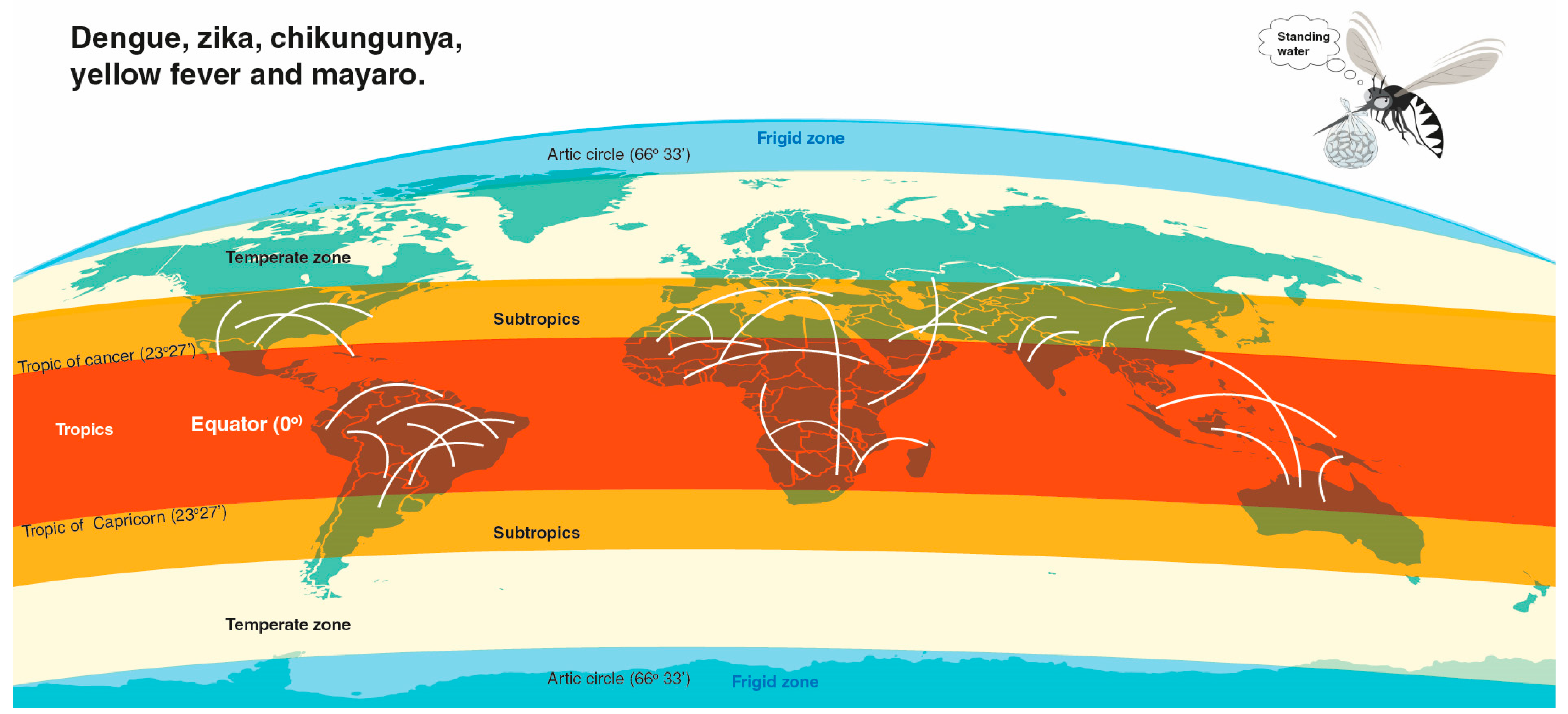
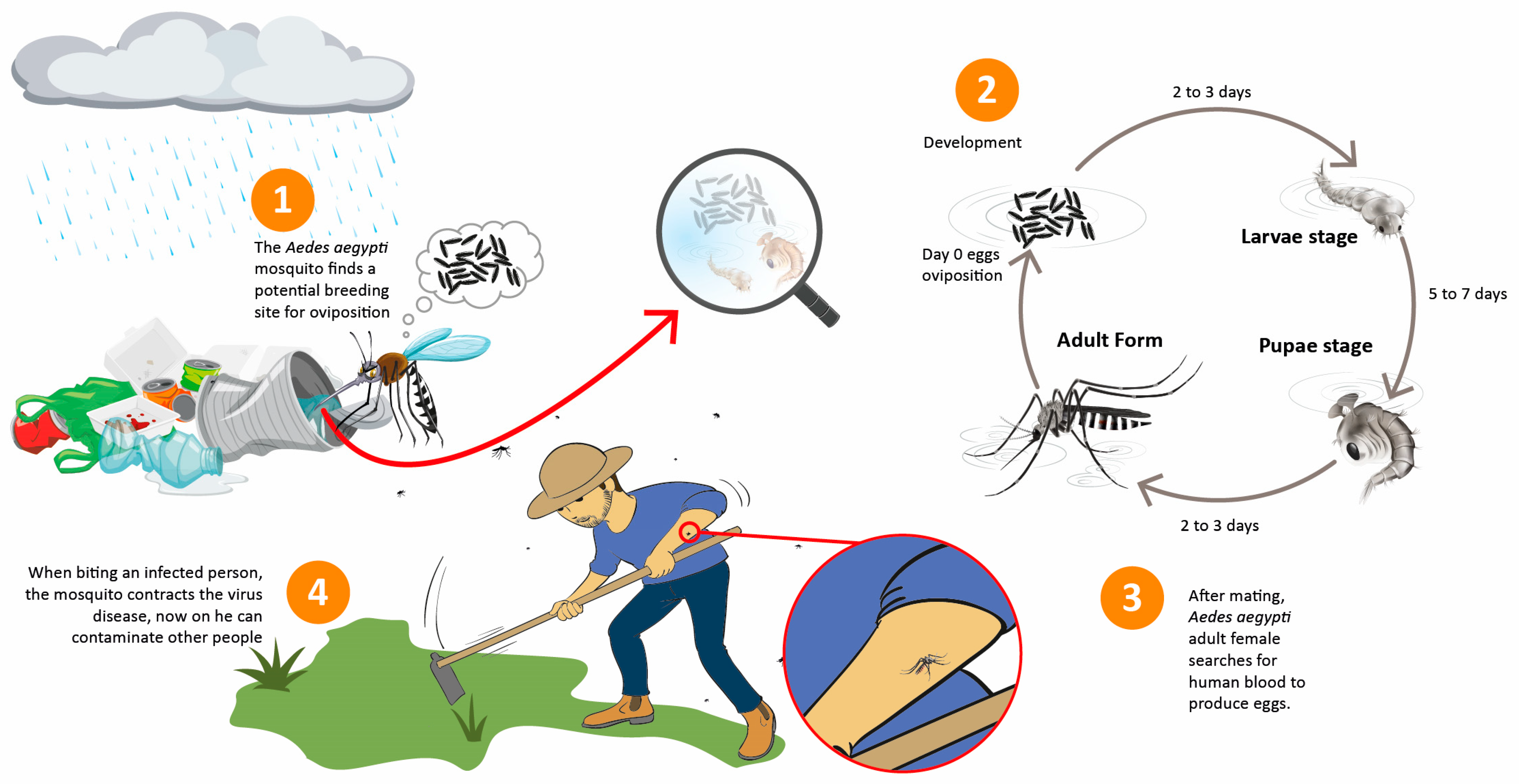
Environmental Variables Related to Aedes aegypti Breeding Spots and the Occurrence of Arbovirus Diseases
Abstract
1. Introduction
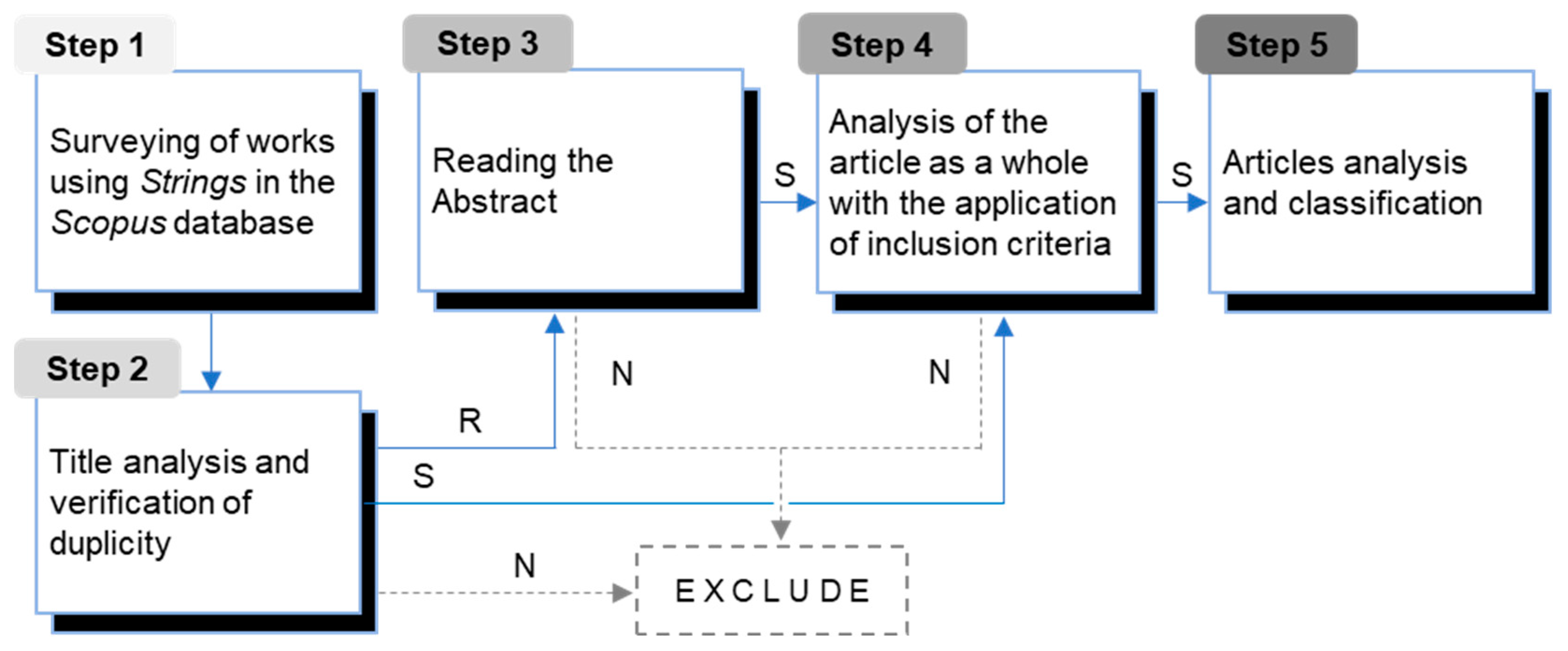
2. Materials and Methods
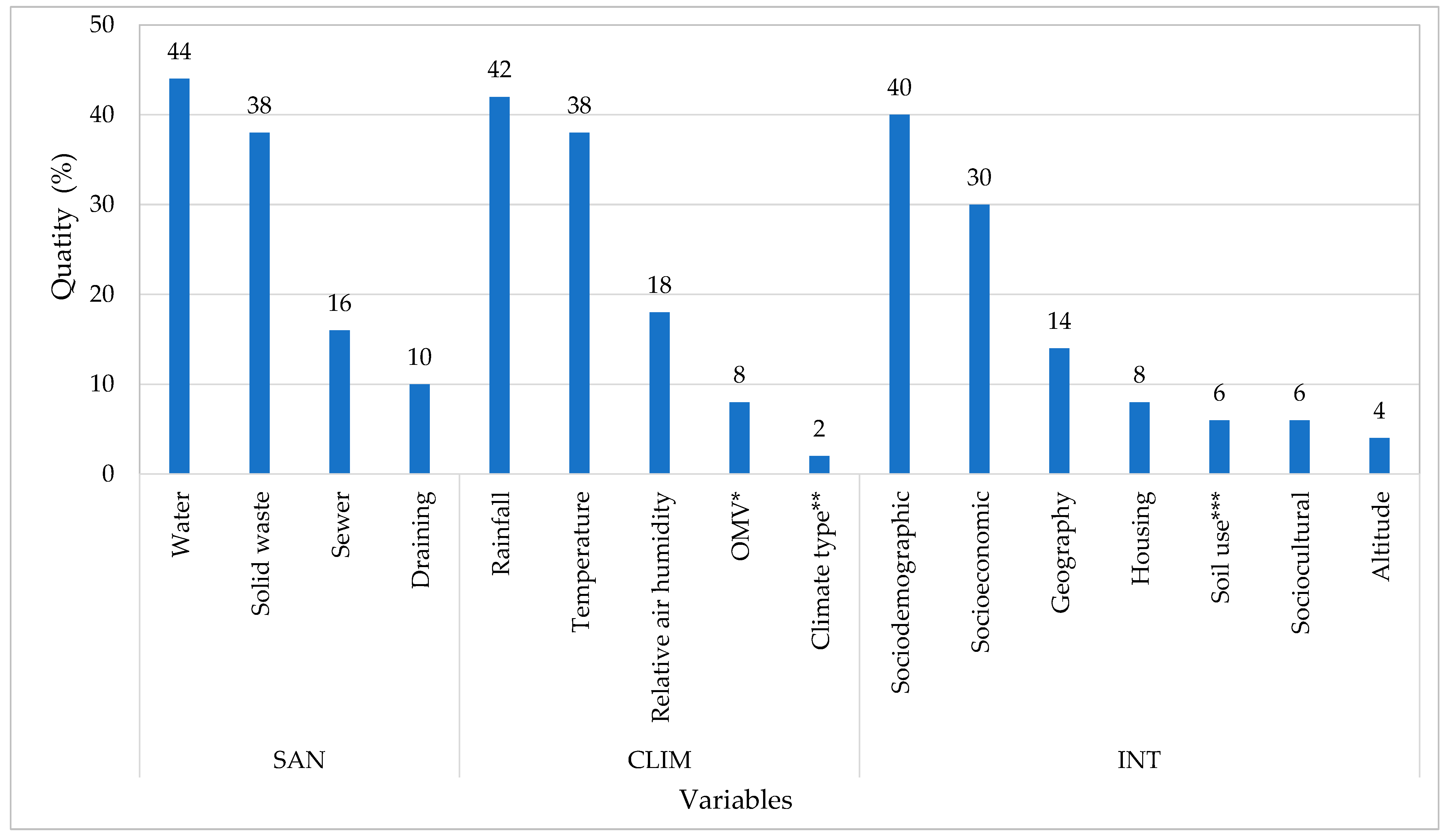
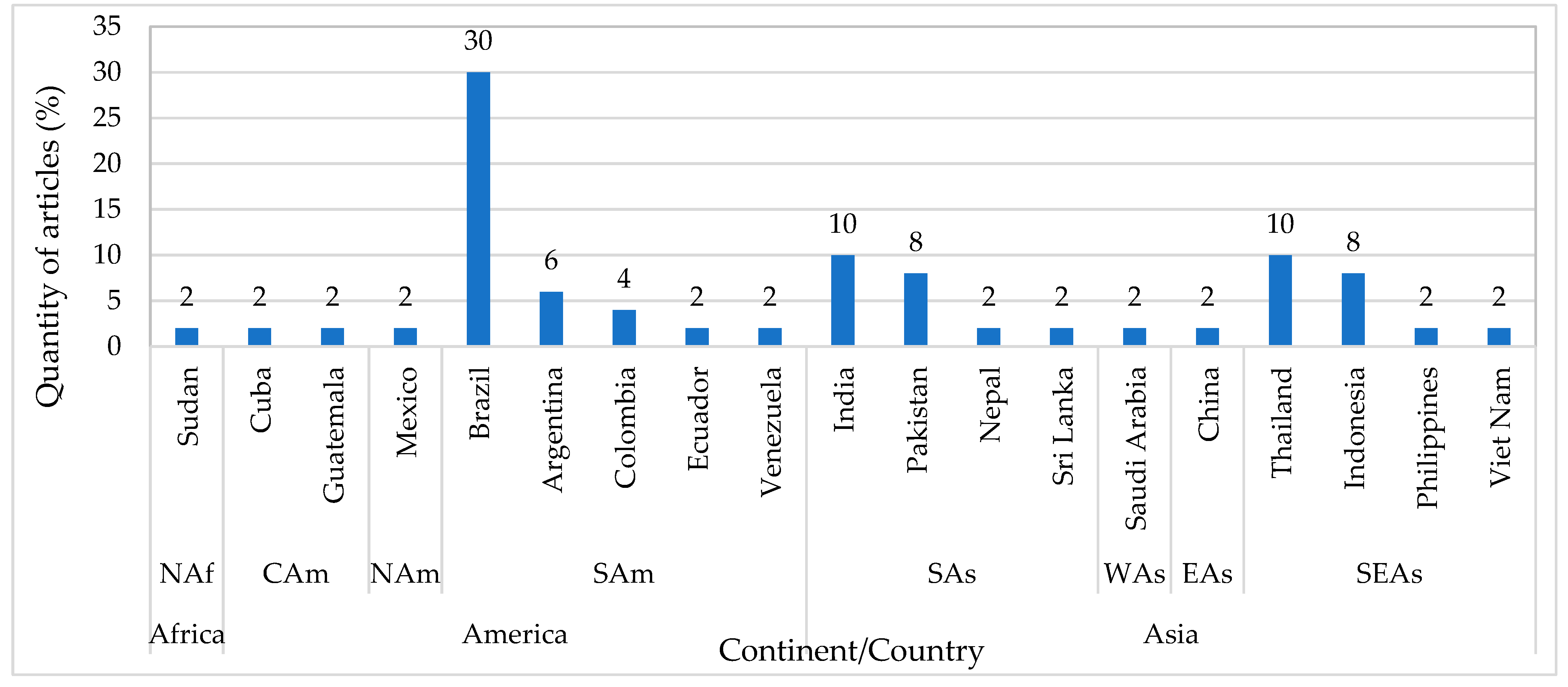
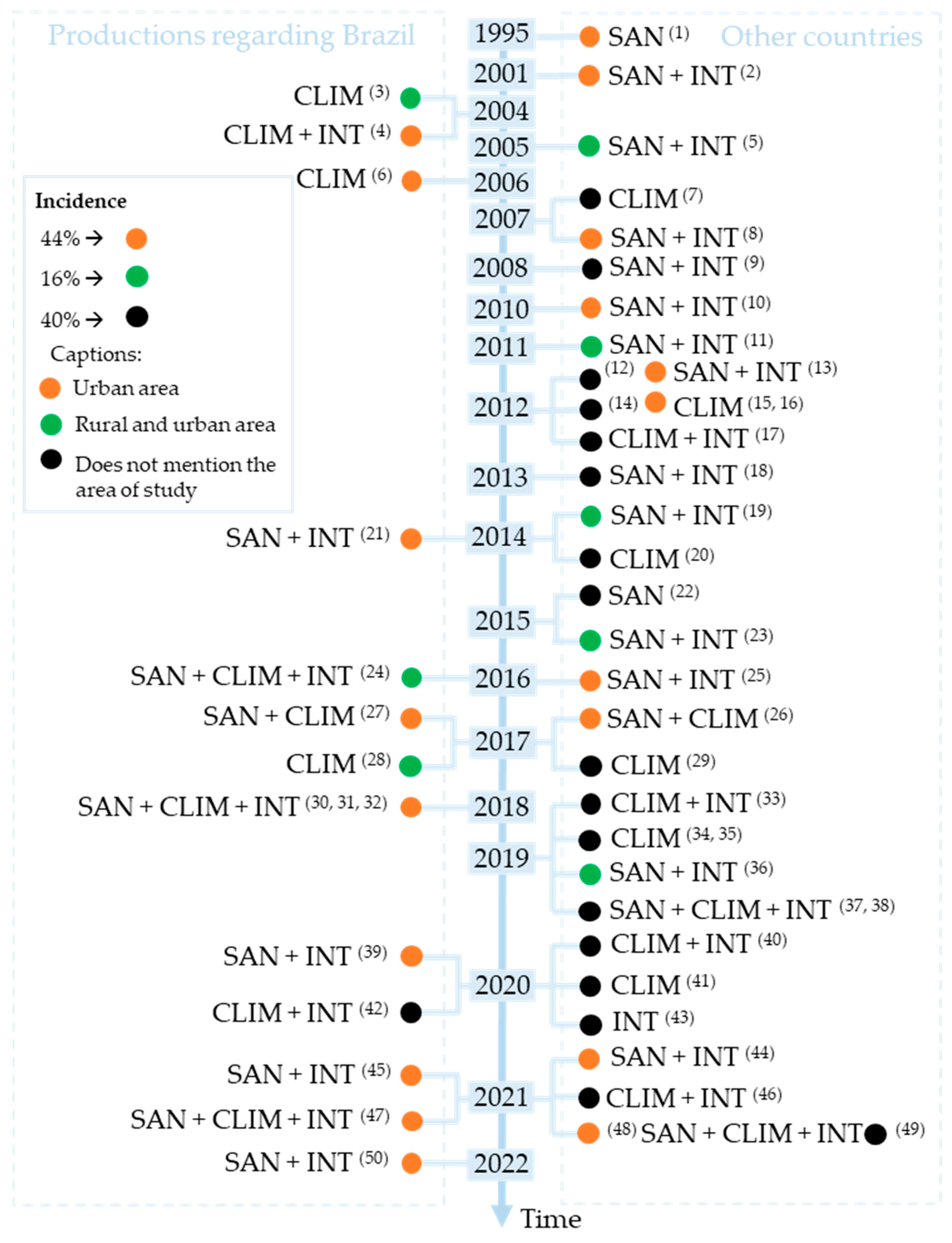
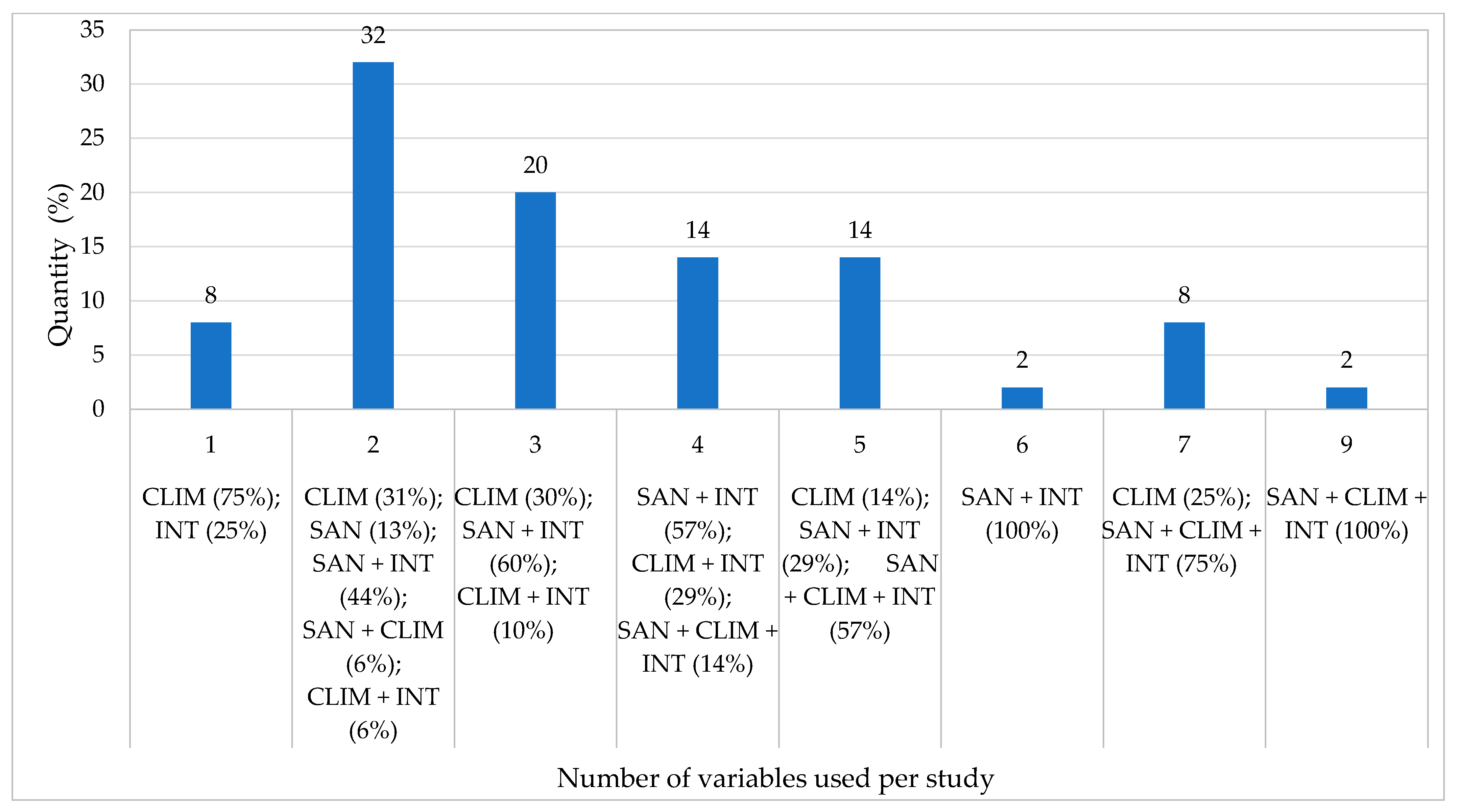
3. Results
4. Discussion
4.1. Significant Relationship between Water and Aedes Breeding Sites
4.2. Sanitation
4.3. Integrative
4.4. Climatologic
5. Tendencies and Gaps
6. Conclusions
- The variable water permeates between the categories (SAN, CLIM and INT) and other research variables, showing notable relevance for public health, especially in the context of basic sanitation.
- The Aedes aegypti breeding spots and occurrence of diseases such as dengue, Zika and chikungunya are related to 16 variables, evaluated individually or jointly within one or more categories defined in this study.
- There are three categories that cover the located variables, these being basic sanitation, climatologic elements and secondary variables, which have been named “integrative” in this work.
- A total of 66% of the works have used some basic sanitation components in the calculation of correlations in this research topic, inferring that sanitation is a crucial indicator that can explain the proliferation of vectors in a given region and, consequently, the increase of incidence of arbovirus diseases.
- From 2010 to the time of this study, 16% of the works have incorporated discussions about Aedes aegypti, arbovirus diseases and environmental variables, the theme of community engagement in health promotion, alerting humanity to join efforts in facing challenges including the prevention of vector-transmitted diseases, especially in the context of climate changes.
- There is a demand for research involving a greater quantity of variables analyzed in tandem, which could better explain the dissemination or lack thereof of arbovirus diseases in a given location.
- In posterior studies, the group of 16 variables may contribute to the creation of indexes that would subsidize a better understanding of the health–disease process, in an analysis of the rural environment concerning measures to prevent diseases and promote health.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zammarchi, L.; Spinicci, M.; Bartoloni, A. Zika virus: A review from the virus basics to proposed management strategies. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016056. [Google Scholar] [CrossRef]
- Lee, G.O.; Vasco, L.; Márquez, S.; Zuniga-Moya, J.C.; Van Engen, A.; Uruchima, J.; Ponce, P.; Cevallos, W.; Trueba, G.; Trostle, J.; et al. A dengue outbreak in a rural community in Northern Coastal Ecuador: An analysis using unmanned aerial vehicle mapping. PLoS Negl. Trop. Dis. 2021, 15, e0009679. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef]
- Moutinho, S.; Rocha, J.; Gomes, A.; Gomes, B.; Ribeiro, A.I. Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review. Sustainability 2022, 14, 8975. [Google Scholar] [CrossRef]
- Alkhaldy, I.; Barnett, R. Explaining Neighbourhood Variations in the Incidence of Dengue Fever in Jeddah City, Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 13220. [Google Scholar] [CrossRef]
- Dieng, I.; Fall, C.; Barry, M.A.; Gaye, A.; Dia, N.; Ndione, M.H.D.; Fall, A.; Diop, M.; Sarr, F.D.; Ndiaye, O.; et al. Re-Emergence of Dengue Serotype 3 in the Context of a Large Religious Gathering Event in Touba, Senegal. Int. J. Environ. Res. Public Health 2022, 19, 16912. [Google Scholar] [CrossRef]
- Lisboa, T.R.; Serafin, I.B.M.; Serafin, J.C.M.; Ramos, A.C.; Nascimento, R.M.; Roner, M.N.B. Relação entre incidência de casos de arboviroses e a pandemia da COVID-19. Rev. Interdiscip. Cienc. Apl. 2022, 6, 31–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Shi, R. Influences of Differentiated Residence and Workplace Location on the Identification of Spatiotemporal Patterns of Dengue Epidemics: A Case Study in Guangzhou, China. Int. J. Environ. Res. Public Health 2022, 19, 13393. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Dengue and Severe Dengue. 2022. Available online: https://www.who.int/news-room/q-a-detail/dengue-and-severe-dengue (accessed on 10 January 2023).
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by EvidenceBased Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Cheng, X.; Hu, H.; Guo, C.; Huang, J.; Chen, Z.; Lu, J. The worldwide seroprevalence of DENV, CHIKV and ZIKV infection: A systematic review and meta-analysis. PLoS Negl. Dis. 2021, 15, e0009337. [Google Scholar] [CrossRef]
- Nelson, M.J.; PAHO—Organizaçion Pan-Americana de la Salud. Aedes Aegypti: Biologia y Ecologia; PAHO: Washington, DC, USA, 1986; 50p, Available online: https://iris.paho.org/handle/10665.2/28513?locale-attribute=pt (accessed on 10 January 2023).
- Ferdousi, F.; Yoshimatsu, S.; Ma, E.; Sohel, N.; Wagatsuma, Y. Identification of Essential Containers for Aedes Larval Breeding to Control Dengue in Dhaka, Bangladesh. Trop. Med. Health 2015, 43, 253–264. [Google Scholar] [CrossRef]
- Buhler, C.; Winkler, V.; Runge-Ranzinger, S.; Boyce, R.; Horstick, O. Environmental methods for dengue vector control—A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007420. [Google Scholar] [CrossRef]
- Aik, J.; Neo, Z.W.; Rajarethinam, J.; Chio, K.; Lam, W.M.; Ng, L.C. The effectiveness of inspections on reported mosquito larval in households: A case-control study. PLoS Negl. Trop. Dis. 2019, 13, e0007492. [Google Scholar] [CrossRef]
- Dalpadado, R.; Amarasinghe, D.; Gunathilaka, N. Water quality characteristics of breeding habitats in relation to the density of Aedes aegypti and Aedes albopictus in domestic settings in Gampaha district of Sri Lanka. Acta Trop. 2022, 229, 106339. [Google Scholar] [CrossRef]
- McBride, W.J.; Mullner, H.; Muller, R.; Labrooy, J.; WRONSKI, I. Determinants of Dengue 2 Infection among Residents of Charters Towers, Queensland, Australia. Am. J. Epidemiol. 1998, 148, 1111–1116. [Google Scholar] [CrossRef]
- Ngugi, H.N.; Mutuku, F.M.; Ndenga, B.A.; Musunzaji, P.S.; Mbakaya, J.O.; Aswani, P.; Irungu, L.W.; Mukoko, D.; Vulule, J.; Kitron, U.; et al. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasites Vectors 2017, 10, 331. [Google Scholar] [CrossRef]
- Wat’senga Tezzo, F.; Fasine, S.; Manzambi Zola, E.; Marquetti, M.C.; Binene Mbuka, G.; Ilombe, G.; Mundeke Takasongo, R.; Smitz, N.; Bisset, J.A.; Van Bortel, W.; et al. High Aedes spp. larval indices in Kinshasa, Democratic Republic of Congo. Parasites Vectors 2021, 14, 92. [Google Scholar] [CrossRef]
- Claro, L.B.L.; Kawa, H.; Cavalini, L.T.; Rosa, M.L.G. Community Participation in Dengue Control in Brazil. Dengue Bull. 2006, 30, 214–222. Available online: https://apps.who.int/iris/handle/10665/170257 (accessed on 15 January 2023).
- Ruiz-Díaz, M.S.; Mora Garcia, G.J.; Salgedo-Madrid, G.I.; Alário, Á.; Gómezcamargo, D.E. Analysis of Health Indicators in Two Rural Communities on the Colombian Caribbean Coast: Poor Water Supply and Education Level Are Associated with WaterRelated Diseases. Am. J. Trop. Med. Hyg. 2017, 97, 1378–1392. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Graziele, I.; Marques, R.C.; Gonçalves, J. Investment in drinking water and sanitation infrastructure and its impact on waterborne diseases dissemination: The Brazilian case. Sci. Total Environ. 2021, 779, 146279. [Google Scholar] [CrossRef]
- Akmal, T.; Jamil, F. Assessing Health Damages from Improper Disposal of Solid Waste in Metropolitan Islamabad–Rawalpindi, Pakistan. Sustainability 2021, 13, 2717. [Google Scholar] [CrossRef]
- Crespo, R.J.; Rogers, R.E. Habitat Segregation Patterns of Container Breeding Mosquitos: The Role of Urban Heat Islands, Vegetation Cover, and Income Disparity in Cemeteries of New Orleans. Int. J. Environ. Res. Public Health 2022, 19, 245. [Google Scholar] [CrossRef]
- Wilke, A.B.; Vasquez, C.; Carvajal, A.; Moreno, M.; Diaz, Y.; Belledent, T.; Gibson, L.; Petrie, W.D.; Fuller, D.O.; Beier, J.C. Cemeteries in Miami-Dade County, Florida are important areas to be targeted in mosquito management and control efforts. PLoS ONE 2020, 15, e0230748. [Google Scholar] [CrossRef]
- Cissé, G. Food-borne and water-borne diseases under climate change in low- and middle-income countries: Further efforts needed for reducing environmental health exposure risks. Acta Trop. 2019, 194, 181–188. [Google Scholar] [CrossRef]
- Ligsay, A.; Telle, O.; Paul, R. Challenges to Mitigating the Urban Health Burden of Mosquito-Borne Diseases in the Face of Climate Change. Int. J. Environ. Res. Public Health 2021, 18, 5035. [Google Scholar] [CrossRef]
- Ahmad Zamzuri, M.’A.I.; Abd Majid, F.N.; Dapari, R.; Hassan, M.R.; Isa, A.M.M. Perceived Risk for Dengue Infection Mediates the Relationship between Attitude and Practice for Dengue Prevention: A Study in Seremban, Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 13252. [Google Scholar] [CrossRef]
- Mulderij-Jansen, V.; Pundir, P.; Grillet, M.E.; Lakiang, T.; Gerstenbluth, I.; Duits, A.; Tami, A.; Bailey, A. Effectiveness of Aedes-borne infectious disease control in Latin America and the Caribbean region: A scoping review. PLoS ONE 2022, 17, e0277038. [Google Scholar] [CrossRef]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- Fernandes, N.A.T.; Souza, A.C.; Simões, L.A.; Reis, G.M.F.; Souza, K.T.; Schwan, R.F. Eco-friendly biosurfactant from Wickerhamomyces anomalus CCMA 0358 as larvicidal and antimicrobial. Microbiol. Res. 2020, 241, 126571. [Google Scholar] [CrossRef]
- Sriklin, T.; Kajornkasirat, S.; Puttinaovarat, S. Dengue Transmission Mapping with Weather-Based Predictive Model in Three Southernmost Provinces of Thailand. Sustainability 2021, 13, 6754. [Google Scholar] [CrossRef]
- Cordeiro, R.; Donaliso, M.R.; Andrande, V.R.; Mafra, A.C.N.; Nucci, L.N.; Brown, J.C.; Stephan, C. Spatial distribuition of the risk of dengue fever in southeast Brazil, 2006–2007. BMC Public Health 2011, 11, 335. [Google Scholar] [CrossRef]
- Khalid, B.; Ghaffar, A. Dengue transmission based on urban environmental gradients in different cities of Pakistan. Int. J. Biometeorol. 2015, 59, 267–283. [Google Scholar] [CrossRef]
- Abd Majid, N.; Muhamad Nazi, N.; Mohamed, A.F. Distribution and Spatial Pattern Analysis on Dengue Cases in Seremban District, Negeri Sembilan, Malaysia. Sustainability 2019, 11, 3572. [Google Scholar] [CrossRef]
- Almeida, S.A.; Cota, A.L.S.; Rodrigues, D.F. Sanitation, Arboviruses, and Environmental Determinants of Disease: Impacts on urban health. Ciên. Saúde Coletiva 2020, 25, 3857–3868. [Google Scholar] [CrossRef]
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.-P.; et al. Climate and Urbanization Drive Mosquito Preference for Humans. Curr. Biol. 2020, 30, 3570–3579. [Google Scholar] [CrossRef]
- Tunali, M.; Radin, A.A.; Başibüyük, S.; Musah, A.; Borges, I.V.G.; Yenigun, O.; Aldosary, A.; Kostkova, P.; Santos, W.S.; Masson, T.; et al. A review exploring the overching burden of Zika virus with emphasis on epidemiological case studies from Brazil. Environ. Sci. Pollut. Res. 2021, 28, 55952–55966. [Google Scholar] [CrossRef]
- Maftei, C.; Bărbulescu, A.; Rugina, S.; Nastac, C.D.; Dumitru, I.M. Analysis of the Arbovirosis Potential Occurrence in Dobrogea, Romania. Water 2021, 13, 374. [Google Scholar] [CrossRef]
- Nagao, Y.; Thavara, U.; Chitnumsup, P.; Tawatsin, A.; Chansan, C.; Campbell-Lendrum, D. Climatic and social risk factors for Aedes infestation in rural Thailand. Trop. Med. Int. Health 2003, 8, 650–659. [Google Scholar] [CrossRef]
- Paul, A.S.; Vicent, J.; Saju, C.R.; Mohamed Rafi, M. A study on larval indices of Aedes and risk for dengue outbreak in a rural area of Thrissur district, Kerala. J. Commun. Dis. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ekalaksananan, T.; Zafar, S.; Poolphol, P.; Shipin, O.; Haque, U.; Paul, R.; Rocklov, J.; Pientong, C.; Overgaard, H.J. Ecological, social and other environmental determinants of dengue vector abundance in urban and rural areas of Northeastern Thailand. Int. J. Environ. Res. Public Health 2021, 18, 5971. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Azami, N.A.; Salleh, S.A.; Neoh, H.M.; Syed Zakaria, S.Z.; Jamal, R. Dengue epidemic in Malaysia: Not a predominantly urban disease anymore. BMC Res. Note 2011, 4, 216. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.Q.; Mai, T.T.X.; Tam, N.L.M.; Nghia, L.T.; Komada, K.; Murakami, H. Prevalence and risk factors of dengue infection in Khanh Hoa Province, Vietnam: A stratified cluster sampling survey. J. Epidemiol. 2018, 28, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Ellis, T.J. A system approach to conduct an effective literature review in support of information systems research. Inf. Sci. J. 2006, 9, 181–212. [Google Scholar] [CrossRef] [PubMed]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Snyder, H. Literature review as a research methodology: An overview and guidelines. J. Bus. Res. 2019, 104, 333–339. [Google Scholar] [CrossRef]
- Barrera, R.; Navarro, J.C.; Mora-Rodrigues, J.D.; Dominguez, D.; Gonzalez-Garcia, J.E. Deficiencia en servicios publicos y cria de Aedes aegypti en Venezuela. Bol. Oficina Sanit. Panam. 1995, 118, 410–423. Available online: https://iris.paho.org/handle/10665.2/15592 (accessed on 20 December 2022).
- Bohra, A.; Andrianasolo, H. Application of GIS in Modeling of Dengue Risk Based on Sociocultural Data: Case of Jalore, Rajasthan, India. Dengue Bull. 2001, 25, 92–102. Available online: https://apps.who.int/iris/handle/10665/163690 (accessed on 12 December 2022).
- Gonçalves Neto, V.S.; Rebêlo, J.M. Aspectos epidemiológicos do dengue no Município de São Luís, Maranhão, Brasil, 1997–2002. Cad. Saúde Pública 2004, 20, 1424–1431. [Google Scholar] [CrossRef]
- Penna, M.L.F. Ecological study of Rio de Janeiro City DEN-3 epidemic, 2001–2002. Dengue Bull. 2004, 28, 20–27. Available online: https://apps.who.int/iris/handle/10665/163977 (accessed on 15 January 2023).
- Thammapalo, S.; Chongsuwiwatwong, V.; Geater, A.; Lim, A.; Choomalee, K. Socio-demographic and environmental factors associated with Aedes breeding places in Phuket, Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36, 426–433. Available online: https://pubmed.ncbi.nlm.nih.gov/15916050/ (accessed on 14 January 2023).
- Favier, C.; Degallier, N.; Vilarinhos, P.D.T.R.; Carvalho, M.D.S.L.; Yoshizawa, M.A.C.; Knox, M.B. Effects of climate and different management strategies on Aedes aegypti breeding sites: A longitudinal survey in Brasília (DF, Brazil). Trop. Med. Int. Health 2006, 11, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Nitatpattana, N.; Singhasivanon, P.; Kiyoshi, H.; Andrianasolo, H.; Yoksan, S.; Gonzalez, J.P.; Barbazan, P. Potential association of dengue hemorrhagic fever incidence and remote senses land surface temperature, Thailand, 1998. Southeast Asian J. Trop. Med. Public Health 2007, 38, 427–433. Available online: https://imsear.searo.who.int/handle/123456789/34750 (accessed on 10 December 2022). [PubMed]
- Brunkard, J.M.; López, J.L.R.; Ramirez, J.; Cifuentes, E.; Rothenberg, S.J.; Hunsperger, E.A.; Moore, C.G.; Brussolo, R.M.; Villarreal, N.A.; Haddad, B.M. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg. Infect. Dis. 2007, 13, 1477–1483. [Google Scholar] [CrossRef]
- Nagao, Y.; Svasti, P.; Tawatsin, A.; Thavara, U. Geographical structure of dengue transmission and its determinants in Thailand. Epidemiol. Infect. 2007, 136, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Peraza, M.C.; Miranda, C.G.; Váldes, L.S.; Chacón, D.P.; Díaz, V.P.; Díaz, D.C.; Rodríguez, C.S.; Van Der Stuyft, P. Encuesta poblacional sobre conocimientos y percepciones acerca de dengue contra prácticas preventivas en el municipio Lisa. Rev. Cuba. Med. Trop. 2010, 62, 245–253. Available online: https://pubmed.ncbi.nlm.nih.gov/23437556/ (accessed on 15 January 2023).
- Anish, T.S.; Vijayakumar, K.; Leela Itty Ammma, K.R. Domestic and environmental factors of chikungunya-affected families in Thiruvananthapuram (Rural) district of Kerala, India. J. Glob. Infect. Dis. 2011, 3, 32–36. [Google Scholar] [CrossRef]
- Kholedi, A.A.N.; Balubaid, O.; Milaat, W.; Kabbash, I.A.; Ibrahim, A. Factors associated with the spread of dengue fever in Jeddah Governorate, Saudi Arabia. East. Mediterr. Health J. 2012, 18, 15–23. [Google Scholar] [CrossRef]
- Mukhtar, F.; Salim, M.; Farooq, A. Outbreak of dengue fever in Lahore: Study of risk factors. J. Ayub Med. Coll. Abbottabad 2012, 24, 99–101. Available online: http://www.ayubmed.edu.pk/JAMC/24-2/Fatima.pdf (accessed on 15 November 2022).
- Rana, M.S.; Latif, A.A.; Akhtar, T. Predictors of dengue fever and dengue haemorrhagic fever in Punjab, Pakistan. Med. Forum Mon. 2012, 23, 26–30. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/emr-124955 (accessed on 15 December 2022).
- Wai, K.T.; Arunachalam, N.; Tana, S.; Espino, F.; Kittayapong, P.; Abeyewickreme, W.; Hapan gama, D.; Tyagi, B.K.; Htun, P.T.; Koyadun, S.; et al. Estimating dengue vector abundance in the wet and dry season: Implications for targeted vector control in urban and peri-urban Asia. Pathog. Glob. Health 2012, 106, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Lin, C.Y.; Wu, Y.T.; Wu, P.C.; Lung, S.C.; Su, H.J. Effects of extreme precipitation to the distribution of infectious diseases in Taiwan, 1994–2008. PLoS ONE 2012, 7, e34651. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, A.E.; Cardo, M.V.; Vezzani, D. Is temperature the main cause of dengue rise in non-endemic countries? The case of Argentina. Int. J. Health Geogr. 2012, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Thakolwiboon, S.; Benjatikul, N.; Sathianvichitr, K.; Prapathrangsee, K.; Tienmontri, T.; Ratanaamonsakul, W.; Assantachai, P.; Homsanit, M. Factors associated with dengue prevention and control in two villages in a central Thai province: A retrospective review. Asian J. Epidemiol. 2013, 96, 984–991. Available online: https://pubmed.ncbi.nlm.nih.gov/23991607/ (accessed on 15 November 2022).
- Suwannapong, N.; Tipayamongkholgul, M.; Bhumiratana, A.; Boonshuyar, C.; Howteerakul, N.; Poolthin, S. Effect of community participation on household environment to mitigate dengue transmission in Thailand. Trop. Biomed. 2014, 31, 149–158. Available online: https://pubmed.ncbi.nlm.nih.gov/24862055/ (accessed on 18 November 2022).
- An, D.T.M.; Rocklöv, J. Epidemiology of dengue fever in hanoi from 2002 to 2010 and its meteorological determinants. Glob. Health Action 2014, 7, 15. [Google Scholar] [CrossRef]
- Honorato, T.; Lapa, P.P.A.; Vendas, C.M.M.; Reis-Santos, B.; Tristão-Sá, R.; Bertolde, A.I.; Maciel, E.L.N. Análise espacial da distribuição dos casos de dengue no Espírito Santo, Brasil, em 2010: Uso do modelo Bayesiano. Rev. Bras. Epidemiol. 2014, 14, 150–159. [Google Scholar] [CrossRef]
- Ballera, J.E.; Zapanta, M.J.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E. Investigation of Chikungunya fever outbreak in Laguna, Philippines, 2012. West. Pac. Surveill. Response J. 2015, 6, 8–11. [Google Scholar] [CrossRef]
- Siregar, F.A.; Abdullah, M.R.; Omar, J.; Sarumpaet, S.M.; Supriyadi, T.; Makmur, T.; Huda, N. Social and environmental determinants of dengue infection risk in North Sumatera Province, Indonesia. Asian J. Epidemiol. 2015, 8, 23–25. [Google Scholar] [CrossRef]
- Rodrigues, N.C.P.; Lino, V.T.S.; Daumas, R.P.; Andrade, M.K.N.; O’Dwyer, G.; Monteiro, D.L.M.; Gerardi, A.; Fernandes, G.H.B.V.; Ramos, J.A.S.; Ferreira, C.E.G.; et al. Temporal and Spatial Evolution of Dengue Incidence in Brazil, 2001–2012. PLoS ONE 2016, 11, e0165945. [Google Scholar] [CrossRef]
- Delmelle, E.; Hagenlocher, M.; Kienberger, S.; Casas, I. A spatial model of socioeconomic and environmental determinants of dengue fever in Cali, Colombia. Acta Trop. 2016, 164, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Beltrán-Ayala, E.; Borbor-Cordova, M.J.; Polhemus, M.E.; Ryan, S.J.; Endy, T.P.; Stewart-Ibarra, A.M. Social-ecological factors and preventive actions decrease the risk of dengue infection at the household-level: Results from a prospective dengue surveillance study in Machala, Ecuador. PLoS Negl. Trop. Dis. 2017, 11, e0006150. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.L.; Calvet, G.; Estevam, C.G.; Angelo, J.R.; Abiodun, G.J.; Halai, U.-A.; Santis, B.; Sequeira, P.C.; Araujo, E.M.; Sampaio, S.A.; et al. Behavioral, climatic, and environmental risk factors for Zika and Chikungunya virus infections in Rio de Janeiro, Brazil, 2015–2016. PLoS ONE 2017, 12, e0188002. [Google Scholar] [CrossRef]
- Correia Filho, W.L.F. Influence of meteorological variables on dengue incidence in the municipality of Arapiraca, Alagoas, Brazil. Rev. Soc. Bras. Med. Trop. 2017, 50, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Yasar, A.; Tabinda, A.B.; Zaheer, I.E.; Malik, K.; Batool, A.; Mahfooz, Y. Assessing spatio-temporal trend of vector breeding and dengue fever incidence in association with meteorological conditions. Environ. Monit. Assess. 2017, 189, 189. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.B.; Machado, C.J.S. Associations between dengue and socio-environmental variables in capitals of the brazilian northeast by cluster analysis. Ambiente Soc. 2018, 21, 22. [Google Scholar] [CrossRef]
- Aguiar, B.S.; Lorenz, C.; Virginio, F.; Suesdek, L.; Chiaravalloti-Neto, F. Potential risks of Zika and chikungunya outbreaks in Brazil: A modeling study. Int. J. Infect. Dis. 2018, 70, 20–29. [Google Scholar] [CrossRef] [PubMed]
- MacCormack-Gelles, B.; Lima Neto, A.S.; Sousa, G.S.; Nascimento, O.J.; Machado, M.M.T.; Wilson, M.E.; Castro, M.C. Epidemiological characteristics and determinants of dengue transmission during epidemic and non-epidemic years in Fortaleza, Brazil: 2011–2015. PLoS Negl. Trop. 2018, 12, e0006990. [Google Scholar] [CrossRef] [PubMed]
- Ishak, H.; Sartika, J.D.; Darmawansyah. Relationship of rainfall, population density, and human behavior with DHF incidence in makassar city. Indian J. Public Health Res. Dev. 2019, 10, 1253–1258. [Google Scholar] [CrossRef]
- Ngweta, L.; Bhanot, K.; Maharaj, A.; Bogle, I.; Munasinghe, T. Identifying the Relationship between Precipitation and Zika Outbreaks in Argentina. In Proceedings of the IEEE International Conference on Big Data, Los Angeles, CA, USA, 9–12 December 2019; pp. 6163–6165. [Google Scholar] [CrossRef]
- Tuladhar, R.; Singh, A.; Banjara, M.R.; Gautam, I.; Dhimal, M.; Varma, A.; Choudhary, D.K. Effect of meteorological factors on the seasonal prevalence of dengue vectors in upland hilly and lowland Terai regions of Nepal. Parasites Vectors 2019, 12, 42. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Sosa, S.; Brouwer, K.C.; Juárez, J.G.; Romero, C.; Lenhart, A.; Cordón-Rosales, C. Associations between household environmental factors and immature mosquito abundance in Quetzaltenango, Guatemala. BMC Public Health 2019, 19, 1729. [Google Scholar] [CrossRef] [PubMed]
- Siregar, F.A.; Makmur, T. Climate risks and environmental determinants on dengue transmission. Indian J. Public Health Res. Dev. 2019, 10, 1242–1247. [Google Scholar] [CrossRef]
- Mala, S.; Jat, M.K. Implications of meteorological and physiographical parameters on dengue fever occurrences in Delhi. Sci. Total Environ. 2019, 650, 2267–2283. [Google Scholar] [CrossRef] [PubMed]
- Mol, M.P.G.; Queiroz, J.T.M.; Gomes, J.; Heller, L. Gerenciamento adequado de resíduos sólidos como fator de proteção contra casos de dengue. Rev. Panam. Salud Publica 2020, 44, e22. [Google Scholar] [CrossRef]
- Sintorini, M.M.; Aliyyah, N.; Sinaga, E.R.K. Environment drivers of DHF disease in Jakarta 2017–2018. Int. J. Sci. Technol. Res. 2020, 9, 3521–3525. Available online: http://www.ijstr.org/final-print/jan2020/Environment-Drivers-Of-Dhf-Disease-In-Jakarta-2017-2018.pdf (accessed on 10 January 2023).
- Shabbir, W.; Pilz, J.; Naeem, A. A spatial-temporal study for the spread of dengue depending on climate factors in Pakistan (2006–2017). BMC Public Health 2020, 20, 995. [Google Scholar] [CrossRef]
- Silva, N.S.; Alves, J.M.B.; da Silva, E.M.; Lima, R.R. Avaliação da relação entre climatologia, condições sanitárias (Lixo) e a ocorrência de arboviroses (Dengue e Chikungunya) em Quixadá-CE no período entre 2016 e 2019. Rev. Bras. Meteorol. 2020, 35, 485–492. [Google Scholar] [CrossRef]
- Elaagip, A.; Alsedig, K.; Altahir, O.; Ageep, T.; Ahmed, A.; Siam, H.A.; Samy, A.M.; Mohamed, W.; Khalid, F.; Gumaa, S.; et al. Seroprevalence and associated risk factors of Dengue fever in Kassala state, eastern Sudan. PLoS Negl. Trop. Dis. 2020, 14, e0008918. [Google Scholar] [CrossRef]
- Roy, P.; Kanga, S.; Sudhanshu; Singh, S.K. Socio-Cultural Practices and Environmental Determinantes for Dengue Incidences: A Casa Study of Jaipur City. Nat. Volatiles Essetial Oils 2021, 8, 2467–2481. Available online: https://www.nveo.org/index.php/journal/article/view/453/419 (accessed on 15 January 2023).
- Costa, S.D.S.B.; Branco, M.R.F.C.; Vasconcelos, V.V.; Queiroz, R.C.S.; Araujo, A.S.; Câmara, A.P.B.; Fushita, A.T.; da Silva, M.D.S.; da Silva, A.A.M.; dos Santos, A.M. Autoregressive spatial modeling of possible cases of dengue, chikungunya, and zika in the capital of northeastern Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e0223. [Google Scholar] [CrossRef]
- Morgan, J.; Strode, C.; Salcedo-Sora, J.E. Climatic and socio-economic factors supporting the co-circulation of dengue, zika and chikungunya in three different ecosystems in Colombia. PLoS Negl. Trop. Dis. 2021, 15, e0009259. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, C.E.; de Medronho, R.A. Association between socio-environmental factors, coverage by family health teams, and rainfall in the spatial distribution of Zika virus infection in the city of Rio de Janeiro, Brazil, in 2015 and 2016. BMC Public Health 2021, 21, 1199. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Díaz, E.; Gleiser, R.M.; Lopez, L.R.; Guzman, C.; Contigiani, M.S.; Spinsanti, L.; Gardenal, C.N.; Gorla, D.E. Oviposition dynamics of Aedes aegypti in Central Argentina. Med. Vet. Entomol. 2022, 36, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Telle, O.; Nikolay, B.; Kumar, V.; Benkimoun, S.; Pal, R.; Nagpal, B.N.; Paul, R.E. Social and environmental risk factors for dengue in delhi city: A retrospective study. PLoS Negl. Trop. Dis. 2021, 15, e0009024. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.T.D.O.; Atanaka, M.; Espinosa, M.M.; Schuler-Faccini, L.; Caldeira, A.D.S.; da Silva, J.H.; Vivi-Oliveira, V.K.; da Paz, R.D.C.; do Nascimento, V.F.; Terças-Trette, A.C.P. Recent dengue virus infection: Epidemiological survey on risk factors associated with infection in a medium-sized city in Mato Grosso. São Paulo Med. J. 2022, 140, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.R.; Rahman, N.; Iqbal, A.; Azad, F.; Titi, S.H.; Udin, M.H.; Mostary, E.U.A.; Tanvir, M.; Tanzim, M.Z.; Mahim, M.A.R. Socio-demographic, environmental and life style factors on the dengue epidemic in Noakhali District, Bangladesh: Evidence from recent outbreak. J. Commun. Dis. 2020, 52, 57–65. [Google Scholar] [CrossRef]
- Nontapet, O.; Jaroenpool, J.; Maneerattanasa, S.; Thongchan, S.; Ponprasert, C.; Khammaneechan, P.; Le, C.N.; Chutipattana, N.; Suwanbamrung, C. Effects of the Developing and Using a Model to Predict Dengue Risk Villages Based on Subdistrict Administrative Organization in Southern Thailand. Int. J. Environ. Res. Public Health 2022, 19, 11989. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Aditya, G.; Saha, G.K. Household Wastes as Larval Habitats of Dengue Vectors: Comparison between Urban and Rural Areas of Kolkata, India. PLoS ONE 2015, 10, e0138082. [Google Scholar] [CrossRef]
- Kampango, A.; Furu, P.; Sarath, D.L.; Haji, K.A.; Konradsen, F.; Schioler, K.L.; Alifrangis, M.; Saleh, F.; Weldon, C.W. Risk factors for occurrence and abundance of Aedes aegypti and Aedes bromeliae at hotel compounds in Zanzibar. Parasites Vectors 2021, 14, 544. [Google Scholar] [CrossRef]
- Scalize, P.S.; Bezerra, N.R.; Baracho, R.O. Safety index of individual basic sanitation systems in rural area. Period. Eletronico Forum Ambient. Alta Paul. 2022, 18, 56–75. [Google Scholar] [CrossRef]
- Braga, D.L.; Bezerra, N.R.; Scalize, P.S. Proposition and application of an environmental salubrity index in rural agglomerations. Rev. Saúde Pública 2022, 56, 44. [Google Scholar] [CrossRef]
- Silva, A.C. Associação entre Variáveis Ambientais Relacionadas a Criadouros de Aedes Aegypti e Doenças Arbovirais em Comunidades Rurais e Tradicionais de Goiás, Brasil. Master’s Thesis, Universidade Federal de Goiás (UFG), Goiânia, Brasil. Available online: https://www.bc.ufg.br/ (accessed on 15 December 2022).
- Schmidt, W.P.; Suzuki, M.; Thiem, V.D.; White, R.G.; Tsuzuki, A.; Yoshida, L.M.; Yanai, h.; Haque, u.; Tho, L.H.; Anh, D.D.; et al. Population Density, Water Supply, and the Risk of Dengue Fever in Vietnam: Cohort Study and Spatial Analysis. PLoS Med. 2011, 8, e1001082. [Google Scholar] [CrossRef] [PubMed]
- Farich, A.; Lipoeto, N.I.; Bachtiar, H.; Hardisman, H. The Effects of Community Empowerment on Preventing Dengue Fever in Lampung Province, Indonesia. Open Access Maced. J. Med. Sci. 2020, 8, 194–197. [Google Scholar] [CrossRef]
- Guad, R.M.; Wu, Y.S.; Aung, Y.N.; Sekaran, S.D.; Wilke, A.B.B.; Low, W.Y.; Sim, M.S.; Carandang, R.R.; Jeffree, M.S.; Taherdoost, H.; et al. Different Domains of Dengue Research in Malaysia: A Systematic Review and Meta-Analysis of Questionnaire-Based Studies. Int. J. Environ. Res. Public Health 2021, 18, 4474. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.S.; Yang, H.-J. Factors That Prevent Mosquito-Borne Diseases among Migrant Workers in Taiwan: Application of the Health Belief Model in a Church-Based Health Promotion Study. Int. J. Environ. Res. Public Health 2022, 19, 787. [Google Scholar] [CrossRef]
- Johansen, I.C.; do Carmo, R.L.; Alves, L.C.; Bueno, M.D.C.D. Environmental and demographic determinants of dengue incidence in Brazil. Rev. Salud Publica 2018, 20, 346–351. [Google Scholar] [CrossRef]
- Flauzino, R.F.; Souza-Santos, R.; Barcelllos, C.; Gracie, R.; Magalhães, M.A.F.M.; de Oliveira, R.M. Heterogeneidade espacial da dengue em estudos locais, Cidade de Niterói, Sudeste do Brasil. Rev. Saúde Pública 2009, 43, 1035–1043. [Google Scholar] [CrossRef]
- dos Santos Souza Marinho, R.; Duro, R.L.S.; de Oliveira Mota, M.T.; Hunter, J.; Diaz, R.S.; Kawakubo, F.S.; Komninakis, S.V. Environmental Changes and the Impact on the Human Infections by Dengue, Chikungunya and Zika Viruses in Northern Brazil, 2010–2019. Int. J. Environ. Res. Public Health 2022, 19, 12665. [Google Scholar] [CrossRef]
- Sheela, A.M.; Ghermandi, A.; Vineetha, P.; Sheeja, R.V.; Justus, J.; Ajayakrishna, K. Assessment of relation of land use characteristics with vector-borne diseases in tropical areas. Land Use Policy 2017, 63, 269–380. [Google Scholar] [CrossRef]
- Gómez Gómez, R.E.; Kim, J.; Hong, K.; Jang, J.Y.; Kisiju, T.; Kim, S.; Chun, B.C. Association between Climate Factors and Dengue Fever in Asuncion, Paraguay: A Generalized Additive Model. Int. J. Environ. Res. Public Health 2022, 19, 12192. [Google Scholar] [CrossRef]
- Li, Z. Forecasting Weekly Dengue Cases by Integrating Google Earth Engine-Based Risk Predictor Generation and Google Colab-Based Deep Learning Modeling in Fortaleza and the Federal District, Brazil. Int. J. Environ. Res. Public Health 2022, 19, 13555. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Herng, L.C.; Sulaiman, L.H.; Wong, S.F.; Jelip, J.; Mokhtar, N.; Harpham, Q.; Tsarouchi, G.; Gill, B.S. The Effects of Meteorological Factors on Dengue Cases in Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 6449. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde; Análise de Situação em Clima e Saúde; Organização Panamericana de Saúde (OPAS); Fundação Instituto Oswaldo Cruz (FIOCRUZ); Instituto de Comunicação e Informação Científica e Tecnológica (ICICT); Observatório Nacional de Clima e Saúde. Doenças e Perguntas. Brasil. 2017. Available online: https://climaesaude.icict.fiocruz.br/doencas/vetores (accessed on 10 January 2023).
| Category | Variable | Indicator |
|---|---|---|
| Sanitation (SAN) | Water (AG) | AG1) Proportion with piped AG; AG2) Scarce access to city AG infrastructure; AG3) Proportion of permanent particular households whose AG supplying method does not pass through the city water system; AG4) Presence of recipient with AG and larvae (tires, pots, flower vases and barrels). |
| Solid Waste (RS) | RS1) Percentage of RS collecting by cleaning service; RS2) Common and selective RS collecting; RS3) Proportion of permanent private households in which the RS is burned, buried, thrown into an empty lot or patio, river, lake, sea or other destination; RS4) Piling of solid waste in the household’s surroundings; RS5) Existence of RS spread or piled in the peridomicile. | |
| Sewage (EG) | EG1) Rate of households with EG system; EG2) Proportion of households with skeptic tank; EG3) Households without EG service. | |
| Draining (DR) | DR1) Amount of households with drainage network; DR2) absence or not of Rainwater collection system; DR3) Presence of stagnant water within internal DR holes. | |
| Integrative (INT) | Sociodemographic (SD) | SD1) Demographic Density; SD2) Percentage of people residing in the rural zone; SD3) Number of family members in the house; SD4) Education level; SD5) Sex; SD6) Color/race; SD7) Age; SD8) Marital Status; SD9) Occupational situation; SD10) Residence time; SD11) Influx of people. |
| Socioeconomic (SE) | SE1) Per capita monthly income; SE2) GDP per capita; SE3) Unemployment rate; SE4) Gini Index; SE5) Percentage of people vulnerable to poverty; SE6) Human Development Index; SE7) Electricity. | |
| Housing (HB) | HB1) Building type; HB2) Screened doors or windows; HB3) Gutter; HB4) Number of rooms; HB5) Use of mosquito net, insect repellent and fumigation inside the house. | |
| Geography (GE) | GE1) Distance of the household top laces with high potential for larval density (graveyards, landfill sites, etc.); GE2) To the closest water bodies or courses. (excluding sea), GE3) to dairy farms, GE4) to other houses, GE5) to tire shops; GE6) to plant nurseries and GE7) abandoned sites. | |
| Soil usage (US) | US1) Urbanized area, forested and open agricultural; US2) Biome type; US3) Vegetation density; US4) Estimated vegetal coverage by satellite image (MODIS and NASA’s Landsat). | |
| Sociocultural (SC) | SC1) History of family members travels; SC2) Frequency of recipient cleaning and water reservoir protection; SC3) Practices regarding water recipient storage, trash disposal and health education; SC4) Knowledge, perceptions, attitudes and community practices in the prevention of diseases (ACP). | |
| Altitude (ALT) | ALT1) Average altitude elevation (Above sea level). | |
| Climatologic (CLIM) | Temperature (TE) | TE1) Average air temperature, annual maximum and minimum; TE2) Dew point temperature; TE3) Terrestrial surface TE. |
| Precipitation (rainfall) (PR) | PR1) Annual rainfall precipitation average; PR2) Estimated rainfall (CHIRPS); PR3) Rainy days; PR4) Great rain events. | |
| Air humidity (UR) | UR1) Relative air humidity (%). | |
| Climate (CL) | CL1) Climate type (tropical, subtropical, semi-arid). | |
| Other Meteorological variables (OMV) | OVM1) Pressure surface; OMV2) Wind speed; OVM3) wind direction; OVM4) Wind gust; OMV5) Dew point; OVM6) Saturation deficit; OVM7) Sunlight hours. | |
| Total of indicators | 70 | |
| Indicator | Recurrence (%) |
|---|---|
| Notified cases of dengue, Zika and/or chikungunya | 50 |
| Incidence of dengue, Zika and/or chikungunya | 10 |
| Positivity for dengue, Zika and/or chikungunya/detection through IgG/IgM/ELISA, NS1 and/or RT-PCR serology | 20 |
| Positivity for dengue, Zika and/or chikungunya/ * | 6 |
| Did not make correlations with arbovirus diseases but with breeding spots of the Aedes aegypti and/or Aedes albopictus transmission vector | 14 |
| Total | 100 |
| Categories | Recurrence | Incidence |
|---|---|---|
| INT | 68 | 2 |
| SAN | 66 | 4 |
| CLIM | 54 | 24 |
| SAN + INT | - | 40 |
| SAN + CLIM + INT | - | 18 |
| CLIM + INT | - | 8 |
| SAN + CLIM | - | 4 |
| Total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.C.; Scalize, P.S. Environmental Variables Related to Aedes aegypti Breeding Spots and the Occurrence of Arbovirus Diseases. Sustainability 2023, 15, 8148. https://doi.org/10.3390/su15108148
da Silva AC, Scalize PS. Environmental Variables Related to Aedes aegypti Breeding Spots and the Occurrence of Arbovirus Diseases. Sustainability. 2023; 15(10):8148. https://doi.org/10.3390/su15108148
Chicago/Turabian Styleda Silva, Adivânia Cardoso, and Paulo Sérgio Scalize. 2023. "Environmental Variables Related to Aedes aegypti Breeding Spots and the Occurrence of Arbovirus Diseases" Sustainability 15, no. 10: 8148. https://doi.org/10.3390/su15108148
APA Styleda Silva, A. C., & Scalize, P. S. (2023). Environmental Variables Related to Aedes aegypti Breeding Spots and the Occurrence of Arbovirus Diseases. Sustainability, 15(10), 8148. https://doi.org/10.3390/su15108148






