Abstract
In the contemporary era, the excessive use of chemical fertilizers in areas where strawberries are intensively cultivated disrupts the balance of nature and reduces economic efficiency. Therefore, using organic and biofertilizers in sustainable agriculture can solve these problems. The effect of organic fertilizer and other treatments on the yield, quality, and plant growth of the Monterey strawberry variety was investigated. Solid farm manure and a liquid organic fertilizer of vegetable origin were used as basic fertilizers, while five different commercial fertilizers containing bacteria and mycorrhiza were used as complementary fertilizers. In addition, this study examined plant growth parameters, including root length, stem diameter, leaf area, yield per plant, fruit weight, pH in the fruit, SSC, acid, SSC-to-acid ratio, and plant nutrient content. The Biofarm+Botanica+Fontera microzone bacterial fertilizer (Azosprillium brasilense, Azotobacter vinelandii, Rhizobium trifollii, Pseudomonas fluorescens, Bacillus subtilis, Bacillus licheniformis, Azotobacter chroococcum, Bacillus amyloliquefaciens, and Bacillus mucilaginosus) treatment provided the best results; namely, it produced the highest total yield (250.17 g plant−1), largest fruits (18.13 g), highest SSC-to-acid ratio (18.05), and best nitrogen, phosphorus, potassium, calcium, and magnesium contents in the leaves. Similarly, the same treatment produced the longest root, thickest stem, and maximum leaf area. As a result of this study, it is recommended to use biofertilizers together with organic fertilizers to ensure high-quality fruit production.
1. Introduction
Strawberries (Fragaria × ananassa Duch.) are one of the most important berry fruits; they are widely grown in the world and are fondly consumed by everyone. Strawberries are vital for health as they contain anticancer components, such as ellagic acid, and are rich in natural antioxidants. Additionally, strawberries contain high amounts of vitamin C (40–120 mg/100 g fruit), protein, and minerals such as potassium, phosphorus, calcium, and iron. Compared with other berry fruits, strawberries contain higher levels of vitamin C, phenolics, and flavonoids [1,2]. In recent years, both plantation areas and the number of strawberries grown have rapidly increased worldwide. According to FAO data, in 2021, the total strawberry production area in the world expanded to 389,665 ha, and the production amount reached 9175.384 tons. Of all the countries producing strawberries, China ranked first with 3,380,478 tons, followed by the United States of America with 1,211,090 tons. Turkey ranked third worldwide with 669,195 tons of strawberries produced (FAO, 2023) [3]. However, despite being one of the key countries for strawberry production, organic strawberry production is relatively low in Turkey, as only 4511.41 tons of organic strawberries were produced in 2021 (Ministry of Agriculture and Forestry, 2023) [4].
Researchers have directed their attention to organic production studies for several reasons, including the negative effects of chemical pesticides and fertilizers on soil, water, air pollution, and human health [5,6,7,8]. In addition to the role played by organic fertilizers, biofertilization is vital due to its environmental friendliness. A variety of bacterial species can positively impact plant growth and improve soil sustainability [9,10,11]. Bacteria with plant-growth-promoting effects that are used in agriculture practices fix the atmospheric nitrogen in the soil and make it available for the plant by increasing the solubility of phosphorus and the intake of water and nutrients; additionally, they also promote plant growth by producing phytohormones (such as IAA and cytokinin) and enzymes. Furthermore, these bacteria suppress the disease factor imposed by the competition for location and nutrients, support plants against both plant pathogens and stress-induced impacts under stressful conditions, and finally reduce the incidence of plant disease and death [12,13,14,15,16].
The importance of sustainable agriculture and food production is increasing each year. Therefore, investigating the effects of organic fertilizers on plants to develop organic agriculture and investigating the dissemination of organic production to minimize the damage to the environment and enhance the soil is important. Additionally, knowing these factors is beneficial for human health. Following this framework, this study investigated the effects of organic fertilizer and other treatments on the yield, quality, and plant growth of the Monterey strawberry variety.
2. Materials and Methods
2.1. Plant Material
This study was conducted in the treatment area of Osmaniye Korkut Ata University in 2019–2020 and used a medium-neutral day Monterey variety as the strawberry variety. Monterey is initially sensitive to mildew. However, compared with Albion, this variety flowers more and has an upright, large plant structure with larger but softer fruits and a distinguished aroma. In addition to its earliness, its plant structure is comparatively strong [17]. In this study, solid and liquid manure were used as basic fertilizers, and the live bacteria and fungi fertilizers described below were used for top fertilization. Biofarm-branded fertilizer is farm manure that is processed, disinfected, and packaged in powder form. It contains 50% organic matter, 2% nitrogen, 2% phosphorus, 2% potassium, and 10% (humic + fulvic) acid. Botanica-branded fertilizer is a liquid organic fertilizer of vegetative origin that contains 50% organic matter, 21.3% organic carbon, 3% nitrogen, and 2.5% potassium (https://www.camli.com.tr/urunler, accessed on 9 January 2023). Biofarm and Botanica-branded fertilizers were obtained from the Camli Feed Fattening Company. Black mulch was used as the mulch.
The living microorganisms contained in the top fertilizers used in this study were as follows: 1-RhizoFill (bacteria): Bacillus subtilis, Bacillus megaterium, and Pseudomonas fluorescens (1 × 109 cfu/mL); 2-Subtima (bacteria): Bacillus subtilis (1 × 109 cfu/mL); 3-Fontera microzon (bacteria): Azosprillium brasilense, Azotobacter vinelandii, Rhizobium trifollii, Pseudomonas fluorescens, Bacillus subtilis, Bacillus licheniformis, Azotobacter chroococcum, Bacillus amyloliquefaciens, and Bacillus mucilaginosus (5 × 108 cfu/g); 4-Endo roods soluble ERS (Mychorriza): Glomus intraradices, Glomus aggregatum, Glomus mosseae, Glomus clarum, Glomus monosporum, Glomus deserticola, Glomus brasilianum, Glomus etunicatum, and Gigaspora margarita (1 × 104 cfu/g); 5-Bontera (bacteria and fungi): Bacillus amyloliquefaciens, Bacillus pumilus, Bacillus subtilis, Bacillus licheniformis, Bacillus megaterium, Trichoderma harzianum, and Tricoderma konigii (1.2 × 108 cfu/mL).
2.2. Soil Sampling, Treatment, and Analyses
The soil parameters of the experimental area were analyzed prior to the experiment according to the recommendations of [18,19,20,21,22]. The treatment area had a loamy soil structure with a pH of 7.9 (Table 1).

Table 1.
Soil properties of the experimental site.
Powder sulfur was applied to the soil with a pH of 7.9 and to the root fungi. Embankments were built with a top width of 60 cm and a height of 30 cm and were covered with black polyethylene mulch after drip irrigation pipes were placed on them. Fresh seedlings were used in the experiment. Biofarm solid farm manure was equally distributed at 300 kg da−1 for all the treatments except the control before planting. When planting the seedlings, 0.2 g of mycorrhiza (endo-roots soluble) was mixed into the soil once for each plant. In November 2019, the seedlings were planted using the triangle seeding method at 30 × 30 cm intervals. One week after planting, at periods of 15 days, Botanica liquid fertilizer was prepared at 8 lt da−1 and applied via dripping, and the other fertilizers were prepared at 1 mL L−1 and applied to the plant roots. Fertilizer treatments were continued weekly from March until the end of the experiment as plant growth and fruit development accelerated as the weather became warmer. Finally, polyethylene was placed in a low tunnel to protect the seedlings from the winter cold. Throughout the experiment, both the Botanica liquid fertilizer and biofertilizer were applied a total of 22 times.
Abbreviations regarding the treatments are defined below:
(T1) Fertilizer-free (control), only water; (T2) Biofarm + Botanica; (T3) Biofarm + Botanica + Rizofil; (T4) Biofarm + Botanica + Subtima; (T5) Biofarm + Botanica + Fontera microzone; (T6) Biofarm + Botanica + Endo Roots Soluble (mycorrhiza); (T7) Biofarm + Botanica + Bontera.
The total yield per plant, fruit weight, pH, soluble acid content (SSC), and acid content were measured in this study. The analysis began in April and lasted until the end of June. The juice pH was determined every 15 days with a pH meter (Hanna Instruments) in juice extracted from 20 randomly selected fruits from each replicate. The soluble acid content (SSC) was manually measured after 15 days with a portable refractometer using the juice obtained from 20 randomly selected fruits from each replication [23,24]. To determine the acid content, 1 mL of fruit juice obtained from 20 randomly selected fruits from each replication was taken and diluted to 50 mL with distilled water. The sample was titrated with 0.1 N sodium hydroxide until the pH was 8.1. The calculations were determined in % of citric acid every 15 days [25,26]. For the leaf analysis, 15 young leaves were randomly selected from each plot in April. Nitrogen, phosphorus, potassium, calcium, magnesium, iron, manganese, zinc, and copper were also analyzed [27]. Three plants from each plot were randomly measured to determine the leaf area. Digimizer version 5.3.5 was used to conduct the leaf area measurements. At the end of the experiment, the root length, root thickness, stem diameter, and stem dry matter content of three plants that were randomly selected from each treatment repetition were measured. The root thickness was measured with a digital caliper. The stem diameter of three plants that were randomly removed from each replication was measured with a digital caliper at the intersection of the root and stem. To determine the amount of dry matter in the roots and stems, their fresh weights were recorded and dried in an oven at 65 °C until they reached a consistent weight; then, the surface dry matter ratios were calculated [28].
2.3. Statistical Analysis
This experiment followed a randomized plot design with seven treatments, whereby each treatment had four replications and 20 plants in each replication. Finally, the MSTAT_C package program (version 1.2, Michigan State University, East Lansing, MI, USA) was used for the statistical analysis, and the difference between the averages was calculated using the standard deviation test.
3. Results and Discussion
3.1. Plant Growth Parameters
The effects of the treatments on root length, root thickness, stem diameter, root dry matter, and stem dry matter were found to be statistically significant (Table 2). The T5 (26.07 cm) and T7 (26.01 cm) treatments produced the longest roots, and the control (T1) treatments produced the shortest root at 21.51 cm. All the treatment groups produced longer roots than the control (T1) treatment. Ciylez and Esitken [29] used a mixture of peat, sand, and perlite at a ratio of 2:1:1:1 as a medium in a study that treated bacteria and mycorrhizae in pots. Glomus etunicatum, Glomus fasciculatum, and Glomus mosseae species mycorrhizae and Bacillus megaterium M3, Agrobacterium rubi A18, and Bacillus subtilis OSU142 bacteria were used as treatments in the production of the Albion strawberry variety, whether alone or in combination. As a result of this study, the authors reported that the longest root was 27.16 cm in G. fasciculatum treatment, while the shortest root was 17.50 cm in Agrobacterium rubi A18 treatment. When the root thickness data were examined, the T5 and T6 treatments produced the highest value at 1.27 mm, whereas the control treatment resulted in the lowest value in root thickness and length (T1: 0.92 mm). The treatments resulted in higher stem-diameter values than the control treatment. The plants with the thickest stems were given the T5 (16.55 mm) treatment, followed by the T4 (13.77 mm), T6 (13.60 mm), and T7 (13.37 mm) treatments. Balci [30], in his study on various organic wastes, demonstrated that the highest trunk diameter was 13 mm.

Table 2.
The effects of treatments on root length, root thickness, stem diameter, root, and stem dry matter amount.
The treatments increased the amount of root dry matter compared with the control (T1). The T5 (46.15%) treatment resulted in the presence of the highest amount of dry matter in the roots, and the roots of the control (T1) group contained the lowest amount (30.54%). When the stem dry matter amount data were examined, it was determined that the T5 (38.08%) treatment gave the highest value, similar to the root dry matter amount. Finally, all the treatments resulted in higher stem dry matter content than the control treatment (T1, 23.07%).
As seen in Figure 1 below, statistically significant differences in the leaf area were observed among the treatments. When the leaf area values were examined, the treatments were found to have resulted in higher values than the control (T1) treatment. Among the treatments, the highest leaf area value was found in the leaves of the plants receiving the T5 treatment (634.30 cm2 plant−1). Tomic et al. [31] observed that the maximum leaf area achieved from biofertilizer 1 (Azotobacter chroococcum, A. vinelandii, Derxia sp., Bacillus megaterium, B. licheniformis, and B. subtilis) and biofertilizer 2 (Klebsiella planticola) treatments in Clery, Joly, and Dely strawberry varieties was 311.7 cm2, a value attained in a biofertilizer 1 treatment. Khalil and Agah [32] stated that the maximum leaf area was 529.66 cm2. Singh et al. [33] suggested that increased vegetative growth might be due to increased biological nitrogen fixation, more efficient root system development, and possibly an increase in plant growth parameters with the direct effect of biofertilizers and the synthesis of plant growth hormones such as IAA, GA, and cytokinins.
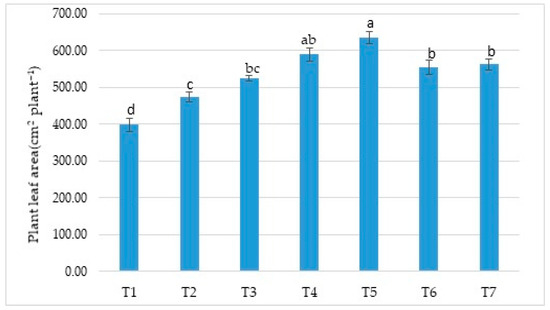
Figure 1.
Effects of treatments on leaf area (cm2 plant−1). Means (±sd) that differ significantly at the 5% level shown with different letters. (T1) No fertilizer (control), only water; (T2) Biofarm + Botanica; (T3) Biofarm + Botanica + Rizofil; (T4) Biofarm + Botanica + Subtima; (T5) Biofarm + Botanica + Fontera microzone; (T6) Biofarm + Botanica + Endo Roots Soluble (mycorrhiza); (T7) Biofarm + Botanica + Bontera.
3.2. Total Yield and Average Fruit Weight per Plant
The effect of the treatments on the total yield per plant was found to be statistically significant (Figure 2). The yield values of all treatments were higher than the control values. Among the treatments, the T5 treatment resulted in the highest total yield at 250.17 g plant−1, and this was followed by the T7 (221.12 g plant−1) and T6 (220.37 g plant−1) treatments. However, the control (T1) treatment resulted in the lowest yield at 127.88 g plant−1. Although the yield values of the T2 treatment were lower than those of the T3, T4, T5, T6, and T7 treatments, they were higher than those of the control (T1). Pesakovic et al. [34] expressed that an increase in yield may be due to the biofertilizers’ hormone production (growth regulator) effects, whereas Singh et al. [33] suggested that increases in yield with the biofertilizer and vermicompost treatments may be due to the increase in fruit set per plant as well as the increase in grain weight with nitrogen fixators. Similarly, Tripathi et al. [35] achieved the highest yield with the Chandler strawberry variety by using a biofertilizer treatment (2008: 180.89 g plant−1; 2009: 178.68 g plant−1). Jain et al. [36] recognized that the increase in yield after using different combinations of various organic fertilizers and biofertilizers for the Sweet Charlie strawberry variety may have resulted from a high amount of carbohydrates following the increase in photosynthesis along with the surge in the number of leaves. By contrast, Srivastav et al. [37] proposed that organic-based treatments could have amplified the activities of beneficial micro-organisms, which in turn promoted the production of growth-promoting substances and augmented the nutrient availability for a longer period during the growing practice; therefore, this may have resulted in a higher yield and overall, more efficient production. Finally, Negi et al. [38] reported that the highest yields observed in organic fertilizers (farmyard manure, vermicompost, and forest litter) and biofertilizers (Azotobacter chroococcum and Pseudomonas fluorescens) treatments in the Chandler strawberry variety were 185.08 g plant−1 in 50 % FYM + 50 % vermicompost + Pseudomonas (T12) treatment and 183.51 g plant−1 in 50 % FYM + 50 % vermicompost + Azotobacter + Pseudomona (T13) treatment. These were equal to the results of T12. The lowest yield was obtained in the control treatment, with 63.37 g plant−1.
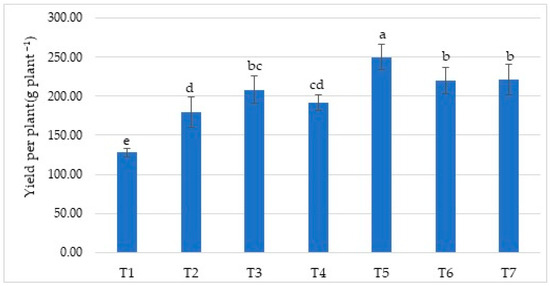
Figure 2.
Effect of treatments on yield per plant (g plant−1). Means (±sd) that differ significantly at the 5% level shown with different letters. (T1) No fertilizer (control), only water; (T2) Biofarm + Botanica; (T3) Biofarm + Botanica + Rizofil; (T4) Biofarm + Botanica + Subtima; (T5) Biofarm + Botanica + Fontera microzone; (T6) Biofarm + Botanica + Endo Roots Soluble (mycorrhiza); (T7) Biofarm + Botanica + Bontera.
The differences in fruit weight obtained according to the treatments were also statistically significant (Figure 3). Firstly, the treatments caused higher fruit weight values than the control (T1) treatment. Among the treatments, the T5 treatment (18.13 g) resulted in the highest value, whereas the control (T1) treatment (15.14 g) resulted in the lowest value. Although fruit size in strawberries is a variety-specific characteristic, environmental factors and practices can also influence it. For example, Jain et al. [36] obtained the highest fruit weight value by using vermicompost + poultry manure + PSB + Azotobacter (12.26 g) in different combinations of organic manure (compost, poultry manure, vermicompost, FYM) and microbial sources of nutrients (Azotobacter and PSB) in the production of the Sweet Charlie strawberry cultivar. Additionally, Seema et al. [39] stated that the maximum fruit weight of the Chandler strawberry variety was 14.62 g when treated with biofertilizer. Biofertilizers have been reported to fix the atmospheric nitrogen in the soil and make it usable for the plant. At the same time, they can dissolve phosphorus, increase the water and nutrients consumed by the plant, and support plant growth by producing phytohormones (such as IAA and cytokinin) and enzymes. They can also fight against some soil-borne diseases and pathogens and provide plant protection against abiotic factors such as drought [12,16].
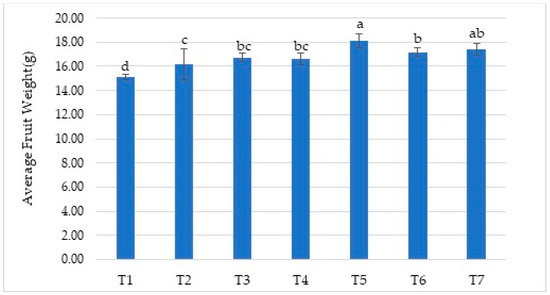
Figure 3.
The effect of treatments on average fruit weight (g). Means (±sd) that differ significantly at the 5% level shown with different letters. (T1) No fertilizer (control), only water; (T2) Biofarm + Botanica; (T3) Biofarm + Botanica + Rizofil; (T4) Biofarm + Botanica + Subtima; (T5) Biofarm + Botanica + Fontera microzone; (T6) Biofarm + Botanica + Endo Roots Soluble (mycorrhiza); (T7) Biofarm + Botanica + Bontera.
3.3. Fruit Juice pH, SSC, Acid, and SSC-to-Acid Ratio
The effects of the treatments on the pH, SSC, acid, and SSC-to-acid ratio were found to be statistically significant (Table 3). Considering the fruit juice pH, the T3 (4.01) and T5 (4.01) treatments resulted in the highest value, whereas the control (T1) treatment resulted in the lowest value at 3.78. Kumar et al. [40] reported that the highest pH value of 4.23 in the Chandler strawberry cultivar was obtained when using the biofertilizer treatment. Additionally, pH values may vary according to the ecological conditions of the region, variety, and treatments. The SSC value was in the range of 9.40 to 10.61%, and the T5 (10.61%) treatment resulted in the highest SSC value. Pesakovic et al. [33] indicated that the highest SSC value was 10.18% when using the biofertilizer treatment, whereas Pradeep and Saravanan [41] acknowledged that the combined treatment of organic and biofertilizer resulted in the highest SSC value of 8.46%. The acid values were between 0.59 and 0.65%. The control treatment resulted in the highest acid value (T1: 0.65%), and it also resulted in the lowest SSC value. Kumar et al. [6] reported that the acidity value was in the range of 0.61 to 0.74%, whereas Pradeep and Saravanan [41] found that the acidity value was in the range of 0.46 to 0.75%. The T5 treatment resulted in the highest SSC-to-acid ratio at 18.05, and the control (T1) treatment resulted in the lowest at a value of 14.48. As a result, all the treatments resulted in higher SSC-to-acid ratio values than the control (T1) treatment. The SSC-to-acid ratio values reported by Kumar et al. [6] were in the range of 12.84 to 17.05 for the Sweet Charlie strawberry variety and 11.3 to 14.0 for the Chandler strawberry variety [40].

Table 3.
The effects of treatments on pH, SSC, acid, and SSC-to-acid ratio values.
3.4. Plant Nutrient Analysis in Leaves
The total amount of nitrogen, phosphorus, potassium, calcium, and magnesium in the leaves after the application of the treatments was statistically significant (Table 4). The leaf nitrogen content in the leaves after the treatments had been applied ranged from 2.66 to 3.94%. The T5 treatment resulted in a higher total nitrogen content (3.94%) than the other treatments. Imriz et al. [12] emphasized that one of the most important features of biofertilizers is that they can bind free nitrogen to the atmosphere. Similarly, Beer and Singh [42] noticed that the vermicompost + Azotobacter treatment resulted in the highest nitrogen content in leaves (3.31%), whereas Jones et al. [43] reported that the nitrogen adequacy level in the leaves was between 2.5 and 4.00%. After the application of the whole battery of treatments, the nitrogen content within the leaves was found to be within the qualification limits and was similar to previous researchers’ findings.

Table 4.
The effects of treatments on the nitrogen, phosphorus, potassium, calcium, and magnesium contents of strawberry leaves (%).
The leaf phosphorus value was between 0.12 and 0.34%, and the T5 (0.34%) treatment resulted in the highest phosphorus value. Imriz et al. [12] proposed that one of the most important features of biofertilizers is their ability to solve phosphorus, and Bacillus polymyxa, Bacillus megatarium, Bacillus circulans, Bacillus Subtilis, Bacillus firmus, Pseudomonas Striata, P. Rathonia, Rhizobium leguminosarum, and R. Meliloti bacteria can dissolve phosphorus by producing organic acid. The combined treatment of organic and biofertilizers produces the highest phosphorus value in the Chandler strawberry variety (0.52%) [38]. Jones et al. [43] outlined that the phosphorus sufficiency level in the leaves was between 0.25 and 1.00%. The treatments resulted in phosphorus content that was within the qualification limits, whereas treatment with the control was found to have insufficient levels of phosphorous content (T1).
The leaf potassium value was found to be between 1.12% and 1.78%. The T5 treatment resulted in the highest potassium value (1.78%), and the control (T1) treatment resulted in the lowest potassium value (1.12%). Negi et al. [38] found that their leaf potassium value was between 0.86 and 1.96%, and the organic and biofertilizer treatments resulted in potassium values that were higher than those produced with the control treatment. Additionally, Jones et al. [43] noted that the potassium sufficiency level in the leaves was between 1.30 and 3.00%. Although the treatments resulted in a sufficient amount of potassium, the control (T1) treatment resulted in an insufficient amount of potassium. The treatments resulted in a leaf calcium value of 1.65–2.47%, and the T5 treatment resulted in the highest calcium content (2.47%). Beer and Singh [42] found that the vermicompost + PSB treatment resulted in the highest calcium value (2.50%). Jones et al. [43] recognized that the calcium sufficiency level in the leaves was between 1.00 and 2.50%. These values show that the calcium values obtained during this study are sufficient. The total magnesium values in the leaf were in the range of 0.21 to 0.94%. Among the treatments, the highest magnesium value was determined in the leaves treated with T5 and T3 to be 0.94% and 0.91%, respectively. Jones et al. [43] reported that the magnesium proficiency level in the leaf was between 0.25 and 1.00%. Although the treatments resulted in a sufficient amount of magnesium, the control (T1) treatment resulted in an insufficient amount of magnesium.
The treatments resulted in a statistically significant total amount of iron, zinc, manganese, and copper in the leaves (Table 5). The total amount of iron in the leaves was in the range of 53.00 ppm to 81.22 ppm. The T5 treatment (81.22 ppm) resulted in the highest amount of iron, and the control (T1) treatment resulted in the lowest amount at 53 ppm. Jones et al. [43] found that the amount of iron in the strawberry leaves was sufficient and was between 50 and 200 ppm. The iron content of the leaves obtained during this study was also determined to be at a sufficient level. The total amount of zinc in the leaves was between 21.22 ppm and 54.21 ppm, and the T5 treatment (54.21 ppm) resulted in the highest amount of zinc. Jones et al. [43] stated that the zinc sufficiency level in leaves was between 20 ppm and 200 ppm. Similarly, all the treatments resulted in a sufficient amount of total zinc in the leaves obtained during this study. The total amount of manganese in the leaves ranged from 30.22 ppm to 74.33 ppm. The T5 treatment resulted in the highest manganese content (74.33 ppm), whereas the control (T1) treatment resulted in the lowest value at 30.22 ppm. Jones et al. [43] claimed that the manganese sufficiency level of leaves was between 50 ppm and 200 ppm. Therefore, when the data obtained were evaluated, it was determined that the total amount of manganese in the leaves was sufficient but that the application of the control (T1) resulted in an insufficient amount. The total copper amount in the leaves was between 2.11 ppm and 3.56 ppm. Similarly, Jones et al. [43] estimated that the copper sufficiency level in leaves was between 6 ppm and 50 ppm. Thus, all the treatments resulted in a low and insufficient amount of copper.

Table 5.
The effects of the treatments on the iron, zinc, manganese, and copper contents of strawberry leaves (ppm).
4. Conclusions
This study obtained higher yields and better-quality fruits via combined treatment with organic fertilizer and biofertilizers compared to the use of organic fertilizer alone. Among the treatments, the T5 (Biofarm + Botanica + Fontera microzone) treatment provided the highest total yield, fruit weight, pH, SSC-to-acid ratio (which affects taste), root length, root thickness, stem diameter, root and stem dry matter amount, and leaf area; additionally, the highest nutrient values were found in the leaves of the Monterey strawberry cultivar. Considering the findings, it can be concluded that the use of fertilizers that contain beneficial bacteria and mycorrhiza increases the efficiency of basic fertilizers and enhances yield and fruit quality by enabling the plant to benefit the soil to a greater extent. In future studies, the effects of the co-treatment of organic fertilizers and biofertilizers on different strawberry varieties should be investigated to assist in the development of sustainable agriculture and food production under global climate conditions.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Kumra, R.R.; Saravanan, S.; Bakshi, P.; Kumar, A.; Singh, M.; Kumar, V. Influence of plant growth regulators on Strawberry: A review. Int. J. Chem. Stud. 2018, 6, 1236–1239. [Google Scholar]
- FAO. 2023. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 February 2023).
- Ministry of Agriculture and Forestry. 2023. Available online: https://www.agricultureforest.gov.tr/Subjects/Crop-Production/Organic-Agriculture/Statistics (accessed on 20 March 2023).
- Joshi, R.; Singh, J.; Vig, A.P. Vermicompost as an effective organic fertilizer and biocontrol agent:effect on growth, yield and quality of plants. Rev. Environ. Sci. Biotechnol 2015, 14, 137–159. [Google Scholar] [CrossRef]
- Kumar, N.; Ram, R.B.; Mishra, P.K. Effect of vermicompost and azotobacter on quality parameters of strawberry (Fragaria × ananassa Duch.) cv. Sweet Charlie. Int. J. Agric. Sci. Res. 2015, 5, 269–276. [Google Scholar]
- Tripathi, V.K.; Jain, A.; Kumar, S.; Dubey, V.; Kumar, A. Efficacy of biofertilizers and mulching on growth, yield and quality of strawberry. (Fragaria × ananassa) cv. Chandler. Indian J. Agric. Sci. 2017, 87, 1179–1183. [Google Scholar]
- Soni, S.; Kanawjia, A.; Chaurasiya, R.; Chauhan, P.S.; Kumar, R.; Dubey, S. Effect of organic manure and biofertilizers on growth, yield and quality of strawberry (Fragaria × ananassa Duch) cv. Sweet Charlie. J. Pharmacogn. Phytochem. 2018, 2, 128–132. [Google Scholar]
- Prasad, H.; Sajwan, P.; Kumari, M.; Solanki, S. Effect of organic manures and biofertilizer on plant growth, yield and quality of horticultural crop: A review. Int. J. Chem. Stud. 2017, 5, 217–221. [Google Scholar]
- Uddin, A.F.M.J.; Rakibuzzaman, M.; naher Wasin, E.W.; Husna, M.A.; Mahato, A.K. Foliar application of Spirulina and Oscillatoria on growth and yield of okra as bio-fertilizer. J. Biosci. Agric. Res. 2019, 22, 1840–1844. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, V.; Johar, V.; Kumar, A.; Kadlag, S.S. Uses of plant growth regulators and biofertilizers in fruit crops: A Review. Int. J. Environ. Clim. Chang. 2022, 12, 314–326. [Google Scholar] [CrossRef]
- Imriz, G.; Ozdemir, F.; Topal, I.; Ercan, B.; Tas, M.N.; Yakısır, E.; Okur, O. Rhizobacteria (PGPRs) promoting plant growth in plant production and their mechanisms of action. Electron. J. Microbiol. 2014, 12, 1–19. [Google Scholar]
- García-Fraile, P.; Menéndez, E.; Rivas, R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioeng. 2015, 2, 183–205. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E. Impact of beneficial microorganisms on strawberry growth, fruit production, nutritional quality, and volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Chawla, R.; Sadawarti, R.K. Effect of bıo-fertılızers and organıc manures on growth, yıeld and fruıt qualıty of fruıt crops. Plant Arch. 2020, 20, 3767–3768. [Google Scholar]
- Turemis, N.; Ağaoğlu, Y.S. Berry Fruits, Chapter II (Strawberry). In Tomurcuk Bag Ltd. Sti. Education Publications 1; Ağaoğlu, S., Gerçekcioğlu, R., Eds.; Tomurcuk Bag Ltd. Sti.: Ankara, Turkey, 2013; pp. 55–100. [Google Scholar]
- Bouyoucus, G.L. A Recalibration of Hydrometer Method for Making Mechanical Analysis of Soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; Agronomy Monographs 9(1); American Society of Agronomy: Madison, WI, USA, 1986; 1188p. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. In U.S. Department of Agriculture Handbook; U.S. Government Printing Office: Washington, DC, USA, 1954; Volume 60, pp. 105–106. [Google Scholar]
- Olsen, S.R.; Cole, V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Lindsay, W.L.; Norvell, W.A. Development of A DTPA Soil Test for Zinc, Iron, Manganese and Copper. Soil Sci. Soc. Am. Proceeding 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Kaska, N.; Yildiz, A.I.; Paydas, S.; Bicici, M.; Turemis, N.; Kuden, A. Effects of winter and early summer plantings and shelter systems on the yield quality and early production of some new strawberry varieties for Turkey under Adana ecological conditions. Nat. Sci. J. 1986, 10, 84–102. [Google Scholar]
- Turemis, N. Adaptation of Some New Strawberry Varieties in Cyprus Conditions; KKTC/TAGEP Project Final Report No. 5.2.3.4; Cukurova University and KKTC Ministry of Agriculture and Natural Resources, KKTC: Lefkosa, Cyprus, 2003. [Google Scholar]
- Ozdemir, E.; Gunduz, K.; Bayazit, S. Determination of yield, quality and precocity of some strawberry cultivars grown under high tunnel by using fresh runners rooted in pots in Amik plain. Bahçe 2001, 30, 65–70. [Google Scholar]
- Adak, N.; Gubbuk, H.; Pekmezci, M. The growing possibilities of some strawberry cultivars grown under protected cultivation in Antalya conditions. In Proceedings of the Turkey IV, National Horticulture Congress, Antalya, Turkey, 8–12 September 2003; pp. 313–315. [Google Scholar]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; 384p. [Google Scholar]
- Turemis, N.; Kaska, N. The effects of planting mother plants at different dates in three regions on arm plant production in strawberries. Turk. J. Agric. For. 1995, 19, 457–463. [Google Scholar]
- Ciylez, S.; Esitken, A. The Effect of Mycorrhiza and PGPR application on strawberry of growth. Selcuk. J. Agric. Food Sci. 2018, 32, 361–365. [Google Scholar] [CrossRef]
- Balcı, G. The Effects of Diffrent Organic Waste on Yıeld and Quality of Organically Grown Strawberry. Ph.D. Thesis, Ondokuz Mayıs University, Samsun, Turkey, 2012; 132p. [Google Scholar]
- Tomic, J.M.; Milivojevic, J.M.; Pesakovic, M.I. The response to bacterial inoculation is cultivar-related in strawberries. Turk. J. Agric. For. 2015, 39, 332–341. [Google Scholar] [CrossRef]
- Khalil, N.H.; Agah, R.J. Effect of chemical, organic and bio fertilization on growth and yield of strawberry plant. Int. J. Adv. Chem. Eng. Biol. Sci. 2017, 4, 5. [Google Scholar] [CrossRef]
- Singh, A.K.; Beer, K.; Pal, A.K. Effect of vermicompost and bio-fertilizers on strawberry ı: Growth, flowering and yield. Ann. Plant Soil Res. 2015, 17, 196–199. [Google Scholar]
- Pešaković, M.; Karaklajić-Stajić, Ž.; Milenković, S.; Mitrović, O. Biofertilizer affecting yield related characteristics of strawberry (Fragaria × ananassa Duch.) and soil micro-organisms. Sci. Hortic. 2013, 150, 238–243. [Google Scholar] [CrossRef]
- Tripathi, V.K.; Kumar, S.; Kumar, K.; Kumar, S.; Dubey, V. Influence of Azotobacter, Azospirillum and PSB on vegetative growth, flowering, yield and quality of strawberry cv. Chandler. Progress. Hortic. 2016, 48, 48–52. [Google Scholar] [CrossRef]
- Jain, N.; Mani, A.; Kumari, S.; Kasera, S.; Bahadur, V. Influence of INM on yield, quality, shelf life and economics of cultivation of strawberry (Fragaria× ananassa Duch.) cv. Sweet Charlie. J. Pharmacogn. Phytochem. 2017, 6, 1178–1181. [Google Scholar]
- Srivastav, A.; Singh, B.K.; Pandey, R.; Singh, K.; Singh, V. Effect of organic manures and bio-fertilizers on vegetative growth and yield of strawberry cv. chandler. J. Pharmacogn. Phytochem. 2018, 7, 2841–2844. [Google Scholar]
- Negi, Y.K.; Sajwan, P.; Uniyal, S.; Mishra, A.C. Enhancement in yield and nutritive qualities of strawberry fruits by the application of organic manures and biofertilizers. Sci. Hortic. 2021, 283, 110038. [Google Scholar] [CrossRef]
- Seema, K.; Mehta, K.; Singh, N. Studies on the effect of plant growth promoting rhizobacteria (PGPR) on growth, physiological parameters, yield and fruit quality of strawberry cv. Chandler. J. Pharmacogn. Phytochem. 2018, 7, 383–387. [Google Scholar]
- Kumar, P.; Sharma, N.; Sharma, S.; Gupta, R. Rizosphere stochiometry, fruit yield, quality attibutes and growth response to PGPR transplant amendments in strawberry (Fragariaxananassa Duch.) growing on solarized soils. Sci. Hortic. 2020, 265, 109215. [Google Scholar] [CrossRef]
- Pradeep, B.; Saravanan, S. Effect of different biofertilizers and organic manures on yield and quality of strawberry (Fragaria× ananassa Duch.) cv. chandler. J. Pharmacogn. Phytochem. 2018, 7, 151–155. [Google Scholar]
- Beer, K.; Singh, A.K. Effect of vermicompost and biofertilizers on strawberry: Chlorophyll and nutrients concentration in leaves. Ann. Plant Soil Res. 2015, 17, 211–214. [Google Scholar]
- Jones, J.R.; Wolf, B.; Mills, H.A. Plant Analysis Handbook; Micro Macro Publishing, Inc.: Athens, GA, USA, 1991; 213p, ISBN 9781878148001. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).