A Comparative Investigation of the Adsorption Characteristics of CO2, O2 and N2 in Different Ranks of Coal
Abstract
1. Introduction
2. Experimental Procedures
2.1. Coal Sample Preparation
2.2. Pore Structure Characterization
2.3. Chemical Structure Characterization
2.4. Measurement of Adsorption Isotherms
3. Adsorption Models and Thermodynamics
3.1. Adsorption Model
3.2. Adsorption Thermodynamics
4. Results and Discussion
4.1. Adsorption Characteristics of CO2, O2 and N2 in Coal
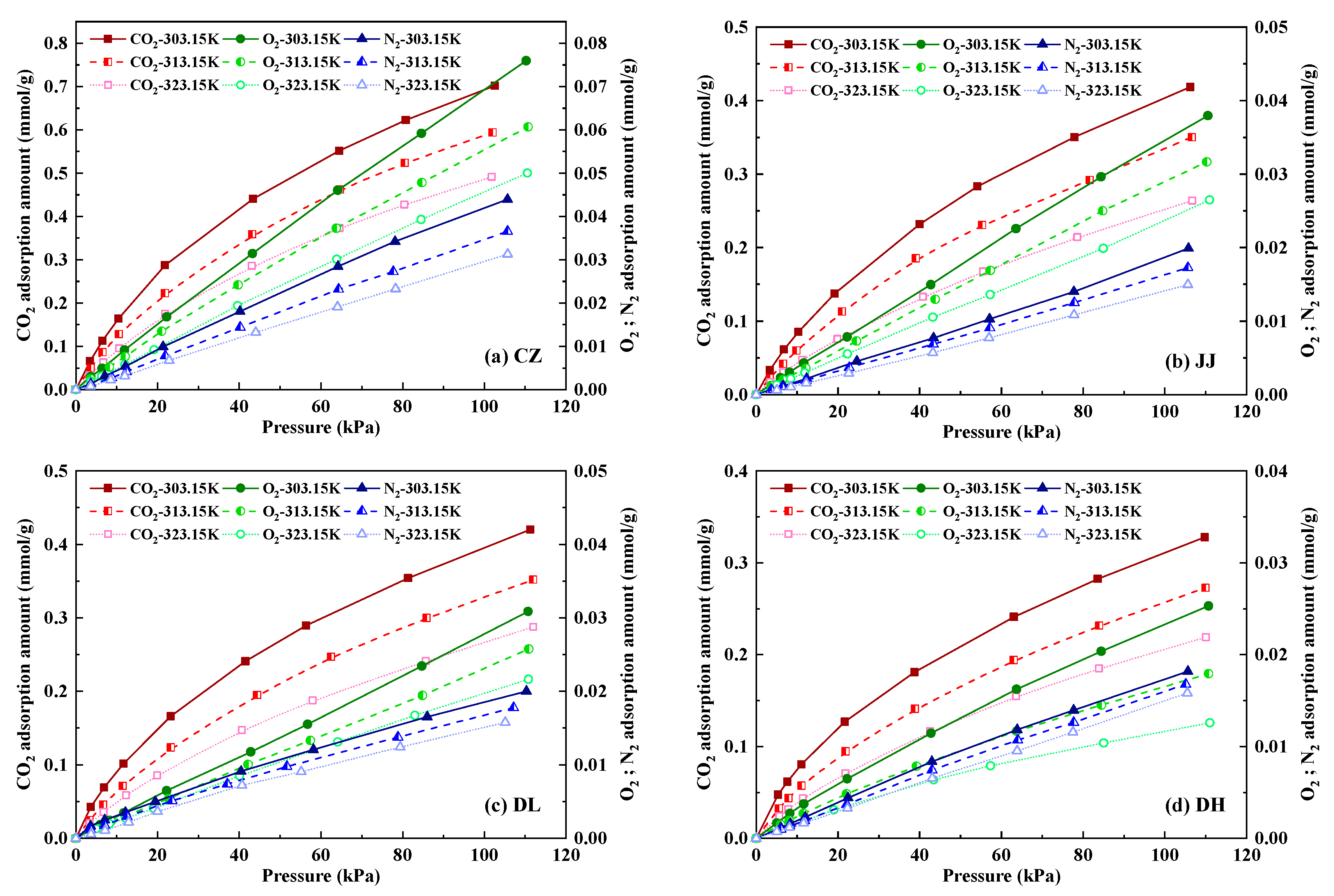
4.1.1. Adsorption Amounts of CO2, O2 and N2 in Coal
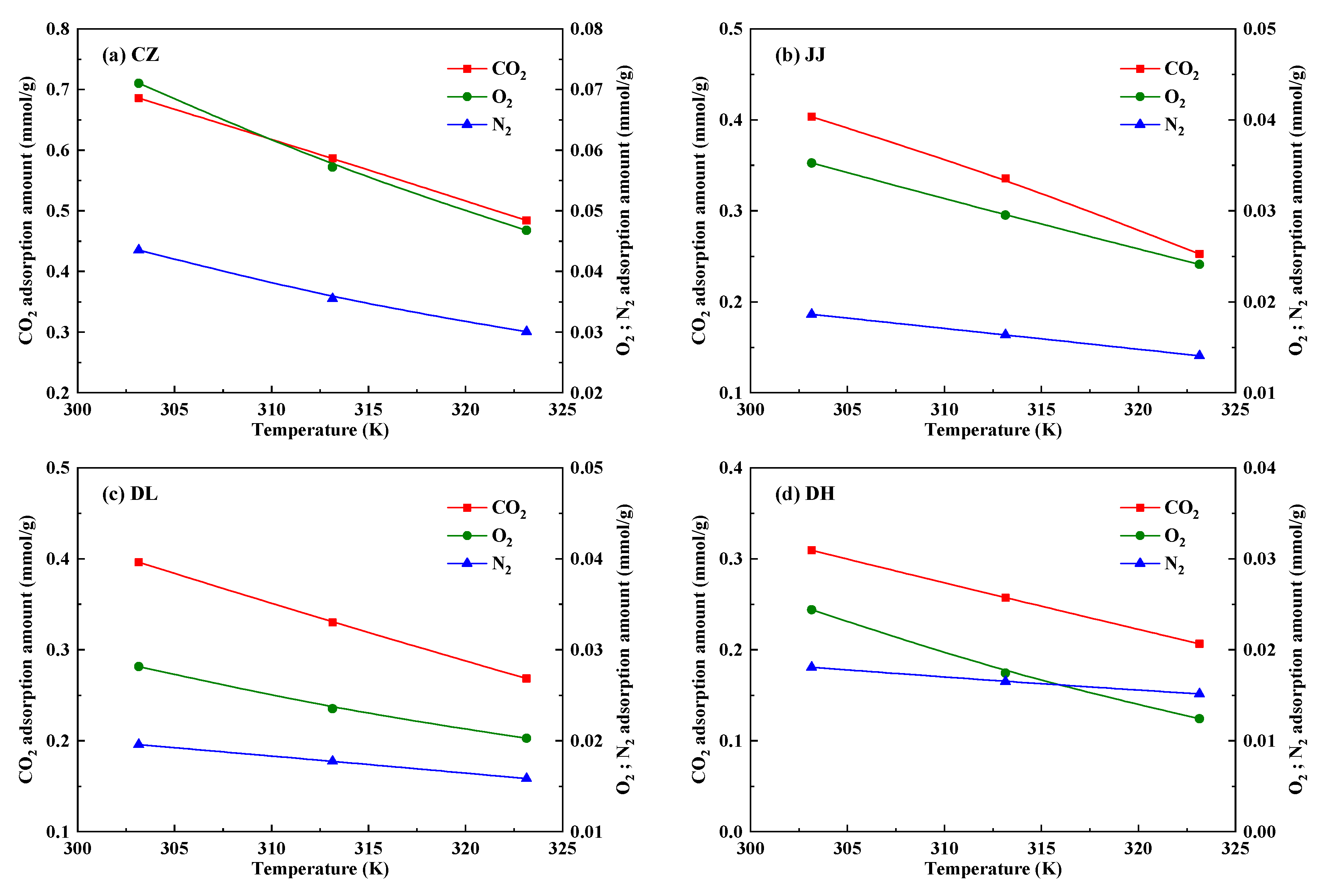
4.1.2. Adsorption Heats of CO2, O2 and N2 in Coal
4.2. Pore and Chemical Structure Characteristics of Coal
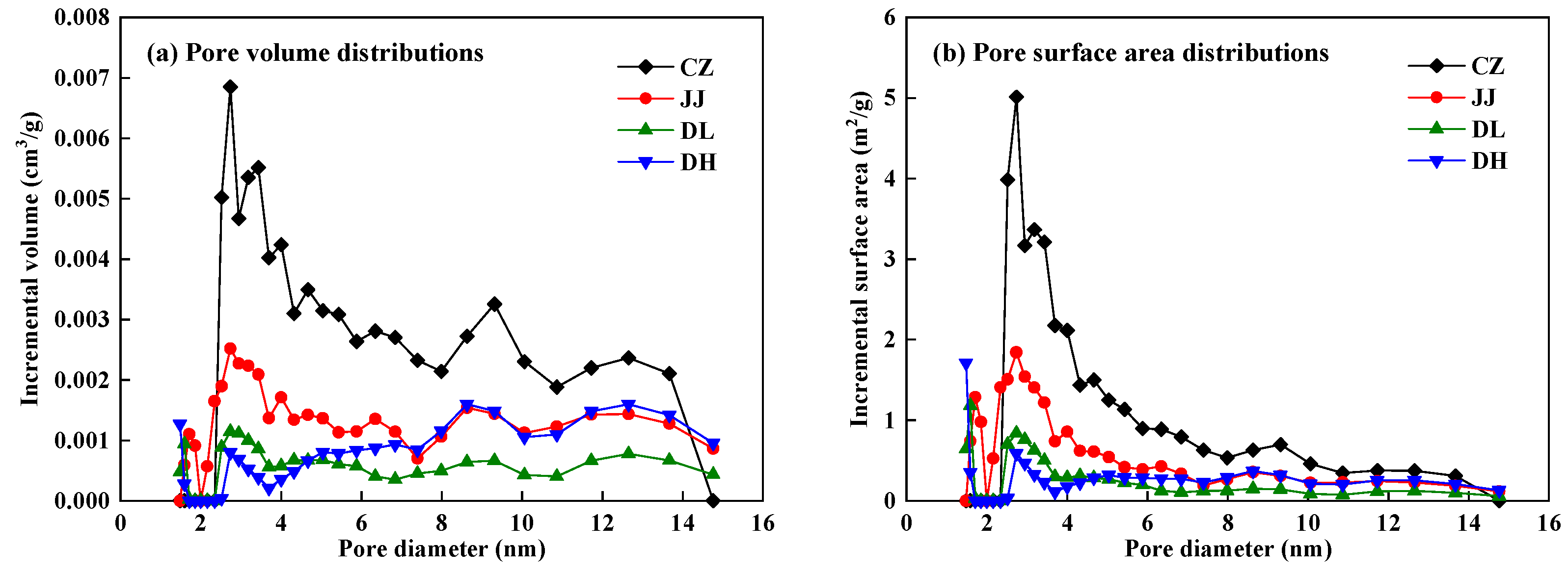
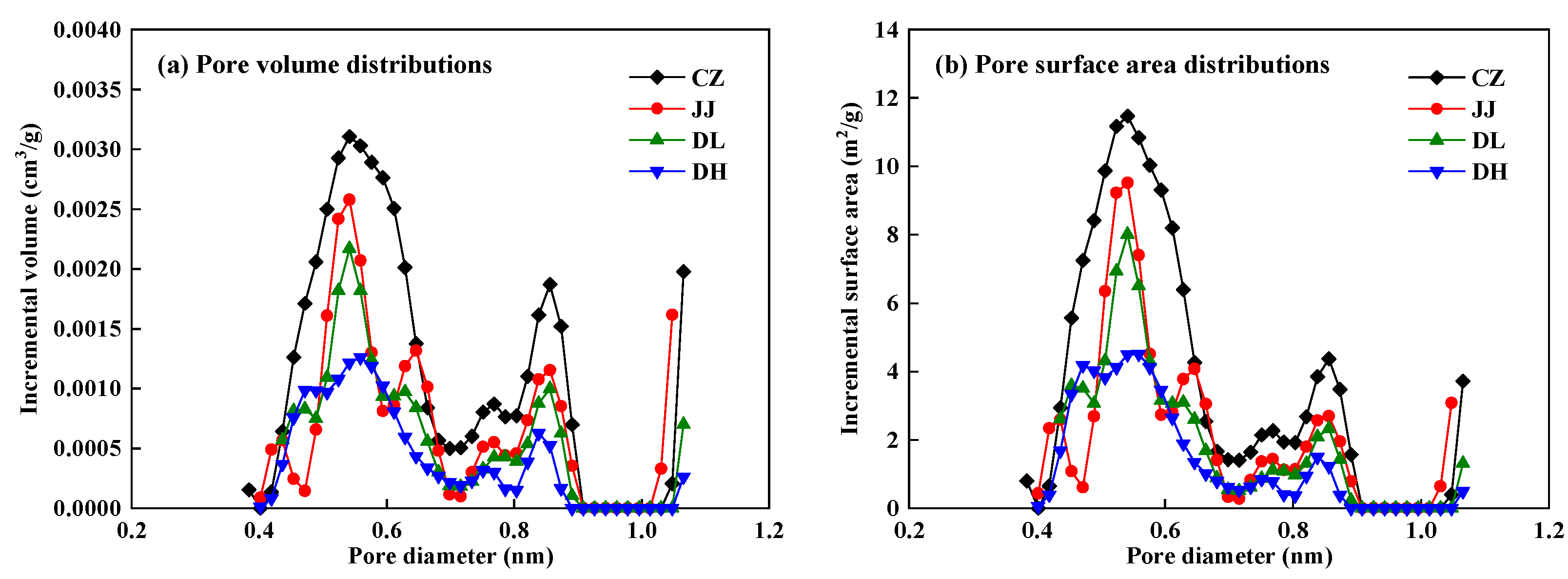
4.2.1. Pore Structure Characteristics of Coal
4.2.2. Chemical Structure Characteristics of Coal
4.3. Influencing Factors Analysis of Gas Adsorption Characteristics
5. Conclusions
- (1)
- In this study, the relationship between gas adsorption amount and adsorption heat in coal and the influencing factors were further clarified. The greater the gas adsorption heat, the greater the change rate of the gas adsorption amount with temperature in coal. The pore structure of the coal, especially the micropore structure, are the main factors that determine the gas adsorption amounts in coal. Gas adsorption heat is affected by the pore structure and chemical composition of the coal.
- (2)
- The differences in the main influencing factors of the adsorption characteristics of CO2, O2, and N2 in coal were explored. For the three gases, the pore structure of coal is the main factor that determines the adsorption amount. The main influencing factors of the adsorption heat of the three gases are different. The N2 adsorption heat is mainly affected by the pore structure of coal, and the CO2 and O2 adsorption heats are mainly affected by the chemical composition of coal. Specifically, the elemental sulfur content of coal is the main influencing factor of CO2 adsorption heat, and the mineral content of coal is the main influencing factor of O2 adsorption heat.
- (3)
- The results of this study are helpful to understand the adsorption mechanism of CO2, O2, and N2 in coal, and the results provide basic gas adsorption data for the fire prevention and control technology of injecting inert gas and the technology of CO2 storage in goaf. The coal properties can be used to predict the gas adsorption parameters and to evaluate the gas adsorption ability of different coals. The gas adsorption parameters can be used to numerically simulate the process of injecting inert gas into a goaf for optimizing the gas injection parameters.
- (4)
- This study has several limitations. In this study, only four types of coal with single particle size were selected, and only the adsorption behaviors of single gases in coals were studied. There are crushed coals with different particle sizes and bulk coals in the field, and competitive adsorption of multi-component gases exists in the goaf. In the future, more types of coals should be collected to study the adsorption behaviors of single and multi-component gases in crushed coals with different particle sizes and bulk coal. A new experimental device should also be constructed to study the mechanism of O2 replacement in coal by injecting CO2 or N2. In addition, the types of functional groups and mineral in coals and their effects on gas adsorption characteristics should be investigated in further research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, F.Q.; Qiu, D.Y. Exploring coal spontaneous combustion by bibliometric analysis. Process Saf. Environ. Prot. 2019, 132, 1–10. [Google Scholar] [CrossRef]
- Han, Z.Y.; Li, D.Y.; Li, X.B. Effects of axial pre-force and loading rate on Mode I fracture behavior of granite. Int. J. Rock Mech. Min. 2022, 157, 105172. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. A review of research on spontaneous combustion of coal. J. Min. Sci. Technol. 2020, 30, 303–311. [Google Scholar] [CrossRef]
- Lu, X.; Deng, J.; Xiao, Y.; Zhai, X.W.; Wang, C.P.; Yi, X. Recent progress and perspective on thermal-kinetic, heat and mass transportation of coal spontaneous combustion hazard. Fuel 2022, 308, 121234. [Google Scholar] [CrossRef]
- Lu, G.B.; Geng, M. Spontaneous combustion of coal mined-out area and its mechanism of control research. J. Liaoning Tech. Univ. Nat. Sci. 2009, 28, 28–30. [Google Scholar]
- Wei, D.Y.; Du, C.F.; Lei, B.A.; Lin, Y.F. Prediction and prevention of spontaneous combustion of coal from goafs in workface: A case study. Case Stud. Therm. Eng. 2020, 21, 100668. [Google Scholar] [CrossRef]
- Han, Z.Y.; Li, D.Y.; Li, X.B. Dynamic mechanical properties and wave propagation of composite rock-mortar specimens based on SHPB tests. Int. J. Min. Sci. Technol. 2022, 32, 793–806. [Google Scholar] [CrossRef]
- Qin, B.T.; Wang, D.M. Present situation and development of mine fire control technology. China Saf. Sci. J. 2007, 17, 80–85. [Google Scholar]
- Liu, S.; Liu, F.Y.; Wang, G.; Wu, D.S.; Xiang, J.W. Development outlook of mine fire control technology by using CO2 in coal mine goaf. Coal Technol. 2014, 33, 7–9. [Google Scholar]
- Li, Q.W.; Xiao, Y.; Zhong, K.Q.; Shu, C.M.; Lü, H.F.; Deng, J.; Wu, S.L. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel 2020, 275, 117981. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H.; Guo, J.; Jin, Y.F.; Wei, G.M.; Yang, Z.W. Coal spontaneous combustion and N2 suppression in triple goafs: A numerical simulation and experimental study. Fuel 2020, 271, 117625. [Google Scholar] [CrossRef]
- Wu, S.Y.; Jin, Z.X.; Deng, C.B. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 2019, 239, 87–96. [Google Scholar] [CrossRef]
- Jing, R.; Wang, X.F.; Qiao, L.; Deng, C.B.; Hao, C.Y.; Kang, Y.L. Research status and future prospect of power plant flue gas sealed in goaf. Coal Eng. 2021, 53, 125–130. [Google Scholar]
- Tan, B.; Cheng, G.; Zhu, X.M.; Yang, X.B. Experimental study on the physisorption characteristics of O2 in coal powder are effected by coal nanopore structure. Sci. Rep. 2020, 10, 6946. [Google Scholar] [CrossRef]
- Wang, X.F.; Qiao, L.; Deng, C.B.; Chu, G.; Li, X.F.; Zhao, Q.; Wang, G.L. Study on the characteristics of nitrogen dioxide adsorption and storage of coal residue in coal fired power plants in goaf. Sci. Rep. 2021, 11, 8822. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Sun, H.T.; Zhang, D.M.; Yang, K.; Wang, D.K.; Li, X.L.; Long, K.; Li, Y.N. Nuclear magnetic resonance study on the influence of liquid nitrogen cold soaking on the pore structure of different coals. Phys. Fluids 2023, 35, 012009. [Google Scholar] [CrossRef]
- Lu, W.; Wang, D.M.; Dai, G.L.; Zhong, X.X. Study on oxygen physisorption of coal. J. Hunan Univ. Sci. Technol. Nat. Sci. Ed. 2005, 20, 6–10. [Google Scholar]
- Ma, H.P.; Lu, W.; Wang, D.M.; Dai, G.L.; Zhong, X.X. Research on physical adsorbed oxygen in coal spontaneous combustion processing. Coal Sci. Technol. 2006, 34, 26–29. [Google Scholar]
- Wu, S.Y.; Deng, C.B.; Dai, F.W.; Gao, F.; Wang, X.F. Differences of ability and competitiveness on coal adsorbing CO2, O2 and N2. Chin. J. Environ. Eng. 2017, 11, 4229–4235. [Google Scholar]
- Kang, J.H.; Zhu, J.P.; Wang, Y.P.; Zhou, F.B.; Liu, Y.K. Dynamical modeling of coupled heat and mass transfer process of coalbed methane desorption in porous coal matrix. Int. J. Heat Mass Transf. 2022, 183, 122212. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery—A review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Chattaraj, S.; Mohanty, D.; Kumar, T.; Halder, G. Thermodynamics, kinetics and modeling of sorption behaviour of coalbed methane-A review. J. Unconv. Oil Gas Resour. 2016, 16, 14–33. [Google Scholar] [CrossRef]
- Chen, L.W.; Zhao, M.Z.; Li, X.H.; Liu, Y. Impact research of CH4 replacement with CO2 in hydrous coal under high pressure injection. Min. Miner. Depos. 2022, 16, 121–126. [Google Scholar] [CrossRef]
- Yang, H.M.; Liu, Y.; Lyu, B.Y. Study on replacement methane with nitrogen from coal by high pressure injection and equal pressure diffusion. J. Saf. Sci. Technol. 2022, 18, 94–99. [Google Scholar]
- Wen, H.; Hao, J.C.; Ma, L.; Zheng, X.Z. Experimental study on replacing coal seam CH4 with CO2 gas. ACS Omega 2022, 7, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.; Tang, G.Q.; Kovscek, A.R. Laboratory and simulation investigation of enhanced coalbed methane recovery by gas injection. Transp. Porous Media 2008, 73, 141–159. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Jiang, C.F.; Liu, D.J.; Chu, W. Microstructure and its influence on CH4 adsorption behavior of deep coal. Chin. Phys. B 2014, 23, 028201. [Google Scholar] [CrossRef]
- Fu, Y.S.; Liu, X.F.; Ge, B.Q.; Liu, Z.H. Role of chemical structures in coalbed methane adsorption for anthracites and bituminous coals. Adsorption 2017, 23, 711–721. [Google Scholar] [CrossRef]
- Liu, X.F.; He, X.Q. Effect of pore characteristics on coalbed methane adsorption in middle-high rank coals. Adsorption 2017, 23, 3–12. [Google Scholar] [CrossRef]
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Li, Q.Z.; Yuan, D.S.; Zhang, G.Y.; Liu, J.F.; Yuan, C.C.; Tao, Q.L. Chemical structure of coal surface and its effects on methane adsorption under different temperature conditions. Adsorption 2018, 24, 613–628. [Google Scholar] [CrossRef]
- Fu, X.X.; Zhang, D.F.; Jiang, W.P.; Lun, Z.M.; Zhao, C.P.; Wang, H.T.; Li, Y.H. Influence of physicochemical properties of coals on pore morphology and methane adsorption: A perspective. Chem. Ind. Eng. Prog. 2019, 38, 2714–2725. [Google Scholar]
- Han, F.S.; Busch, A.; Krooss, B.M.; Liu, Z.Y.; Yang, J.L. CH4 and CO2 sorption isotherms and kinetics for different size fractions of two coals. Fuel 2013, 108, 137–142. [Google Scholar] [CrossRef]
- Zhang, L.; Aziz, N.; Ren, T.; Nemcik, J.; Tu, S.H. Influence of coal particle size on coal adsorption and desorption characteristics. Arch. Min. Sci. 2014, 59, 807–820. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Kwiatek, L.M. Physical properties of selected block Argonne Premium bituminous coal related to CO2, CH4, and N2 adsorption. Int. J. Coal Geol. 2009, 77, 2–9. [Google Scholar] [CrossRef]
- Song, Y.C.; Xing, W.L.; Zhang, Y.; Jian, W.W.; Liu, Z.Y.; Liu, S.Y. Adsorption isotherms and kinetic characteristics of methane on block anthracite over a wide pressure range. Adsorption 2015, 21, 53–65. [Google Scholar] [CrossRef]
- Zhou, F.B.; Liu, S.Q.; Pang, Y.Q.; Li, J.L.; Xin, H.H. Effects of coal functional groups on adsorption microheat of coal bed methane. Energy Fuels 2015, 29, 1550–1557. [Google Scholar] [CrossRef]
- Li, H.J.; Kang, J.H.; Zhou, F.B.; Qiang, Z.Y.; Li, G.H. Adsorption heat features of coalbed methane based on microcalorimeter. J. Loss Prev. Process Ind. 2018, 55, 437–449. [Google Scholar] [CrossRef]
- Liu, Y.K.; Kang, J.H.; Zhou, F.B.; Fan, Y.M.; Li, H.J. Effects of maceral compositions of coal on methane adsorption heat. Geofluids 2018, 2018, 7596138. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S. Relationships between the critical properties of gases and their high pressure sorption behavior on coals. Energy Fuels 2010, 24, 1781–1787. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S. Relationships between the sorption behaviour of methane, carbon dioxide, nitrogen and ethane on coals. Fuel 2012, 97, 725–729. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.J.P.; Sakurovs, R.; Grigore, M.; Bunt, J.R. The carbon dioxide, methane and nitrogen high-pressure sorption properties of South African bituminous coals. Int. J. Coal Geol. 2019, 209, 40–53. [Google Scholar] [CrossRef]
- Tan, B.; Cheng, G.; Fu, S.H.; Wang, H.Y.; Li, Z.X.; Zhang, X.D. Molecular simulation for physisorption characteristics of O2 in low-rank coals. Energy 2022, 242, 122538. [Google Scholar] [CrossRef]
- Deng, C.B.; Deng, H.Z.; Wang, J.R.; Lin, H. Coal surface containing phosphorus group physical adsorption to oxygen molecules mechanism. Coal Convers. 2008, 31, 1–5. [Google Scholar]
- Deng, C.B.; Wang, X.F.; Wang, J.R.; Deng, H.Z.; Ye, B. Physical adsorption mechanism of coal surface containing sulfur group adsorption to more oxygen molecule. J. China Coal Soc. 2008, 33, 556–560. [Google Scholar]
- GB/T 5751-2009; Chinese Classification of Coals. The National Standards Compilation Group of Peoples Republic of China. Standards Press of China: Beijing, China, 2009.
- GB/T 482-2008; Sampling of Coal Seams. The National Standards Compilation Group of Peoples Republic of China. Standards Press of China: Beijing, China, 2008.
- GB/T 6948-2008; Method of Determining Microscopically the Reflectance of Vitrinite in Coal. The National Standards Compilation Group of Peoples Republic of China. Standards Press of China: Beijing, China, 2008.
- ICCP. The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. The National Standards Compilation Group of Peoples Republic of China. Standards Press of China: Beijing, China, 2008.
- GB/T 214-2007; Determination of Total Sulfur in Coal. The National Standards Compilation Group of Peoples Republic of China. Standards Press of China: Beijing, China, 2007.
- Everett, D.H. Manual of symbols and terminology for physicochemical quantities and units. Pure Appl. Chem. 1972, 31, 579–638. [Google Scholar] [CrossRef]
- Gao, F.; Deng, C.B.; Wang, X.F.; Dai, F.W.; Wu, S.Y. Adsorption of power plant flue gas by abandoned coal at normal temperature and pressure. Chin. J. Environ. Eng. 2016, 10, 1907–1912. [Google Scholar]
- Wang, D.C.; Yang, Z.Y.; Liu, J.P.; Liao, H.B.; Li, Z.H. Fitting results comparison of different CH4/N2 adsorption models and its adsorption thermodynamics analysis. Coal Sci. Technol. 2016, 44, 192–198. [Google Scholar]
- Tang, X.; Wang, Z.F.; Ripepi, N.; Kang, B.; Yue, G.W. Adsorption affinity of different types of coal: Mean isosteric heat of adsorption. Energy Fuels 2015, 29, 3609–3615. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Stadie, N.P.; Yu, L.J. Thermodynamic analysis of high pressure methane adsorption in Longmaxi shale. Fuel 2017, 193, 411–418. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Auroux, A. Calorimetry and Thermal Methods in Catalysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Siperstein, F.; Gorte, R.J.; Myers, A.L. A new calorimeter for simultaneous measurements of loading and heats of adsorption from gaseous mixtures. Langmuir 1999, 15, 1570–1576. [Google Scholar] [CrossRef]
- Pan, H.H.; Ritter, J.A.; Balbuena, P.B. Examination of the approximations used in determining the isosteric heat of adsorption from the Clausius-Clapeyron equation. Langmuir 1998, 14, 6323–6327. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 88th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Dou, H.R.; Ni, G.H.; Sun, G.S.; Li, Z.; Yin, X.L.; Huang, Q.M.; Wang, Z.Y. Study on dominant structural factors and laws of combustion performance of acidified coal. Energy 2023, 266, 126413. [Google Scholar] [CrossRef]
- Hao, M.; Wei, C.M.; Zhang, H. Adsorption and diffusion of methane in coal slit pores: Insights into the molecular level. Energy Fuels 2022, 36, 880–886. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.J.; Liang, L.X. Molecular simulation study on the adsorption behaviors of methane in slit-like clay mineral pore. J. China Coal Soc. 2017, 42, 959–968. [Google Scholar]
- Wang, F.S.; Zhang, Z.M.; Sun, C.; Dong, X.W.; Wang, C. Study on influence of coal composition and structure on spontaneous combustion tendency. Coal Technol. 2019, 38, 75–77. [Google Scholar]
- Deng, J.; Ma, X.F.; Zhang, Y.T.; Li, Y.Q.; Zhu, W.W. Effects of pyrite on the spontaneous combustion of coal. Int. J. Coal Sci. Technol. 2015, 2, 306–311. [Google Scholar] [CrossRef]
- Chen, S.H.; Bai, X.F.; Fang, Q.G. Research on positive effect mechanism of pyrite on coal oxidation at low temperature. Clean Coal Technol. 2018, 24, 14–19. [Google Scholar]
- Zhang, A.H.; Tao, M.X.; Liu, P.Y.; Chen, X.R.; Li, D.D. Advance of research on the occurrence state and content of nitrogen in coal. Coal Geol. Explor. 2016, 44, 9–13. [Google Scholar]
- Fan, C.J.; Li, S.; Luo, M.K.; Zhou, L.J.; Zhang, H.H.; Yang, Z.H. Effects of N and S functionalities on binary gas co-adsorption on a coal macromolecule. Energy Fuels 2019, 33, 3934–3946. [Google Scholar] [CrossRef]

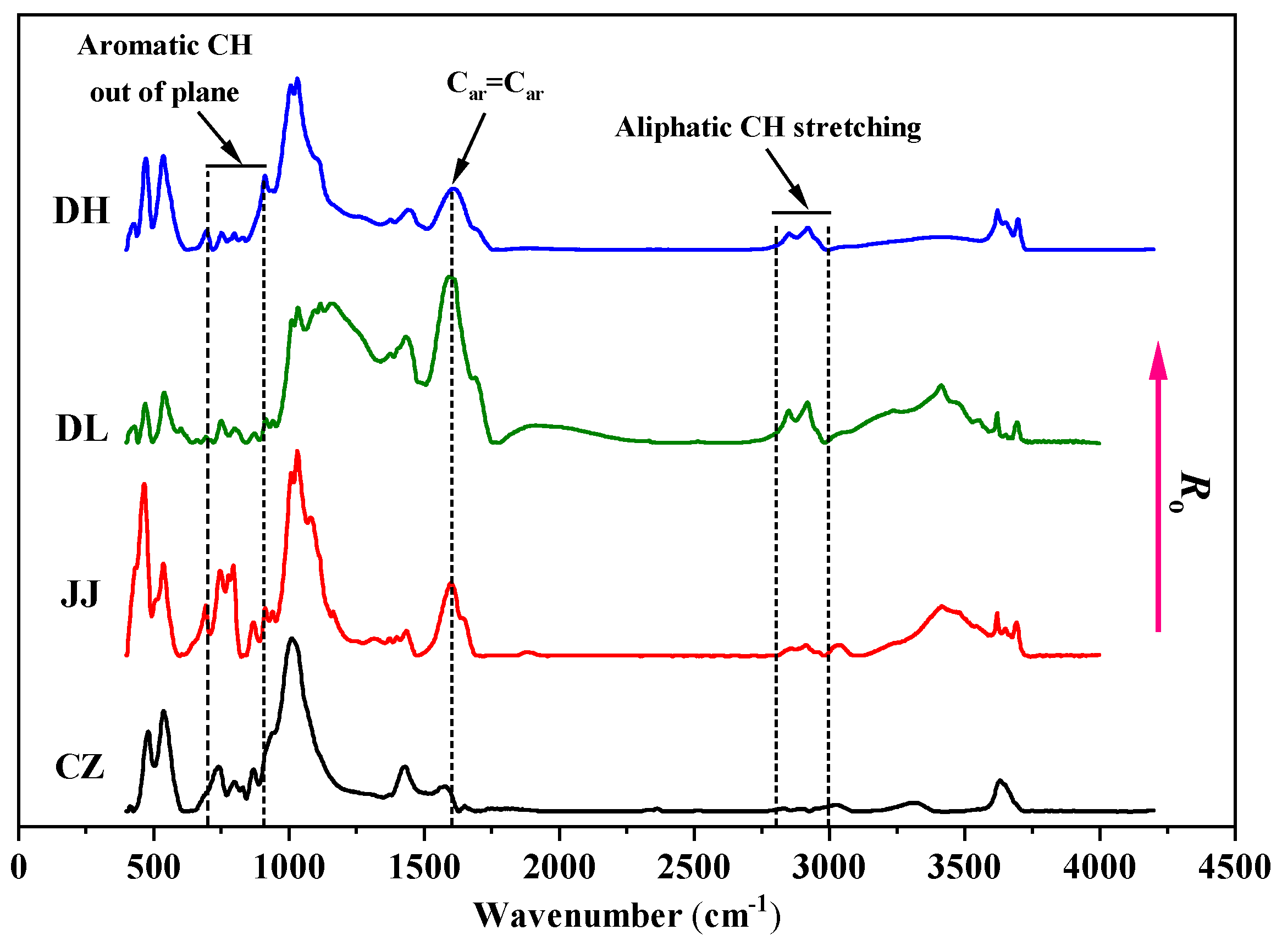
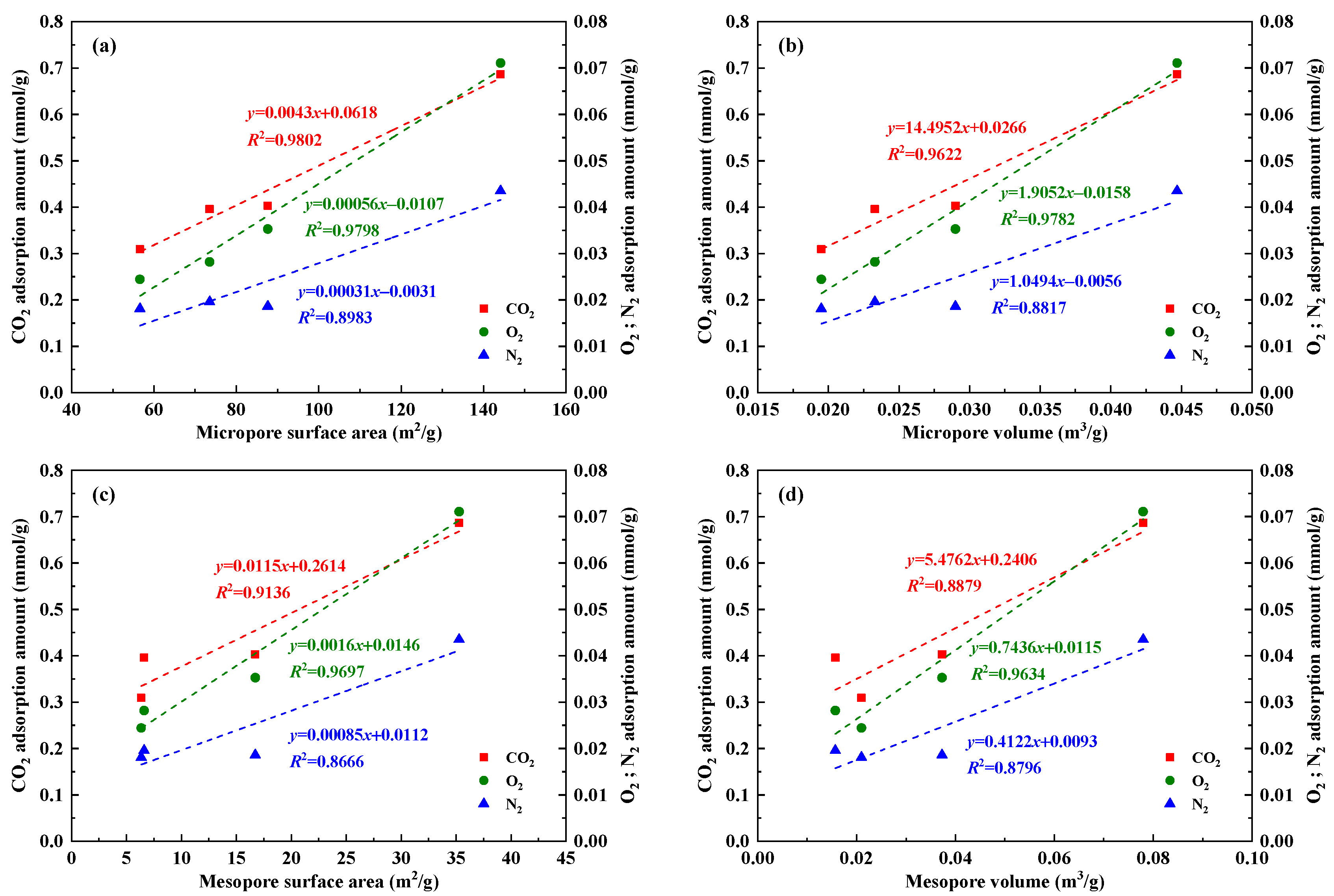
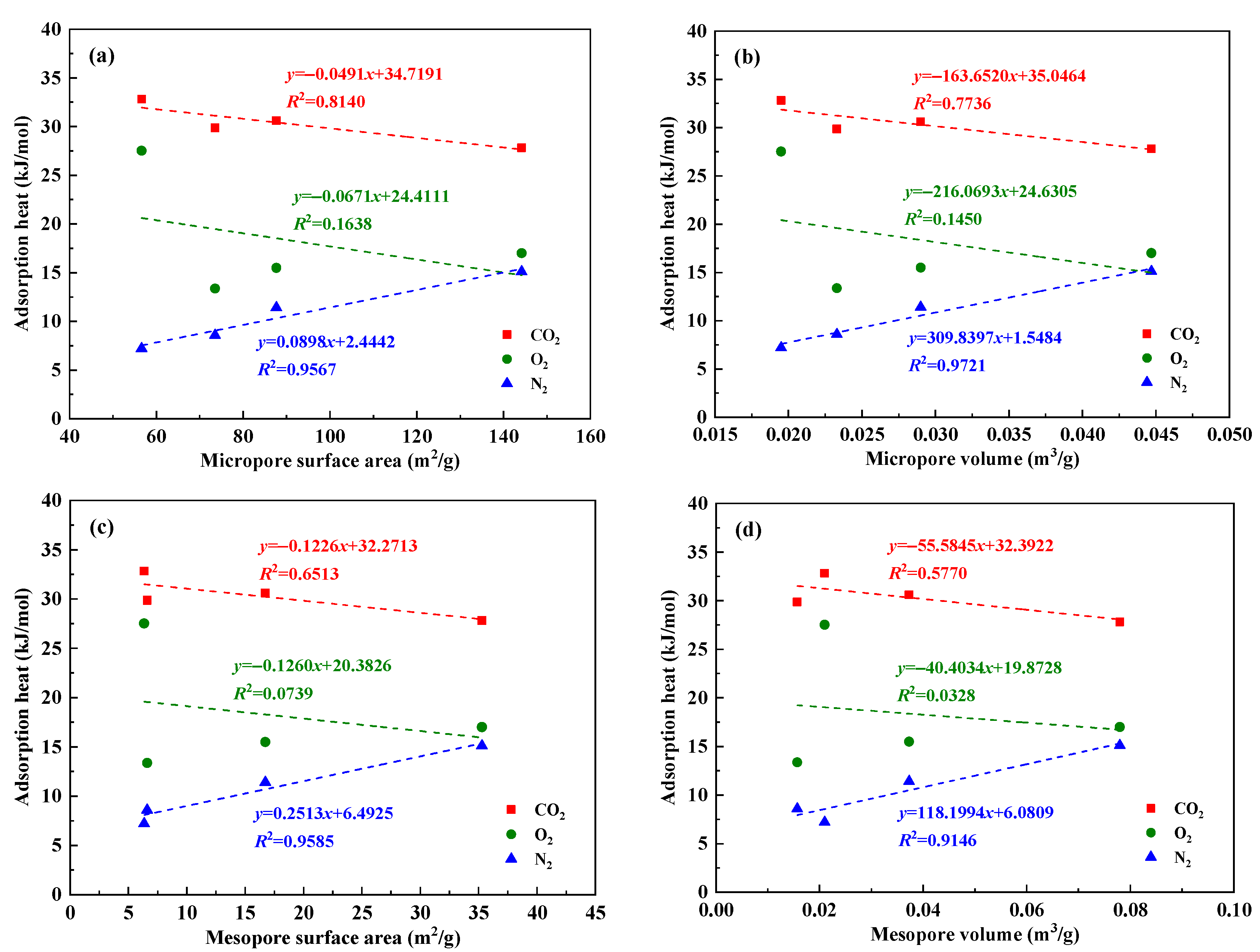
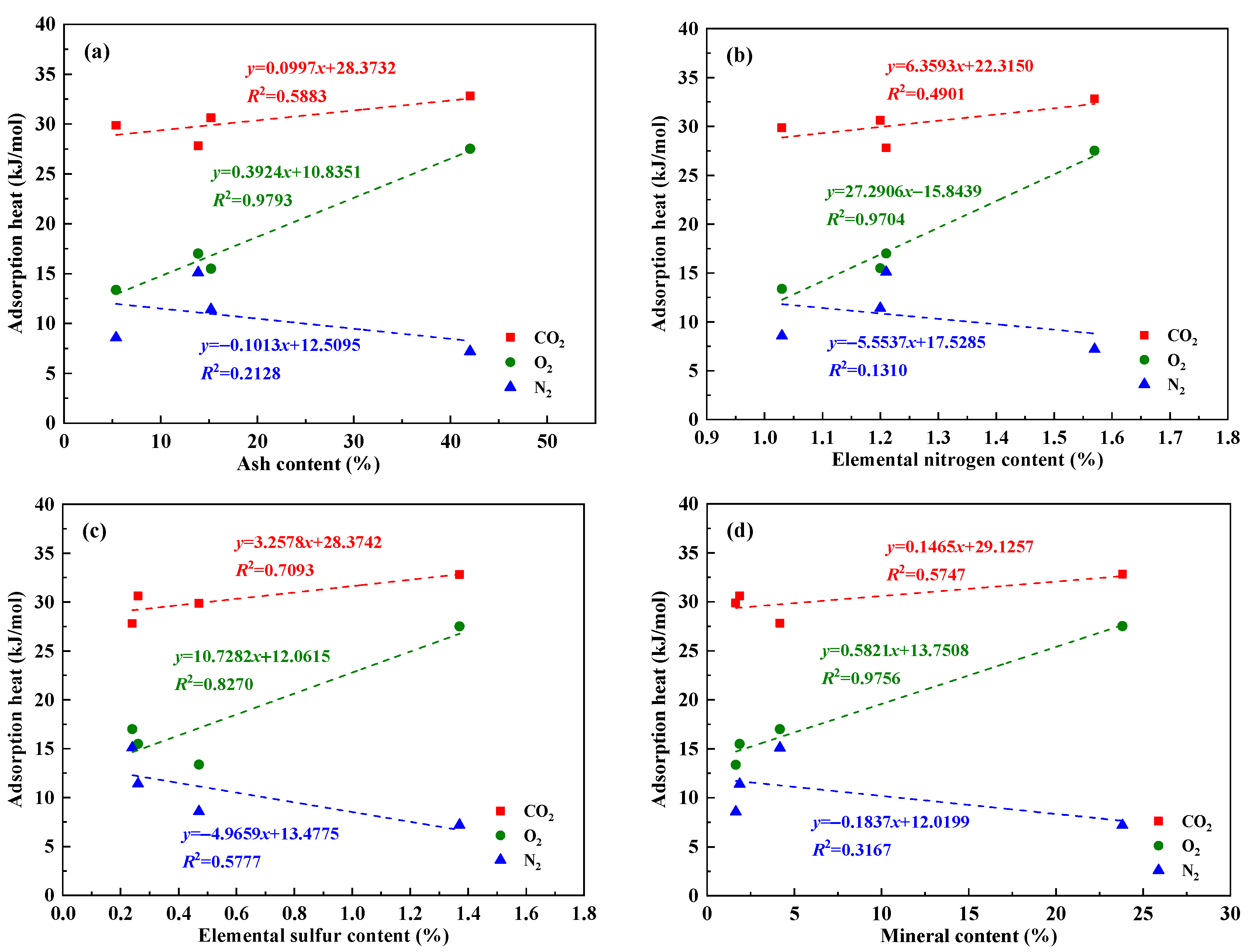
| Sample | Coal Mine | Coal Seam | Coal Field | Geological Formation |
|---|---|---|---|---|
| CZ | Chengzhuang | 3 | Qinshui | Shanxi formation of the lower Permian system |
| JJ | Jinjia | 18 | Liupanshui | Longtan formation of the upper Permian system |
| DL | Daliuta | 5 | Dongsheng | Yan’an formation of the middle Jurassic system |
| DH | Donghuai | 1 | Baise | Nadu formation of the lower Tertiary system |
| Properties | CZ | JJ | DL | DH |
|---|---|---|---|---|
| Coal Type | Anthracite | Lean coal | Fat coal | Lignite |
| Ro,m (%) | 2.37 | 2.04 | 0.76 | 0.58 |
| Proximate Analysis (%, air-drying base) | ||||
| M | 0.72 | 0.54 | 3.62 | 3.17 |
| A | 13.88 | 15.20 | 5.38 | 42.02 |
| V | 7.54 | 8.88 | 31.84 | 27.96 |
| FC | 77.59 | 75.38 | 59.16 | 26.85 |
| Ultimate Analysis (%, air-drying base) | ||||
| C | 77.59 | 76.63 | 73.30 | 39.33 |
| H | 2.89 | 3.30 | 4.48 | 3.48 |
| O | 3.48 | 2.89 | 11.72 | 9.06 |
| N | 1.21 | 1.20 | 1.03 | 1.57 |
| S | 0.24 | 0.26 | 0.47 | 1.37 |
| Maceral Composition (%) | ||||
| VI | 67.26 | 68.12 | 49.45 | 72.02 |
| IN | 28.56 | 30.00 | 47.80 | 1.19 |
| EX | – | – | 1.10 | 2.98 |
| MI | 4.18 | 1.88 | 1.65 | 23.81 |
| Sample | Temperature (K) | CO2-Langmuir Model | CO2-Virial Model | O2-Henry Model | N2-Henry Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a (mmol·g−1) | b (kPa−1) | R2 | A0 | A1 | R2 | KH (×10−4 mmol ·g−1·kPa−1) | R2 | KH (×10−4 mmol ·g−1·kPa−1) | R2 | ||
| CZ | 303.15 | 1.1287 | 0.0153 | 0.9991 | −3.8830 | −1.5816 | 0.9995 | 7.0127 | 0.9990 | 4.2964 | 0.9978 |
| 313.15 | 1.0616 | 0.0122 | 0.9997 | −4.2133 | −1.5789 | 0.9985 | 5.6471 | 0.9969 | 3.5041 | 0.9993 | |
| 323.15 | 0.9769 | 0.0097 | 0.9997 | −4.5665 | −1.5652 | 0.9996 | 4.6192 | 0.9989 | 2.9663 | 0.9997 | |
| JJ | 303.15 | 0.7525 | 0.0114 | 0.9991 | −4.5798 | −2.3537 | 0.9934 | 3.4816 | 0.9995 | 1.8373 | 0.9988 |
| 313.15 | 0.7037 | 0.0090 | 0.9993 | −4.9591 | −2.2248 | 0.9962 | 2.9138 | 0.9996 | 1.6147 | 0.9996 | |
| 323.15 | 0.6249 | 0.0067 | 0.9994 | −5.3317 | −2.6907 | 0.9812 | 2.3799 | 0.9996 | 1.3883 | 0.9985 | |
| DL | 303.15 | 0.6794 | 0.0138 | 0.9980 | −4.4371 | −2.8189 | 0.9930 | 2.7785 | 0.9997 | 1.9320 | 0.9768 |
| 313.15 | 0.6767 | 0.0094 | 0.9992 | −4.8843 | −2.5881 | 0.9920 | 2.3230 | 0.9994 | 1.7510 | 0.9852 | |
| 323.15 | 0.5838 | 0.0084 | 0.9991 | −5.1692 | −2.8890 | 0.9919 | 2.0020 | 0.9980 | 1.5646 | 0.9935 | |
| DH | 303.15 | 0.5102 | 0.0152 | 0.9966 | −4.6033 | −3.8698 | 0.9876 | 2.4098 | 0.9897 | 1.7860 | 0.9961 |
| 313.15 | 0.4964 | 0.0106 | 0.9983 | −5.0551 | −3.6292 | 0.9875 | 1.7204 | 0.9844 | 1.6283 | 0.9987 | |
| 323.15 | 0.4437 | 0.0086 | 0.9990 | −5.4084 | −3.8621 | 0.9867 | 1.2260 | 0.9762 | 1.4962 | 0.9999 | |
| Properties/Gases | CO2 | O2 | N2 |
|---|---|---|---|
| Molecular Mass, m (g/mol) | 44 | 32 | 28 |
| Critical Temperature, Tc (K) | 304.2 | 154.6 | 126.2 |
| Critical Pressure, Pc (MPa) | 7.4 | 5.0 | 3.4 |
| Van der Waals Attractive Constant, a (kJ2/mol2Pa) | 0.365 | 0.139 | 0.137 |
| Kinetic Diameter, σk (nm) | 0.330 | 0.346 | 0.364 |
| Sample | Gas | Fitting Formula | R2 | −ΔH0 (kJ·mol−1) |
|---|---|---|---|---|
| CZ | CO2 | lnKH = 3345.6/T − 14.912 | 0.9986 | 27.82 |
| O2 | lnKH = 2045.1/T − 14.009 | 0.9999 | 17.00 | |
| N2 | lnKH = 1815.9/T − 13.747 | 0.9984 | 15.10 | |
| JJ | CO2 | lnKH = 3682.0/T − 16.723 | 0.9998 | 30.61 |
| O2 | lnKH = 1861.5/T − 14.098 | 0.9969 | 15.48 | |
| N2 | lnKH = 1370.9/T − 13.119 | 0.9960 | 11.40 | |
| DL | CO2 | lnKH = 3593.2/T − 16.312 | 0.9882 | 29.87 |
| O2 | lnKH = 1606.5/T − 13.491 | 0.9988 | 13.36 | |
| N2 | lnKH = 1032.1/T − 11.953 | 0.9967 | 8.58 | |
| DH | CO2 | lnKH = 3947.3/T − 17.636 | 0.9973 | 32.82 |
| O2 | lnKH = 3308.9/T − 19.242 | 0.9996 | 27.51 | |
| N2 | lnKH = 867.33/T − 11.492 | 0.9999 | 7.21 |
| Sample | St (m2/g) | Smic (m2/g) | Smes (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) |
|---|---|---|---|---|---|---|
| CZ | 179.42 | 144.14 | 35.28 | 0.1227 | 0.0447 | 0.0780 |
| JJ | 104.37 | 87.65 | 16.72 | 0.0663 | 0.0290 | 0.0373 |
| DL | 80.15 | 73.54 | 6.61 | 0.0390 | 0.0233 | 0.0157 |
| DH | 62.94 | 56.60 | 6.34 | 0.0405 | 0.0195 | 0.0210 |
| Sample | fa | Hal/H | A(CH2)/A(CH3) | ’C’ |
|---|---|---|---|---|
| CZ | 0.9786 | 0.0862 | 0.7471 | 0.3013 |
| JJ | 0.9703 | 0.1036 | 0.2468 | 0.2458 |
| DL | 0.6904 | 0.7599 | 3.6485 | 0.4616 |
| DH | 0.7114 | 0.4892 | 2.3060 | 0.4972 |
| X/Y | Adsorption Amount | Adsorption Heat | ||||
|---|---|---|---|---|---|---|
| CO2 | O2 | N2 | CO2 | O2 | N2 | |
| Smic | 0.9901 | 0.9899 | 0.9478 | −0.9022 | −0.4048 | 0.9781 |
| Vmic | 0.9809 | 0.9890 | 0.9390 | −0.8796 | −0.3808 | 0.9859 |
| Smes | 0.9558 | 0.9847 | 0.9309 | −0.8071 | −0.2718 | 0.9790 |
| Vmes | 0.9423 | 0.9815 | 0.9379 | −0.7596 | −0.1811 | 0.9563 |
| fa | 0.6784 | 0.7359 | 0.5838 | −0.5750 | −0.3322 | 0.8859 |
| Hal/H | −0.5778 | −0.6737 | −0.5371 | 0.3740 | 0.0478 | −0.7888 |
| A(CH2)/A(CH3) | −0.4565 | −0.5570 | −0.4019 | 0.2720 | 0.0441 | −0.7070 |
| ‘C’ | −0.5544 | −0.5899 | −0.4090 | 0.5481 | 0.5031 | −0.8035 |
| M | −0.6142 | −0.6815 | −0.5224 | 0.4994 | 0.2815 | −0.8420 |
| A | −0.4528 | −0.3324 | −0.2636 | 0.7670 | 0.9896 | −0.4613 |
| V | −0.6737 | −0.7406 | −0.5942 | 0.5413 | 0.2620 | −0.8777 |
| FC | 0.7070 | 0.6660 | 0.5294 | −0.8417 | −0.8307 | 0.8391 |
| C | 0.6209 | 0.5438 | 0.4272 | −0.8427 | −0.9434 | 0.7109 |
| H | −0.5558 | −0.6762 | −0.6052 | 0.2349 | −0.2882 | −0.6816 |
| O | −0.5594 | −0.6455 | −0.4923 | 0.3976 | 0.1458 | −0.7921 |
| N | −0.3603 | −0.2322 | −0.1701 | 0.7001 | 0.9851 | −0.3620 |
| S | −0.6498 | −0.5861 | −0.4592 | 0.8422 | 0.9094 | −0.7601 |
| VI | 0.0474 | 0.2009 | 0.1542 | 0.3160 | 0.6837 | 0.1920 |
| IN | 0.2893 | 0.1539 | 0.1050 | −0.6459 | −0.9661 | 0.2728 |
| MI | −0.4757 | −0.3811 | −0.2679 | 0.7581 | 0.9877 | −0.5628 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zeng, Q.; Kang, J.; Cheng, G.; Cheng, J.; Wang, S. A Comparative Investigation of the Adsorption Characteristics of CO2, O2 and N2 in Different Ranks of Coal. Sustainability 2023, 15, 8075. https://doi.org/10.3390/su15108075
Li H, Zeng Q, Kang J, Cheng G, Cheng J, Wang S. A Comparative Investigation of the Adsorption Characteristics of CO2, O2 and N2 in Different Ranks of Coal. Sustainability. 2023; 15(10):8075. https://doi.org/10.3390/su15108075
Chicago/Turabian StyleLi, Haijian, Qiang Zeng, Jianhong Kang, Gang Cheng, Jianwei Cheng, and Shengcheng Wang. 2023. "A Comparative Investigation of the Adsorption Characteristics of CO2, O2 and N2 in Different Ranks of Coal" Sustainability 15, no. 10: 8075. https://doi.org/10.3390/su15108075
APA StyleLi, H., Zeng, Q., Kang, J., Cheng, G., Cheng, J., & Wang, S. (2023). A Comparative Investigation of the Adsorption Characteristics of CO2, O2 and N2 in Different Ranks of Coal. Sustainability, 15(10), 8075. https://doi.org/10.3390/su15108075









