Abstract
The increase in oil production from petroleum reservoirs has led to studies examining the effects of these activities on groundwater quality. Oily wastewater associated with oil production is often reinjected through abandoned wells into the unproductive portions of the reservoir to avoid its discharge on the surface. The reinjection process is designed to be environmentally friendly and to exclude direct interactions between injected fluids and the surrounding groundwater; nevertheless, the evaluation of the compatibility between this process and the protection of the surrounding environment is of utmost importance when oilfields are located within sensitive and protected areas. The present work aimed to evaluate the impact of the oily wastewater reinjection into a long-term and high-rate disposal well in the Val d’Agri oilfield (Southern Italy). Previous preliminary investigations carried out at the study site led researchers to hypothesize the possible hydrocarbon contamination of the shallower aquifer caused by reinjection well integrity issues. Our strategy is based on an integrated and multidisciplinary approach involving isotopic (stable isotopes 2H and 18O), chemical, and microbiological (characterization of bacterial and archaeal communities) analyses. After a comprehensive and meticulous examination of the research data, it has been ascertained that significant discrepancies exist between the shallow and reinjection water systems. This allowed us to clarify the area’s complex flow dynamics and exclude hydrocarbon contamination of spring waters caused by the reinjection process.
1. Introduction
Oily wastewaters associated with oil production are often reinjected into unproductive portions of petroleum reservoirs through abandoned wells. The reinjection process is recognized worldwide and is environmentally friendly, without any impact on groundwater flowing within the shallow geological sequence through which the reinjection wells have been drilled. Obviously, the screened interval of the reinjection wells involves only the deep petroleum reservoir to avoid direct interactions between the injected fluids and the surrounding groundwater.
The compatibility between the wastewater reinjection process and the protection of the surrounding environment is of utmost importance, primarily when oilfields are located within a sensitive and protected area, such as the Agri Valley in Southern Italy. At this site, wastewater has been reinjected since 2006 into the Costa Molina 2 (CM2) injection well, with a well-head pressure ranging from 13 to 14 MPa and an injection rate of approximately 2000 m3/day. Since then, no significant interruptions have been recorded; therefore, the CM2 injection well can be classified, on the whole, as a long-term and high-rate disposal well [1].
The impact of the oily wastewater reinjection process has been studied at the Val d’Agri oilfield [2,3,4,5] and worldwide [6] in terms of induced seismicity. However, few studies have been conducted to assess the possible interactions between oily wastewater and the local shallow environment [7,8]. Abou-Sayed et al. [7] performed a three-day injection test, by measuring quantitative parameters, such as surface and downhole pressures, ground surface deflections, and wellbore hydraulic impedance. Differently, in Kholy et al. [8] analyzed the feasibility of wastewater injection by assessing the subsurface geology and the petrophysical features of rocks to create a geomechanical earth model and a three-dimensional fracture simulator. The simulations allowed forecasting fracture growth within a candidate injection site at different injection scenarios, propaedeutic to forecast possible interactions between deep and shallow environments. In relation to the Val d’Agri oilfield, one published contribution [9] hypothesized that reinjected wastewaters in the CM2 well could contaminate shallow aquifers, probably due to well integrity issues. Nevertheless, this conference paper synthesized very preliminary results, in contrast to those obtained in more recent research carried out by Avagliano et al. [10] at the same study site.
In light of the differences that emerged between the two previous papers that focused on the Val d’Agri oilfield [9,10], a more detailed and exhaustive study was needed to experimentally verify whether the reinjection process can influence shallow groundwater and/or affect its quality. With the aim of obtaining conclusive evidence, a purpose-designed multidisciplinary approach based on chemical, isotopic, and biomolecular analyses has been developed.
In a wider context, the present study also provides a significant contribution from a methodological perspective, introducing new arguments into the discussion related to the experimental (and not only numerical) approaches that can be effective in analyzing the possible environmental impacts of oily wastewaters injection.
In the following paragraphs, after the description of the main geological and hydrogeological features of the study area, the main results of all investigations (chemical, isotopic, and biomolecular) are presented and then discussed, in order to achieve a final conclusion for the considered study site and to verify the effectiveness of the proposed multidisciplinary approach from a methodological point of view.
Study Area
The Val d’Agri is a Quaternary NW–SE trending intramountain basin located within the southern Apennines thrust belt (Southern Italy) (Figure 1), whose formation and evolution are controlled by brittle tectonics. The intense and recent deformation is testified by seismic activity in the last 40 ka, such as the M7, 1857 Basilicata earthquake [10,11,12,13].
The presence of petroleum in Val d’Agri is well known. Some explorations have been carried out in the area of Tramutola’s municipality, in which there are still natural hydrocarbon springs, recently studied in terms of bioremediation and migration of fluids [14,15,16], but the main Val d’Agri oilfield is hosted in a reservoir made of fractured, low-porosity carbonates belonging to the buried inner Apulian Platform belt [17,18,19]. Light-to-medium crude oil and gas are stored in limestone and dolomite (Miocene to Cretaceous ages) [20]. The carbonate reservoir lies below the Pliocene siliciclastic foredeep deposits and a thick mélange layer (Figure 1). Hydrocarbons have been extracted in large quantities since the mid-1990 through several wells at depths ranging from 1.8 to 3.5 km below sea level. The actual production rates are up to 65,000 barrels (bbl)/d with 2.6 × 105 m3/d of gas.
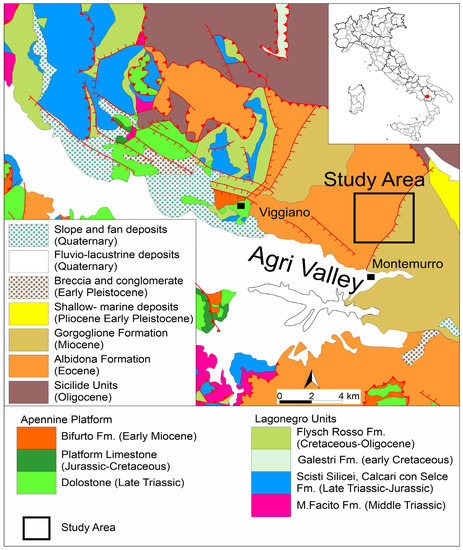
Figure 1.
Schematic map of the Agri Valley showing the location of the study area (modified by Rizzo et al. [21]).
The Apulian Platform is about 7000 m thick with a bottom part made up of evaporites, sandstones, and conglomerates (Triassic age), and lies above the crystalline basement. Well data were used to establish the progressive movement of the front of the chain towards NE during the Pliocene and early Pleistocene.
The study area is located on the left orographic side in the southeastern edge of the valley, near the municipality of Montemurro, and includes reinjection well CM2 (Costa Molina 2) (screened into the petroleum reservoir, between 2890 and 3096 m below ground). On top of the carbonate reservoir, formations belonging to the Lagonegro basin (lower Triassic–lower Cretaceous), the Liguridi–Sicilidi basin (upper Jurassic–lower Miocene), and middle-late Miocene turbiditic siliciclastic successions can be found [19,22,23,24,25,26,27] (Figure 2).
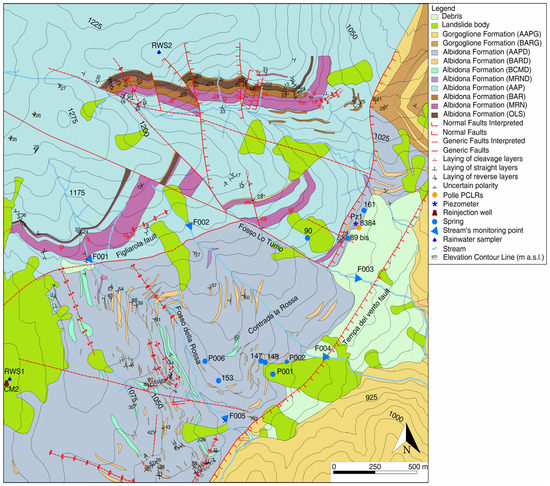
Figure 2.
Geological map and monitored springs/streams (see location in Figure 1) (modified by Rizzo et al. [21]).
In the study area, the sedimentary successions belong to the so-called turbiditic siliciclastic hydrogeological complex (sensu [28]). A detailed hydrogeological study of the outcropping formations [21] showed the absence of vertical heterogeneity due to rock alteration and/or stress-release fracturing, thereby excluding the existence of a shallower and widespread horizon characterized by higher rock permeability. On the whole, the presence of perched groundwater due to the permeability contrast with depth was excluded, and all the analyzed springs were related to unique groundwater flowing within a low-permeability continuum at the basin scale. The hydraulic continuity at this scale is enhanced by the fracture network associated with folds and faults, which can interrupt the spatial continuity of the low-permeability layers within the heterogeneous sedimentary succession. As for the location of the springs, an overall adaptation of the potentiometric surface to the ground morphology was observed. As a matter of fact, the spatial variations in the topographic gradient and the thin unsaturated aquifer cause the shallower groundwater to flow out where the phreatic surface intersects the topographic gradient. In greater detail, Rizzo et al. [21] hypothesized that the relatively high hydraulic gradient observed within the heterogeneous system is due to the back-dipping low-permeability layers (opposite to the main groundwater flow lines) that act as hydraulic barriers. According to findings related to low-permeability fault zones [29], the relatively high head loss observed within the turbidite succession would be distributed across several aquitards characterized by relatively low integrity.
2. Materials and Methods
The investigations were based on an integrated chemical-isotopic-microbiological approach.
The research was developed from December 2017 to March 2019 and involved (i) oily wastewaters before their injection in well CM2 and (ii) groundwater flowing towards the former two outflows (the so-called “Polle” of C.da La Rossa; PCLRs) associated with methane emission [9]. A purpose-designed piezometer (Pz1; 14 m deep, screened between 8 and 13 m below ground) was drilled in early November 2017, a few meters upgradient from the PCLRs. During this study, no measurable or collectible water outflow was observed at the two PCLRs after an alteration made by the area’s owner. Nevertheless, the data obtained were compared with those available for both PCLRs acquired after previous surveys (carried out in 2016; unpublished data) before they were tampered with.
2.1. Isotopic Analyses
A monthly monitoring campaign was carried out from December 2017 to March 2019 by collecting Pz1 groundwater and oily wastewaters before reinjection. The data were analyzed together with those obtained from previous surveys of the PCLRs and other spring waters. Unfortunately, due to the inability to access the study area, the campaign that started in December 2017 stopped until May 2018, when it resumed with monthly continuity.
Rainwater samples for δ(18/16O) (indicated as δ18O) and δ(2/1H) (indicated as δ2H) analyses were collected monthly in two rain samplers, RWS1 and RWS2, located within the Val d’Agri oilfield (Figure 2) at 1047 and 1290 m above sea level (m a.s.l.), respectively from May 2016 to April 2017 during a different study on the same area. Rainfall was collected using ten-liter polyethylene bottles containing about 300 mL of vaseline oil to prevent evaporation. In addition, oil contamination was carefully avoided by syringing the water samples out of the bottle.
For stable isotope (δ18O, δ2H) analysis, oily wastewater and groundwater from Pz1 were collected to compare the isotopic signature detected in the deeper and shallower aquifer.
After collection, the samples were transported in a refrigerated box to the laboratory.
Electrical conductivity, temperature, and pH were measured in situ using portable equipment (Hanna Instruments 9829, Roccafranca Padovana, Italy).
Stable isotope (δ18O and δ2H) analyses were carried out at the Isotope Geochemistry Laboratory of the University of Parma, Italy, using a water equilibrator at 18 °C online with a Finnigan Delta XP spectrometer. For the oxygen isotope determination, 5 cm3 of water was equilibrated with pure CO2, while for the hydrogen isotope, 5 cm3 of water was equilibrated with pure H2 (platinum wire was used as a catalyzer for gas–liquid water equilibration). The isotope ratios were expressed as
where A is 18O or 2H, B is 16O or 1H, R is the ratio of the isotopic abundances, s is the sample of interest, ‰ = 10−3, and RF indicates that the data refer to the VSMOW-SLAP scale.
2.2. Chemical Analyses: Organic Compounds
Oily wastewaters before their injection into well CM2 and groundwater in Pz1 were collected for chemical analyses, together with samples for isotopic determinations. The analyses were carried out at the Analytical Chemistry Laboratory of the University of Parma, Italy, using the following methods: EPA 5021A + 8260C, EPA 3511 + 8270D, Internal method + ISO 17943, and APAT CNR IRSA 5160B Man 29 2003 [30,31,32,33,34,35].
2.3. Preliminary Characterization of Microbial Communities through Biomolecular Analyses Carried Out in 2016: 16S rDNA Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (PCR-DGGE) and Next-Generation Sequencing (NGS)
Water samples for microbiological investigations were first collected in April 2016 to preliminarily analyze the bacterial and archaeal communities of PCLRs (sample codes 83 and 84), before being tampered with, other springs (sample codes P001, P002, P006, 89BIS, 90, 147, 148, 153, and 161) and stream monitoring points (sample codes F001, F002, F003, F004, and F005).
2.3.1. DNA Extraction
One L water samples were filtered through mixed esters of cellulose filters (S-PakTM Membrane Filters, 47 mm diameter, 0.22 µm pore size, Millipore Corporation, Billerica, MA, USA) within eight hours after collection. Subsequently, the filters were stored at −80 °C until DNA extraction, which was performed using a PowerWater® DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA).
2.3.2. 16S rDNA Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (PCR-DGGE)
Nested PCR was carried out to amplify bacterial 16S rDNA fragments. The primer pairs 27F/1492R and 341F-GC (with a 40 bp GC-clamp)/907R [36] were used for the primary and secondary amplifications, respectively. PCRs were performed according to the protocols reported by Hernàndez-Diaz et al. [37] using 5 µL of the template.
Nested PCR was carried out to amplify also archaeal 16S rDNA fragments, by using the primer pairs 21F/958R [38] and Parch519F/ARC915R-GC (with 40 bp GC-clamp) [39]. Reagent concentrations in the PCR mixtures were the same as those reported by Hernàndez-Diaz et al. [37], but 5 µL and 6 μL of the template were used in the primary and secondary amplifications, respectively.
Primary amplification of archaeal 16S rDNA fragments was performed using the following process: 94 °C for 2 min, 30 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min and 30 s, and a single final extension at 72 °C for 4 min. The secondary amplification consisted of an initial denaturation step at 94 °C for 2 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 40 s, extension at 72 °C for 40 s, and a final elongation step at 72 °C for 4 min.
DGGE and cluster analysis of microbial community patterns were performed as reported by Hernàndez-Diaz et al. [37].
2.3.3. Next-Generation Sequencing (NGS) Carried Out in 2016 for Samples Collected before the PCLRs Were Tampered with
A preliminary NGS sequencing was performed at the BMR Genomics srl (Padova, Italy) for water samples collected at PCLRs, other springs (sample codes 89BIS, 90, 148, 153, 161), and one stream monitoring point (sample code F003) by amplifying the V3-V4 regions of the 16S rRNA genes of Bacteria and Archaea, using the primers Pro341F and Pro805R [40], modified with universal tails.
PCR products were purified with Agencourt XP 0.8× magnetic beads and amplified using the Nextera XT Index (Illumina, 5200 Illumina Way, San Diego, CA 92122, USA) in the second PCR step. Amplicons were then normalized using SequalPrep (Thermo Fisher Scientific, 168 Third Avenue, Waltham, MA 02451, USA) and multiplexed. The pool was purified using Agencourt XP 1× magnetic beads. The library was run on an Illumina MiSeq and sequenced with V3 chemistry—300PE strategy. The forward and reverse sequences (R1 and R2 reads) were merged using the software FLASH v.1.2.11 [41] and filtered for quality (Q > 30). The clustering of the Operational Taxonomic Units (OTUs) (cluster of similar sequences with 97% identity) was based on the method pick_closed_reference_otus.py of Qiime 1.9.1 [42] and on the database Greengenes v.13–8. OTUs representing fewer than 0.005% of all sequences were filtered as recommended by Bokulich et al. [43].
2.4. Characterization of Bacterial Communities through Next-Generation Sequencing (NGS) after the PCLRs Were Tampered with
For microbial community analyses carried out after the PCLRs were tampered with, one L water samples were filtered through sterile mixed esters of cellulose filters (S-PakTM Membrane Filters, 47 mm diameter, 0.22 μm pore size, Millipore Corporation, Billerica, MA, USA) within 24 h of collection. Subsequently, DNA was extracted using the commercial kit FastDNA SPIN Kit for soil (MP Biomedicals, LLC, Solon, OH, USA) and the FastPrep®Instrument (MP Biomedicals, LLC, Solon, OH, USA). After the extraction, DNA integrity and quantity were evaluated by electrophoresis on 0.8% agarose gel containing 1 μg/mL of Gel-RedTM (Biotium, Inc., Fremont, CA, USA). The microbial community profiles in the samples were generated using NGS (Next-Generation Sequencing) technologies at Genprobio S.r.l. Laboratory, following the protocol reported by Rizzo et al. [14,21].
3. Results
3.1. Isotopic Analyses
The isotopic content of local precipitation gives the following regression δ2H on δ18O:
where s(yx) is the standard error of the regression, n is the number of couples of data, and A is the regression line’s intercept.
δ2H = [6.48 (±0.66) δ18O + 4.58 (±5.19)] ‰
s(yx) = 4.56 ‰, n = 21, p (A = 0) = 0.39
s(yx) = 4.56 ‰, n = 21, p (A = 0) = 0.39
The regression calculated at the study site is very close to that calculated by Longinelli and Selmo [44] using precipitation collected in Southern Italy, as well as those calculated at other study sites in the same macro-area [45,46,47].
All the isotopic data of groundwaters and PCLRs fall close to regression (1), demonstrating a meteoric origin of the analyzed waters (Figure 3). On the contrary, the oily wastewaters are far from the same regression line, being enriched more in 18O than in 2H (Figure 3). The data for CM2 water are consistent with a fossil source being modified by lithology-dependent diagenetic processes. This lithology is represented by the carbonate formation of a deep petroleum reservoir at high temperatures [48].
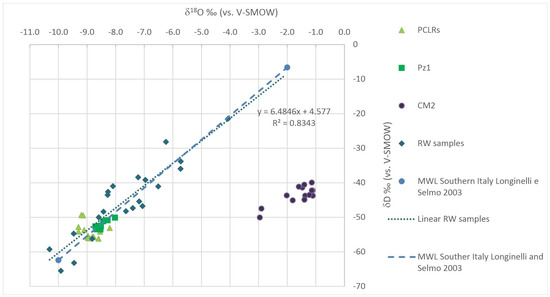
Figure 3.
O versus δ2H relationship in water samples collected from rainwater samplers at PCLRs, at the Pz1, and the oily wastewaters before the reinjection. Linear RW is the local meteoric water line obtained by local rainwater. MWL is the Meteoric Water Line of Southern Italy obtained from Longinelli and Selmo [44].
3.2. Chemical Analyses of Organic Compounds
Chemical analyses of organic compounds in the oily wastewaters before their injection in well CM2 and in waters collected at Pz1 revealed, as shown in Table 1 and Table 2 (example of available data), that the content of benzene, toluene, ethylbenzene, xylenes, and styrene was as follows: (i) always lower than the detection limit in groundwater sampled at the piezometer drilled immediately upstream of PCLRs and, as expected, (ii) much higher in the reinjection waters.

Table 1.
Results of the chemical analyses carried out on the CM2 well reinjection waters of the March 2019 sampling campaign.

Table 2.
Results of the chemical analyses carried out on the Pz1 groundwater of the March 2019 sampling campaign.
3.3. Preliminary Investigations on Microbial Communities through PCR-DGGE and Next-Generation Sequencing
A preliminary assessment of the bacterial and archaeal communities of PCLRs (sample codes 83 and 84), other springs (sample codes P001, P002, P006, 89BIS, 90, 147, 148, 153, and 161), and stream monitoring points (sample codes F001, F002, F003, F004, and F005) was conducted in April 2016 using a PCR-DGGE-based approach (Figure 4). In addition, NGS was also performed to obtain a more comprehensive identification of microorganisms constituting the microbial communities of PCLRs, some of the springs, and one stream monitoring point analyzed through PCR-DGGE (sample codes 89BIS, 90, 148, 153, 161, and F003). Unfortunately, the use of the primer pair Pro341F and Pro805R did not allow a detailed resolution of archaeal communities, unlike the bacterial ones. Nevertheless, taken together, the results of the preliminary biomolecular investigations provided an interesting contribution to further clarify if there could be hydrocarbon contamination of groundwater due to issues linked to the reinjection process. In general, the main bacterial phyla found after NGS data analysis were Proteobacteria, Bacteroidota, and Verrucomicrobiota. These taxa were present in almost all the samples, although with different relative abundance values. On the other hand, Archaea belonging to the genera Methanosarcina, Methanosaeta, and Nitrosopumilus were detected only in PCLRs and springs 90, 161, and 153. In detail, Methanosarcina and Methanosaeta are genera comprising species that are able to perform methanogenesis, an anaerobic process that generates methane as the final product of metabolism [49]. Their presence in PCLRs and in spring 161 suggests the following: (i) the methane emissions recorded in PCLRs have a biological origin (further supporting the suggestions made by Avagliano et al. [10], using the isotopic signature of methane sampled in PCLRs), and (ii) methanogenesis is not limited to PCLRs within the studied hydrogeological system. From a wider perspective, the DGGE results suggest that there is a hydraulic interconnection between the sub-basin hosting PCLRs and the larger hydrostructure to which this sub-basin belongs, in agreement with the whole hydrogeological model pointed out by Rizzo et al. [21] at the same site. In fact, the analysis of the DGGE profiles (Figure 5) shows, on the whole, a rather high similarity among the aquatic microbial communities.
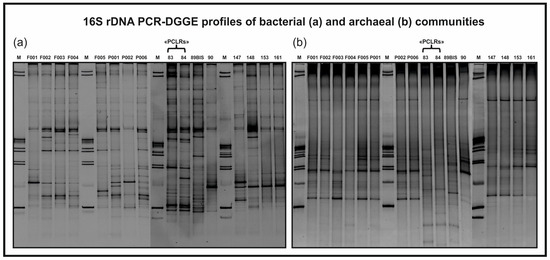
Figure 4.
Bacterial (a) and archaeal (b) 16S rDNA PCR-DGGE community profiles of PCLRs (sample codes 83 and 84) before being tampered with (April 2016), other springs (sample codes P001, P002, P006, 89BIS, 90, 147, 148, 153, and 161), and stream monitoring points (sample codes F001, F002, F003, F004, and F005).
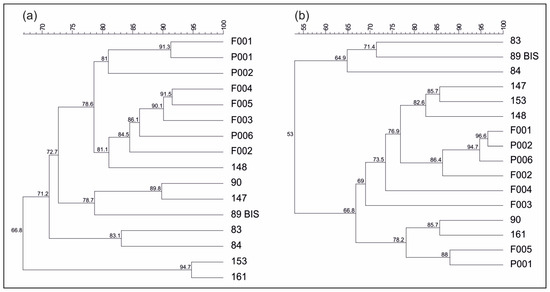
Figure 5.
Cluster analysis of bacterial (a) and archaeal (b) 16S rDNA PCR-DGGE profiles of PCLRs (sample codes 83 and 84) before being tampered with (April 2016), other springs (sample codes P001, P002, P006, 89BIS, 90, 147, 148, 153, and 161), and stream monitoring points (sample codes F001, F002, F003, F004, and F005) (Dice coefficient with the UPGMA clustering algorithm). Similarity values are shown.
3.4. Bacterial Community Analysis through Next-Generation Sequencing (NGS) after the PCLRs Were Tampered with
The results of the characterization of microbial communities of the springs through Next-Generation Sequencing have already been reported by Rizzo et al. [21]; accordingly, only the data relating to CM2 and PZ1, used as a representative of the groundwater formerly feeding the PCLRs, are discussed here. The final read numbers of the sequences obtained for each sample are shown in Table 3. Unfortunately, the PZ1 sample taken in January 2019 was damaged during transport and could not be analyzed in the laboratory.

Table 3.
The number of 16S rDNA bacterial sequences obtained after NGS analysis for the four sampling campaigns.
The 16S rRNA gene sequences have been deposited in the NCBI Sequence Read Archive (SRA: accession numbers PRJNA629324 and PRJNA877189).
The rarefaction analysis, a measure used to estimate the alpha diversity in samples and gauge whether or not sequencing efforts captured the microbial diversity, revealed Shannon index values ranging from 3.6 and 4.0 for the CM2 samples. For the Pz1 samples, lower biodiversity was recorded in September 2018 (Shannon index value of 4.0) compared to June 2018 and March 2019 (Shannon index values of 7.3 and 7.0, respectively) (Figure 6).

Figure 6.
Rarefaction curves with Shannon index for Bacteria domain.
Bacterial community analyses at the genus level (Figure 7) revealed that the waters of the CM2 well were characterized by a microbial signature very different from that found in the PZ1 waters; the most abundant genera were Desulfomicrobium (relative abundance values ranging from 38.40% to 46.75%), Thermoanaerobacterium (relative abundance values ranging from 10.88% to 28.92%), Acetomicrobium (relative abundance values ranging from 6.39% to 16.71%), and Thermovirga (relative abundance values ranging from 3.23% to 14.61%). These results testify to the presence of thermophilic bacteria in the reinjection waters, with anaerobic metabolism (fermentation or anaerobic respiration with sulfate, sulfite, and thiosulfate as electron acceptors) compatible with the characteristics of the waters in which they were found [50,51,52]. These four genera alone represented more than 67% of all the sequences obtained using NGS analyses in the different monitoring campaigns.
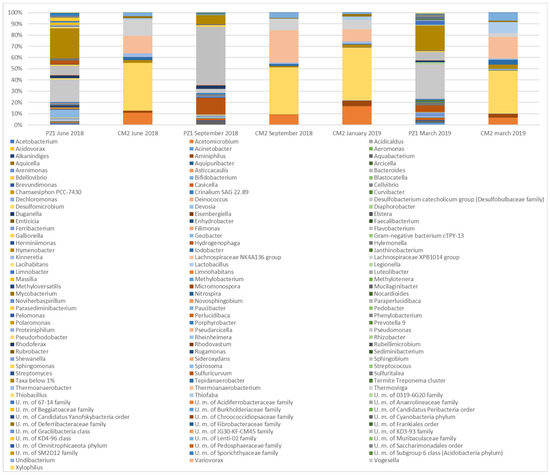
Figure 7.
Genus level bacterial community composition.
On the other hand, a predominance of entirely different genera, comprising species living at lower temperatures and under aerobic conditions, such as Sphingobium (relative abundance values ranging from 7.25% to 51.56%) or Pseudomonas (relative abundance values ranging from 3.70% to 30.64%), was detected in PZ1.
4. Discussion and Conclusions
The isotopic content (δ2H on δ18O) of the ground- (PZ1) and springwaters (including the PCLRs) is exclusively compatible with the isotopic signature of local rainwater, suggesting the absence of mixing between the shallow groundwater and possible deep fluids typical of the petroleum reservoir (in the case study, the reinjection waters). This hydrogeological scenario is in agreement with the conceptual model pointed out by Rizzo et al. [21]. As a matter of fact, the PCLRs (as well as the other springs) were located along an abrupt variation in the topographic gradient, where the hydraulic head intercepts the ground surface, thereby causing shallow groundwater to flow out. Their location was not anomalous within the local hydrogeological system, so much so that both PCLRs flowed out very close to springs 89 bis and 161. Moreover, the reinjection waters had an isotopic signature incompatible with that of the local precipitations, whereas they were consistent with a fossil source being modified by lithology-dependent diagenetic processes.
Biomolecular and chemical analyses further support this scenario. In fact, the biomolecular investigations showed very different microbial communities between the shallow and deep fluids. CM2 reinjection waters were characterized by thermophilic genera with anaerobic metabolism, whereas shallow ground- and springwaters were characterized by psychrophilic or mesophilic aerobic communities. Moreover, methanogens were found not only in the former PCLRs, but also in another spring (161) flowing out close to the PCLRs, further demonstrating that methane at the study site is biogenic (and not thermogenic), and is typical of some of the sub-basins coexisting within the whole hydrogeological system analyzed in the present study.
As for the chemical analyses, hydrocarbons have never been detected in the PZ1-waters in any monitoring campaign. Conversely, the reinjection fluids in CM2 were always characterized by high concentrations of hydrocarbons. In oil contaminant plumes, the analysis of only total petroleum hydrocarbons (TPH) could be ineffective due to the potential biological effects of degradation [53]; in the case study, a set of 25 different analytes were analyzed as possible tracers.
On the whole, the synergic analysis of the chemical, isotopic, and biomolecular data obtained through the developed multidisciplinary approach made it possible to demonstrate the total absence of interaction between the reinjection water in CM2 and the shallow groundwater system, including the former PCLRs.
From a methodological point of view, this research (together with that carried out by Rizzo et al. [21]) further demonstrates the importance of applying interdisciplinary approaches when studying the possible hydraulic interconnections between deep and shallow fluids in heterogeneous geological systems, with an emphasis on those systems where reinjection processes of oily wastewaters can potentially cause the contamination of groundwater and aquatic ecosystems. The synergy between different disciplines and the utilization of experimental data coming from independent investigations is the only way to obtain an exhaustive and effective response.
Author Contributions
Conceptualization, P.R., A.B., A.M.S., G.N., P.I., F.B., C.M. and F.C. (Fulvio Celico); methodology, P.R., A.B., P.I., F.B. and F.C. (Fulvio Celico); software, P.R. and A.B.; validation, P.R., A.B., P.M. and M.R.; formal analysis, P.R., A.B., P.I., F.B. and N.R.; investigation, P.R., A.B., P.M., N.R. and A.C.; resources, A.M.S., P.I., F.B., C.M., G.N., D.A., F.C. (Francesco Coraggio), A.C. and F.C. (Fulvio Celico); data curation, P.R., A.B. and M.R.; writing—original draft preparation, P.R. and A.B.; writing—review and editing, P.R., A.B., D.A., F.C. (Francesco Coraggio) and F.C. (Fulvio Celico); visualization, P.R. and A.B.; supervision, A.M.S., P.I., F.B., C.M. and F.C. (Fulvio Celico); project administration, F.C. (Fulvio Celico); funding acquisition, F.C. (Fulvio Celico). All authors have read and agreed to the published version of the manuscript.
Funding
This work has benefited from the equipment and framework of the COMP-HUB and COMP-R Initiatives, funded by the ‘Departments of Excellence’ program of the Italian Ministry for University and Research (MIUR, 2018–2022, and MUR, 2023–2027).
Data Availability Statement
The data presented in this study are all the available data.
Acknowledgments
We are thankful to three anonymous reviewers for their interesting comments/questions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frohlich, C. Two-year survey comparing earthquake activity and injection-well locations in the Barnett Shale, Texas. Proc. Natl. Acad. Sci. USA 2012, 109, 13934–13938. [Google Scholar] [CrossRef] [PubMed]
- Buttinelli, M.; Improta, L.; Bagh, S.; Chiarabba, C. Inversion of inherited thrusts by wastewater injection induced seismicity at the Val d’Agri oilfield (Italy). Sci. Rep. 2016, 6, 37165. [Google Scholar] [CrossRef] [PubMed]
- Improta, L.; Bagh, S.; De Gori, P.; Valoroso, L.; Pastori, M.; Piccinini, D.; Chiarabba, C.; Anselmi, M.; Buttinelli, M. Reservoir structure and wastewater-induced seismicity at the Val d’Agri Oilfield (Italy) shown by three-dimensional Vp and Vp/Vs local earthquake tomography. J. Geophys. Res. Solid Earth 2017, 122, 9050–9082. [Google Scholar] [CrossRef]
- Hager, B.H.; Dieterich, J.; Frohlich, C.; Juanes, R.; Mantica, S.; Shaw, J.H.; Bottazzi, F.; Caresani, F.; Castineira, D.; Cominelli, A.; et al. A process-based approach to understanding and managing triggered seismicity. Nature 2021, 595, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Stabile, T.A.; Vlček, J.; Wcisło, M.; Serlenga, V. Analysis of the 2016–2018 fluid-injection induced seismicity in the High Agri Valley (Southern Italy) from improved detections using template matching. Sci. Rep. 2021, 11, 20630. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, J.L.; Ellsworth, W.L.; McGarr, A.; Benz, H.M. The 2001–present induced earthquake sequence in the Raton Basin of northern New Mexico and southern Colorado. Bull. Seismol. Soc. Am. 2014, 104, 2162–2181. [Google Scholar] [CrossRef]
- Abou-Sayed, A.S.; Andrews, D.E.; Buhidma, I.M. Evaluation of oily waste injection below the permafrost in Prudhoe Bay field. In Proceedings of the SPE California Regional Meeting, Bakersfield, CA, USA, 5–7 April 1989. [Google Scholar] [CrossRef]
- Kholy, S.M.; Sameh, O.; Mounir, N.; Shams, M.; Mohamed, I.M.; Abou-Sayed, A.; Abou-Sayed, O. Evaluating the Feasibility of Waste Slurry Injection in an Oil Prospect in the Western Desert, Egypt. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 18–21 March 2019. [Google Scholar] [CrossRef]
- Colella, A. Anomalous deep waters gurgling to the surface and impacting soils in the Val d’Agri oil field, Southern Italy. Int. J. Ecosyst. Ecol. Sci. 2014, 4, 533–542. [Google Scholar]
- Avagliano, D.; Coraggio, F.; Martinenghi, C.P.; Barbieri, C.; Previde Massara, E.; Musto, M.A.; Caputi, A.; Palladino, G.; Prosser, G.; Celico, F. L’importanza del modello geologico-concettuale negli studi ambientali: Il caso delle manifestazioni sorgive nell’area di Contrada La Rossa (Montemurro—Potenza). In Geologia dell’Ambiente; SIGEA Italian Society of Environmental Geology: Rome, Italy, 2022; ISSN 1591-5352. (In Italian) [Google Scholar]
- Giano, S.I.; Maschio, L.; Alessio, M.; Ferranti, L.; Improta, S.; Schiattarella, M. Radiocarbon dating of active faulting in the Agri high valley, Southern Italy. J. Geodyn. 2000, 29, 371–386. [Google Scholar] [CrossRef]
- Daddezio, G.; Karner, D.B.; Burrato, P.; Insinga, D.; Maschio, L.; Ferranti, L.; Renne, P.R. Tephrochronology in faulted Middle Pleistocene tephra layer in the Val d’Agri area (Southern Italy). Ann. Geophys. 2009, 49, 1029–1040. Available online: http://hdl.handle.net/2122/2122 (accessed on 31 March 2023).
- Giocoli, A.; Stabile, T.A.; Adurno, I.; Perrone, A.; Gallipoli, M.R.; Gueguen, E.; Norelli, E.; Piscitelli, S. Geological and geophysical characterization of the south-eastern side of the High Agri Valley (southern Apennines, Italy). Nat. Hazards Earth Syst. Sci. Discuss 2014, 2, 6271–6294. [Google Scholar] [CrossRef]
- Rizzo, P.; Bucci, A.; Sanangelantoni, A.M.; Iacumin, P.; Celico, F. Coupled Microbiological–Isotopic Approach for Studying Hydrodynamics in Deep Reservoirs: The Case of the Val d’Agri Oilfield (Southern Italy). Water 2020, 12, 1483. [Google Scholar] [CrossRef]
- Rizzo, P.; Malerba, M.; Bucci, A.; Sanangelantoni, A.M.; Remelli, S.; Celico, F. Potential enhancement of the in-situ bioremediation of contaminated sites through the isolation and screening of bacterial strains in natural hydrocarbon springs. Water 2020, 12, 2090. [Google Scholar] [CrossRef]
- Remelli, S.; Rizzo, P.; Celico, F.; Menta, C. Natural surface hydrocarbons and soil faunal biodiversity: A bioremediation perspective. Water 2020, 12, 2358. [Google Scholar] [CrossRef]
- Merlini, S.; Mostardini, F. Appennino centro-meridionale: Sesioni geologiche e proposta di modello strutturale. In Geologia Dell’Italia; Centrale Congresso Nazionale: Rome, Italy, 1986; Volume 73, Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=8170990 (accessed on 31 March 2023).
- Butler, R.W.H.; Corrado, S.; Mazzoli, S.; De Donatis, M.; Di Bucci, D.; Naso, G.; Scrocca, D.; Nicolai, C.; Zucconi, V. Time and space variability of «thin-skinned» and «thick-skinned» thrust tectonics in the Apennines (Italy). Rend. Lincei 2000, 11, 5–39. [Google Scholar] [CrossRef]
- Menardi Noguera, A.; Rea, G. Deep structure of the Campanian–Lucanian arc (southern Apennine, Italy). Tectonophysics 2000, 324, 239–265. [Google Scholar] [CrossRef]
- Wavrek, D.A.; Mosca, F. Compositional grading in the oil column: Advances from a mass balance and quantitative molecular analysis. Geol. Soc. Lond. Spec. Publ. 2004, 237, 207–220. [Google Scholar] [CrossRef]
- Rizzo, P.; Severini, E.; Bucci, A.; Bocchia, F.; Palladino, G.; Riboni, N.; Sanangelantoni, A.M.; Francese, R.; Giorgi, M.; Iacumin, P.; et al. How do turbidite systems behave from the hydrogeological point of view? New insights and open questions coming from an interdisciplinary work in Southern Italy. PLoS ONE 2022, 17, e0268252. [Google Scholar] [CrossRef]
- Boenzi, F.; Ciaranfi, N. Stratigrafia di dettaglio del Flysch di Gorgoglione (Lucania). Mem. Della Soc. Geol. Ital. 1970, 9, 65–79. [Google Scholar]
- Lentini, F.; Carbone, S.; Di Stefano, A.; Guarnieri, P. Stratigraphical and structural constraints in the Lucanian Apennines (Southern Italy): Tools for reconstructing the geological evolution. J. Geodyn. 2002, 34, 141–158. [Google Scholar] [CrossRef]
- Tavarnelli, E.; Prosser, G. The complete Apennines orogenic cycle preserved in a transient single outcrop near San Fele, Lucania, Southern Italy. J. Geol. Soc. 2003, 160, 429–434. [Google Scholar] [CrossRef]
- Giannandrea, P.; Loiacono, F.; Maiorano, P.; Liler, F. Carta Geologica del Settore Orientale del Bacino di Gorgoglione (Eastern Sector); Litografia Artistica Cartografica Srl: Florence, Italy, 2009. [Google Scholar]
- Maffione, M.; Speranza, F.; Cascella, A.; Longhitano, S.G.; Chiarella, D. A~125 post-early Serravallian counterclockwise rotation of the Gorgoglione Formation (Southern Apennines, Italy): New constraints for the formation of the Calabrian Arc. Tectonophysics 2013, 590, 24–37. [Google Scholar] [CrossRef]
- Romano, G.; Balasco, M.; Siniscalchi, A.; Gueguen, E.; Petrillo, Z.; Tripaldi, S. Geological and geo-structural characterization of the Montemurro area (Southern Italy) inferred from audiomagnetotelluric survey. Geomat. Nat. Hazards Risk 2018, 9, 1156–1171. [Google Scholar] [CrossRef]
- Pantaleone, D.V.; Vincenzo, A.; Fulvio, C.; Silvia, F.; Cesaria, M.; Giuseppina, M.; Ilaria, M.; Vincenzo, P.; Rosa, S.A.; Gianpietro, S.; et al. Hydrogeology of continental Southern Italy. J. Maps 2018, 14, 230–241. [Google Scholar] [CrossRef]
- Petrella, E.; Aquino, D.; Fiorillo, F.; Celico, F. The effect of low-permeability fault zones on groundwater flow in a compartmentalized system. Experimental evidence from a carbonate aquifer (Southern Italy). Hydrol. Process. 2014, 29, 1577–1587. [Google Scholar] [CrossRef]
- U.S. EPA. Method 5021A (SW-846 Update V): Volatile Organic Compounds in Various Sample Matrices Using Equilibrium Headspace Analysis; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- U.S. EPA. Method 8260C: Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- U.S. EPA. Method 3511 (SW-846 Update V): Organic Compounds in Water by Microextraction; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- U.S. EPA. Method 8270D (SW-846 Update V): Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- ISO 17943; Water quality—Determination of Volatile Organic Compounds in Water—Method Using Headspace Solid-Phase Micro-Extraction (HS-SPME) followed by Gas Chromatography-Mass Spectrometry (GC-MS). International Organization for Standardization: Geneva, Switzerland, 2016.
- APAT CNR IRSA 5160B Man 29/2003; Metodi Analitici per le Acque, Vol. 2—Sez. 5000: Costituenti Organici. The Agency for the Protection of the Environment and for Technical Services: Roma, Italy, 2004; ISBN 88-448-0083-7. Available online: https://www.irsa.cnr.it/wp/wp-content/uploads/2022/04/Vol2_Sez_5000_Organici.pdf (accessed on 31 March 2023). (In Italian)
- Chong, C.W.; Dunn, M.J.; Convey, P.; Tan, G.Y.A.; Wong, R.C.S.; Tan, I.K.P. Environmental influences on bacterial diversity of soils on Signy Island, maritime Antarctic. Polar Biol. 2009, 32, 1571–1582. [Google Scholar] [CrossRef]
- Hernàndez-Diaz, R.; Petrella, E.; Bucci, A.; Naclerio, G.; Feo, A.; Sferra, G.; Chelli, A.; Zanini, A.; Gonzalez-Hernandez, P.; Celico, F. Integrating hydrogeological and microbiological data and modelling to characterize the hydraulic features and behaviour of coastal carbonate aquifers: A case in western Cuba. Water 2019, 11, 1989. [Google Scholar] [CrossRef]
- DeLong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef]
- Coolen, M.J.; Hopmans, E.C.; Rijpstra, W.I.C.; Muyzer, G.; Schouten, S.; Volkman, J.K.; Damsté, J.S.S. Evolution of the methane cycle in Ace Lake (Antarctica) during the Holocene: Response of methanogens and methanotrophs to environmental change. Org. Geochem. 2004, 35, 1151–1167. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Longinelli, A.; Selmo, E. Isotopic composition of precipitation in Italy: A first overall map. J. Hydrol. 2003, 270, 75–88. [Google Scholar] [CrossRef]
- Paternoster, M.; Liotta, M.; Favara, R. Stable isotope ratios in meteoric recharge and groundwater at Mt. Vulture volcano, Southern Italy. J. Hydrol. 2008, 348, 87–97. [Google Scholar] [CrossRef]
- Petrella, E.; Capuano, P.; Carcione, M.; Celico, F. A high-altitude temporary spring in a compartmentalized carbonate aquifer: The role of low-permeability faults and karst conduits. Hydrol. Process. 2009, 23, 3354–3364. [Google Scholar] [CrossRef]
- Petrella, E.; Celico, F. Mixing of water in a carbonate aquifer, Southern Italy, analysed through stable isotope investigations. Int. J. Speleol. 2013, 42, 25–33. [Google Scholar] [CrossRef]
- Candela, S.; Mazzoli, S.; Megna, A.; Santini, S. Finite element modelling of stress field perturbations and interseismic crustal deformation in the Val d’Agri region, southern Apennines, Italy. Tectonophysics 2015, 657, 245–259. [Google Scholar] [CrossRef]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef]
- Guan, J.; Xia, L.-P.; Wang, L.-Y.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Diversity and distribution of sulfate-reducing bacteria in four petroleum reservoirs detected by using 16S rRNA and dsrAB genes. Int. Biodeterior. Biodegrad. 2012, 76, 58–66. [Google Scholar] [CrossRef]
- Grassia, G.S.; McLean, K.M.; Glénat, P.; Bauld, J.; Sheehy, A.J. A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol. Ecol. 1996, 21, 47–58. [Google Scholar] [CrossRef]
- Dahle, H.; Birkeland, N.K. Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Int. J. Syst. Evol. Microbiol. 2006, 56, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Bekins, B.A.; Brennan, J.C.; Tillitt, D.E.; Cozzarelli, I.M.; Illig, J.M.; Martinović-Weigelt, D. Biological effects of hydrocarbon degradation intermediates: Is the total petroleum hydrocarbon analytical method adequate for risk assessment? Environ. Sci. Technol. 2020, 54, 11396–11404. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).