Comparative Evaluation of Microbially-Produced Biostimulants on Peanut Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
- CL (patent number: CN108893435A) mainly contains 1–10% Pantoea alhagi culture (1 × 109–9 × 1010 CFU/mL), 0.1–10% polyglutamic acid, 50–70% organic matter. The Pantoea alhagi was grown in LB liquid medium for 40 h under 30 °C (>1 × 1010 CFU/mL). The polyglutamic acid was produced by Bacillus Subtilis according to patent CN202010945386 and CN107881124A. The organic matter was a mixture of fulvic acid, sugars, and yeast extracts.
- P1 (patent number: CN111903702A) mainly contains 0.02–5% chito-oligosaccharide and 0.003–0.5% brassinolide. The Pantoea alhagi was grown in LB liquid medium for 12 h under 37 °C, and then the Pantoea alhagi culture was transferred into the chito-oligosaccharide medium (35 g/L sucrose, 5.5 g/L peptone, 1 g/L MgSO4, and 2 g/L K2PO4; pH 6.5). After 700 rpm, 24 h growth, the fermentation broth was heated to 121 °C for 20 min in an oil bath. Then, the chito-oligosaccharide supernatant was collected through a plate and frame filter. After distillation and rotary evaporation in 3-times volume ethanol, the chito-oligosaccharide precipitation was collected by centrifugation. P1 were the mixtures of 0.02–5% chito-oligosaccharide, 0.003–0.5% brassinolide, 6% lignosulfonate, 1.5% tween-60, 0.6% benzoic acid, 0.6% organic silicone, and 0.4% urea.
- Both T4 (CN109824430A) and T6 (CN113412247A) are mixtures of P1 and polyglutamic acid. Although the proportions are different, the components of T4 and T6 are in the same range as a result of the flexible components in P1. According to the information of related patents, T4 and T6 mainly contain 5–35 g/L polyglutamic acid, 8–15 g/L chito-oligosaccharide, and 80–120 g/L organic matter. The polyglutamic acid was produced by Bacillus Subtilis (patent number: CN107881124A). The chito-oligosaccharide was made according to patent CN108865951B.
2.2. Phenotypic Investigation
2.3. Determination of Nitrogen Concentration of Each Tissue
2.4. Statistical Analysis
3. Result
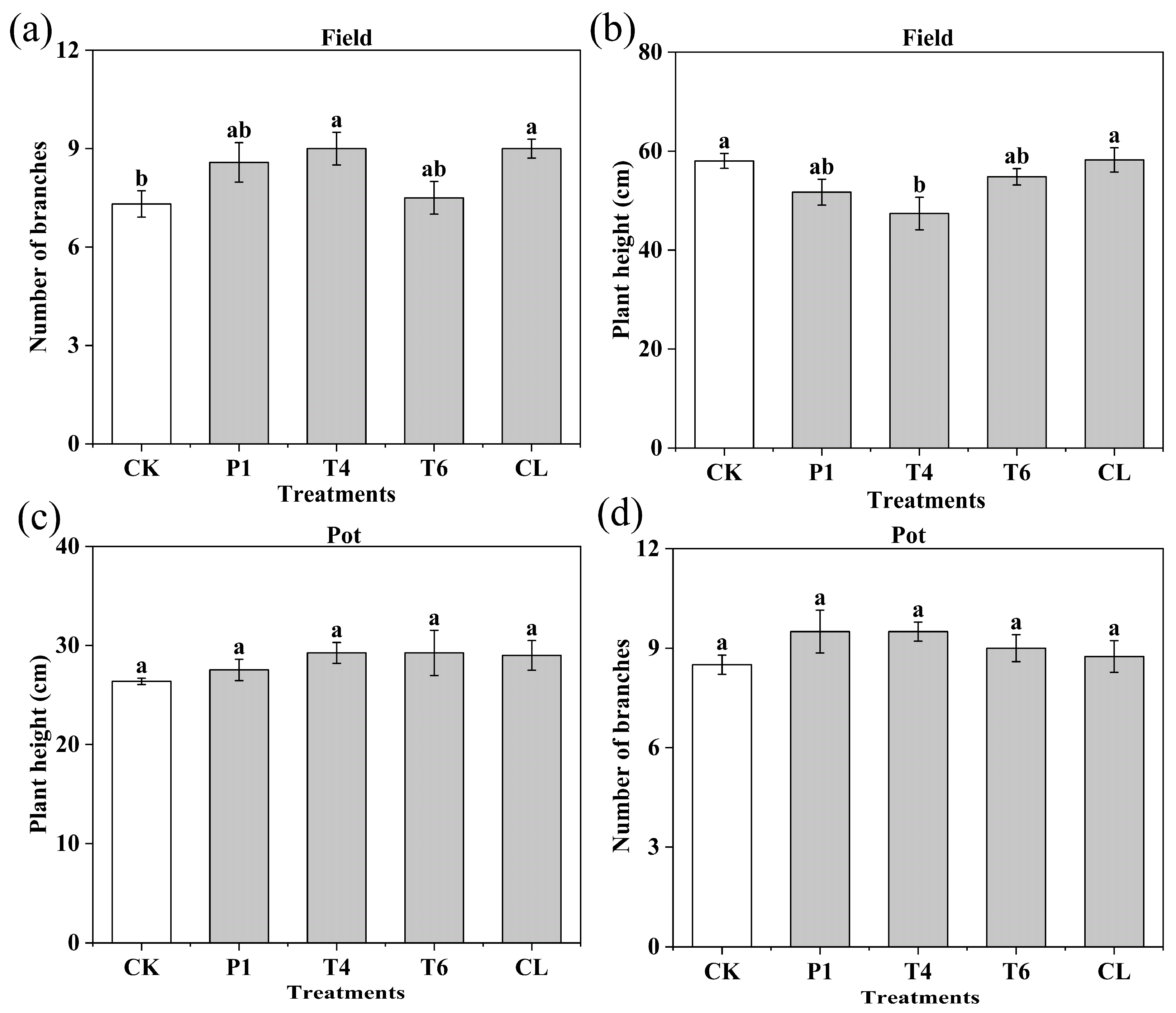
3.1. Effect of Different Microbial Additives on the Phenotype of Peanut Plants
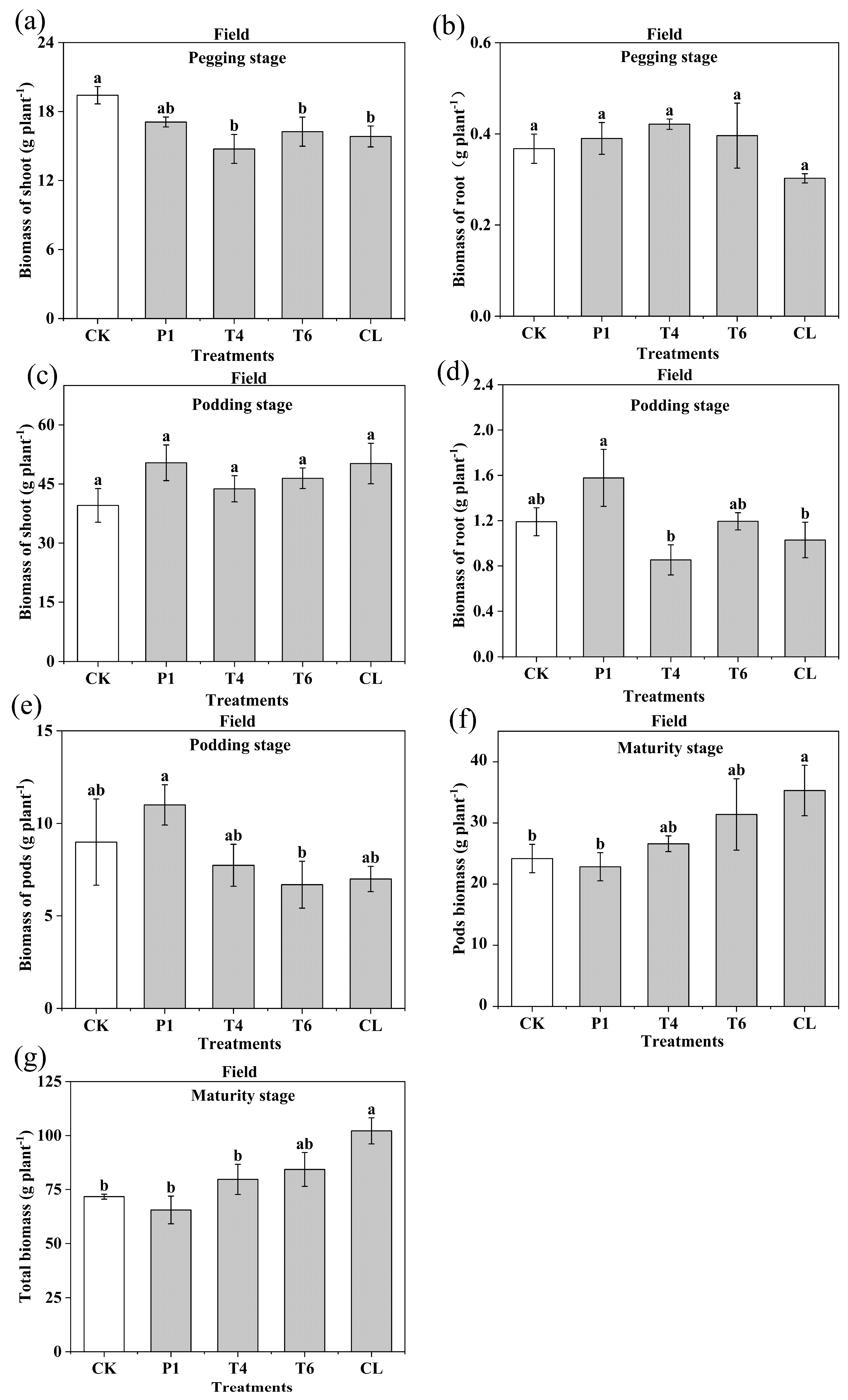
3.2. Effect of Different Fertilizer Additives on Plant Biomass and Yield of Peanut
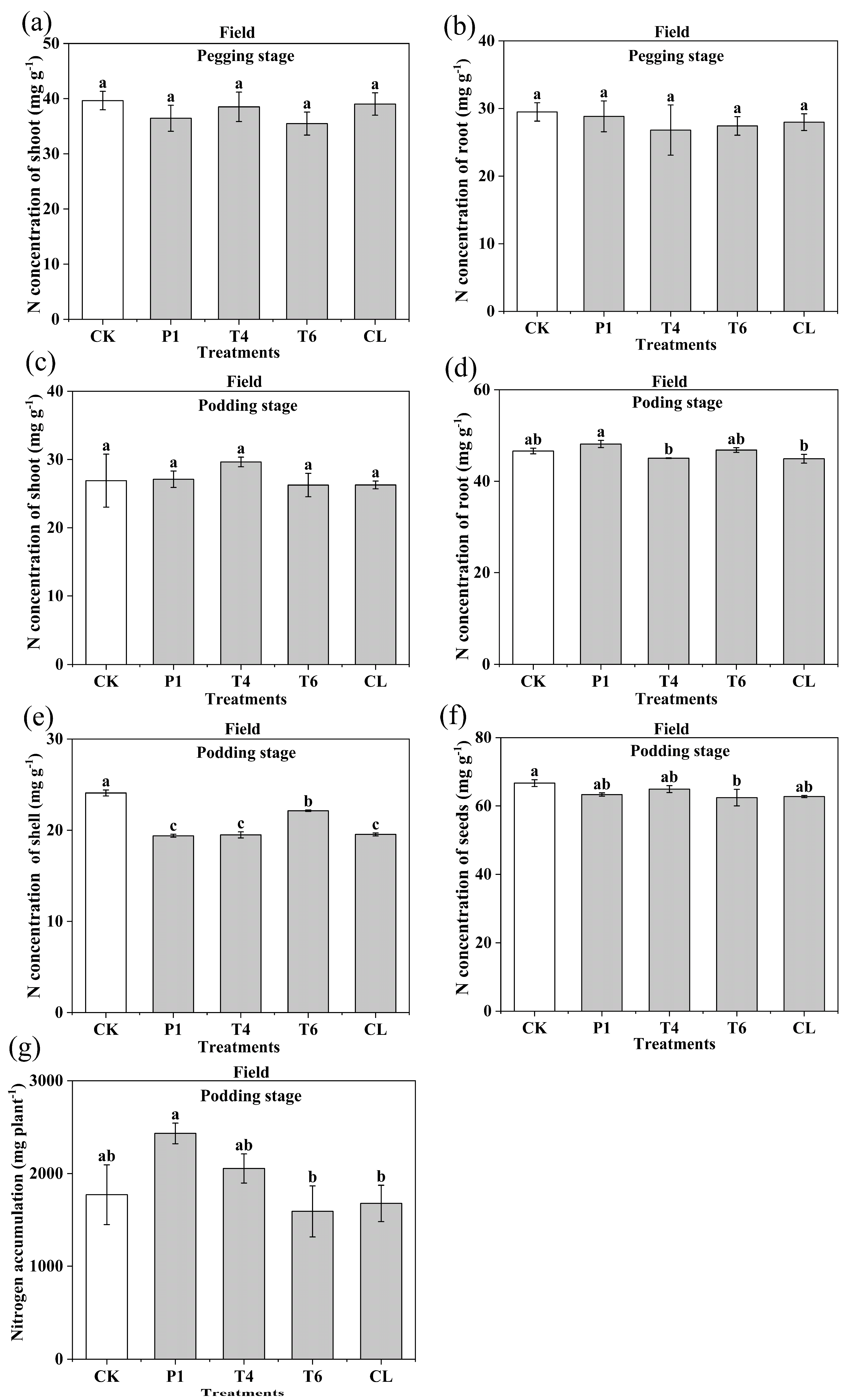
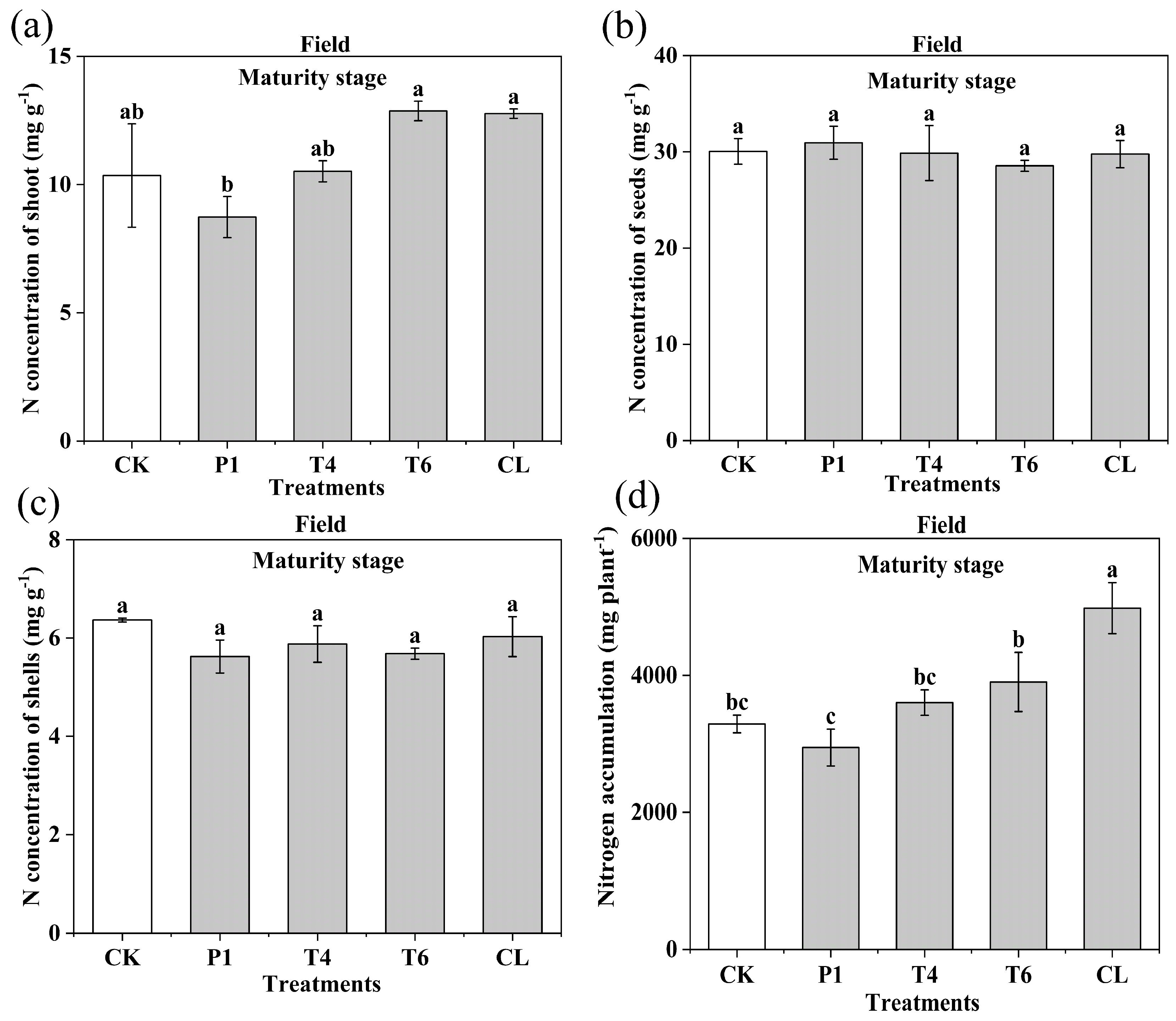
3.3. Effect of Different Fertilizer Additives on the Nitrogen Concentration of Peanut Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Food and Agriculture Organization Statistics; FAO: Rome, Italy, 2022. [Google Scholar]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Action Plan for Zero Growth in Fertilizer Use by 2025. 2022; p. 07B100307202200999. Available online: www.moa.gov.cn/govpublic/ZZYGLS/202212/t20221201_6416398 (accessed on 22 March 2023).
- Fu, X.; Zheng, Z.; Sha, Z.; Cao, H.; Yuan, Q.; Yu, H.; Li, Q. Biorefining waste into nanobiotechnologies can revolutionize sustainable agriculture. Trends Biotechnol. 2022, 40, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lian, F.; Han, Y.; Bao, Q.-l.; Wang, Z.; Xing, B. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: Implications for agronomic benefits and potential risk. Sci. Total Environ. 2019, 656, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and nanofertilizers for sustainable agriculture: Phycoprospects and challenges. Sci. Total Environ. 2021, 803, 149990. [Google Scholar] [CrossRef] [PubMed]
- Mitter, E.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking Crop Nutrition in Times of Modern Microbiology: Innovative Biofertilizer Technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Stamenković, S.O.; Beškoski, V.P.; Karabegović, I.T.; Lazić, M.L.; Nikolić, N.Č. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, 21. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.M.; Diaz, P.A.E.; Lobo, L.L.B.; Rigobelo, E.C. Use of Plant Growth-Promoting Rhizobacteria in Maize and Sugarcane: Characteristics and Applications. Front. Sustain. Food Syst. 2020, 4, 136. [Google Scholar] [CrossRef]
- Kumar, M.; Giri, V.P.; SP, P.; Gupta, A.; Patel, M.K.; Bajpai, A.B.; Jenkins, S.N.; Siddique, K.H.M. Plant-Growth-Promoting Rhizobacteria Emerging as an Effective Bioinoculant to Improve the Growth, Production, and Stress Tolerance of Vegetable Crops. Int. J. Mol. Sci. 2021, 22, 12245. [Google Scholar] [CrossRef]
- Koryagin, Y.; Kulikova, E.V.; Koryagina, N.; Sharunov, O. Biotechnology of biological bacterial preparations used in resource-saving farming. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 613. [Google Scholar]
- Vassilev, N.; Vassileva, M.; Martos, V.; García del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Horiike, T.; Hamada, K.; Kanaya, S.; Shinozawa, T. Origin of eukaryotic cell nuclei by symbiosis of Archaea in Bacteria is revealed by homology-hit analysis. Nat. Cell Biol. 2001, 3, 210–214. [Google Scholar] [CrossRef]
- Lind, A.E.; Lewis, W.H.; Spang, A.; Guy, L.; Embley, T.M.; Ettema, T.J.G. Genomes of two archaeal endosymbionts show convergent adaptations to an intracellular lifestyle. ISME J. 2018, 12, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Hug, J.J.; Krug, D.; Müller, R. Bacteria as genetically programmable producers of bioactive natural products. Nat. Rev. Chem. 2020, 4, 172–193. [Google Scholar] [CrossRef] [PubMed]
- El-Baroty, G.S.; Moussa, M.Y.; Shallan, M.A.; Ali, M.A.; Sabh, A.Z.; Shalaby, E.A. Contribution to the Aroma, Biological Activities, Minerals, Protein, Pigments and Lipid Contents of the Red Alga: Asparagopsis taxiformis (Delile) Trevisan. J. Appl. Sci. Res. 2007, 3, 1825–1834. [Google Scholar]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lei, P.; Wang, Q.; Ma, J.; Zhan, Y.; Jiang, K.; Xu, Z.; Xu, H. The Endophyte Pantoea alhagi NX-11 Alleviates Salt Stress Damage to Rice Seedlings by Secreting Exopolysaccharides. Front. Microbiol. 2020, 10, 3112. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Wang, R.; Li, S.; Qiu, Y.; Lei, P.; Gao, J.; Xu, H.; Zhang, F.; Lv, Y. Effects of exopolysaccharide derived from Pantoea alhagi NX-11 on drought resistance of rice and its efficient fermentation preparation. Int. J. Biol. Macromol. 2020, 162, 946–955. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, L.; Ma, Y.; Lei, P.; Wang, R.; Gu, Y.; Li, S.; Zhang, F.; Xu, H. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int. J. Biol. Macromol. 2022, 209, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Bengyella, L.; Iftikhar, S.; Nawaz, K.; Fonmboh, D.J.; Yekwa, E.L.; Jones, R.C.; Njanu, Y.M.T.; Roy, P. Biotechnological application of endophytic filamentous bipolaris and curvularia: A review on bioeconomy impact. World J. Microbiol. Biotechnol. 2019, 35, 69. [Google Scholar] [CrossRef]
- Quezada, J.C.; Lenssen, A.W.; Moore, K.J.; Sawyer, J.E.; Summer, P. Amino acid biosynthesis byproducts are a suitable source of nitrogen for corn production. Field Crops Res. 2015, 184, 123–132. [Google Scholar] [CrossRef]
- Larskaya, I.A.; Gorshkova, T.A. Plant oligosaccharides—Outsiders among elicitors? Biochemistry 2015, 80, 881–900. [Google Scholar] [CrossRef]
- Li, J.; Li, C. Effect of EM compost on development and yield of peanut. J. Peanut Sci. 2014, 43, 52–55. [Google Scholar] [CrossRef]
- Arnold, J.A.; Beasley, J.P.; Harris, G.H.; Grey, T.L.; Cabrera, M.L. Effect of Gypsum Application Rate, Soil Type, and Soil Calcium on Yield, Grade and Seed Quality of Runner Type Peanut Cultivars. Peanut Sci. 2017, 44, 13–18. [Google Scholar]
- LI, F.; Zhang, T.; Wang, M.; Si, T.; Wang, Y.; Zhang, X.; Yu, X.; Zhang, H.; Zhou, X.-x. Effect of Chemical Fertilizer Reduction Combined with Microbial Fertilizer on Peanut Senescence Characteristics, Dry Matter Accumulation and Fertilizer Utilization Efficiency. J. Peanut Sci. 2022, 51, 9–16. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, H.; Zhang, W. Effects of five biofertilizers on peanut growth and development. In Proceedings of the Third National Green Fertilizer New Technology and New Products Exchange Meeting, Guangzhou, China, 10 December 2003; p. 5. [Google Scholar]
- Zhang, Y.; Song, X.; Wei, T.; Wang, H. Effect of seaweed fertilizer on growth and development of peanut and soil physicochemical properties. South China Agric. 2021, 15, 4–6. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Feng, X.-h.; Xu, X.; Liang, J.; Chi, B.; Xu, H. Calcium involved in the poly(γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol. Biochem. PPB 2014, 80, 144–152. [Google Scholar] [CrossRef]
- Quan, J.; Zheng, W.-w.; Tan, J.-F.; Li, Z.; Wu, M.-H.; Hong, S.-B.; Zhao, Y.; Zhu, Z.; Zang, Y. Glutamic Acid and Poly-γ-glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling. Int. J. Mol. Sci. 2022, 23, 11671. [Google Scholar] [PubMed]
- Xu, Z.; Lei, P.; Pang, X.; Li, H.; Feng, X.-h.; Xu, H. Exogenous application of poly-γ-glutamic acid enhances stress defense in Brassica napus L. seedlings by inducing cross-talks between Ca2+, H2O2, brassinolide, and jasmonic acid in leaves. Plant Physiol. Biochem. PPB 2017, 118, 460–470. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.M.; Gao, D.; Wang, L.; Li, J.; Wei, Z.; Shi, Y. Effects of poly-γ-glutamic acid (γ-PGA) on plant growth and its distribution in a controlled plant-soil system. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Catford, J.G.; Staehelin, C.; Lerat, S.; Piché, Y.; Vierheilig, H. Suppression of arbuscular mycorrhizal colonization and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with Nod factors. J. Exp. Bot. 2003, 54, 1481–1487. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Souleimanov, A.; Zhou, X.G.; Smith, D.L. Differential response of soybean (Glycine max (L.) Merr.) genotypes to lipo-chito-oligosaccharide Nod Bj V (C(18:1) MeFuc). J. Exp. Bot. 2000, 51, 2045–2051. [Google Scholar] [CrossRef]
- Volpe, V.; Carotenuto, G.; Berzero, C.; Cagnina, L.; Puech-Pagès, V.; Genre, A. Short chain chito-oligosaccharides promote arbuscular mycorrhizal colonization in Medicago truncatula. Carbohydr. Polym. 2020, 229, 115505. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Hundred Pods Weight (g) | Hundred Seeds Weight (g) | Kernel Yield (%) | Pod Fullness | Yield (kg acres−1) | Full Fruit Weight Rate (%) | Full Kernel Weight Rate (%) |
|---|---|---|---|---|---|---|---|
| CK | 145.90 b | 59.40 a | 64.29 a | 0.43 a | 156.16 c | 63.54 b | 67.47 a |
| P1 | 151.85 ab | 59.35 a | 65.48 a | 0.48 a | 165.55 abc | 68.7 ab | 72.58 a |
| T4 | 154.45 ab | 61.25 a | 67.04 a | 0.47 a | 196.62 bc | 67.64 ab | 70.55 a |
| T6 | 167.80 a | 61.50 a | 67.32 a | 0.48 a | 211.92 a | 72.03 a | 70.82 a |
| CL | 155.10 ab | 63.55 a | 66.88 a | 0.46 a | 200.20 ab | 71.47 a | 69.29 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Dai, J.; Li, N.; Zhao, H.; Chang, H.; Liao, X.; Sheng, F.; Qin, L. Comparative Evaluation of Microbially-Produced Biostimulants on Peanut Growth. Sustainability 2023, 15, 8025. https://doi.org/10.3390/su15108025
Zheng W, Dai J, Li N, Zhao H, Chang H, Liao X, Sheng F, Qin L. Comparative Evaluation of Microbially-Produced Biostimulants on Peanut Growth. Sustainability. 2023; 15(10):8025. https://doi.org/10.3390/su15108025
Chicago/Turabian StyleZheng, Wuyong, Jing Dai, Ning Li, Hongtao Zhao, Haibin Chang, Xing Liao, Feng Sheng, and Lu Qin. 2023. "Comparative Evaluation of Microbially-Produced Biostimulants on Peanut Growth" Sustainability 15, no. 10: 8025. https://doi.org/10.3390/su15108025
APA StyleZheng, W., Dai, J., Li, N., Zhao, H., Chang, H., Liao, X., Sheng, F., & Qin, L. (2023). Comparative Evaluation of Microbially-Produced Biostimulants on Peanut Growth. Sustainability, 15(10), 8025. https://doi.org/10.3390/su15108025





