and a Clinoptilolite–Fe oxide system. Water Res. 2006, 40, 3167–3176. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Abdel-Hamid, M.I.; Rashad, M.; Ali, A.S.M.; Azab, Y.A. New calcareous soil–alginate composites for efficient uptake of Fe(III), Mn(II) and As(V) from water. Carbohydr. Polym. 2013, 96, 450–459. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Li, Z.; Tian, X. Removal of sulfate and heavy metals by sulfate-reducing bacteria in an expanded granular sludge bed reactor. Environ. Technol. 2018, 39, 3379–3389. [Google Scholar] [CrossRef] [PubMed]


| Number | Variable | Response Value | ||||
|---|---|---|---|---|---|---|
| A: Coal-Gangue Dosage (g) | B: SRB Inoculation Concentration (%) | C: Temperature (°C) | SO42− Removal Rate (%) | Fe2+ Removal Rate (%) | Mn2+ Removal Rate (%) | |
| 1 | 40 | 5 | 30 | 63.61 | 90.65 | 80.13 |
| 2 | 60 | 5 | 30 | 70.87 | 93.94 | 81.44 |
| 3 | 40 | 15 | 30 | 78.21 | 96.82 | 81.43 |
| 4 | 60 | 15 | 30 | 79.54 | 98.34 | 80.72 |
| 5 | 40 | 10 | 20 | 66.95 | 89.87 | 62.19 |
| 6 | 60 | 10 | 20 | 68.18 | 91.26 | 70.22 |
| 7 | 40 | 10 | 40 | 64.58 | 94.33 | 85.55 |
| 8 | 60 | 10 | 40 | 82.08 | 99.21 | 83.09 |
| 9 | 50 | 5 | 20 | 68.23 | 93.87 | 64.23 |
| 10 | 50 | 15 | 20 | 69.73 | 98.34 | 67.75 |
| 11 | 50 | 5 | 40 | 73.02 | 96.12 | 73.11 |
| 12 | 50 | 15 | 40 | 85.17 | 98.22 | 79.17 |
| 13 | 50 | 10 | 30 | 88.57 | 98.54 | 87.63 |
| 14 | 50 | 10 | 30 | 87.23 | 98.25 | 86.85 |
| 15 | 50 | 10 | 30 | 89.71 | 99.69 | 86.31 |
| 16 | 50 | 10 | 30 | 88.16 | 99.24 | 85.51 |
| 17 | 50 | 10 | 30 | 87.13 | 98.32 | 85.97 |
| Dynamic Model | Parameter | Adsorbent Fe2+ | Adsorbent Mn2+ |
|---|---|---|---|
| First-order dynamic model | k1 | 7.33171 × 10−4 | 3.76372 × 10−4 |
| qe | 0.10081 | 0.03716 | |
| R2 | 0.99038 | 0.85009 | |
| Second-order dynamic model | k2 | 0.01197 | 0.00792 |
| qe | 0.11027 | 0.04740 | |
| R2 | 0.99371 | 0.95363 | |
| Intraparticle diffusion model | k3 | 5.86623 × 10−4 | 3.41275 × 10−4 |
| c | 0.05164 | 0.00569 | |
| R2 | 0.67433 | 0.91709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Gao, Z.; Di, J.; Wang, D.; Yang, Z.; Guo, X.; Li, Y.; Zhu, X.; Wang, G. Remediation of Acid Mine Drainage in the Haizhou Open-Pit Mine through Coal-Gangue-Loaded SRB Experiments. Sustainability 2023, 15, 9375. https://doi.org/10.3390/su15129375
Dong Y, Gao Z, Di J, Wang D, Yang Z, Guo X, Li Y, Zhu X, Wang G. Remediation of Acid Mine Drainage in the Haizhou Open-Pit Mine through Coal-Gangue-Loaded SRB Experiments. Sustainability. 2023; 15(12):9375. https://doi.org/10.3390/su15129375
Chicago/Turabian StyleDong, Yanrong, Ziqing Gao, Junzhen Di, Dong Wang, Zhenhua Yang, Xuying Guo, Ying Li, Xiaotong Zhu, and Guixian Wang. 2023. "Remediation of Acid Mine Drainage in the Haizhou Open-Pit Mine through Coal-Gangue-Loaded SRB Experiments" Sustainability 15, no. 12: 9375. https://doi.org/10.3390/su15129375
APA StyleDong, Y., Gao, Z., Di, J., Wang, D., Yang, Z., Guo, X., Li, Y., Zhu, X., & Wang, G. (2023). Remediation of Acid Mine Drainage in the Haizhou Open-Pit Mine through Coal-Gangue-Loaded SRB Experiments. Sustainability, 15(12), 9375. https://doi.org/10.3390/su15129375







