Abstract
Cleaning is one of the most important, essential, and delicate operation which has to be handled by conservators before applying new materials to any substrates. In past decades, nanotechnology introduced new concepts and materials in the conservation field, which have been providing many advantageous performances, especially higher than older materials. As a result, the conservators have already started to use nanomaterials in the cleaning processes of artifacts. Taking into consideration this new approach, our study has focused on using nano-structured emulsions (NSE) as smart cleaning materials for removing polymer coatings (e.g., acrylic polymers). For this purpose, Paraloid B-72 was applied on three different substrates (glass, Lecce stone, and Arenaria stone) and cleaning was performed by a specific nano-structured emulsion (NSE) based on an eco-friendly surfactant (EcoSurf) and two organic solvents in different proportions. In order to better understand the interaction of surfactant and organic solvents with polymer coating, plain EcoSurf in water was also used for comparison. In this study, the decay process of the considered polymer was also deeply studied, because it directly affects the cleaning effectiveness. Coated specimens of the different substrates were artificially aged and examined by different techniques: chromatic variations and contact angle measurements, optical microscopy, FTIR, and SEM-EDS. This material characterization process is important to understand the colour, morphology, and micro-structural difference, and the changes of hydrophobic behaviour as well as chemical composition of Paraloid B-72 polymer due to different ageing processes. After that, substrates coated with both unaged and aged polymer were cleaned by NSE according to the direct-contact procedure and cellulose pulp method. Preliminary analyses suggested that the direct-contact cleaning performed by nano-emulsion (i.e., NSE) induced a complete removal of the acrylic polymer, despite that this method is not recommended for the artifacts and can be hardly applicable in real cases. On the other hand, experimental results showed that satisfactory cleaning of stone substrates can be obtained by using NSE/the cellulose pulp system.
1. Introduction
Cleaning of artifacts represents one of the most essential and delicate operations in the conservation field because it may affect the original materials in a different way, as it could be (i) potentially invasive, (ii) aggressive, and (iii) completely irreversible [1,2,3]. If this process is not correctly performed, it may induce irreversible effects and even damage the artifacts. Synthetic polymers have been largely employed in the traditional restoration practice as adhesives and conservation agents [4,5,6,7]. Porous substrates treated with these types of polymers commonly underwent a drastic reduction of water capillary absorption and surface wettability. At the same time, the hydrophobic treatment often caused a considerable decrease of water vapor permeability, with consequent enhancement of decay, up to a substantial loss of the artifact surface layers [2,3,8]. Therefore, the removal of aged polymeric coatings from the artifacts’ surface is becoming a quite common cleaning operation in the conservation field. Traditionally, several organic solvents have been used for dissolving or swelling these types of aged polymers as well as other unwanted materials [2,9,10]. However, as reported in the literature, this approach has several non-negligible drawbacks: the presence of unremoved residues, poor control, environmental issues, safety problems for the users, uncontrolled penetration of organic solvents to the artifacts, damaging the original materials, and poor selectivity [2,3]. Therefore, conservation scientists and restorers have been investigating alternative products that could correctly clean the artifacts, overcoming those limitations.
Emulsions (aqueous swollen micelles and oil-in-water microemulsions) have been used as alternatives to non-confined organic solvents [2,11]. In the last decade, the effectiveness of these types of cleaning materials has been studied, but their performances are not always satisfactory depending on several factors, such as substrate and coating features, ageing processes of materials, thickness of the coating, the penetration depth of the coating, and so on. In addition, the removing of other undesired materials (deposits of pollutants, grime due to natural ageing, and graffiti) from the artifacts without affecting the original materials is one of the most important issues for conservation scientists [3,6]. Hence, high performance systems and ensuring a controlled and selective cleaning action must be considered in order to overcome the above-mentioned drawbacks.
For this purpose, nano-structured emulsions, which showed low toxicity compared to traditional solvents, have been proposed in the last decades for porous inorganic substrates [2,8]. The nano-fluids are water-based systems containing surfactants with or without limited amounts of organic solvents, in which nano-sized droplets are stabilized by the surfactants. Surfactants and solvents, owing also to the large surface area induced by the nano-droplets, are responsible for enhancing the cleaning effect. At the same time, aqueous medium limits the re-deposition of the disconnected hydrophobic materials on the hydrophilic surfaces [2]. However, the cleaning process of nano-structured fluids is still under investigation, because it depends on several factors, including the amount of cleaning materials, substrate features, type of polymer and its ageing degree, and thickness and penetration depth of the coating.
The present study is focused on the removal process of an acrylic polymer (Paraloid B-72) applied on two different porous stone substrates (Lecce stone and Arenaria stone) by nano-structured emulsion (NSE). The removal of polymer coating from the glass surface was also studied for comparison.
Lecce stone (LS) is a fossiliferous bio-calcarenite (calcite as the main component 95 ± 2%, with very high open porosity >30%), which has been frequently used in the past by architects and sculptors in the south of Italy, mainly during the Baroque period [12,13]. Moreover, as reported in our previous papers, LS is a soft stone material displaying low surface cohesion and has a quite heterogeneous morphology (irregular structure) due to its chemical composition and high porosity [6,13,14]. The pores, whose size ranges between 0.003 and 40 μm in diameter, can be classified as mesopores (0.002–0.05 μm) and macropores (>0.05 μm), according to IUPAC’s classification [13,14].
It is still diffusely used in the construction of contemporary buildings and also in restoration interventions. It was selected as a target substrate for this study because polymer materials were largely applied in the traditional conservation practices on buildings, churches, bas-reliefs, and other monuments made by LS [6,7,8,9,10,11,12,13,14]. Moreover, this type of stone is subject to drastic damage due to several weathering mechanisms (physical and chemical agents) because of its high porosity and high content of calcite, as reported in previous papers [13,14,15]. On the other hand, the selected Arenaria stone (AS, Province of Pavia, North Italy) is a clastic sedimentary rock composed mainly by sand-sized minerals or rock grains with a yellow colour, and the open porosity is around 15% (it is also a soft stone material with a low surface cohesion, and it shows heterogeneous behaviour (irregular structure) due to its chemical composition and high porosity). It has also been a highly employed material in northern Italy since XI-XII centuries, especially in Pavia and its surroundings (e.g., Basilica of San Michele Maggiore) [16,17]. This church underwent many conservation interventions during the last decades, which also involved polymeric agents. In addition to these two lithotypes, glass substrate was used as a reference material to better evaluate the behaviour of the polymer. In this experimental work, all the considered substrates were treated with Paraloid B-72 after dissolving it in ethyl acetate.
Before investigating the cleaning process, the effects of artificial ageing (heating as well as salt crystallization) on the Paraloid B-72 coating were deeply studied to understand the possible correlation between the decay processes of the polymer and the effectiveness of the cleaning treatment.
Then, an NSE containing an eco-friendly surfactant (EcoSurf™ EH-6) and a limited amount of organic solvents (2-butanol and butanone) was selected after performing different preliminary trials and was used for the cleaning procedures. The cleaning effectiveness of a plain surfactant (EcoSurf™ EH-6) in water was also considered for comparison.
The polymer-removing ability of considered liquid systems was evaluated using, at first, the direct contact method. For instance, all the considered samples were directly exposed to an NSE and EcoSurf-H2O. The comparative investigation was conducted, considering aged samples with respect to the unaged samples (glass, LS, and AS). Despite that direct contact with nano-emulsion provides good results, the method cannot be recommended for the heritage building materials and can be hardly applied in real cases [2]. As reported in the literature, the traditional cellulose pulp method is still used in this field [2,3,8]. Hence, in this study, cellulose pulp was used as a supporting medium for the application of nano-structured emulsion. It is based on a natural bio-degradable material, and, at the same time, it can be more safely applied in the conservation of cultural heritage items. The polymer removal performances by nano-structured emulsion and EcoSurf-H2O loaded on cellulose pulp were investigated both on unaged and aged samples by different experiments: colour differences before and after cleaning process were examined by chromatic measurements, contact angle measurements were carried out to understand the surface wettability after cleaning actions, morphological and micro-structural changes were observed by optical microscopy and scanning electron microscopy, together with energy dispersive X-ray spectra (SEM-EDS) analyses, the variations of chemical composition were examined by Fourier transform infrared (FTIR) and micro-FTIR analyses, and the presence of residues on the surfaces after cleaning was detected by an iodine vapour test together with micro-FTIR mapping analyses.
2. Materials and Methods
2.1. Chemicals and Substrates
The acrylic polymer commercially known as Paraloid B-72 (Bresciani s.r.l., Milan, Italy) was used for stone as well as glass coating. A surfactant (EcoSurf™ EH-6, C8H18O.(C3H6O)x.(C2H4O)y, non-ionic surfactant, Sigma Aldrich, St. Louis, MO, USA) was used for preparing nano-structured emulsions. 2-butanol (C2H5CH(OH)CH3, Sigma Aldrich), 2-butanone (CH3C(O)CH2CH3, Sigma Aldrich), ethyl acetate (CH3COOC2H5, Sigma Aldrich), n-butyl acetate (CH3(CH2)3O2CCH3, Sigma Aldrich), and ethanol (absolute, 99.8%, C2H5OH, Sigma Aldrich) were used as solvents. Sodium sulphate (Na2SO4, Sigma Aldrich) was used for the salt crystallization test. Iodine (crystals, Sigma Aldrich) was used for the iodine vapour staining test. All the chemicals were used as received, without any further purification. Water was purified using a Millipore Organex system: R ≥18 M cm (Burlington, MA, USA) and was used for preparing the nano-structured emulsions and surfactant solution.
Before applying the polymer coating, all the considered stone specimens were cleaned following the standard method (UNI 10,921 Protocol) [18]. Lecce stone (LS, provided by Tarantino, and Lotriglia, Nardò, Lecce, Italy) and Arenaria stone (AS, taken from a quarry in the area of Monte Arzolo, Province of Pavia, North Italy) specimens (5 × 5 × 1 and 5 × 5 × 2 cm) were smoothed by abrasive, carbide paper (No: 180 mesh), washed with deionized water, dried in an oven at 60 °C, and stored in a desiccator until they reached room temperature. Then, their dry weight was measured until it was constant [19,20]. Moreover, glass samples (glass slides: 2.5 × 7.5 × 0.1 cm) were also used as a reference substrate.
2.2. Application of Polymer Coating and Ageing Procedures
First of all, the standardized stone samples were collected from the desiccator, and one of their square surfaces was divided into two parts (one part for being coated and the other part was used as an untreated reference surface). After that, one part of the stone surface (2.5 × 5 × 1 cm of LS, and AS) as well as the glass supports were treated with appropriate amounts of Paraloid B-72 dissolved in ethyl acetate (10% w/w). For instance, 600 µL and 200 µL of polymer solutions were applied to stone specimens and glass slides by a small pipette, respectively. The amount of protective material for the stone substrates was chosen on the basis of several preliminary trials and was also suggested by experiments reported in a previous paper [6]. Polymer-coated stone samples were named as LSPB and ASPB.
The artificial ageing of coated samples was performed in an oven (temperature = 70 ± 2 °C; relative humidity = 15 ± 2%), and the progress of polymer decay was evaluated after 12, 25, and 35 days.
Furthermore, another group of samples underwent chemical weathering induced by salt crystallization. For this test, all the surfaces of the stone specimens were fully coated with Paraloid B-72, and then, they were exposed to a salt solution (by immersion) following the Spanish standard (UNE-EN, 12370) as reported in our previous papers [13,14,21]. In brief, the stone specimens were immersed in a sodium sulphate aqueous solution (Na2SO4, 14% w/w) for 4 h at room temperature (20 ± 2 °C), and then, they were dried in an oven at 60 °C for 16 h. In order to complete the cycle (24 h), as the last step, they were cooled at the laboratory temperature (20 ± 2 °C) for 4 h. The same procedure was repeated for up to 10 cycles, and finally, the dry weight loss (DWL%) [21] was calculated. The same procedure was repeated on specimens that were previously artificially aged at different times in the oven (T = 70 ± 2 °C; RH = 15 ± 2%; 12, 25, and 35 days). The untreated stones were similarly tested as references.
The chemical weathering affected to damage the polymer material was assessed by DWL% measurements together with chromatic variations, an optical microscope, and FTIR analysis, unaged and aged (aged in the oven).
2.3. Preparation of Nano-Structured Emulsions and Their Applications in Polymer Cleaning Process
For this experimental work, after doing several trials, two different liquid systems were chosen with different compositions (v/v ratio): (i) EcoSurf-H2O: 95.0% H2O and 5.0% surfactant (EcoSurf™ EH-6, non-ionic surfactant); and (ii) NSE: 65.9% H2O, 3.5% surfactant, 9.7% BuOH, and 20.9% butanone.
First of all, the direct contact method (direct interaction between the polymer coating and the cleaning fluid, without any supporting agent) was employed to examine the performances of considered emulsions. For an example, a set of coated samples (unaged and aged stone samples) was partially immersed in about 60 mL of NSE and EcoSurf-H2O emulsions for around 10 min (100 mL plastic tubs with lids were used). The same procedure was used for coated glass samples, but these samples were partially immersed in about 30 mL emulsions due to the smaller sample size than the stone specimens (50 mL Falcon tubes were used). At the end of the test (after removing samples from containers), the exposed surfaces were smoothly cleaned with a wet cotton swab as reported in the literature [2]. Even though this method provides very good results, it is not recommended for the cultural heritage materials, and it can be hardly applied to real cases. Hence, as an appropriate option, the traditional cellulose pulp cleaning method was involved in our study, and all the polymer removing performances were evaluated using a pulp-supported NSE.
The nano-structured emulsion was transported to the specimen surfaces by cellulose pulp poultice. The minimum amount of nano-emulsion and cellulose were used, taking into account the coated area of all the considered supports to be treated. Typically, the poultice to be applied on a stone sample (12.5 cm2) was prepared by a well, mixing 2.1 mL of emulsion with 0.5 g of cellulose powder. The poultice was applied to all the coated substrates, interposing a Japanese paper between the nano-emulsion-loaded pulp and the specimen surface. The thickness of the pulp was around 5 mm, and it was kept on the sample surface for 15 min before removal. After that, a gentle mechanical action was performed on the cleaned surfaces using a wet cotton swab in order to remove poultice residues, as recommended by the literature [2]. The procedure was repeated twice in order to obtain the complete removal of polymer coatings. All the performances were evaluated by different experimental analyses as well as instrumental techniques, as indicated in Section 2.4.
2.4. Experimental Analyses and Instrumental Techniques
Chromatic differences induced by ageing as well by cleaning processes were examined by the instrument of Konica Minolta CM-2600D spectrophotometer (Konica Minolta, Inc., Tokyo, Japan), determining three different coordinates (L*, a*, and b*) of the CIELAB space and the global chromatic variations, expressed as ΔE* according to the UNI EN 15886 protocol [22]. The mean values were calculated from fifteen different measurements as reported in previous works [23,24].
The deviation of the hydrophobic property of coated surfaces due to ageing was analysed by static contact angle measurements. It was performed by a Lorentzen and Wettre instrument (Zurich, Switzerland) according to the UNI EN 15802 Protocol [25]. As in the chromatic variation measurements, the average values were taken from fifteen different measurements of each type of the samples [23,24].
Infrared spectra were collected by two different instruments: PerkinElmer Spectrum 100 Fourier transform infrared (FT-IR) spectrometer in the attenuated total reflectance (ATR) mode (PerkinElmer, Waltham, MA, USA) and Nicolet iN10 Thermo Fischer μ-FT-IR spectrometer in attenuated total reflectance mode (ATR, germanium crystal, Thermo Fisher Scientific, Waltham, MA, USA).
The morphological and micro-structural features of the coated samples due to different phenomena were observed by optical microscopy (light polarized microscope Olympus BX51TF, equipped with the Olympus TH4-200 lamp: Olympus Corporation, Tokyo, Japan) and scanning electron microscopy (SEM), together with energy dispersive X-ray spectra (EDS): Tescan FE-SEM, MIRA XMU series (TESCAN, Brno, Czech Republic), equipped with a Schottky field emission source and with a Bruker Quantax 200 energy-dispersive X-ray spectrometer (Bruker, Billerica, MA, USA), operating in both low and high vacuums (located at the Arvedi Laboratory, CISRiC, University of Pavia, Pavia, Italy).
The iodine vapour staining test (I2 react with many functional groups: its violet colour changes to a red-brown colour) was a quick and easy experiment to examine the presence of organic residues on the cleaned surfaces. The test was performed on all the cleaned specimens as follows [8]: 1 g of iodine crystals was placed on the glass chamber and heated until it produced the iodine vapour. The cleaned samples (polymer-removed specimens) were exposed, for 5 min, to this vapor, keeping the distance around 5 cm from the stone surface. Since the reaction with iodine is weak and reversible, the test has to be repeated several times on the same specimens in order to acquire the correct results.
3. Results and Discussion
3.1. Artificial Ageing
The stone specimens (LS and AS) and glass slides treated with Paraloid B-72 underwent artificial ageing processes to evaluate the progress of polymer decay before investigating the performances of the cleaning method on both unaged and aged coatings. In fact, the changes experienced by the polymer due to the ageing process may directly affect its removing process.
The artificial ageing was performed by exposing the specimens to an oven (70 °C, 15% RH) for up to 35 days. It has been reported that effects induced by this artificial ageing procedure after 35 days can be comparable to those observed after 50 years of natural ageing [8].
The effects induced by the ageing on the coating were investigated by chromatic change and contact angle measurements, optical microscopy observations, SEM-EDS experiments, and FTIR spectra.
Oven ageing induced a lightening decrease of the treated surfaces, which increased with exposure time (Figure 1). In fact, the chromatic coordinate L*, that is related to surface brightness, was much more affected than a* and b*, and its value distinctly decreased with the ageing time (ΔL* < 0, Table 1, Figure 1a). The brightness lowering of organic coatings is a visible effect that is usually suggestive of their degradation due to ageing processes [26,27].
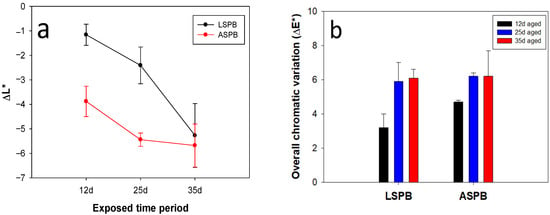
Figure 1.
ΔL* variations (a) and overall chromatic changes (ΔE*) (b) observed on coated stones after oven-ageing cycles (variations referred to coated unaged surfaces).

Table 1.
Chromatic coordinates of treated stones after oven-ageing cycles.
The overall chromatic variations also increased with ageing time (Figure 1b) for both coated stone materials. In particular, ΔE* value increasing was observed for up to 25 days of exposure. Then, they did not undergo significant changes after longer ageing times.
Ageing also affected the coating surface behaviour towards water for both of the examined stones. Contact angle (α) values underwent a gradual decrease with ageing time (by 16.5% and 21.6% for LSPB and ASPB, respectively), indicating that the hydrophobic character of the polymer was partially lost with its decay (Figure 2 and Table S1). This effect was particularly pronounced in the case of the treated Arenaria stone whose α value dropped from about 111° (distinct hydrophobic character) to about 87°.
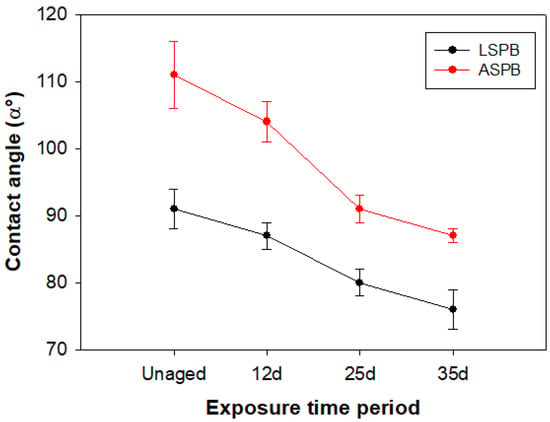
Figure 2.
Contact angle (α) measurements of coated stones after oven-ageing cycles.
The images of the coating surface taken by an optical microscope on LS and AS before and after artificial ageing are reported in Figure 3. The prolonged exposure to the artificial ageing conditions (T = 70 ± 2 °C, RH = 15 ± 2%) clearly affected the physical appearance and the morphology (texture) of the Paraloid B-72 coating. The polymer layer on the stone surface appeared quite damaged (matt looks with removal in some areas too), and sometimes its adhesion to the substrate was partially lost. The damaging effects were more and more evident as the ageing time increased (Figure 3a–d and Figure 3e–h for LS, and AS, respectively). Similar results were observed also in artificially weathered coating films obtained from polymer deposition on the glass slides.
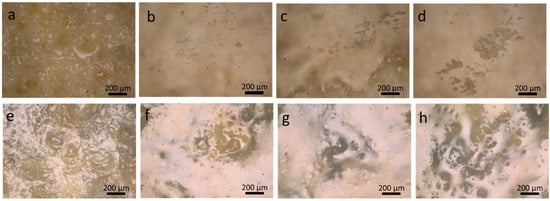
Figure 3.
Optical microscope images of both of the coated stones, taken before and after different ageing cycles: (a) unaged LSPB; (b) 12 d LSPB; (c) 25 d LSPB; (d) 35 d LSPB; (e) unaged ASPB; (f) 12 d ASPB; (g) 25 d ASPB; and (h) 35 d ASPB.
SEM-EDS analyses were carried out to compare the morphological as well as compositional changes underwent by all the considered samples due to the oven ageing process. The samples from unaged to aged (12, 25, and 35 days) showed that the damage and/or adhesion loss clearly depend on the exposition time (ageing time), and they gradually increased by the period of ageing, as discussed above for the optical microscope observations. Therefore, the most aged samples (35 days) showed the highest changes with respect to the corresponding unaged coated samples (0 days), as can be seen in Figure 4. In both stone specimens, the artificial ageing induced changes in the surface morphology, although they occurred differently on the two treated stones due to their different features (e.g., different porosity). For instance, the polymer, which completely covered the surface of Arenaria after treatment, was still quite evenly distributed on the stone after ageing, as also confirmed by the EDS micro-analysis. The EDS spectrum of the unaged ASPB showed the peaks corresponding to C and O, i.e., the main detectable elements of the polymer composition. After ageing, only very low intensity peaks corresponding to Si and Ca (due to stone elemental composition; 1–2% (w/w), according to the semi-quantitative analysis) appeared in the EDS spectrum, suggesting that only small areas of the stone surface resulted in not being covered by the polymer layer at the end of the ageing process (see the inset in Figure 4c,d).
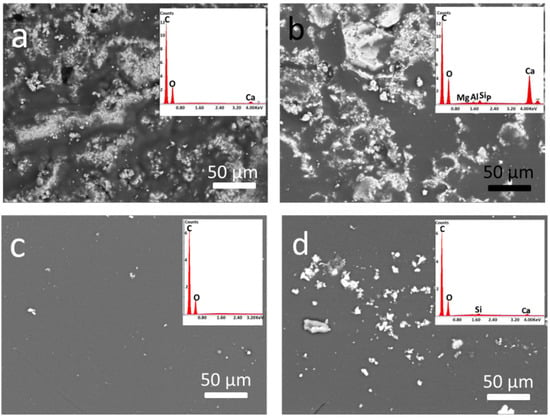
Figure 4.
SEM images of coated stones before and after 35 d ageing: (a) unaged LSPB; (b) 35 d LSPB; (c) unaged ASPB; and (d) 35 d ASPB. Corresponding EDS spectra (performed over a surface area 50 ) are in the inset.
On the contrary, the polymer distribution on the surface of the Lecce stone significantly changed after the prolonged oven exposure. At the end of the ageing process, the details of the stone morphology are more visible than before. Some peaks appear in the EDS spectrum of the aged sample, which corresponds to elements (Mg, Al, Si, P) typical of the stone’s composition. Moreover, the peaks of Ca, which had a very low intensity in unaged LSPB (about 2–3% w/w), distinctly increased its intensity (up to 17–18%) after ageing (see inset in Figure 4a,b).
The different behaviours of the two examined litotypes can be explained considering their different features. LS is a fossiliferous biocalcarenite displaying very high porosity and surface inhomogeneity. Therefore, the polymer is less regularly distributed on its surface than on AS, which is less porous. During the ageing process, the polymer undergoes softening (due to the heating) and possible chain degradation. Therefore, it could be adsorbed more deeply into the porous LS matrix and be more dispersed on the surface due to its roughness and unevenness. As a consequence, a considerable surface portion of the LS resulted in being uncovered (and unprotected) after ageing. This phenomenon occured at a distinctly lower extent in the case of the less porous AS.
The chemical changes experienced by Paraloid B-72 after the artificial ageing were also investigated by the FTIR technique. In particular, infrared spectra were taken on coating films before and after ageing in ATR mode (Figure 5). The spectra measured on unaged specimens displayed the typical peak at 1731 cm−1 due to the C=O stretching of polyacrylate ester groups. Other peaks at about 1100 and 1020 cm−1 can be ascribed to C-O asymmetric and symmetric stretching vibrations, respectively [2,28]. At the end of the ageing cycle (35 days), the intensity of carbonyl absorptions strongly decreases and almost disappear, while other bands related to stone components become stronger in the spectra, taken both on LSPB and ASPB. In particular, the peaks at about 1400 and 875 cm−1 can be ascribed to carbonate components in both stones, and the intense peak between 1000–1100 cm−1 is due to silicate in AS [14,29]. These results confirm that the polymer undergoes degradation due to the ageing process and/or is partially lost from the surface, resulting in a drastic reduction of the protecting effect of the coating layer.
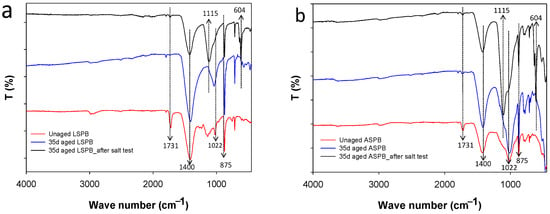
Figure 5.
FTIR spectra (ATR mode) of unaged and aged coated samples after oven-artificial-ageing cycles and after the salt crystallization test: (a) LSPB and (b) ASPB.
The decay of coating protecting performance due to its chemical weathering was also pointed out by evaluating the resistance of coated stones to salt crystallization after artificial ageing. Salt crystallization is one of the main weathering processes that drastically affects stone materials, especially the porous ones [13,14]. As soluble salts are carried into the stone by water, the effectiveness of the polymer coating against water penetration can be assessed by considering the effect induced by repeated crystallizations. Therefore, the salt crystallization test (10 cycles) was performed on fully coated stone specimens, both unaged and during the artificial ageing (after 12, 25, and 35 exposure days). Uncoated stones were also similarly tested for comparison.
At the end of any crystallization test, dry weight loss (DWL%) values were determined, with respect to the corresponding uncoated not tested stone. Results are summarized in Table 2. As expected, the surface treatment with Paraloid B-72 strongly prevents the absorption of the salt solution by the stone specimens and, as a consequence, the DWL% values observed for unaged coated samples are extremely lower than the uncoated corresponding ones. When considering the aged specimens, the weight loss induced by salt crystallization cycles increases with the ageing time. In fact, the decay underwent by the polymer coating during the artificial weathering makes the salt solution easier to penetrate the stone, with consequently more pronounced damage due to the repeated crystallization process. It is worth noting that (i) the polymer coating still behaves as a partial barrier against water penetration even after the longest considered ageing time; and (ii) the protecting effectiveness of the coating is lower on the more porous LS both on unaged and on aged specimens.

Table 2.
Dry weight loss (DWL%) values after salt crystallization test (10 cycles).
After the salt crystallization cycles, the stone samples also showed clear differences in the infrared spectra taken on their surface (Figure 5, black spectra). The new strong peaks that appeared at 1115 and 604 cm−1 are due to absorptions of sulphate (SO42−) [30,31], whose salts crystallized on the stone surface. These results are in good agreement with the whitish appearance of the specimens at the end of the salt crystallization test. Pictures of the tested stone specimens before and after the test are reported in Figure S1 (Supplementary Information).
3.2. Cleaning Tests
The tests for removing the acrylic polymer coating (both unaged and artificially aged in the oven) were performed at first with different pure solvents (ethyl acetate, 2-butanone, butyl acetate, 2-butanol, ethanol, and water were considered). The direct immersion method was applied, as a preliminary analysis, on the coated glass and stone (LS and AS) specimens, in order to evaluate the effectiveness of each solvent to remove the coating. The results are resumed in Tables S2 and S3. The less polar solvents (ethyl acetate, 2-butanone, and butyl acetate) efficiently remove (or dissolve) the polymer even from the aged samples, while ethanol and water do not induce any change on the coating. 2-butanol (BuOH) displays middle behaviour.
However, the use of organic solvents in conservation practices has several limitations: (i) they are dangerous for health, especially if they are volatile; (ii) considerable amounts of organic solvents would be necessary for cleaning purposes with consequent environmental issues; (iii) the action of organic solvents is poorly controllable on porous substrates; and (iv) once the coating is dissolved, the polymer may penetrate the pores of the substrate and re-deposit after solvent evaporation [8].
In order to overcome these limitations, nano-structured emulsions (NSEs) have been proposed in the last years as a viable alternative to plain organic solvents [2]. In fact, the minimum amount of volatile solvents required to prepare NSEs reduces health risks as well as environmental impacts. At the same time, NSEs induce limited spreading of the dissolved polymer. Therefore, after doing several trials, a nano-structured emulsion, labelled as NSE, was selected. It is based on a non-ionic eco-friendly surfactant (EcoSurf™ EH-6) and contains limited amounts of 2-butanol and butanone as reported in the experimental section. The cleaning performance of the NSE was properly compared with the plain surfactant in water (EcoSurf-H2O). In order to assess the cleaning properties of these two liquid systems, all the considered samples were directly exposed to them for about 10 min, and then a gentle mechanical action was performed by wet cotton swabs as explained above. EcoSurf-H2O, which does not contain any organic solvent, does not provide any removal of Paraloid B-72 from all the substrates (glass, LS, and AS). A partial swelling was observed only on unaged and 12 day-aged coating layers on glass and AS (Table 3 and Table 4). In the case of LS, treatment with EcoSurf-H2O had no effect on both unaged and aged coatings.

Table 3.
The dissolving efficiency of nano-emulsions towards Paraloid B-72 polymer on glass supports. (The removability reported as: ×, no removal; ±, partial removal; ✓, complete removal).

Table 4.
The dissolving efficiency of nano-emulsions towards Paraloid B-72 polymer on stone substrates. (The removability reported as: ×, no removal; ±, partial removal; ✓, complete removal).
On the contrary, an NSE that contains also BuOH and butanone induces swelling and/or de-wetting of coating on all the substrates. Therefore, the polymer removal is almost complete for all the examined samples (Table 3 and Table 4). The surface appearance of all the considered substrates was also observed by an optical microscope before and after cleaning (Figure 6). It is particularly worth noting that the treatment by using NSE emulsion allows for the satisfactory removal of even the most aged coating (35 days). After cleaning, the surface of the stone specimens recovered their original optical appearance as also confirmed by the negligible chromatic variation (ΔE* < 5) compared to the starting uncoated materials. Moreover, the fairly hydrophilic character of the stone surface (αLS = 55 ± 2°, and αAS = 70 ± 3°) observed after cleaning confirms the satisfactory removal of the organic polymer.
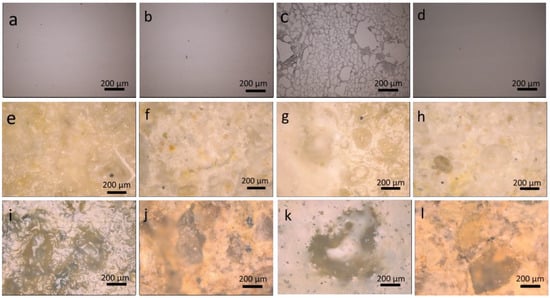
Figure 6.
Unaged, and 35 day-aged polymer coated samples before, and after cleaning with NSE, respectively: (a,b) unaged glass samples; (c,d) 35 day-aged glass samples; (e,f) unaged LS; (g,h) 35 day-aged LS; (i,j) unaged AS; and (k,l) 35 day-aged AS.
Although the above-described cleaning tests showed that the combination of surfactant and organic solvents (NSE) is distinctly more effective for the removal of acrylic coatings than a plain surfactant, further investigations were needed in order to assess the most appropriate method for the application of an NSE to the heritage building materials. In fact, the actions of organic solvents on the stone surface, even if they are emulsified in aqueous media, can be poorly controlled and, as a consequence, they could be hardly applied directly to real cases [2]. As reported in the literature, the most recommended methods to properly control the effects of cleaning agents when applied to heritage material surfaces are the cellulose pulp method and hydrogel cleaning method [2,8]. The present work mainly focused on the cellulose pulp cleaning method since it is extensively used in conservation practices and allows for the use of bio-degradable and eco-friendly products.
Therefore, further experiments were carried out on the different coated substrates (glass, LS, and AS, both unaged and aged) by applying cellulose pulp poultice previously loaded with the appropriate amount of NSE (see the experimental Section 2.3 for details). A satisfactory removal of the polymer coating was observed after repeating the procedure twice. The cleaning effectiveness was confirmed by SEM observations of the stone surface morphology (Figure 7). In all cases, the treatment makes the stone surface appear to be fully recovered. Only rarely were polymer residues observed inside large pores of LS (Figure 7c).
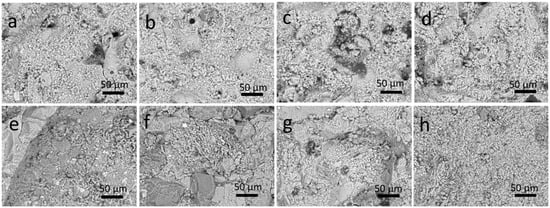
Figure 7.
SEM images of stones after cleaning by the NSE-cellulose pulp method (2 poultice applications): (a–d) LS; and (e–h) AS from unaged to 12 day-aged, 25 day-aged, and 35 day-aged, respectively.
The EDS semi-quantitative analysis confirmed that the chemical composition of the considered stones after the cleaning process was very similar to their original material compositions. For instance, the values of C% (by weight) determined on the surface of stone specimens before and after the cleaning by NSE are reported in Table 5. Carbon is the most abundant element detected by micro-analysis before cleaning due to the presence of organic coating on the stone surface. After cleaning, its abundance drastically decreases, and the C% values obtained for both unaged and 35 day-aged samples are finely comparable with the C% values observed for the plain uncoated stones, i.e., with the values corresponding to the carbonate inorganic components. Although these EDS experiments cannot be considered as quantitative analyses, the huge variations of carbon contents on the stone surface are strongly suggestive of a quite complete removal of the organic polymer layer.

Table 5.
The results of the EDS semi-quantitative analysis of both LS and AS (Wt% of carbon): uncoated, coated, and cleaned (unaged and 35 day-aged).
Chromatic variation measurements were also performed to monitor the cleaning effect of the poultice application on the coated stones. Colour changes were measured on the unaged and 35 day-aged LSPB and ASPB specimens after the first and the second applications. The overall chromatic variations (referred to original uncoated stones) are graphically presented in Figure 8. For all the examined specimens, the observed E* values suggest that residual polymer is still present on the stone surface after the first cleaning step. The second NSE-cellulose pulp application drastically reduces E* values (lower or close to 5), and the colour differences between the cleaning and uncoated stone surface are almost undetectable by the naked eye [6], confirming that the polymer removal is almost exhaustive.
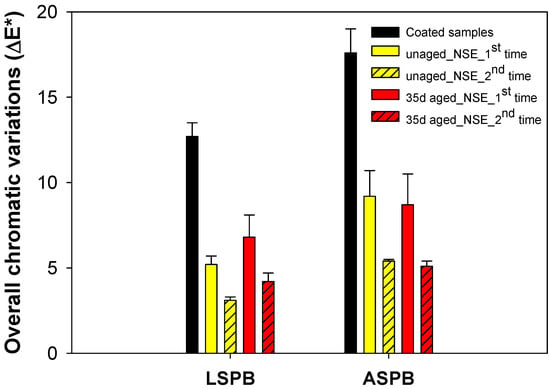
Figure 8.
The overall chromatic variations observed for both types of coated stones before (black bars) and after exposure to the NSE-loaded cellulose pulp cleaning method (coloured bars; difference vs. plain uncoated stones).
In addition, -FTIR analyses were conducted directly on the treated surfaces of the LSPB as well as the ASPB specimens before and after cleaning with the NSE-cellulose pulp poultice, and the results are in good agreement with those obtained by the experiments (SEM and chromatic variations) described above. Spectra obtained for LSPB and ASPB are reported in Figure 9. The characteristic peaks of polyacrylate (C=O stretching at 1731 cm−1 and C-O stretching at 1000–1200 cm−1) [2,32,33] progressively disappear after the first and the second cleaning steps. At the same time, the relative intensity of the bands due to the main stone components (1400 and 875 cm−1 due to carbonate in both LS and AS, and about 1100 cm−1 corresponding to silicate in AS [14,29]) increase over the cleaning process.
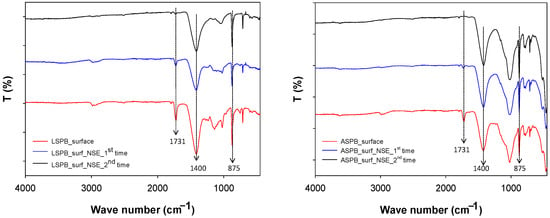
Figure 9.
Micro-FTIR (ATR mode) spectra of the LSPB (left) and ASPB (right) surface, before and after cleaning steps by the NSE-cellulose pulp (all the spectra were average from many collected data).
The possible presence of polymer residues inside the stone (under the surface) was also taken into consideration. Powder samples were collected from the cross-section (distance from the surface between 0.2 and 1 mm) of the LSPB and ASPB before cleaning and after each cleaning step. The powder samples were analysed by ATR-FTIR, and the results are in accordance with those obtained from surface analyses. Spectra of the ASPB show that the peak at 1731 cm−1 already disappears after the first applications of the NSE-cellulose pulp (Figure 10). In the case of LSPB, a very weak peak corresponding 1731 cm−1 is still present, in agreement with the previously mentioned hypothesis that some polymer residue may persist inside the larger pores of the Lecce stone.
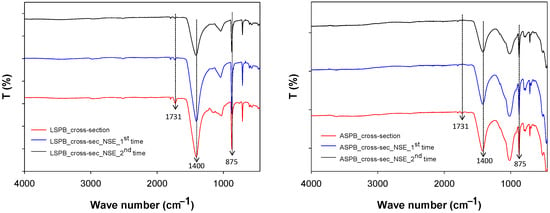
Figure 10.
FTIR spectra (ATR mode) on powder samples taken from cross-sections of the LSPB (left) and ASPB specimens (right) before and after cleaning steps by the NSE-cellulose pulp (all the spectra were average from many collected data).
The cleaned samples were stored for around three months in the laboratory in order to obtain natural degradation of any surfactant material remained in the stones. After that, the specimens underwent a final check by an iodine vapour staining test and a -FTIR mapping analysis in order to detect any possible residual compound on the stone surface.
Iodine vapor tests did not induce any significant colour change on the surface of the cleaned stones (LS and AS). Quite rare spots of red-brown colour can be ascribed to the presence of the small residue of polymer and/or cellulose.
Micro-FTIR maps were collected in different areas of the cleaned surfaces. As an example, the average map and the corresponding spectra taken on the LS surface is illustrated in Figure 11 (an analogous example for AS is reported in Figure S3). Surfaces of cleaned specimens were examined along a distance of 0.5 mm and spectra were taken every 100 μm. The spectra registered by using the ATR technique in different points of the same area can be quite different due both to the surface roughness as well as to the compositional differences at a microscopic level. For instance, in the case of AS (Figure S3), some spectra do not show any intense band (poor contact of ATR crystal with the surface) or display mainly peaks related to carbonate or silicate components. At any rate, no peak due to absorption of the polymer or other organic material can be detected in all the examined areas.
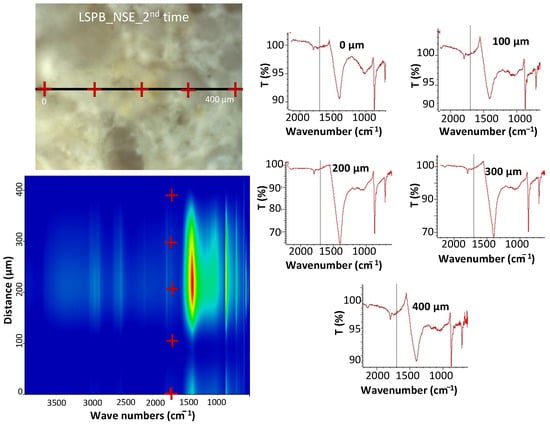
Figure 11.
Micro-FTIR (ATR mode) mapping analysis of a cleaned LS surface.
These results confirm again that the investigated cleaning procedure, consisting of a double application of NSE-cellulose pulp on a coated stone surface, is a promising method for the removal of aged polyacrylate from heritage stone materials.
4. Conclusions
The present work is focused on testing the effectiveness of nano-structured emulsions (NSE) supported by cellulose pulp in the removal of Paraloid B-72 from stone surfaces (LS and AS). Before performing the cleaning tests, coated stone specimens were artificially aged in an oven (70 ± 2 °C, 15 ± 2% R.H.) to simulate the natural weathering of the organic polymer. The chromatic changes and wettability decrease experienced by both stone surfaces following the artificial ageing suggest a physico-chemical decay process, which was confirmed by optical microscopy, SEM-EDS, and FTIR analyses. All the experiments also showed that the effects of polymer degradation increase with ageing time (up to a 35-day exposition of specimens in the oven).
In particular, the resistance to salt crystallization distinctly decreases with ageing time, as demonstrated by a salt crystallization test performed on specimens with different levels of artificial ageing (from 0 to 35 days). This also confirmed that the protecting ability of Paraloid B-72 is significantly endangered by the decay process.
A nano-structured emulsion (NSE) based on the eco-friendly surfactant named EcoSurf™ and containing limited amounts of poorly polar solvents (2-butanol and butanone) showed high effectiveness in removing the polymer coating. Hence, NSE was also tested after loading it in the cellulose pulp. The resulting poultice was applied to the coated specimens, and the polymer removal was monitored by different techniques. The laboratory tests showed that two successive applications provided satisfactory removal of the unaged as well as the weathered polymer coating from the LS and AS surfaces. After the two-step cleaning process, the stone surface features are almost completely recovered and almost undetectable residues of polymer (and/or cellulose) remained.
These results suggest that a cleaning approach based on the NSE-cellulose pulp system is a very promising method for the removal of old polymer treatments from stone substrates. This method could be safely applied to cultural heritage items as it is safe from the environmental and health points of view (limited amount of solvents and eco-friendly surfactants), and it provide a good spatial control besides an effective cleaning action. The work is in progress in our laboratory to further investigate all these properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15108117/s1. Table S1. Contact angle (α) measurements of coated stones after oven ageing cycles; Table S2. The dissolving efficiency of organic solvents towards Paraloid B-72 polymer on glass supports; Table S3. The dissolving efficiency of organic solvents towards Paraloid B-72 polymer on LS and AS specimens; Figure S1. Different samples of LS before and after salt crystallization test; Figure S2. Unaged, and 35 d aged polymer coated samples before, and after cleaning with EcoSurf-H2O, respectively: (a,b) unaged glass samples; (c,d) 35 d aged glass samples; (e,f) unaged LS; (g,h) 35 d aged LS; (i,j) unaged AS; (k,l) 35d aged AS; Figure S3. Micro-FTIR (ATR mode) mapping on cleaned surfaces of AS.
Author Contributions
Conceptualization, M.L.W.; Methodology, M.L.W.; Validation, M.L.W. and M.L.; Formal analysis, A.G., M.F. and D.S.; Investigation, D.S. and M.L.; Resources, M.L.; Data curation, M.L.W.; Writing—original draft, M.L.W.; Writing—review & editing, M.L.W. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge support from the Ministero dell’Università e della Ricerca (MUR) and the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this research study are available in the present article and in the related Supplementary Information.
Acknowledgments
The authors gratefully acknowledge Carlo Mangano, Department of Chemistry, University of Pavia, for his kind support during the experimental work in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Bouaziz, J.; Leo, F.D.; Licchelli, M. Ag-TiO2/PDMS Nanocomposite Protective Coatings: Synthesis, Characterization, and Use as a Self-Cleaning and Antimicrobial Agent. Prog. Org. Coat. 2021, 158, 106342. [Google Scholar] [CrossRef]
- Baglioni, M.; Alterini, M.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Removing Polymeric Coatings with Nanostructured Fluids: Influence of Substrate, Nature of the Film, and Application Methodology. Front. Mater. 2019, 6, 1–18. [Google Scholar] [CrossRef]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogel Based on Semi-Intrepenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Sianesi, D.; Marchionni, G.; De Pasquale, R.J. Perfluoropolyethers (PFPEs) from perfluoroolefin photooxidation. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R.E., Ed.; Plenum Press: New York, NY, USA, 1994; pp. 431–461. [Google Scholar]
- Ameduri, B.; Boutevina, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.L.; Zanchi, C. Water-repellent properties of fluoroelastomers on a very porous stone: Effect of the application procedure. Prog. Org. Coat. 2013, 76, 495–503. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Brajer, I.; Rouzic, M.F.; Shashoua, Y.; Taube, M.; Chelazzi, D.; Baglioni, M.; Giorgi, R.; Baglioni, P. The Removal of Aged Acrylic Coatings from Wall Paintings using Microemulsions. In Proceedings of the ICOM-CC, 17th Triennial Conference 2014 Melbourne, Melbourne, Australia, 15–19 September 2014. [Google Scholar]
- Baglioni, M.; Giorgi, R.; Berti, D.; Baglioni, P. Smart cleaning of cultural heritage: A new challenge for soft nanoscience. Nanoscale 2012, 4, 42–53. [Google Scholar] [CrossRef]
- Burnstock, A.; White, R. A preliminary assessment of the aging/degradation of ethomeen C-12 residues from solvent gel formulations and their potential for inducing changes in resinous paint media. Stud. Conserv. 2000, 45, 34–38. [Google Scholar] [CrossRef]
- Carretti, E.; Giorgi, R.; Berti, D.; Baglioni, P. Oil-in-water nano-containers as low environmental impact cleaning tools for works of art: Two case studies. Langmuir 2007, 23, 6396–6403. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Crivelli, F.; Galimberti, C.; Malagodi, M.; Licchelli, M. Evaluation of commercial consolidating agents on very porous biocalcarenite. Int. J. Conserv. Sci. 2020, 11, 251–260. [Google Scholar]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; Russa, M.L. Consolidation of Bio-Calcarenite Stone by Treatment Based on Diammonium Hydrogenphosphate and Calcium Hydroxide Nanoparticles. Measurement 2018, 127, 396–405. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for conservation of bio-calcarenite stone. Appl. Phys. A 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Bugani, S.; Camaiti, M.; Morselli, L.; De Casteele, E.V.; Janssens, K. Investigation on porosity changes of Lecce stone due to conservation treatments by means of x-ray nano- and improved micro-computed tomography: Preliminary results. X-ray Spectrom. 2007, 36, 316–320. [Google Scholar] [CrossRef]
- Riganti, V.; Perotti, A.; Fiumara, A.; Veniale, F.; Zezza, U. Applicazione di tecniche strumentali al controllo del degrade delle pietre nei monumenti: Il caso della Basilica di S. Michele in Pavia. Atti. Soc. Ital. Sci. Nat. Museo Civ. Stor. Nat. Milano. 1981, 122, 109–138. [Google Scholar]
- Molina, E.; Cultrone, G.; Sebastián, E.; Alonso, F.J.; Carrizo, L.; Gis-bert, J.; Buj, O. The pore system of sedimentary rocks as a key factor in the durability of building materials. Eng. Geol. 2011, 118, 110–121. [Google Scholar] [CrossRef]
- UNI 10921:2001; Beni Culturali—Materiali Lapidei Naturali Ed Artificiali—Prodotti Idrorepellenti—Applicazione Su Provini e Determinazione in Laboratorio Delle Loro Caratteristiche. UNI: Milan, Italy, 2001. Available online: https://www.biblio.units.it/sebinaopac/resource/beni-culturali-materiali-lapidei-naturali-ed-artificiali-prodotti-idrorepellenti-applicazione-su-pro/tsa1388731 (accessed on 30 September 2021).
- la Russa, M.F.; Rovella, N.; de Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Weththimuni, M.; Ben Chobba, M.; Tredici, I.; Licchelli, M. ZrO2-Doped ZnO-PDMS Nanocomposites as Protective Coatings for the Stone Materials. Acta IMEKO 2022, 11, 5. [Google Scholar] [CrossRef]
- Zedef, V.; Kocak, K.; Doyen, A.; Ozsen, H.; Kekec, B. Effect of salt crystallization on stones of historical buildings and monuments, Konya, Central Turkey. Build. Environ. 2007, 42, 1453–1457. [Google Scholar] [CrossRef]
- UNI EN 15886:2010; Conservazione dei Beni Culturali, Metodi di Prova, Misura del Colore Delle Superfici. UNI Ente Italiano di Normazione: Milan, Italy, 2010.
- Weththimuni, M.L.; Ben Chobba, M.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Sacchi, D.; Bouaziz, J.; De Leo, F.; Urzi, C.; Licchelli, M. Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites. Sustainability 2021, 13, 11033. [Google Scholar] [CrossRef]
- UNI EN 15802:2010; Conservazione dei Beni Culturali—Metodi di Prova—Determinazione Dell’angolo di Contatto Statico. UNI: Milan, Italy, 2010.
- Sanmartín, P.; Cappitelli, F. Evaluation of Accelerated Ageing Tests for Metallic and Non-Metallic Graffiti Paints Applied to Stone. Coatings 2017, 7, 180. [Google Scholar] [CrossRef]
- Feller, R.L. Accelerated Aging: Photochemical and Thermal Aspects; Getty Conservation Institute: Los Angeles, CA, USA, 1994. [Google Scholar]
- Oguchi, C.T.; Yu, S. A review of theoretical salt weathering studies for stone heritage. Prog. Earth Planet. Sci. 2021, 8, 32. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G.; Sarmah, N.C. Fourier transform infrared spectroscopic estimation of crystallinity in SiO2 based rocks. Bull. Mater. Sci. 2008, 31, 775–779. [Google Scholar] [CrossRef]
- Periasamy, A.; Muruganand, S.; Palaniswamy, M. Vibrational Studies of Na2SO4, K2SO4, NaHSO4 and KHSO4 crystals. Rasayan J. Chem. 2009, 2, 981–989. [Google Scholar]
- Denecker, M.; Hébert, R.; Bourgès, A.; Menendez, B.; Doehne, E. Mirabilite and Heptahydrate Characterization from Infrared Microscopy and Thermal data. In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone Columbia University, New York, NY, USA, 22–26 October 2012. [Google Scholar]
- Weththimuni, M.L.; Canevari, C.; Legnani, A.; Licchelli, M.; Malagodi, M.; Ricca, M.; Zeffiro, A. Experimental characterization of oil-colophony varnishes: A preliminary study. Int. J. Conserv. Sci. 2016, 2, 813–826. [Google Scholar]
- Weththimuni, M.L.; Milanese, C.; Licchelli, M.; Malagodi, M. Improving the Protective Properties of Shellac-Based Varnishes by Functionalized Nanoparticles. Coatings 2021, 11, 419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).