A Brief Insight into the Toxicity Conundrum: Modeling, Measuring, Monitoring and Evaluating Ecotoxicity for Water Quality towards Environmental Sustainability
Abstract
1. Introduction
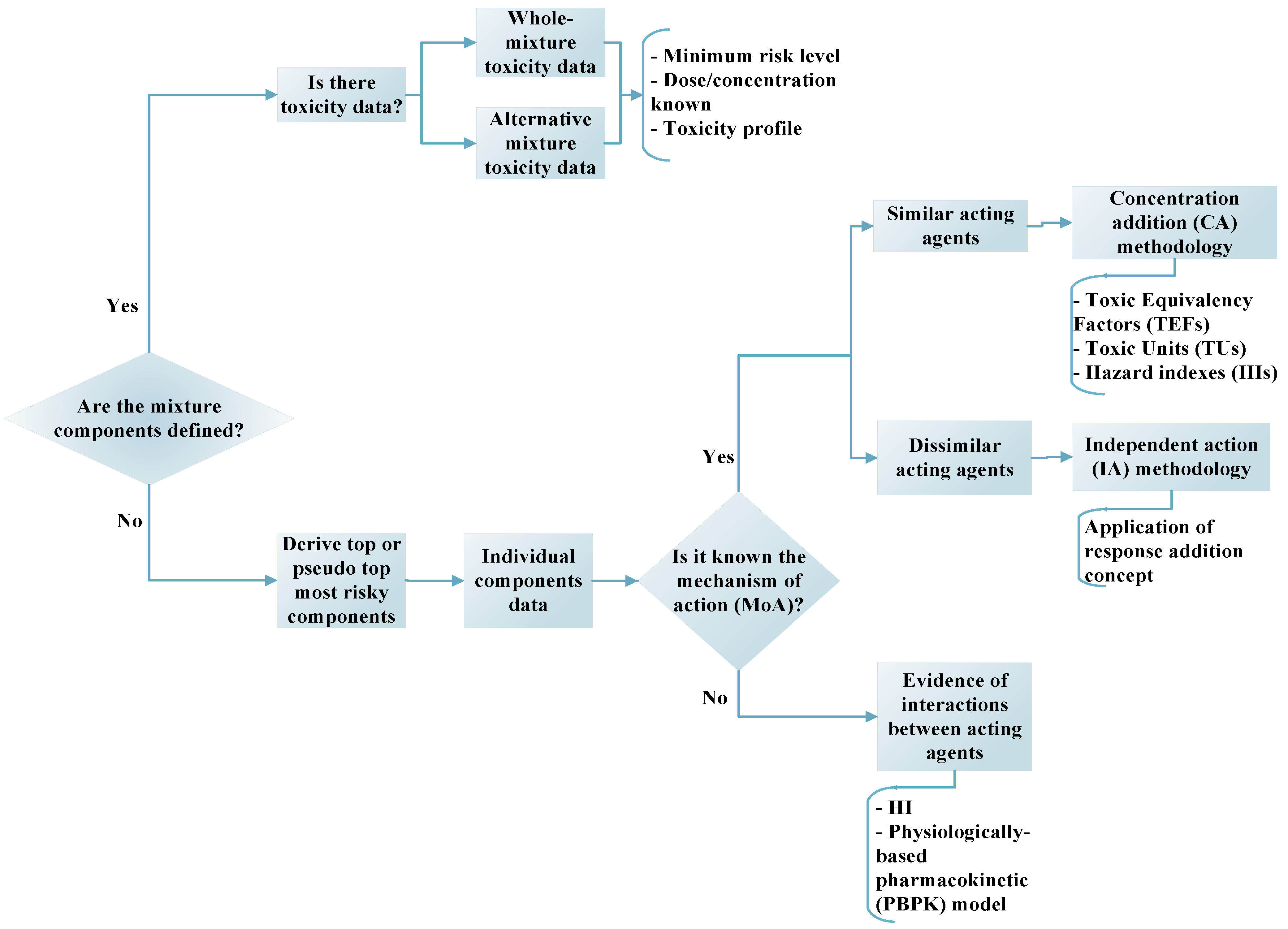
2. Evaluation of Ecotoxicity Modeling Methodologies
2.1. Individual Chemical Modeling
2.1.1. Similar Mode of Action Approach
2.1.2. Dissimilar Mode of Action Approach
2.1.3. Selection between the CA and IA Modeling Approaches
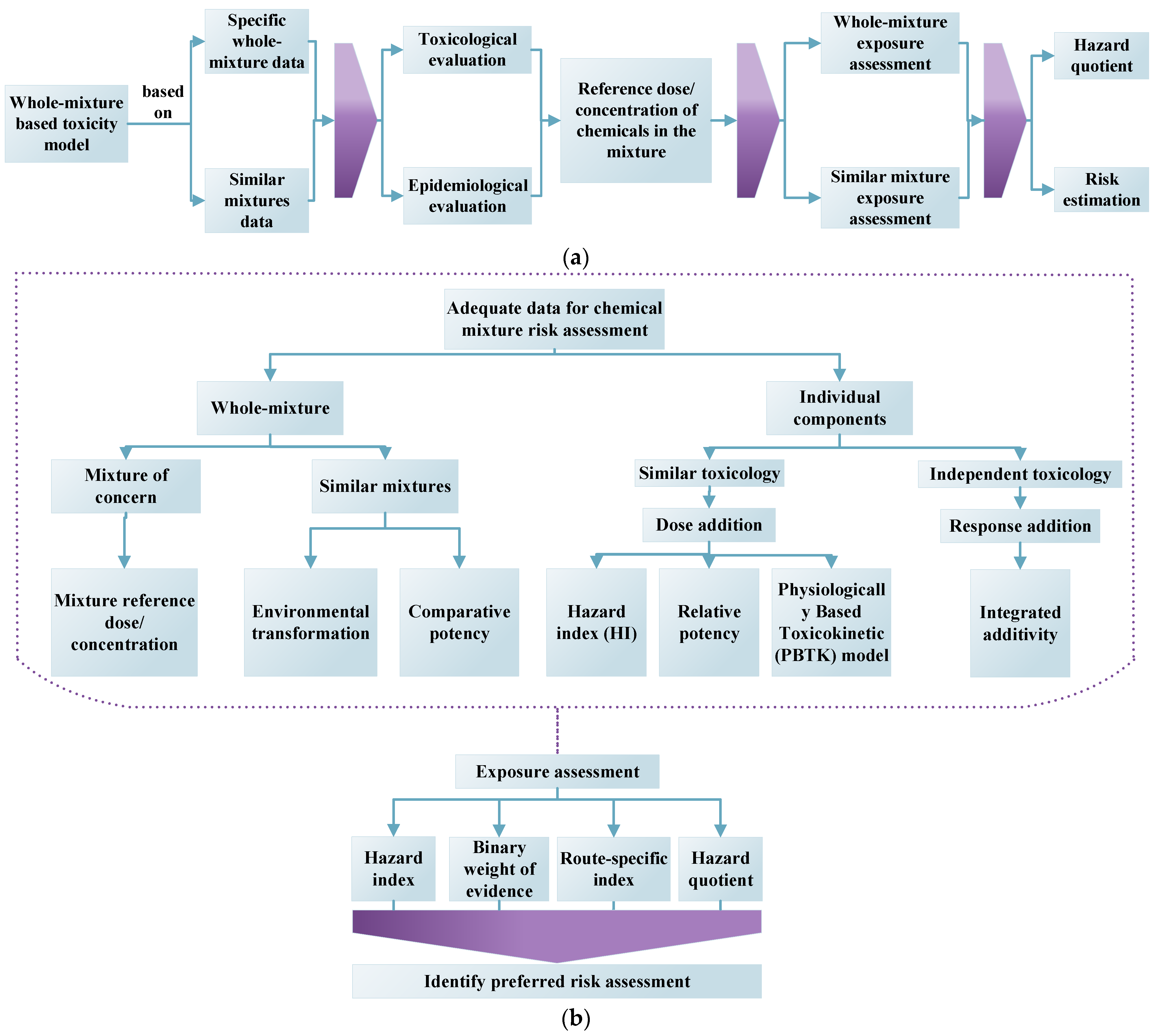
2.2. Whole-Mixture Based Modeling
Integrated Model Approach
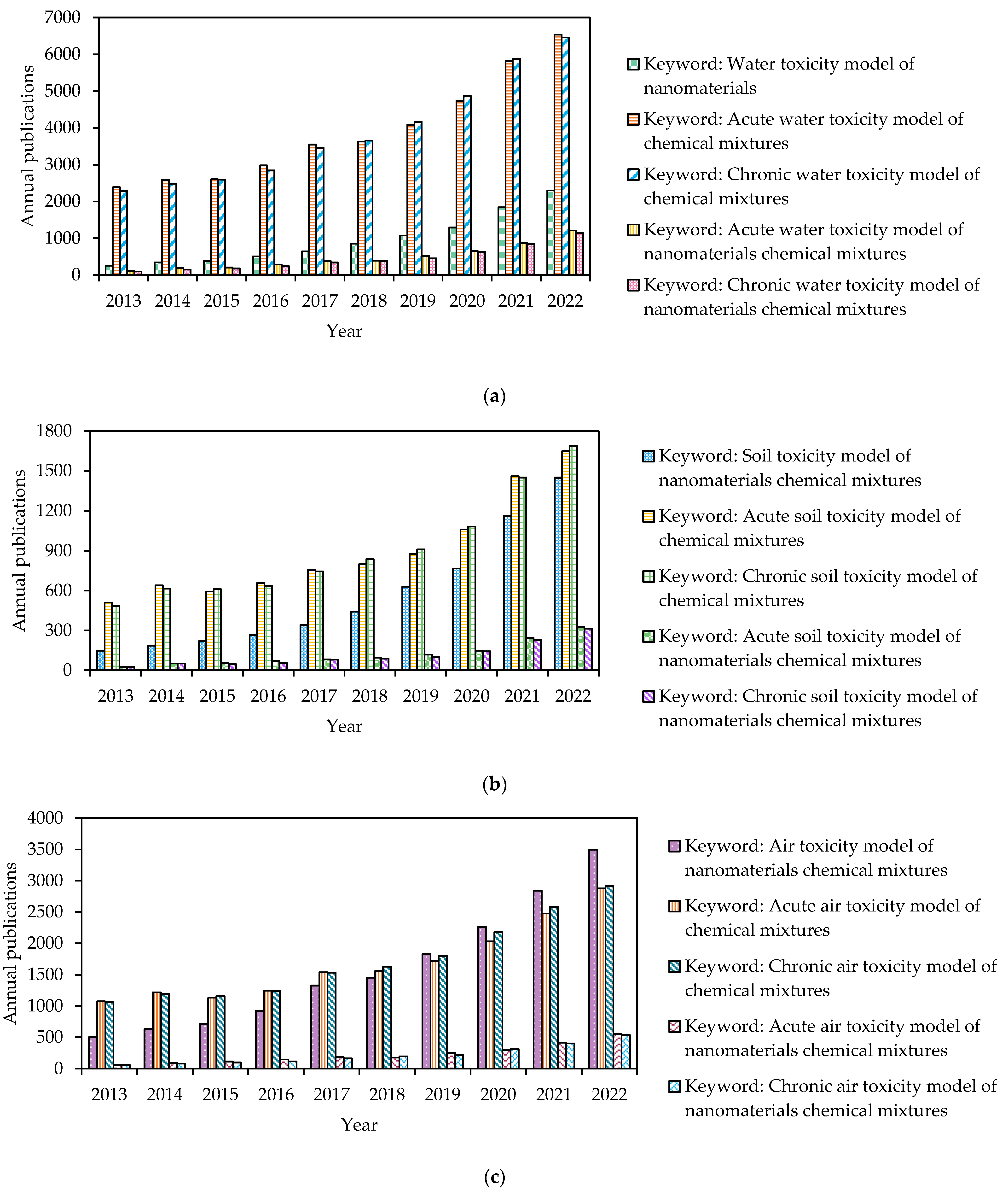
3. Case Studies of Toxicity Modeling Applications
3.1. Nanoparticles Toxicity Studies
3.2. Water Toxicity Modeling Studies
3.3. Soil Toxicity Modeling Evaluation for Water Quality
4. Limitations and Future Research Studies
- Conservative risk of chemical mixtures and greater accuracy could be achieved with the implementation of up-to-date monitoring techniques such as sensor integration in endangered regions worldwide.
- To assist with predictive approach implementation, mechanistic data must be included in the assessment given that the modeling design needs to be based on real data to be validated. In addition, given the lack of understanding of the nanomaterial in chemical mixtures, the effects of the corona and colloid characteristics of chemicals need to be deeply accounted for future studies.
- The interactions between multiple stressors, their alternative usage, and the stress they present to the ecosystem-expanding populations should be equally integrated into the combined model analysis to cover global problems such as the increasing number of industries and climate change.
- Ecotoxicity of chemical mixtures in environmental compartments could be better applied to endangered species with appropriate biomonitoring of living organisms, which would additionally benefit national economies.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, V.; Muratov, E.; Capuzzi, S.; Politi, R.; Low, Y.; Braga, R.; Zakharov, A.V.; Sedykh, A.; Mokshyna, E.; Andrade, C.; et al. Alarms about Structural Alerts. Green Chem. 2017, 18, 4348–4360. [Google Scholar] [CrossRef]
- Tennekes, H.A.; Sánchez-Bayo, F. The Molecular Basis of Simple Relationships between Exposure Concentration and Toxic Effects with Time. Toxicology 2013, 309, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, Z.; Hollert, H.; Zhou, S.; Deutschmann, B.; Seiler, T.B. Toxicity of 10 Organic Micropollutants and Their Mixture: Implications for Aquatic Risk Assessment. Sci. Total Environ. 2019, 666, 1273–1282. [Google Scholar] [CrossRef]
- Rhind, S.M. Anthropogenic Pollutants: A Threat to Ecosystem Sustainability? Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Kovalishyn, V.; Abramenko, N.; Kopernyk, I.; Charochkina, L.; Metelytsia, L.; Tetko, I.V.; Peijnenburg, W.; Kustov, L. Modelling the Toxicity of a Large Set of Metal and Metal Oxide Nanoparticles Using the OCHEM Platform. Food Chem. Toxicol. 2018, 112, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Önlü, S.; Saçan, M.T. Toxicity of Contaminants of Emerging Concern to Dugesia Japonica: QSTR Modeling and Toxicity Relationship with Daphnia Magna. J. Hazard. Mater. 2018, 351, 20–28. [Google Scholar] [CrossRef]
- Tlili, S.; Mouneyrac, C. New Challenges of Marine Ecotoxicology in a Global Change Context. Mar. Pollut. Bull. 2021, 166, 112242. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Petersen, K.; Song, Y.; Ruus, A.; Grung, M.; Bakke, T.; Tollefsen, K.E. Environmental Risk Assessment of Combined Effects in Aquatic Ecotoxicology: A Discussion Paper. Mar. Environ. Res. 2014, 96, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, C.; Shi, J.; Chen, L. Evaluation on Joint Toxicity of Chlorinated Anilines and Cadmium to Photobacterium Phosphoreum and QSAR Analysis. J. Hazard. Mater. 2014, 279, 156–162. [Google Scholar] [CrossRef]
- Son, J.; Lee, Y.-S.; Kim, Y.; Shin, K.-I.; Hyun, S.; Cho, K. Joint Toxic Action of Binary Metal Mixtures of Copper, Manganese and Nickel to Paronychiurus Kimi (Collembola). Ecotoxicol. Environ. Saf. 2016, 132, 164–169. [Google Scholar] [CrossRef]
- Walter, H.; Consolaro, F.; Gramatica, P.; Scholze, M.; Altenburger, R. Mixture Toxicity of Priority Pollutants at No Observed Effect Concentrations (NOECs). Ecotoxicology 2002, 11, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. Chemical Transformations in the Environment. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2016; pp. 305–353. ISBN 9780128044926. [Google Scholar]
- Moore, M.N.; Wedderburn, R.J.; Clarke, K.R.; McFadzen, I.R.B.; Lowe, D.M.; Readman, J.W. Emergent Synergistic Lysosomal Toxicity of Chemical Mixtures in Molluscan Blood Cells (Hemocytes). Environ. Pollut. 2018, 235, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Ghosh, S.; Leszczynski, J. Single or Mixture Halogenated Chemicals? Risk Assessment and Developmental Toxicity Prediction on Zebrafish Embryos Based on Weighted Descriptors Approach. Chemosphere 2018, 210, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Zhang, Q.; Carmichael, P.L.; Boekelheide, K.; Andersen, M.E. Toxicity Testing in the 21st Century: Defining New Risk Assessment Approaches Based on Perturbation of Intracellular Toxicity Pathways. PLoS ONE 2011, 6, e20887. [Google Scholar] [CrossRef]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The Eco-Toxic Effects of Pesticide and Heavy Metal Mixtures towards Earthworms in Soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Boraschi, D.; Swartzwelter, B.J.; Italiani, P. Interaction of Engineered Nanomaterials with the Immune System: Health-Related Safety and Possible Benefits. Curr. Opin. Toxicol. 2018, 10, 74–83. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in Food and Agriculture: An Overview on Their Safety Concerns and Regulatory Issues. Crit. Rev. Food Sci. Nutr. 2018, 58, 297–317. [Google Scholar] [CrossRef]
- Li, X.; Peng, L.; Yao, X.; Cui, S.; Hu, Y.; You, C.; Chi, T. Long Short-Term Memory Neural Network for Air Pollutant Concentration Predictions: Method Development and Evaluation. Environ. Pollut. 2017, 231, 997–1004. [Google Scholar] [CrossRef]
- Zhu, X.W.; Ge, H.L.; Cao, Y. Bin Mixture Cytotoxicity Assessment of Ionic Liquids and Heavy Metals in MCF-7 Cells Using Mixtox. Chemosphere 2016, 163, 544–551. [Google Scholar] [CrossRef]
- Ben Ghanem, O.; Mutalib, M.I.A.; Lévêque, J.M.; El-Harbawi, M. Development of QSAR Model to Predict the Ecotoxicity of Vibrio Fischeri Using COSMO-RS Descriptors. Chemosphere 2017, 170, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Giesen, D.; Van Gestel, C.A.M. QSAR Development and Bioavailability Determination: The Toxicity of Chloroanilines to the Soil Dwelling Springtail Folsomia Candida. Chemosphere 2013, 90, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Peng, T.; Yang, X.; Liu, H. Development of QSAR Models for Predicting the Binding Affinity of Endocrine Disrupting Chemicals to Eight Fish Estrogen Receptor. Ecotoxicol. Environ. Saf. 2018, 148, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, W.; Luo, J.; Pan, Y.; Yang, M.; Hua, M.; Chu, W.; Shuang, C.; Li, A. Comparative Toxicity Analyses from Different Endpoints: Are New Cyclic Disinfection Byproducts (DBPs) More Toxic than Common Aliphatic DBPs? Environ. Sci. Technol. 2022, 56, 194–207. [Google Scholar] [CrossRef]
- Roy, K.; Kar, S.; Das, R.N. Chapter 1—Background of {QSAR} and Historical Developments. In Understanding the Basics of {QSAR} for Applications in Pharmaceutical Sciences and Risk Assessment; Roy, K., Kar, S., Das, R.N., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1–46. ISBN 978-0-12-801505-6. [Google Scholar]
- Klüver, N.; Vogs, C.; Altenburger, R.; Escher, B.I.; Scholz, S. Development of a General Baseline Toxicity QSAR Model for the Fish Embryo Acute Toxicity Test. Chemosphere 2016, 164, 164–173. [Google Scholar] [CrossRef]
- Wang, D.; Shi, J.; Xiong, Y.; Hu, J.; Lin, Z.; Qiu, Y.; Cheng, J. A QSAR-Based Mechanistic Study on the Combined Toxicity of Antibiotics and Quorum Sensing Inhibitors against Escherichia Coli. J. Hazard. Mater. 2018, 341, 438–447. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Quantitative Structure-Activity Relationships (QSARs) for the Transformation of Organic Micropollutants during Oxidative Water Treatment. Water Res. 2012, 46, 6177–6195. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Calvin, J.; Amy, G.L. QSAR Models for the Removal of Organic Micropollutants in Four Different River Water Matrices. Chemosphere 2012, 87, 144–150. [Google Scholar] [CrossRef]
- Bucur, A.I.; Bucur, R.A.; Szabadai, Z.; Mosoarca, C.; Linul, P.A. Influence of Small Concentration Addition of Tartaric Acid on the 220 °C Hydrothermal Synthesis of Hydroxyapatite. Mater. Charact. 2017, 132, 76–82. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Qian, Y.; Zhao, X.; Wang, Q. The Synergistic Toxicity of the Multiple Chemical Mixtures: Implications for Risk Assessment in the Terrestrial Environment. Environ. Int. 2015, 77, 95–105. [Google Scholar] [CrossRef]
- Feng, L.; Liu, S.S.; Li, K.; Tang, H.X.; Liu, H.L. The Time-Dependent Synergism of the Six-Component Mixtures of Substituted Phenols, Pesticides and Ionic Liquids to Caenorhabditis Elegans. J. Hazard. Mater. 2017, 327, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, A.; Bopp, S.K.; van der Linden, S.; Berggren, E.; Worth, A. Regulatory Assessment of Chemical Mixtures: Requirements, Current Approaches and Future Perspectives. Regul. Toxicol. Pharmacol. 2016, 80, 321–334. [Google Scholar] [CrossRef]
- Lopes, S.; Pinheiro, C.; Soares, A.M.V.M.; Loureiro, S. Joint Toxicity Prediction of Nanoparticles and Ionic Counterparts: Simulating Toxicity under a Fate Scenario. J. Hazard. Mater. 2016, 320, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, S.S.; Qu, R.; Liu, H.L. Global Concentration Additivity and Prediction of Mixture Toxicities, Taking Nitrobenzene Derivatives as an Example. Ecotoxicol. Environ. Saf. 2017, 144, 475–481. [Google Scholar] [CrossRef]
- Wang, D.; Gu, Y.; Zheng, M.; Zhang, W.; Lin, Z.; Liu, Y. A Mechanism-Based QSTR Model for Acute to Chronic Toxicity Extrapolation: A Case Study of Antibiotics on Luminous Bacteria. Sci. Rep. 2017, 7, 6022. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, C.; Wang, Y.; Peng, Q.; Zhao, H.; Guo, D.; Wang, Q.; Qian, Y. Mixture Toxicity of Four Commonly Used Pesticides at Different Effect Levels to the Epigeic Earthworm, Eisenia Fetida. Ecotoxicol. Environ. Saf. 2017, 142, 29–39. [Google Scholar] [CrossRef]
- Hsieh, N.H.; Chen, Z.; Rusyn, I.; Chiu, W.A. Risk Characterization and Probabilistic Concentration–Response Modeling of Complex Environmental Mixtures Using New Approach Methodologies (NAMs) Data from Organotypic in Vitro Human Stem Cell Assays. Environ. Health Perspect. 2021, 129, 1–13. [Google Scholar] [CrossRef]
- Nweke, C.O.; Orji, J.C.; Ahumibe, N.C. Prediction of Phenolic Compounds and Formulated Glyphosate Toxicity in Binary Mixtures Using Rhizobium Species Dehydrogenase Activity. Adv. Life Sci. 2015, 5, 27–38. [Google Scholar] [CrossRef]
- Thienpont, B.; Barata, C.; Raldúa, D. Modeling Mixtures of Thyroid Gland Function Disruptors in a Vertebrate Alternative Model, the Zebrafish Eleutheroembryo. Toxicol. Appl. Pharmacol. 2013, 269, 169–175. [Google Scholar] [CrossRef]
- Qin, L.T.; Liu, S.S.; Zhang, J.; Xiao, Q.F. A Novel Model Integrated Concentration Addition with Independent Action for the Prediction of Toxicity of Multi-Component Mixture. Toxicology 2011, 280, 164–172. [Google Scholar] [CrossRef]
- Tyagi, D.; Couillard, D. Toxic Effects of Inhibitors in Biological Wastewater Treatment Processes. Can. J. Chem. Eng. 1998, 66, 97–106. [Google Scholar] [CrossRef]
- Petersen, B.; Gernaey, K.; Ottoy, J.; Vanrolleghem, P. Application of Biosensors in Wastewater Treatment. In Proceedings of the 2e Symposium sur les Eaux usées et 11e Atelier sur L’eau Potable, Montréal, Canada, 20–21 October 1999; pp. 204–210. [Google Scholar]
- Cai, B.; Xie, L.; Yang, D.; Arcangeli, J.P. Application of QSARs Model in Toxicity Evaluation of Wastewater to Bio-Treatment System in WWTP. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, iCBBE 2008, Shanghai, China, 16–18 May 2008; pp. 3027–3030. [Google Scholar]
- De Schepper, W.; Dries, J.; Geuens, L.; Blust, R. Wastewater Treatment Plant Modeling Supported Toxicity Identification and Evaluation of a Tank Truck Cleaning Effluent. Ecotoxicol. Environ. Saf. 2010, 73, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Balistrieri, L.S.; Todd, A.S. Using Biotic Ligand Models to Predict Metal Toxicity in Mineralized Systems. Appl. Geochem. 2015, 57, 55–72. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, L.; Ye, M.; Guan, Z.; Huang, J.; Liu, J.; Li, L.; Huang, S.; Sun, S. Evaluation of the Dewaterability, Heavy Metal Toxicity and Phytotoxicity of Sewage Sludge in Different Advanced Oxidation Processes. J. Clean. Prod. 2020, 265, 121839. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W.; Yang, L.; Chen, Y.; Hu, Y. Co-N-Doped MoO2 Modified Carbon Felt Cathode for Removal of EDTA-Ni in Electro-Fenton Process. Environ. Sci. Pollut. Res. 2018, 25, 22754–22765. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hua, L.; Ma, Y. A Biotic Ligand Model Predicting Acute Copper Toxicity for Barley (Hordeum Vulgare): Influence of Calcium, Magnesium, Sodium, Potassium and PH. Chemosphere 2012, 89, 89–95. [Google Scholar] [CrossRef]
- Lofts, S.; Criel, P.; Janssen, C.R.; Lock, K.; McGrath, S.P.; Oorts, K.; Rooney, C.P.; Smolders, E.; Spurgeon, D.J.; Svendsen, C.; et al. Modelling the Effects of Copper on Soil Organisms and Processes Using the Free Ion Approach: Towards a Multi-Species Toxicity Model. Environ. Pollut. 2013, 178, 244–253. [Google Scholar] [CrossRef]
- Smolders, E.; Oorts, K.; Peeters, S.; Lanno, R.; Cheyns, K. Toxicity in Lead Salt Spiked Soils to Plants, Invertebrates and Microbial Processes: Unraveling Effects of Acidification, Salt Stress and Ageing Reactions. Sci. Total Environ. 2015, 536, 223–231. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Gao, H.; Zhang, Q.; Lv, M.-C.; Wang, S.; Liu, G.; Pan, Y.-Y.; Christie, P.; Sun, W. Improving Prediction of Metal Uptake by Chinese Cabbage (Brassica pekinensis L.) Based on a Soil-Plant Stepwise Analysis. Sci. Total Environ. 2016, 569–570, 1595–1605. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Chen, H.; Chen, L.; Tang, D.-D.; Miao, H.; Zhao, Y.-Y. Metabolomic Application in Toxicity Evaluation and Toxicological Biomarker Identification of Natural Product. Chem. Biol. Interact. 2016, 252, 114–130. [Google Scholar] [CrossRef]
- Zaitsu, K.; Hayashi, Y.; Kusano, M.; Tsuchihashi, H.; Ishii, A. Application of Metabolomics to Toxicology of Drugs of Abuse: A Mini Review of Metabolomics Approach to Acute and Chronic Toxicity Studies. Drug Metab. Pharmacokinet. 2016, 31, 21–26. [Google Scholar] [CrossRef]
- Pauluhn, J. Risk Assessment in Combustion Toxicology: Should Carbon Dioxide Be Recognized as a Modifier of Toxicity or Separate Toxicological Entity? Toxicol. Lett. 2016, 262, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, C.; Li, T.; Xu, S.; Liu, L.; Ju, M. A Water Quality Management Strategy for Regionally Protected Water through Health Risk Assessment and Spatial Distribution of Heavy Metal Pollution in 3 Marine Reserves. Sci. Total Environ. 2017, 599–600, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Banton, M.; Klapacz, J.; Shen, H. A Toxicological Review of the Ethylene Glycol Series: Commonalities and Differences in Toxicity and Modes of Action. Toxicol. Lett. 2017, 278, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk Assessment of Environmental Mixture Effects. RSC Adv. 2016, 6, 47844–47857. [Google Scholar] [CrossRef]
- Farley, K.J.; Meyer, J.S.; Balistrieri, L.S.; De Schamphelaere, K.A.C.; Iwasaki, Y.; Janssen, C.R.; Kamo, M.; Lofts, S.; Mebane, C.A.; Naito, W.; et al. Metal Mixture Modeling Evaluation Project: 2. Comparison of Four Modeling Approaches. Environ. Toxicol. Chem. 2015, 34, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, J.; Wang, C.; Zhu, L. Modeling Interactions and Toxicity of Cu-Zn Mixtures to Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2017, 138, 146–153. [Google Scholar] [CrossRef]
- Rider, C.V.; LeBlanc, G.A. An Integrated Addition and Interaction Model for Assessing Toxicity of Chemical Mixtures. Toxicol. Sci. 2005, 87, 520–528. [Google Scholar] [CrossRef]
- Ra, J.S.; Lee, B.C.; Chang, N.I.; Kim, S.D. Estimating the Combined Toxicity by Two-Step Prediction Model on the Complicated Chemical Mixtures from Wastewater Treatment Plant Effluents. Environ. Toxicol. Chem. 2006, 25, 2107–2113. [Google Scholar] [CrossRef]
- Watt, J.; Webster, T.F.; Schlezinger, J.J. Generalized Concentration Addition Modeling Predicts Mixture Effects of Environmental PPARγ Agonists. Toxicol. Sci. 2016, 153, 18–27. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.C.; Ma, X. Characteristics of Concentration-Inhibition Curves of Individual Chemicals and Applicability of the Concentration Addition Model for Mixture Toxicity Prediction. Ecotoxicol. Environ. Saf. 2015, 113, 176–182. [Google Scholar] [CrossRef]
- Hong, Y.; Tan, Y.; Meng, Y.; Yang, H.; Zhang, Y.; Warren, A.; Li, J.; Lin, X. Evaluation of Biomarkers for Ecotoxicity Assessment by Dose-Response Dynamic Models: Effects of Nitrofurazone on Antioxidant Enzymes in the Model Ciliated Protozoan Euplotes Vannus. Ecotoxicol. Environ. Saf. 2017, 144, 552–559. [Google Scholar] [CrossRef]
- Yang, A.; Liu, S.; Cheng, Z.; Pu, H.; Cheng, N.; Ding, J.; Li, J.; Li, H.; Hu, X.; Ren, X.; et al. Dose-Response Analysis of Environmental Exposure to Multiple Metals and Their Joint Effects with Fasting Plasma Glucose among Occupational Workers. Chemosphere 2017, 186, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tada, M. Generalized Concentration Addition Approach for Predicting Mixture Toxicity. Environ. Toxicol. Chem. 2017, 36, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.A.; Leusch, F.D.L.; Escher, B.I. Applying Mixture Toxicity Modelling to Predict Bacterial Bioluminescence Inhibition by Non-Specifically Acting Pharmaceuticals and Specifically Acting Antibiotics. Chemosphere 2017, 173, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Di Nica, V.; Gallet, J.; Villa, S.; Mezzanotte, V. Toxicity of Quaternary Ammonium Compounds (QACs) as Single Compounds and Mixtures to Aquatic Non-Target Microorganisms: Experimental Data and Predictive Models. Ecotoxicol. Environ. Saf. 2017, 142, 567–577. [Google Scholar] [CrossRef]
- Loewe, S.; Muischnek, H. Effect of Combinations: Mathematical Basis of Problem. N-S. Arch. Ex. Path. Ph. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- van Gestel, C.; Jonker, M.; Kammenga, J.; Laskowski, R.; Svendsen, C. Mixture Toxicity: Linking Approaches from Ecological and Human Toxicology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Hadrup, N.; Taxvig, C.; Pedersen, M.; Nellemann, C.; Hass, U.; Vinggaard, A.M. Concentration Addition, Independent Action and Generalized Concentration Addition Models for Mixture Effect Prediction of Sex Hormone Synthesis In Vitro. PLoS ONE 2013, 8, e70490. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Peijnenburg, W.J.G.M. Prediction of Joint Algal Toxicity of Nano-CeO2/Nano-TiO2and Florfenicol: Independent Action Surpasses Concentration Addition. Chemosphere 2016, 156, 8–13. [Google Scholar] [CrossRef]
- Brinkmann, M.; Hecker, M.; Giesy, J.P.; Jones, P.D.; Ratte, H.T.; Hollert, H.; Preuss, T.G. Generalized Concentration Addition Accurately Predicts Estrogenic Potentials of Mixtures and Environmental Samples Containing Partial Agonists. Toxicol. In Virto 2018, 46, 294–303. [Google Scholar] [CrossRef]
- Gosset, A.; Wiest, L.; Fildier, A.; Libert, C.; Giroud, B.; Hammada, M.; Hervé, M.; Sibeud, E.; Vulliet, E.; Polomé, P.; et al. Ecotoxicological Risk Assessment of Contaminants of Emerging Concern Identified by “Suspect Screening” from Urban Wastewater Treatment Plant Effluents at a Territorial Scale. Sci. Total Environ. 2021, 778. [Google Scholar] [CrossRef]
- Cedergreen, N.; Christensen, A.M.; Kamper, A.; Kudsk, P.; Mathiassen, S.K.; Streibig, J.C.; Sørensen, H. A Review of Independent Action Compared to Concentration Addition as Reference Models for Mixtures of Compounds with Different Molecular Target Sites. Environ. Toxicol. Chem. 2008, 27, 1621–1632. [Google Scholar] [CrossRef]
- Faust, M.; Altenburger, R.; Backhaus, T.; Blanck, H.; Boedeker, W.; Gramatica, P.; Hamer, V.; Scholze, M.; Vighi, M.; Grimme, L.H. Joint Algal Toxicity of 16 Dissimilarly Acting Chemicals Is Predictable by the Concept of Independent Action. Aquat. Toxicol. 2003, 63, 43–63. [Google Scholar] [CrossRef]
- Hertzberg, R.C.; Pan, Y.; Li, R.; Haber, L.T.; Lyles, R.H.; Herr, D.W.; Moser, V.C.; Simmons, J.E. A Four-Step Approach to Evaluate Mixtures for Consistency with Dose Addition. Toxicology 2013, 314, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kamo, M.; Yokomizo, H. Explanation of Non-Additive Effects in Mixtures of Similar Mode of Action Chemicals. Toxicology 2015, 335, 20–26. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Backhaus, T.; Faust, M. State of the Art Report on Mixture Toxicity. 2009. Available online: https://www.pan-europe.info/old/Campaigns/pesticides/documents/cum_syn_effects/Kortenkamp%20state%20of%20the%20art%20mixture%20toxicity.pdf (accessed on 23 May 2023).
- Altenburger, R.; Boedeker, W.; Faust, M.; Grimme, L.H. Regulations for Combined Effects of Pollutants: Consequences from Risk Assessment in Aquatic Toxicology. Food Chem. Toxicol. 1996, 34, 1155–1157. [Google Scholar] [CrossRef]
- Feron, V.J.; Groten, J.P. Toxicological Evaluation of Chemical Mixtures. Food Chem. Toxicol. 2002, 40, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.; Woutersen, R.A.; Feron, V.J. Toxicity of Mixtures of Nephrotoxicants with Similar or Dissimilar Mode of Action. Food Chem. Toxicol. 1996, 34, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Wolansky, M.J.; Gennings, C.; DeVito, M.J.; Crofton, K.M. Evidence for Dose-Additive Effects of Pyrethroids on Motor Activity in Rats. Environ. Health Perspect. 2009, 117, 1563–1570. [Google Scholar] [CrossRef]
- Rizzati, V.; Briand, O.; Guillou, H.; Gamet-Payrastre, L. Effects of Pesticide Mixtures in Human and Animal Models: An Update of the Recent Literature. Chem. Biol. Interact. 2016, 254, 231–246. [Google Scholar] [CrossRef]
- Howdeshell, K.L.; Hotchkiss, A.K.; Gray, L.E. Cumulative Effects of Antiandrogenic Chemical Mixtures and Their Relevance to Human Health Risk Assessment. Int. J. Hyg. Environ. Health 2017, 220, 179–188. [Google Scholar] [CrossRef]
- Walker, N.J.; Crockett, P.W.; Nyska, A.; Brix, A.E.; Jokinen, M.P.; Sells, D.M.; Hailey, J.R.; Easterling, M.; Haseman, J.K.; Yin, M.; et al. Dose-Additive Carcinogenicity of a Defined Mixture of “Dioxin-like Compounds”. Environ. Health Perspect. 2005, 113, 43–48. [Google Scholar] [CrossRef]
- Charles, G.D.; Gennings, C.; Zacharewski, T.R.; Gollapudi, B.B.; Carney, E.W. An Approach for Assessing Estrogen Receptor-Mediated Interactions in Mixtures of Three Chemicals: A Pilot Study. Toxicol. Sci. 2002, 68, 349–360. [Google Scholar] [CrossRef]
- Payne, J.; Scholze, M.; Kortenkamp, A. Mixtures of Four Organochlorines Enhance Human Breast Cancer Cell Proliferation. Environ. Health Perspect. 2001, 109, 391–397. [Google Scholar] [CrossRef]
- Tichý, M.; Borek-Dohalský, V.; Rucki, M.; Reitmajer, J.; Feltl, L. Risk Assessment of Mixtures: Possibility of Prediction of Interaction between Chemicals. Int. Arch. Occup. Environ. Health 2002, 75, 133–136. [Google Scholar] [CrossRef]
- Jobling, S.; Burn, R.W.; Thorpe, K.; Williams, R.; Tyler, C. Statistical Modeling Suggests That Antiandrogens in Effluents from Wastewater Treatment Works Contribute to Widespread Sexual Disruption in Fish Living in English Rivers. Environ. Health Perspect. 2009, 117, 797–802. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Huang, L.; Wang, Y.; Cai, X.; Qiao, X.; Dong, Y. Integrated Fuzzy Concentration Addition–Independent Action (IFCA–IA) Model Outperforms Two-Stage Prediction (TSP) for Predicting Mixture Toxicity. Chemosphere 2009, 74, 735–740. [Google Scholar] [CrossRef]
- Lambert, J.C.; Lipscomb, J.C. Mode of Action as a Determining Factor in Additivity Models for Chemical Mixture Risk Assessment. Regul. Toxicol. Pharmacol. 2007, 49, 183–194. [Google Scholar] [CrossRef]
- Gruiz, K.; Fekete-Kertész, I.; Kunglné-Nagy, Z.; Hajdu, C.; Feigl, V.; Vaszita, E.; Molnár, M. Direct Toxicity Assessment — Methods, Evaluation, Interpretation. Sci. Total Environ. 2016, 563–564, 803–812. [Google Scholar] [CrossRef]
- Landrum, P.F.; Chapman, P.M.; Neff, J.; Page, D.S. Evaluating the Aquatic Toxicity of Complex Organic Chemical Mixtures: Lessons Learned from Polycyclic Aromatic Hydrocarbon and Petroleum Hydrocarbon Case Studies. Integr. Environ. Assess. Manag. 2012, 8, 217–230. [Google Scholar] [CrossRef]
- Lebrun, J.D.; Uher, E.; Fechner, L.C. Behavioural and Biochemical Responses to Metals Tested Alone or in Mixture (Cd-Cu-Ni-Pb-Zn) in Gammarus Fossarum: From a Multi-Biomarker Approach to Modelling Metal Mixture Toxicity. Aquat. Toxicol. 2017, 193, 160–167. [Google Scholar] [CrossRef]
- Menz, J.; Baginska, E.; Arrhenius, Å.; Haiß, A.; Backhaus, T.; Kümmerer, K. Antimicrobial Activity of Pharmaceutical Cocktails in Sewage Treatment Plant Effluent—An Experimental and Predictive Approach to Mixture Risk Assessment. Environ. Pollut. 2017, 231, 1507–1517. [Google Scholar] [CrossRef]
- Petit, P.; Maître, A.; Persoons, R.; Bicout, D.J. Modeling the Exposure Functions of Atmospheric Polycyclic Aromatic Hydrocarbon Mixtures in Occupational Environments. Sci. Total Environ. 2017, 584–585, 1185–1197. [Google Scholar] [CrossRef]
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The Consequences of Exposure to Mixtures of Chemicals: Something from ‘Nothing’ and ‘a Lot from a Little’ When Fish Are Exposed to Steroid Hormones. Sci. Total Environ. 2018, 619–620, 1482–1492. [Google Scholar] [CrossRef]
- Baas, J.; Jager, T.; Kooijman, S.A.L.M. A Model to Analyze Effects of Complex Mixtures on Survival. Ecotoxicol. Environ. Saf. 2009, 72, 669–676. [Google Scholar] [CrossRef]
- Roth, N.; Ciffroy, P. A Critical Review of Frameworks Used for Evaluating Reliability and Relevance of (Eco)Toxicity Data: Perspectives for an Integrated Eco-Human Decision-Making Framework. Environ. Int. 2016, 95, 16–29. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Wang, F.; Liu, Q. Environmental Behavior and Eco-Toxicity of Xylene in Aquatic Environments: A Review. Ecotoxicol. Environ. Saf. 2017, 145, 324–332. [Google Scholar] [CrossRef]
- Zhou, C.; Ge, S.; Yu, H.; Zhang, T.; Cheng, H.; Sun, Q.; Xiao, R. Environmental Risk Assessment of Pyrometallurgical Residues Derived from Electroplating and Pickling Sludges. J. Clean. Prod. 2018, 177, 699–707. [Google Scholar] [CrossRef]
- Choudhury, H.; Hertzberg, R.; Rice, G.; Cogliano, J.; Mukerjee, D.; Teuschler, L.; Doyle, E.; Woo, Y.; Schoeny, R. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. 2000. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20533 (accessed on 23 May 2023).
- Nys, C.; Janssen, C.R.; De Schamphelaere, K.A.C. Development and Validation of a Metal Mixture Bioavailability Model (MMBM) to Predict Chronic Toxicity of Ni-Zn-Pb Mixtures to Ceriodaphnia Dubia. Environ. Pollut. 2017, 220, 1271–1281. [Google Scholar] [CrossRef]
- Hassan, S.H.A.; Van Ginkel, S.W.; Hussein, M.A.M.; Abskharon, R.; Oh, S.E. Toxicity Assessment Using Different Bioassays and Microbial Biosensors. Environ. Int. 2016, 92–93, 106–118. [Google Scholar] [CrossRef]
- Giubilato, E.; Radomyski, A.; Critto, A.; Ciffroy, P.; Brochot, C.; Pizzol, L.; Marcomini, A. Modelling Ecological and Human Exposure to POPs in Venice Lagoon. Part I — Application of MERLIN-Expo Tool for Integrated Exposure Assessment. Sci. Total Environ. 2016, 565, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Gadaga, L.L.; Tagwireyi, D. Critical Review of the Guidelines and Methods in Toxicological Research in Africa; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780128004753. [Google Scholar]
- Autrup, H.; Calow, P.; Dekant, W.; Greim, H.; Hanke, W.; Janssen, C.; Jansson, B.; Komulainen, H.; Ladefoged, O.; Linders, J.; et al. Opinion on Risk Assessment on Indoor Air Quality. 2007. Available online: https://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_055.pdf (accessed on 23 May 2023).
- Moya, W.; Jácome, G.; Yoo, C.K. Past, Current, and Future Trends of Red Spiny Lobster Based on PCA with MaxEnt Model in Galapagos Islands, Ecuador. Ecol. Evol. 2017, 7, 4881–4890. [Google Scholar] [CrossRef] [PubMed]
- Jácome, G.; Vilela, P.; Yoo, C.K. Present and Future Incidence of Dengue Fever in Ecuador Nationwide and Coast Region Scale Using Species Distribution Modeling for Climate Variability’s Effect. Ecol. Model. 2019, 400, 60–72. [Google Scholar] [CrossRef]
- Vilela, P.; Jácome, G.; Kim, S.Y.; Nam, K.; Yoo, C. Population Response Modeling and Habitat Suitability of Cobitis Choii Fish Species in South Korea for Climate Change Adaptation. Ecotoxicol. Environ. Saf. 2020, 189. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.M.; Sommerville, D.R.; Goodwin, M.R.; James, R.A.; Channel, S.R. Acute Toxicity When Concentration Varies with Time: A Case Study with Carbon Monoxide Inhalation by Rats. Regul. Toxicol. Pharmacol. 2016, 80, 102–115. [Google Scholar] [CrossRef]
- Ashauer, R.; Escher, B. Advantages of Toxicokinetic and Toxicodynamic Modeling in Aquatic Ecotoxicology and Risk Assessment. J. Environ. Monit. 2010. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Ruiz, P.; Veber, P. Robust Fit of Toxicokinetic–Toxicodynamic Models Using Prior Knowledge Contained in the Design of Survival Toxicity Tests. Environ. Sci. Technol. 2017, 51, 4038–4045. [Google Scholar] [CrossRef]
- Diepens, N.J.; Arts, G.H.P.; Brock, T.C.M.; Smidt, H.; Van Den Brink, P.J.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Sediment Toxicity Testing of Organic Chemicals in the Context of Prospective Risk Assessment: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 255–302. [Google Scholar] [CrossRef]
- Trisciuzzi, D.; Alberga, D.; Mansouri, K.; Judson, R.; Novellino, E.; Mangiatordi, G.F.; Nicolotti, O. Predictive Structure-Based Toxicology Approaches to Assess the Androgenic Potential of Chemicals. J. Chem. Inf. Model. 2017, 57, 2874–2884. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging Patterns for Engineered Nanomaterials in the Environment: A Review of Fate and Toxicity Studies. J. Nanoparticle Res. 2014, 16, 2503. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wang, Y.; Gong, Z.; Fan, X.; Ni, B. Toxic Effects of Copper Nanoparticles on Paramecium Bursaria–Chlorella Symbiotic System. Front. Microbiol. 2022, 13, 834208. [Google Scholar] [CrossRef]
- Auclair, J.; Peyrot, C.; Wilkinson, K.J.; Gagné, F. The Influence of Silver Nanoparticle Form on the Toxicity in Freshwater Mussels. Appl. Sci. 2022, 12, 1429. [Google Scholar] [CrossRef]
- Fernández-Pampín, N.; González Plaza, J.J.; García-Gómez, A.; Peña, E.; Rumbo, C.; Barros, R.; Martel-Martín, S.; Aparicio, S.; Tamayo-Ramos, J.A. Toxicology Assessment of Manganese Oxide Nanomaterials with Enhanced Electrochemical Properties Using Human in Vitro Models Representing Different Exposure Routes. Sci. Rep. 2022, 12, 20991. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.; Chhabra, V. Nanotechnology: Its Scope in Agriculture. J. Phys. Conf. Ser. 2022, 2267, 012112. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of Engineered Nanoparticles as Fertilizers for Increasing Agronomic Productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Malysheva, A.; Lombi, E.; Voelcker, N.H. Bridging the Divide between Human and Environmental Nanotoxicology. Nat. Nanotechnol. 2015, 10, 835–844. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-Milled Carbon Nanomaterials for Energy and Environmental Applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity Assessment of Nanoparticles in Various Systems and Organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Stapleton, P.A. Engineered Nanomaterial Applications in Perinatal Therapeutics. Pharmacol. Res. 2018, 130, 36–43. [Google Scholar] [CrossRef]
- Guggenheim, E.J.; Milani, S.; Röttgermann, P.J.F.; Dusinska, M.; Saout, C.; Salvati, A.; Rädler, J.O.; Lynch, I. Refining in Vitro Models for Nanomaterial Exposure to Cells and Tissues. NanoImpact 2018, 10, 121–142. [Google Scholar] [CrossRef]
- Li, D.; Lai, W.-Y.; Zhang, Y.-Z.; Huang, W. Printable Transparent Conductive Films for Flexible Electronics. Adv. Mater. 2018, 30, 1704738. [Google Scholar] [CrossRef] [PubMed]
- Vilela, P.; Liu, H.; Lee, S.C.; Hwangbo, S.; Nam, K.J.; Yoo, C.K. A Systematic Approach of Removal Mechanisms, Control and Optimization of Silver Nanoparticle in Wastewater Treatment Plants. Sci. Total Environ. 2018, 633, 989–998. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science (80-) 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, B.; Al-Doaiss, A.; Shati, A.; Al-Kahtani, M.; Jarrar, Q. Behavioural Alterations Induced by Chronic Exposure to 10 Nm Silicon Dioxide Nanoparticles. IET Nanobiotechnol. 2021, 15, 221–235. [Google Scholar] [CrossRef]
- Brown, D.M.; Johnston, H.J.; Gaiser, B.; Pinna, N.; Caputo, G.; Culha, M.; Kelestemur, S.; Altunbek, M.; Stone, V.; Roy, J.C.; et al. A Cross-Species and Model Comparison of the Acute Toxicity of Nanoparticles Used in the Pigment and Ink Industries. NanoImpact 2018, 11, 20–32. [Google Scholar] [CrossRef]
- Sarkheil, M.; Johari, S.A.; An, H.J.; Asghari, S.; Park, H.S.; Sohn, E.K.; Yu, I.J. Acute Toxicity, Uptake, and Elimination of Zinc Oxide Nanoparticles (ZnO NPs) Using Saltwater Microcrustacean, Artemia Franciscana. Environ. Toxicol. Pharmacol. 2018, 57, 181–188. [Google Scholar] [CrossRef]
- Conine, A.L.; Rearick, D.C.; Xenopoulos, M.A.; Frost, P.C. Variable Silver Nanoparticle Toxicity to Daphnia in Boreal Lakes. Aquat. Toxicol. 2017, 192, 1–6. [Google Scholar] [CrossRef]
- Rossi, L.; Bagheri, M.; Zhang, W.; Chen, Z.; Burken, J.G.; Ma, X. Using Artificial Neural Network to Investigate Physiological Changes and Cerium Oxide Nanoparticles and Cadmium Uptake by Brassica Napus Plants. Environ. Pollut. 2019, 246, 381–389. [Google Scholar] [CrossRef]
- Carafa, R.; Faggiano, L.; Real, M.; Munné, A.; Ginebreda, A.; Guasch, H.; Flo, M.; Tirapu, L.; der Ohe, P.C. von Water Toxicity Assessment and Spatial Pollution Patterns Identification in a Mediterranean River Basin District. Tools for Water Management and Risk Analysis. Sci. Total Environ. 2011, 409, 4269–4279. [Google Scholar] [CrossRef]
- Papadakis, E.-N.; Tsaboula, A.; Kotopoulou, A.; Kintzikoglou, K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Pesticides in the Surface Waters of Lake Vistonis Basin, Greece: Occurrence and Environmental Risk Assessment. Sci. Total Environ. 2015, 536, 793–802. [Google Scholar] [CrossRef]
- Movahedian, H.; Bina, B.; Asghari, G.H. Toxicity Evaluation of Wastewater Treatment Plant Effluents Using Daphnia Magna. Iran. J. Environ. Health Sci. Eng. 2005, 2, 1–4. [Google Scholar]
- Rose, S.; Altenburger, R.; Sturm, A. Mixture Toxicity Effects of Sea Louse Control Agents in Daphnia Magna. Chemosphere 2016, 144, 599–606. [Google Scholar] [CrossRef]
- Claessens, M.; Vanhaecke, L.; Wille, K.; Janssen, C.R. Emerging Contaminants in Belgian Marine Waters: Single Toxicant and Mixture Risks of Pharmaceuticals. Mar. Pollut. Bull. 2013, 71, 41–50. [Google Scholar] [CrossRef]
- Wang, B.; Bai, Z.; Jiang, H.; Prinsen, P.; Luque, R.; Zhao, S.; Xuan, J. Selective Heavy Metal Removal and Water Purification by Microfluidically-Generated Chitosan Microspheres: Characteristics, Modeling and Application. J. Hazard. Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef]
- Yang, R.; Zhong, S.; Zhang, L.; Liu, B. PW12/CN@Bi2WO6 Composite Photocatalyst Prepared Based on Organic-Inorganic Hybrid System for Removing Pollutants in Water. Sep. Purif. Technol. 2020, 235, 2–11. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, Z.; Hu, C.; Zhong, S.; Zhang, L.; Liu, B.; Wang, W. One-Step Preparation (3D/2D/2D) BiVO4/FeVO4@rGO Heterojunction Composite Photocatalyst for the Removal of Tetracycline and Hexavalent Chromium Ions in Water. Chem. Eng. J. 2020, 390, 124522. [Google Scholar] [CrossRef]
- Altenburger, R.; Brack, W.; Burgess, R.M.; Busch, W.; Escher, B.I.; Focks, A.; Mark Hewitt, L.; Jacobsen, B.N.; de Alda, M.L.; Ait-Aissa, S.; et al. Future Water Quality Monitoring: Improving the Balance between Exposure and Toxicity Assessments of Real-World Pollutant Mixtures. Environ. Sci. Eur. 2019, 31, 12. [Google Scholar] [CrossRef]
- Yaseen, Z.M. An Insight into Machine Learning Models Era in Simulating Soil, Water Bodies and Adsorption Heavy Metals: Review, Challenges and Solutions. Chemosphere 2021, 277, 130126. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, L.; Dong, J.; Wu, J.; Ye, Z.; Zhao, W.; Ding, L.; Fu, W. Risk Assessment, Spatial Patterns and Source Apportionment of Soil Heavy Metals in a Typical Chinese Hickory Plantation Region of Southeastern China. Geoderma 2020, 360, 114011. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A.; Chandra, R.; Mishra, B.K. Potential Human Health Hazard Due to Bioavailable Heavy Metal Exposure via Consumption of Plants with Ethnobotanical Usage at the Largest Chromite Mine of India. Environ. Geochem. Health 2020, 42, 4213–4231. [Google Scholar] [CrossRef]
- Ahmadi, M.; Jorfi, S.; Azarmansuri, A.; Jaafarzadeh, N.; Mahvi, A.H.; Darvishi Cheshmeh Soltani, R.; Akbari, H.; Akhbarizadeh, R. Zoning of Heavy Metal Concentrations Including Cd, Pb and As in Agricultural Soils of Aghili Plain, Khuzestan Province, Iran. Data Br. 2017, 14, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ghayoraneh, M.; Qishlaqi, A. Concentration, Distribution and Speciation of Toxic Metals in Soils along a Transect around a Zn/Pb Smelter in the Northwest of Iran. J. Geochem. Explor. 2017, 180, 1–14. [Google Scholar] [CrossRef]
- Rai, N.; Sjöberg, V.; Forsberg, G.; Karlsson, S.; Olsson, P.E.; Jass, J. Metal Contaminated Soil Leachates from an Art Glass Factory Elicit Stress Response, Alter Fatty Acid Metabolism and Reduce Lifespan in Caenorhabditis Elegans. Sci. Total Environ. 2019, 651, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Darabi-Golestan, F.; Hezarkhani, A. High Precision Analysis Modeling by Backward Elimination with Attitude on Interaction Effects on Au (Ag)-Polymetallic Mineralization of Glojeh, Iran. J. Afr. Earth Sci. 2016, 124, 505–516. [Google Scholar] [CrossRef]
| Research Area | Methodology | Toxic Compounds | Chemical Concentration | Reference |
|---|---|---|---|---|
| Wastewater treatment | Adenosine triphosphate (ATP) analysis to study the response of cells to their environment. |
|
| [43] |
| Combined respirometric–titrimetric method for characterization of activated sludge and wastewater. | Creoline | 25 mg/L | [44] | |
| QSARs models for estimated bio-toxicity of chemicals. |
| Mid-term toxicity: 211–23,000 mg/L Short-term toxicity: 2–4996 mg/L | [45] | |
| Simulation-aided TIE in a wastewater treatment plant simulation model. |
|
| [46] | |
| Numerical approach as a geochemical speciation, metal–organic binding, and toxicological model. |
|
| [47] | |
| Chinese standard GB/T 23486 method carried out on phytotoxicity tests conducted with plant species Brassica rapa chinensis (Chinese cabbage) and Lactuca sativa (Lettuce). Control standard of pollutants in sludge for agricultural (GB 4284-2018, China) from municipal wastewater treatment plant. |
| Liquid-phase contents of As, Cd, Pb and Zn were 0.0514, 0.0088, 0.0053 and 0.2350 mg/L, respectively. | [48] | |
| Electrochemical advanced oxidation process (EAOP) focused on the wastewater treatment for metal ions removal (EDTA-Ni complex) containing ethylenediaminetetraacetic acid (EDTA). Nickel ion concentration was measured by atomic absorption spectroscopy (AAS, PinAAcle 900 T, Perkin Elmer), standard method. | EDTA-Ni complex | 10 mg/L | [49] | |
| Soil | Biotic ligand model for prediction of acute copper toxicity. | Copper | NR | [50] |
| Free ion approach for derivation of critical limits for copper and other metals. | Copper | NR | [51] | |
| Toxicity tests in lead salt-spiked soils applied to potentially different exposure routes of plants, invertebrates, and microbial processes. | Lead | NR | [52] | |
| Diffusive gradients in thin film (DGT) method for correlation between the metal of the shoots and metal concentrations. |
| Varied in different soil types. | [53] | |
| Acute toxicology | LC-MS technology in metabolomics and the chromatographic method. |
| NR | [54] |
| NR | NR | 2.5–50 mg/kg | [55] | |
| FED approach for non-toxicologist. | NR | NR | [56] | |
| Nemerow index andUSEPA model methodology. |
| NR | [57] | |
| NR |
| NR | [58] |
| Toxicity Model | Methodology | Toxic Compounds | Compound Concentration | Reference |
|---|---|---|---|---|
| Concentration addition (CA) | Approach to incorporate interactions among chemical constituents. |
|
| [62] |
| Two-step prediction (TSP) method. |
| NR | [63] | |
| CA model based on an index from the concentration–response curves (CRCs). |
| NR | [64] | |
| Dose–response dynamic models. | Nitrofurazone | NR | [65] | |
| NR |
| NR | [66] | |
| Generalized concentration addition (GCA) model. |
| NR | [67] | |
| Independent action (IA) | NR |
| NR | [68] |
| Biotic ligand-based TK-TD model for aquatic systems. |
| NR | [61] | |
| Microtox® test to investigate the toxicity effects of chemical compounds and mixtures. | NR | NR | [69] | |
| Bioavailability model (MMBM) to predict chronic toxicity. |
| NR | [70] |
| Topic | Methodology | Major Highlights | Reference |
|---|---|---|---|
| The interaction model for assessing the toxicity of chemical mixtures | Integrated model (IAI). | Toxicokinetic interactions could be incorporated into mixture assessments by qualitative weight of evidence or a quantitative approach. | [62] |
| Toxicity by chemical mixtures from WWTP effluents | Two-step prediction (TSP) method. | The combined toxicity could be predicted appropriately by the TSP model for chemicals with similar modes of action by the CA model in the first stage and for chemicals with dissimilar modes of action by the IA model in the second stage. | [63] |
| Mixture effects using different additivity models | Integrated fuzzy concentration addition-independent action (IFCA-IA) model. | TEF overestimated the mixture response but had the advantage of easy interpretability and use. | [106] |
| Estrogenic potentials of mixtures and environmental samples containing partial agonists | Generalized concentration addition (GCA) model. | The heuristic assumption of the GCA approach that the cumulative effect of all components in a particular mixture is subject to a particular toxic interaction rule (TIR) regardless of the number of components. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, P.; Jácome, G.; Moya, W.; Ifaei, P.; Heo, S.; Yoo, C. A Brief Insight into the Toxicity Conundrum: Modeling, Measuring, Monitoring and Evaluating Ecotoxicity for Water Quality towards Environmental Sustainability. Sustainability 2023, 15, 8881. https://doi.org/10.3390/su15118881
Vilela P, Jácome G, Moya W, Ifaei P, Heo S, Yoo C. A Brief Insight into the Toxicity Conundrum: Modeling, Measuring, Monitoring and Evaluating Ecotoxicity for Water Quality towards Environmental Sustainability. Sustainability. 2023; 15(11):8881. https://doi.org/10.3390/su15118881
Chicago/Turabian StyleVilela, Paulina, Gabriel Jácome, Wladimir Moya, Pouya Ifaei, Sungku Heo, and Changkyoo Yoo. 2023. "A Brief Insight into the Toxicity Conundrum: Modeling, Measuring, Monitoring and Evaluating Ecotoxicity for Water Quality towards Environmental Sustainability" Sustainability 15, no. 11: 8881. https://doi.org/10.3390/su15118881
APA StyleVilela, P., Jácome, G., Moya, W., Ifaei, P., Heo, S., & Yoo, C. (2023). A Brief Insight into the Toxicity Conundrum: Modeling, Measuring, Monitoring and Evaluating Ecotoxicity for Water Quality towards Environmental Sustainability. Sustainability, 15(11), 8881. https://doi.org/10.3390/su15118881











