Abstract
Rare earth elements, particularly middle and heavy rare earth, are among the most valuable resources in the pursuit of a greener economy. The production of middle and heavy rare earth elements heavily relies on ion adsorption, which constitutes over 80% of global output and is centered in southern China. Unfortunately, the extensive mining activities have led to severe environmental pollution, resource depletion, and risks to human health. In contrast, biochar application offers a cost-effective and efficient phytoremediation solution. However, existing literature on the biochar application in IAT-Res mine tailings is limited. In this paper, we conducted a literature review and summarized the contaminations in the ion adsorption mine tailings, as well as explored the potential of using biochar to remediate contaminations. We aim to raise interest and encourage further research on utilizing biochar for pollution remediation in ion adsorption rare earth mine tailings. By effectively managing contamination, this approach can contribute to the sustainable supply of ion adsorption rare earth elements while ensuring their long-term viability.
1. Introduction
1.1. Background
Rare Earth Elements (REE) have received considerable interest due to their vital applications in various areas, including agriculture, electronics, green energy, the national defense industry, and precision equipment [1,2]. They are defined by the International Union of Pure and Applied Chemistry as a group of 17 elements that naturally occur in earth-parent materials, including scandium, yttrium, and the lanthanide series [3]. Although REE are present in more than 250 minerals (silicates, carbonates, oxides, and phosphates), their distribution is unpredictable and scarce, which makes extraction difficult as they are not found in concentrated mineral structures [4,5,6]. The total crustal abundance of REE is 169 mg/kg, with 137.8 mg/kg of light rare earth elements (LREE: La to Gd) and 31.34 mg/kg of heavy rare earth elements (HREE: Tb to Lu, plus Sc and Y), where LREE are 4.39 times higher than HREE [7]. Mid-heavy REE (including Gd) are considered critical resources, and nearly 80% of the world’s supplies are from ion-adsorption type rare-earth (IAT-Res) ores in South China [8,9,10].
IAT-Res deposit is a unique REE resource. REE-oxide are trivalent cations that REE are adsorbed onto clay minerals during weathering [11,12]. In the past few decades, unregulated and intensive extraction for REE in the IAT-Res ore consequence ecological pollutant has been pronounced in these areas [10,13]. Because of the uncertain bioaccumulation mechanisms, there have been only a few successful plant restoration occurrences on IAT-Res mine tailings so far [7]. The crucial lack of HREE resources, however, makes contamination control and IAT-Res recovery from contaminations increasingly crucial. This paper aims to provide an overview of the IAT-Res mining-related contaminations and look into a potential contamination remediation strategy by biochar application.
1.2. Ion-Adsorption Type Rare Earths (IAT-Res)
REE-containing minerals are classified into two main types: mineral type REE (MT-Res) ores and ion adsorption type REE (IAT-Res) ores, with either HREE or LREE dominating [14,15]. The world reserves of different types of REE are shown in Figure 1. The MT-Res ores are rich in LREE and are primarily composed of bastnaesite and monazite, whereas the IAT-Res ores which are weathering profiles developed on protoliths, are primarily composed of middle-heavy REE [1,8,16]. Clay minerals such as kaolinite, halloysite, illite, and montmorillonite are the primary components (40–70%) of the weathering crust throughout the weathering process [12,16]. Meanwhile, REE trivalent cations can bind to clay minerals to form REE-oxide (hydrated or hydroxyl-hydrated ions) [8,14,17]. In this circumstance, an IAT-Res deposit develops.
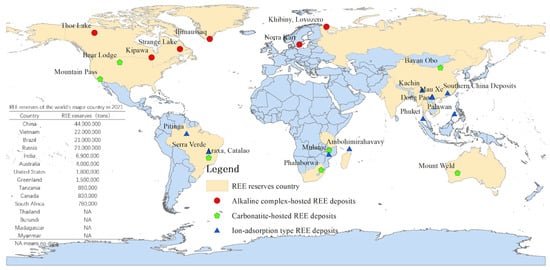
Figure 1.
REE types and distributions in the world (data obtained from Borst et al. [8] and USGS [18]).
The world’s most important source of HREE are IAT-Res deposits, which are primarily found in the southern Chinese provinces, such as Fujian, Guangdong, Guangxi, Hunan, and Jiangxi [8,19,20]. Since the discovery of IAT-Res ores in Jiangxi, China, in 1969, a series of hydrometallurgy methods, including barrel/pool leaching, heap leaching, and in-situ leaching, have been developed by scientists and engineers [16,21]. Pool leaching is similar to leap leaching. They both require complete stripping of the subsoil in the mining area, causing physical problems with the territory’s geography, such as landslides and loss of vegetation cover, soil erosion, crop production damage, and biodiversity loss [22]. Over the last few decades of exploration, mining for IAT-Res has caused many environmental problems in these areas. Since 2011, China has restricted REE exports because of environmental protection concerns [23]. The exploration of IAT-Res resources has expanded to Brazil, Laos, Thailand, Indonesia, Madagascar, the Philippines, and the United States [18,24,25]. Thus, environmental management in southern China will be a critical example in the developing of IAT-Res mining.
1.3. Usages and Demands
The application of REE takes place in different sectors, such as agriculture and modern technologies. Global REE demand has spiked in recent years and is expected to increase from 167,000 tons in 2020 to 280,000 tons in 2030 [18,26,27]. In agriculture, REE are utilized as micro-fertilizers to promote the growth and yield of plants and livestock [5,28]. Since they are used in so many modern conveniences (including lighter flints, fluorescent lights, batteries, lasers, and permanent magnets), REE are now considered to be strategic components [15,23,29]. REE are applied in relative amounts, yet they are critical components of devices in green economics, high-precision medical equipment, and defense industries [15]. Most REE is used in magnetic field generators, which are in turn used in a broad variety of low-carbon technologies (such as wind turbines and electric and hybrid automobiles) [30,31]. Figure 2 shows a breakdown of their holdings.
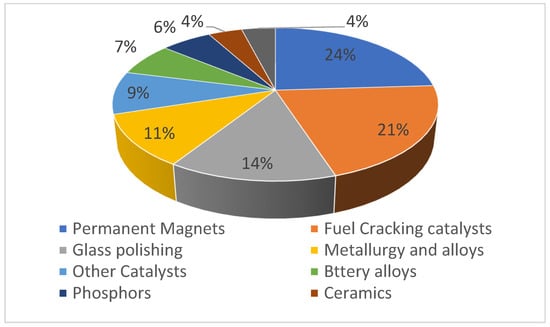
Figure 2.
Share of REE application in different sectors (data obtained from Asadollahzadeh et al. (2021) [30]).
2. Contaminations in IAT-Res Tailings
In contrast to the fact that REE, especially HREE, are critical resources in many countries, aggressive mining and manufacturing are causing severe environmental contamination and overlaid REE are considered as hazards in these areas [31,32]. Compared to the open-pit mining technologies for bastnaesite (MT-Res), fewer environmental impactions occurred from manufacturing IAT-Res [31,33].
The contamination in the IAT-Res mining areas can be categorized into soil physicochemical properties, ammonium pollution, and REE pollution. Pool and heap leaching methods have been abandoned for IAT-Res mining since 2008, leading to the widespread adoption of in-situ leaching, which does not require the removal of surface vegetation [9,21]. However, contaminations left behind from the early production of IAT-Res by pool or heap leaching are still apparent, trees do not grow at these tailings [22,30]. In-situ leaching, which is considered an eco-friendly method requires a higher amount of ammonium leachate during processing. As the extraction of IAT-Res follows the mechanism, [34,35], the ammonium-containing leakage could further lead to greater terrestrial and aquatic eco-toxicity ratings [21,22,30]. Nevertheless, the toxicity of discharged REE to the environment and humans is usually underestimated because it is commonly evaluated as a group, but their toxicity coefficients can be higher than heavy metals, such as Lu, Eu, and Tb are higher than Pb and Cu [36,37,38].
2.1. Vegetation Loss and Soil Degeneration
IAT-Res only has 0.05–0.3 wt% REO but extracting REE from IAT-Res is simple and inexpensive [14,17,21]. To produce one ton of REO using pool or heap leaching, one needs to strip off 150–200 m2 of vegetation and 1500–2000 m3 of topsoil, leading to 150–200 m2 and 1700 t of tailings [39]. In recent decades, intensive exploration of IAT-Res resources has resulted in more than 100 km2 of REE mine tailings in Southern China [10,17]. Because of the serious environmental consequences of unregulated small-scale extraction for IAT-Res resources, China is stepping up efforts to limit illegal mining and conduct artificial vegetation restoration in mine tailings [23,40,41,42].
As IAT-Res are scattered across the distribution, surface mining has become the predominant method. This has changed how land is used and caused soil degradation in Southern China. Further contamination caused irreparable harm to the ecosystem, including soil erosion, air pollution, biodiversity loss, and human health issues [17,38]. Moreover, red soil predominates in these regions, making natural restoration after extensive vegetation loss problematic. For example, Figure 3 shows a satellite image of IAT-Res mine regions and mine tailings where no grass grows even after many years of rehabilitation.
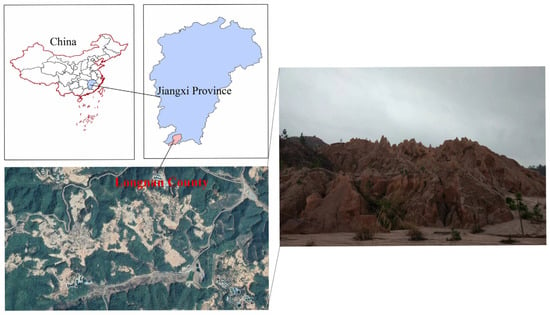
Figure 3.
IAT-Res mine tailing in Southern China.
2.2. Ammonium Contamination
In-situ leaching has replaced pool leaching and heap leaching in IAT-Res mining since 2008, as it does not result in severe soil deterioration, such as landslides and soil erosion, nor does it leave behind a barren wasteland [43,44]. Nevertheless, in-situ leaching requires a substantial quantity of the leaching reagent ammonium sulfate [], which, after leaching, enters the soil and groundwater [10,45]. Around seven to ten tons of ammonium sulfate are required to manufacture one ton of REO; hence, the excessive amount of ammonium sulfate at a mining site leads to changes in the surface soil and groundwater characteristics [17,43,46]. In the IAT-Res mine tailing soil profiles, the ammonia nitrogen concentration ranged from 49.8 to 77.7 mg/kg, whereas it was between 5 and 6 mg/kg in the original mine soil [47]. According to the Pollution Discharge Standards for the Rare Earth Industry, the limits for water or air in the form of ammonia pollution should not be higher than 25 mg/L [31,48]. However, after in-situ leaching, the wastewater in the IAT-Res mine tailings was found to contain ammonia-nitrogen up to 3000–4000 mg/L [10]. Ammonium pollution extends to nearby habitats; Hao et al. (2016) [49] evaluated different surface and shallow aquatic systems from the REE mining area in southern Jiangxi Province. They stated that the concentration of paddy field water (1.28 mg/L), pond water (4.53 mg/L), and stream water (8.31 mg/L) all exceeded the standard (0.02 mg/L). In addition, the leachate produces a large amount of ions and lead to soil acidification [50]. In conclusion, leaching solutions containing ammonium sulfate not only lead to severe ammonia-nitrogen pollution and soil acidification, but they also contain low levels of REE and flow off into rivers when it rains, resulting in incalculable REE loss and contamination.
2.3. REE Contamination
REE enter the environment through various pathways, including mining, atmospheric deposition, and end-life product disposal [5]. In the IAT-Res resources reserved surroundings, mining is the majority means of exceeding REE in the ecological environment and is considered an emerging contaminant. Mining for the IAT-Res in Southern China has left a large area of tailings that contain 409–1035 mg/kg of REE [51,52]. In the majority of these sites, the REE level exceeds the mining-grade threshold (500 mg/kg) [53]. For instance, the total REE level of 976.94 mg/kg found by Jin et al. (2019) [2] in a mine tailing in China’s Jiangxi province, is 4.53 times and 5.09 times greater than the soil in Jiangxi province and the entire country, respectively. Liu et al. (2019) [52] also found the total REE in a mine tailing soil (392 mg/kg) in Jiangxi province was two times higher than the control sites (192 mg/kg). Similarly, soil samples from 118 locations around Fujian province were analyzed for their Rare Earth Element (REE) composition by Chen et al. (2019) [54]. With an average of 255.34 mg/kg, the total REE content was significantly higher than the Fujian province background value of 186.76 mg/kg and the HREE (77.07 mg/kg) was two times higher than the background value. This also agreed with the study of Li et al. (2013) [38] in Hetian County, Fujian; the soil REE (242.92 mg/kg) was higher than the background soil (135.85 mg/kg). Studies that claim soil REE contamination in IAT-Res mining tailings are listed in Table 1. Nevertheless, the content of REE in the soil is also determined by the soil pH, sorption capacity, and salt content and studies focusing on REE contaminations are spare, thus more systematic studies and comprehensive information still need to be conducted.

Table 1.
REE content in the soil of IAT-Res mining tailings (mg/kg).
Despite the soil contaminations, overloaded REE also spread to the surrounding water system. The concentration of REE in rivers in the mining area is 55.72 mg/L, which is 8974.7 times higher than the contrast area [2]. Studies by Liu et al. (2019) [52] and Hao et al. (2016) [49] also found that the total REE concentrations in the stream water (4.46 mg/L and 1.596 mg/L), pond water (0.069 mg/L), paddy field water (0.031 mg/L), spring water (0.035 mg/L), and well water (0.03 mg/L) all exceeded the recommended contents (0.02 mg/L). Although it is applied in agriculture as a micronutrient, studies show that REEs also cause adverse effects in plants and animals [55,56,57]. Furthermore, Li et al. (2013) [38] documented higher REE in human blood and hair in the mine tailing surrounding areas than in control sites. To date, the determination of REE in the environment is limited because more sensitive analytical techniques allow a quantitative assessment of the trace concentrations in various biological materials [4].
2.4. Current Problems
The shortage of REE is a global problem. They are considered the most critical raw materials group with the highest supply risk by the European Commission, the US Department of Energy and the British Geological Survey [18,58,59]. Although the only REE element estimated to have a resource life of fewer than 1000 years is Eu, which has a resource life of about 600 years [15], the dramatic growing consumption of particular REE still marks the importance of the recovery potential.
On the other hand, the extensive mining for IAT-Res has led to severe environmental contamination and toxic effects on surrounding habits. REE mine tailings contain high concentrations of REE even after years of abandonment, and these REE are highly exchangeable (>20% of total REE) [10,52,54]. The contradiction between limited resources and resource demand growth has shifted governments’ awareness of the importance of resource conservation. A new concept has been promoted: from waste to valuable resources. However, in contrast to the emerging studies focusing on REE pollution and biochar as promising adsorbents for toxic contamination, the information on biochar application in IAT-Res tailings remains sparse.
3. Biochar
3.1. What Is Biochar
Biochar is made when biomass, such as wood, grass, dairy manure, broiler litter, and crop residues are processed by pyrolysis [60,61,62,63]. Pyrolysis is the thermal (usually not over 700 °C) decomposition of biomass in the partial or complete absence of oxygen [64]. Producing biochar requires less energy and cost than active carbon generation since biochar is generally obtained at lower temperatures and without additional activation processing [65,66,67]. It consists primarily of cellulose, hemicellulose and lignin [64]. Because of its adaptable physicochemical properties, high adsorption capacity, and chemical stability, biochar is a low-cost carbonaceous material that has been widely used in environmental applications, either to neutralize greenhouse gas emissions or as a replacement for fossil carbon carriers [64,68,69]. When investigating the removal of a given component through the adsorption process, the selection of adsorbent material is a crucial step [24]. Trace element adsorption is significantly affected by biochar’s porous structure and the presence of functional groups [70,71]. The reaction states of biochar in soil include dissolution (1–3 weeks), reactive surface development (1–6 months), and aging (beyond 6 months) [72].
In the first state, the major influence factors are the selection of adsorbent materials and the soil conditions [24,71]. After application to soil, biochar dissolves soluble organic and mineral compounds by pores, increasing dissolved organic carbon, cations, and anions in the soil solution, increasing electrical conductivity and pH and reducing redox potential [72,73]. After the rapid dissolution, biochar interacts with plant roots and microorganisms. In this state, the porous structure of biochar and surface functional groups (e.g., carboxyl, carbonyl, hydroxyl) absorb trace elements, which results in trace element precipitation [65,70,71]. In the long-term reaction stage, biochar aging in the soil, along with cultivation and soil fauna, causes further fragmentation of biochar particles and the oxidation of biochar surfaces exposed by micro agglomerate detachment [72,73].
3.2. Environment Effects
Applying biochar in trace element remediation has been proposed as a novel approach to sustainable environmental development. Evidence shows biochar is effective in adsorbing organic pollutants from wastewater [74], improves soil fertility, reduces soil bulk density, increases crop production [13,62], is a long-term sink for atmospheric CO2 in terrestrial ecosystems [61,75], and provides a habitat for microorganisms [13,76]. Biochar possesses the necessary qualities of permeable porosity and pore size in wastewater treatment [69]. When applied as a soil amendment, it is expected to function as long-term carbon storage while enhancing soil characteristics [68,76,77]. More examples of biochar are used in conjunction with traditional phytoremediation procedures to stabilize trace elements in soil and reduce phytotoxicity, according to studies by Paz-Ferreiro et al. (2012) [78], Sarwar et al. (2020) [79], and Wei et al. (2019) [80].
3.2.1. Improve Soil Chemical Properties
Applying biochar increases soil phytochemical properties, such as pH, electrical conductivity, and organic matter, further influences REE remediation [81]. Biochar application increases soil acidity in the short term by releasing acidifying chemicals via chemical and microbiological processes but raises soil pH in the long term as the carbonates and hydroxides contained in biochar degrade [72,77,82]. A low pH is typical of the red soil found in southern China. Heavy mining for REE has caused the soil in IAT-Res mining wastelands to have a loose texture, poor aggregation, low water-holding capacity and fertility, and less microbial variety; biochar, on the other hand, with vast surface area and large functional groups (e.g., carboxyls and phenolic hydroxyls) can improve soil cation exchange capacity, water-holding capacity, and keep fertilizer from washing away [13].
In addition to leachate absorption, biochar also absorbs organic matter and nutrients, resulting in increased concentrations of water-extractable organic carbon, total soluble nitrogen, plant-available phosphorus, and plant-available potassium, therefore, increasing the nutrient retention capacity of the soil [75,77,83]. In the study of Chen et al. (2018) [84], an additional 2.5–5% of biochar can largely promote plant growth on IAT-Res mine tailings by improving soil physicochemical properties. Moreover, the effect of biochar is a long-term retention, thus it will be promising in decreasing soil acidity and soil amendment.
3.2.2. Buffer Ammonia Contamination
In the nitrogen cycle, nitrate nitrogen ( is the final product of nitrogen compounds in soil [47]. To degrade pollution, nitrification and denitrification are the two important processes; it is first oxidized to and , then reduced to or under anaerobic conditions [47,85]. Biochar can efficiently adsorb ammonia and act as an ammonia buffer in soil [72,78,82].
Biological immobilization of nitrogen occurs in both captured particles in the reaction state and naturally loaded particles during the biochar aging phase [86]. The surfaces of biochar form redox-active layers that physically or chemically bind nitrogen molecules during the medium-term reactions, while at the aging stage, biochar adsorbed nitrogen onto the organo-mineral micro agglomerates [72,86]. Some studies have explored the potential of biochar to adsorb ammonium nitrate ( from IAT-Res tailings [85,87]. Keeping in mind that IAT-Res mining produced a large amount of leachate, applying biochar will potentially decrease ammonia volatilization from the IAT-Res mine tailings.
3.2.3. Remove REE Contamination
Among the several methods (precipitation, filtration, and solvent extraction) to recover REE, adsorption has been recognized as one of the most promising because of its simplicity, high efficiency, and wide availability [29,88,89]. To adsorb REE elements from aqueous solutions, a variety of organic and inorganic adsorbents, including zeolite, silica, graphene nanomaterials, activated carbon (AC) and its modifications, and low-cost materials, have been used [30,32]. Biochar has a relatively structured carbon matrix with a medium-to-large surface area, suggesting that it may act as a surface absorbent before being suspended by bioturbation and hydraulic drift; it may absorb pollutants on a solid matrix, such as sediments [60,90]. Studies prove that applying biochar is effective in adsorbing REE from wastewater [91,92,93]. This is mainly contributed to by the process of ion exchange with elements such as Ca, Mg, K, and Na and the carboxyl, hydroxyl, and related phenolic groups, then REE adsorbed on the surface of biochar [91,92].
Most studies explored the possibility of remediation of total REE elements from the mine tailings, such as Zhang et al. (2020) [50], Jin et al. (2018) [94], Zhao et al. (2021) [95] and Liu et al. (2020) [96]. Some studied the remediation of a single REE element [92,97]. You et al. (2019) [98] evaluated the possibility of remediating from IAT-Res mine tailings. We summarized articles that applied biochar in REE contaminant remediation and listed them in Table 2. Considering REE’s critical role in modern techniques and South China’s weathered IAT-Res ores supply the majority of total world HREE production [9]. Applying biochar in the IAT-Res contamination reduction will lead to its resources’ sustainable development. Since lanthanide ions reside in solutions in the third oxidation state, Kołodyńska et al. (2018) [93] found that the maximum sorption capacity of biochar is at pH = 4 in their study. As mentioned previously, IAT-Res mine tailings in south China are in naturally acidic red soil, which will point to the success of the application of biochar in phytoremediation.

Table 2.
Biochar utilization in REE contaminants remediation.
The REE are cation adsorped via electrostatic bonds on the surface of IAT-Res ores, and the exterior of mineral clays has a permanent negative charge [100]. Therefore, biochar can absorb REE through ion cation attraction. Pourret and Houben (2018) [101] examined REE adsorption onto biochar from pH 3–9 and ionic strength mol/L to mol/L and found that increasing the pH from acidic to neutral values increases the amount of adsorbed REE as well as the relative contribution of carboxy-phenolic and phenolic groups to REE sorption. At low ionic strength and acidic pH, REE are mainly absorbed by carboxylic groups, while at high ionic strength and alkaline pH, REE are primarily absorbed by carboxy-phenolic groups.
As described previously, many REE occur as physically adsorbed species. In most mining methods, they can be recovered by ion exchange leaching. Thus, biochar can remove REE contamination by ion exchange. Biochar’s high sorption is not only the result of surface adsorption but also the precipitation with phosphate due to the high P content of biochar that would react with metals to form stable minerals [102].
Trace element biosorption is a non-metabolically mediated passive process of metal binding by biosorbents [103,104]. It is based on physicochemical interactions, and binding is independent of metabolism and hence a physical process [89,104]. To date, biochar is considered one of the most cost-effective adsorbents and has been widely used in heavy metal remediation. Since REE and heavy metals are both trace elements and biochar is more efficient for long-term metal immobilization at high ionic strength conditions [99,101] it is promising to use biochar in IAT-Res remediation. Various types of biochar have been demonstrated to be effective for REE removal [93]. The mechanisms of applying biochar in IAT-Res remediation involve precipitation, ion exchange, and electrostatic attraction [105,106] (as shown in Figure 4). Nevertheless, the interaction processes between biochar and target toxins, as well as the environmental restrictions that govern biochar application, are poorly known. Before biochar may be used to its full potential as a remediation agent, its limiting constraints and properties, along with its soil-environmental interactions, must be better understood.
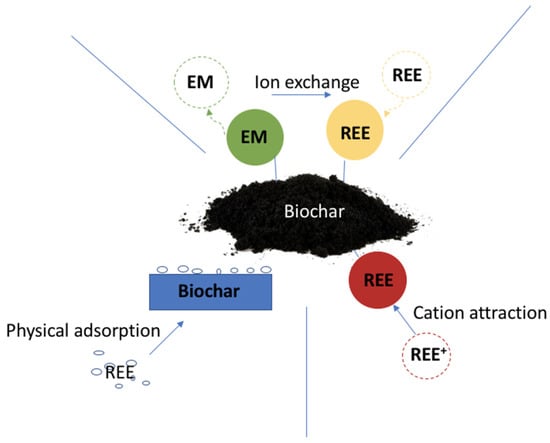
Figure 4.
Postulated mechanisms of biochar interaction with REE. Note: EM—exchangeable metal, REE—rare earth elements, REE+—rare earth elements with positive charge, →—pathways.
3.2.4. Modified Biochar
Since biochar’s ability to immobilize trace elements relies on the number of functional groups at its surface [101]. Adsorbents can be more selective and have a stronger attraction to REE in order to increase the efficiency of biochar applications. Modified biochar is biochar loaded with different elements by impregnation and in situ synthesis methods [89]. Various modified biochar can be prepared by loading them with target elements, impregnating the biochar in a solution containing a modifier, such as Fe-Mn-Ce-modified in a mixed solution with , to create a suitable environment for microbial growth and increase remediation effects [107,108]. In the in-situ synthesis method, the target modification reagent is added directly to the raw material, followed by pyrolysis, chemical precipitation, and activation to obtain the modified biochar [89].
Chemically modified adsorbents have also been explored and shown high adsorption efficiency (greater than 99.5%) [105], i.e., the adsorption capacity of La by modified biochar (363.32 mg/g) was approximately 1.3 times higher than the raw biochar [109]. Thus, modified biochar enhanced trace elements’ retention. In addition to contamination remediation, modified biochar provided other environmental impacts, such as enhancing catalytic capacity [110], changing biochar pore structure and enhancing antibiotics adsorption [111], increasing crop biomass and reducing trace element accumulation in crops [107], and enhancing antibiotics and trace element adsorbent from the aqua system [109,112,113]. However, attempts of using modified biochar in REE mine tailings are very scarce (Table 3).

Table 3.
Modified biochar in REE mine tailing remediation.
3.3. Disadvantages and Questions of Biochar Application
Several factors, such as feedstock, highest treatment temperature (300 °C to over 750 °C), residence time at the highest treatment temperature, and treatments applied before and after pyrolysis, influence biochar properties and effects in contaminant amendment [72,102]. Thus, applying biochar is not without its drawbacks.
Biochar’s ability to absorb elements is not universal, some biochars may have weak remediation effects on contaminated soils [89], which makes the selection of raw biochar material critical. In the study of Yao et al. (2012) [87], for instance, nine out of thirteen (69%) examined biochar did not exhibit nitrate removal ability and even released nitrate into the solution; four out of thirteen (31%) did not show ammonium sorption ability and five (38%) had the ability to remove phosphate from aqueous solutions and the rest released phosphate into the solution [87]. In addition, unlike biochar application for heavy metal retention, which has been intensively investigated [63,113,114,115,116], biochar application in REE remediation is still in its infancy. Few biomass materials are evaluated for REE retention. Moreover, biochar application might have negative effects on the activity of certain soil microbial communities [117,118,119]. As the application of biochar is a long-term reaction, applying large quantities of biochar can lead to a nutrient imbalance in the soil [89], including its priming effect on soil native carbons [106,120]. Therefore, secondary contamination formed by biochar application should be avoided. Lastly, nitrogen (both and ) that captured biochar particles is neither easily extracted by conventional procedures nor readily available to plants under field conditions [86]. This will apply biochar to mitigate from IAT-Res mine tailings in question.
In this paper, we reviewed the mechanisms of plant-biochar-IAT-Res contamination to attract future interest in the IAT-Res conservation of resources and pollution reduction. Consider the naturally acidic soil and fragile environmental conditions in the IAT-Res mine tailings, as well as the overloaded , and REE. To ensure the viability of biochar application in IAT-Res residues remediation, additional research must be conducted in the future.
4. Conclusions
With the rapid development of current technologies, REE demand continues to rise. Urgent ecological restoration of the IAT-Res mining region is required. On the other hand, biochar is promising for improving soil fertility and reclaiming degraded soil. Therefore, we conducted a literature review to assess the potential of using biochar as a method of reducing REE-related pollution mitigating their environmental hazards, and conserving natural resources. Although the current literature is lacking systematic knowledge on the application of biochar in IAT-Res mine tailing remediation, the obtained results from studies indicate that the use of biochar or modified biochar in IAT-Res mine tailing contamination remediation is promising. With this paper, we hope to address the interests of researchers in this field. The incorporation of biochar into the IAT-Res tailing pollution reduction strategies will provide critical theoretical support for determining an economical, efficient, and practical joint remediation approach for promoting the IAT-Res tailings remediation process and, thus, achieving a sustainable supply of IAT-REs.
Author Contributions
H.C. (Haimei Chen): conceptualization, formal analysis and investigation, writing—original draft, writing—review and editing; H.C. (Haibin Chen): conceptualization, methodology, formal analysis and investigation, writing—original draft preparation, writing—review and editing, funding acquisition; L.K.: methodology, supervision; V.S.: methodology, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Project to Guide Social Development of Fujian Province, China (No. 2020N5007), the Education Research for Young and middle-aged Teachers of Fujian Educational Bureau, China (No. JAT220217), and the Minnan Normal University President’s Fund (No. KJ2021022).
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
We are thankful for the support from Minnan Normal University and the Hungarian University of Agriculture and Life Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Jin, S.; Hu, Z.; Man, B.; Pan, H.; Kong, X.; Jin, D. Application of Phosphate-Containing Materials Affects Bioavailability of Rare Earth Elements and Bacterial Community in Soils. Sci. China Technol. Sci. 2019, 62, 1616–1627. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry (IUPAC). Nomenclature of Inorganic Chemistry. Chem. Int. Newsmag. IUPAC 2005, 27. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-415-33340-5. [Google Scholar]
- Hu, Z.; Haneklaus, S.; Sparovek, G.; Schnug, E. Rare Earth Elements in Soils. Commun. Soil Sci. Plant Anal. 2006, 37, 1381–1420. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of Rare Earth Elements in Regolith-Hosted Clay Deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef]
- Chi, R.; Tian, J.; Li, Z.; Peng, C.; Wu, Y.; Li, S.; Wang, C.; Zhou, Z. Existing State and Partitioning of Rare Earth on Weathered Ores. J. Rare Earths 2005, 23, 756. [Google Scholar]
- Liu, J.; Wu, X.; Huang, K.; Liu, W.; Zhao, Z.; Liu, H. Instability Behavior of Bubble Supported Organic Liquid Membrane in Extraction of Low-Concentration Rare Earths from in-Situ Leaching Solutions of Ion-Adsorption Ores. Miner. Eng. 2020, 159, 106645. [Google Scholar] [CrossRef]
- Ou, X.; Chen, Z.; Chen, X.; Li, X.; Wang, J.; Ren, T.; Chen, H.; Feng, L.; Wang, Y.; Chen, Z.; et al. Redistribution and Chemical Speciation of Rare Earth Elements in an Ion–Adsorption Rare Earth Tailing, Southern China. Sci. Total Environ. 2022, 821, 153369. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, L.; Wang, D.; Hou, X.; Sun, Y.; Zhou, X.; Zhou, X.; Li, Y. Evaluating the Fractionation of Ion-Adsorption Rare Earths for in-Situ Leaching and Metallogenic Mechanism. J. Rare Earths 2018, 36, 1333–1341. [Google Scholar] [CrossRef]
- Luo, C.; Deng, Y.; Inubushi, K.; Liang, J.; Zhu, S.; Wei, Z.; Guo, X.; Luo, X. Sludge Biochar Amendment and Alfalfa Revegetation Improve Soil Physicochemical Properties and Increase Diversity of Soil Microbes in Soils from a Rare Earth Element Mining Wasteland. Int. J. Environ. Res. Public Health 2018, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Onel, O.; Council-Troche, M.; Noble, A.; Yoon, R.-H.; Morris, J.R. A Study of Rare Earth Ion-Adsorption Clays: The Speciation of Rare Earth Elements on Kaolinite at Basic PH. Appl. Clay Sci. 2021, 201, 105920. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of Mining, Mineralogy, Uses, Sustainability and Environmental Impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Nie, W.; Zhang, R.; He, Z.; Zhou, J.; Wu, M.; Xu, Z.; Chi, R.; Yang, H. Research Progress on Leaching Technology and Theory of Weathered Crust Elution-Deposited Rare Earth Ore. Hydrometallurgy 2020, 193, 105295. [Google Scholar] [CrossRef]
- Yang, X.J.; Lin, A.; Li, X.-L.; Wu, Y.; Zhou, W.; Chen, Z. China’s Ion-Adsorption Rare Earth Resources, Mining Consequences and Preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- United States Geological Survey (USGS). Mineral Commodity Summaries; United States Geological Survey (USGS): Reston, VA, USA, 2022; 202p. [CrossRef]
- Kynicky, J.; Smith, M.P.; Xu, C. Diversity of Rare Earth Deposits: The Key Example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Hu, G.; Huang, L.; Huang, X.; Chen, Y.; Long, Z. Reduction Leaching of Rare Earth from Ion-Adsorption Type Rare Earths Ore with Ferrous Sulfate. J. Rare Earths 2016, 34, 917–923. [Google Scholar] [CrossRef]
- Huang, X.-W.; Long, Z.-Q.; Wang, L.-S.; Feng, Z.-Y. Technology Development for Rare Earth Cleaner Hydrometallurgy in China. Rare Met. 2015, 34, 215–222. [Google Scholar] [CrossRef]
- Chen, H.B.; Chen, H.M.; Chen, Z.B.; Chen, Z.Q. The Ecological Impacts of Residues from the Heap Leaching of Ion-Adsorption Rare Earth Clays. Int. J. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese Policies on Rare Earth Supply Chain Resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- da Costa, T.B.; Silva, M.G.C.d.; Vieira, M.G.A. Recovery of Rare-Earth Metals from Aqueous Solutions by Bio/Adsorption Using Non-Conventional Materials: A Review with Recent Studies and Promising Approaches in Column Applications. J. Rare Earths 2020, 38, 339–355. [Google Scholar] [CrossRef]
- Estrade, G.; Marquis, E.; Smith, M.; Goodenough, K.; Nason, P. REE Concentration Processes in Ion Adsorption Deposits: Evidence from the Ambohimirahavavy Alkaline Complex in Madagascar. Ore Geol. Rev. 2019, 112, 103027. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Tommasi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of Rare Earth Elements as Fertilizers and Feed Additives: A Knowledge Gap Analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of Rare Earth Metals: A Review of Recent Literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Extraction and Separation of Rare Earth Elements by Adsorption Approaches: Current Status and Future Trends. Sep. Purif. Rev. 2021, 50, 417–444. [Google Scholar] [CrossRef]
- Bailey, G.; Joyce, P.J.; Schrijvers, D.; Schulze, R.; Sylvestre, A.M.; Sprecher, B.; Vahidi, E.; Dewulf, W.; Van Acker, K. Review and New Life Cycle Assessment for Rare Earth Production from Bastnäsite, Ion Adsorption Clays and Lateritic Monazite. Resour. Conserv. Recycl. 2020, 155, 104675. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic Footprints of Rare Earth Elements on Natural Resources and Living Organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Schreiber, A.; Marx, J.; Zapp, P.; Hake, J.-F.; Voßenkaul, D.; Friedrich, B. Environmental Impacts of Rare Earth Mining and Separation Based on Eudialyte: A New European Way. Resources 2016, 5, 32. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Wen, Q.; Jia, Q.; Liu, Q. Interspecific Associations of Plant Populations in Rare Earth Mining Wasteland in Southern China. Int. Biodeterior. Biodegrad. 2017, 118, 82–88. [Google Scholar] [CrossRef]
- Qiu, T.; Zhu, D.; Fang, X.; Zeng, Q.; Gao, G.; Zhu, H. Leaching Kinetics of Ionic Rare-Earth in Ammonia-Nitrogen Wastewater System Added with Impurity Inhibitors. J. Rare Earths 2014, 32, 1175–1183. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Chen, Z.; Ou, X.; Chen, J. Calculation of Toxicity Coefficient of Potential Ecological Risk Assessment of Rare Earth Elements. Bull. Environ. Contam. Toxicol. 2020, 104, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. The Biological Hormesis Effects of Rare Earths and Potential Effects of Application of Rare Earths on Agricultural Eco-Environment. Rural Eco-Environ. 2004, 20, 1–5. (In Chinese) [Google Scholar]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A Human Health Risk Assessment of Rare Earth Elements in Soil and Vegetables from a Mining Area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, Q. Contamination from Rare Earth Ore Strip Mining and Its Impacts on Resources and Ecovironment. Chin. J. Ecol. 2011, 30, 2915–2922. (In Chinese) [Google Scholar]
- Chen, Z.; Chen, Z. Effects of Ecological Restoration Measures on the Distribution of Dicranopteris Dichotoma at the Microscale in the Red Soil Hilly Region of China. PLoS ONE 2018, 13, e0204743. [Google Scholar] [CrossRef]
- Ou, Z.; Pang, S.; He, Q.; Peng, Y.; Huang, X.; Shen, W. Effects of Vegetation Restoration and Environmental Factors on Understory Vascular Plants in a Typical Karst Ecosystem in Southern China. Sci. Rep. 2020, 10, 12011. [Google Scholar] [CrossRef]
- Szamałek, K.; Konopka, G.; Zglinicki, K.; Marciniak-Maliszewska, B. New Potential Source of Rare Earth Elements. Gospod. Surowcami Min. 2013, 29, 59–76. [Google Scholar] [CrossRef]
- Chen, S.; Zha, X.; Bai, Y.; Wang, L. Evaluation of Soil Erosion Vulnerability on the Basis of Exposure, Sensitivity, and Adaptive Capacity: A Case Study in the Zhuxi Watershed, Changting, Fujian Province, Southern China. Catena 2019, 177, 57–69. [Google Scholar] [CrossRef]
- Wen, X.; Duan, C.; Zhang, D. Effect of Simulated Acid Rain on Soil Acidification and Rare Earth Elements Leaching Loss in Soils of Rare Earth Mining Area in Southern Jiangxi Province of China. Environ. Earth Sci. 2013, 69, 843–853. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.; Li, C.; Sun, Y.; Zhou, X.; Li, Y. Searching High Efficiency and Environmentally Benign Leaching Reagent for Ion-Adsorption Rare Earth Based on the Zeta Potential of Clay Particles. Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Feng, Z.; Huang, X.; Huang, L.; Long, Z.; Cui, D. Leaching Characteristics of Ion-Adsorption Type Rare Earths Ore with Magnesium Sulfate. Trans. Nonferrous Met. Soc. China 2015, 25, 3784–3790. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, F.; Li, F.; Chen, G.; Yang, G.; Wang, J.; Du, K.; Liu, S.; Li, Z. Ammonia Nitrogen Sources and Pollution along Soil Profiles in an In-Situ Leaching Rare Earth Ore. Environ. Pollut. 2020, 267, 115449. [Google Scholar] [CrossRef] [PubMed]
- GB 26451-2011; China Rare Earth Emission Standards of Pollutants from Rare Earths Industry. National Standard of the People’s Republic of China: Beijing, China, 2011. Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=71F772D7DFD1D3A7E05397BE0A0AB82A (accessed on 8 May 2023). (In Chinese)
- Hao, X.; Wang, D.; Wang, P.; Wang, Y.; Zhou, D. Evaluation of Water Quality in Surface Water and Shallow Groundwater: A Case Study of a Rare Earth Mining Area in Southern Jiangxi Province, China. Environ. Monit. Assess. 2016, 188, 24. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, G.; Zhou, C.; Luo, J.; Lin, J.; Zhao, X. Rehabilitation Effect of the Combined Application of Bamboo Biochar and Coal Ash on Ion-Adsorption-Type Rare Earth Tailings. J. Soils Sediments 2020, 20, 3351–3357. [Google Scholar] [CrossRef]
- Chao, Y.; Liu, W.; Chen, Y.; Chen, W.; Zhao, L.; Ding, Q.; Wang, S.; Tang, Y.-T.; Zhang, T.; Qiu, R.-L. Structure, Variation, and Co-Occurrence of Soil Microbial Communities in Abandoned Sites of a Rare Earth Elements Mine. Environ. Sci. Technol. 2016, 50, 11481–11490. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-S.; Guo, M.-N.; Liu, C.; Yuan, M.; Chen, X.-T.; Huot, H.; Zhao, C.-M.; Tang, Y.-T.; Morel, J.L.; Qiu, R.-L. Water, Sediment and Agricultural Soil Contamination from an Ion-Adsorption Rare Earth Mining Area. Chemosphere 2019, 216, 75–83. [Google Scholar] [CrossRef]
- Ding, J.; Deng, G. Main Problems in the Current Ionic Adsorption Rare Earth Exploration Specifications and Their Amendment Proposals. Nonferrous Met. Sci. Eng. 2013, 4, 96–102. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Chen, Z.; Ma, Q.; Zhang, Q. Rare Earth Elements in Paddy Fields from Eroded Granite Hilly Land in a Southern China Watershed. PLoS ONE 2019, 14, e0222330. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.E.; Pourret, O.; Faucon, M.-P.; Dian, C. Effect of Rare Earth Elements on Rice Plant Growth. Chem. Geol. 2018, 489, 28–37. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health Effects and Toxicity Mechanisms of Rare Earth Elements—Knowledge Gaps and Research Prospects. Ecotoxicol. Environ. Saf. 2015, 115, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare Earth Elements (REEs): Effects on Germination and Growth of Selected Crop and Native Plant Species. Chemosphere 2014, 96, 57–66. [Google Scholar] [CrossRef]
- British Geological Survey. An Update to the Supply Risk Index for Elements or Element Groups That Are of Economic Value; British Geological Survey: Nottingham, UK, 2015. Available online: https://www2.bgs.ac.uk/mineralsuk/download/statistics/risk_list_2015.pdf (accessed on 8 May 2023).
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Brussels, 3.9.2020 COM(2020) 474 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0474 (accessed on 8 May 2023).
- Cao, X.; Harris, W. Properties of Dairy-Manure-Derived Biochar Pertinent to Its Potential Use in Remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Kong, L.-L.; Liu, W.-T.; Zhou, Q.-X. Biochar: An Effective Amendment for Remediating Contaminated Soil. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2014; Volume 228, pp. 83–99. ISBN 978-3-319-01618-4. [Google Scholar]
- Lehmann, J. Bio-Energy in the Black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Tack, F.M.G.; Ippolito, J.A.; Rinklebe, J. Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2803–2843. [Google Scholar] [CrossRef]
- Breunig, H.M.; Amirebrahimi, J.; Smith, S.; Scown, C.D. Role of Digestate and Biochar in Carbon-Negative Bioenergy. Environ. Sci. Technol. 2019, 53, 12989–12998. [Google Scholar] [CrossRef]
- Shinogi, Y.; Yoshida, H.; Koizumi, T.; Yamaoka, M.; Saito, T. Basic Characteristics of Low-Temperature Carbon Products from Waste Sludge. Adv. Environ. Res. 2003, 7, 661–665. [Google Scholar] [CrossRef]
- Matuštík, J.; Hnátková, T.; Kočí, V. Life Cycle Assessment of Biochar-to-Soil Systems: A Review. J. Clean. Prod. 2020, 259, 120998. [Google Scholar] [CrossRef]
- Monga, D.; Shetti, N.P.; Basu, S.; Raghava Reddy, K.; Badawi, M.; Bonilla-Petriciolet, A.; Aminabhavi, T.M. Engineered Biochar: A Way Forward to Environmental Remediation. Fuel 2022, 311, 122510. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M.; Lošák, T.; Spandel, A.; Kuc, K. The Effectiveness of Biochar in Mitigating Changes in the Chemical Properties of Sandy Soil Treadted with Various Chemicals. J. Elem. 2019, 25, 1045–1058. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of Sewage Sludge-Derived Biochars from Different Feedstocks and Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Joseph, S.; Husson, O.; Graber, E.; Van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The Electrochemical Properties of Biochars and How They Affect Soil Redox Properties and Processes. Agronomy 2015, 5, 322–340. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of Biochar for Resource Recovery from Water: A Review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Bovsun, M.A.; Castaldi, S.; Nesterova, O.V.; Semal, V.A.; Sakara, N.A.; Brikmans, A.V.; Khokhlova, A.I.; Karpenko, T.Y. Effect of Biochar on Soil CO2 Fluxes from Agricultural Field Experiments in Russian Far East. Agronomy 2021, 11, 1559. [Google Scholar] [CrossRef]
- Song, Y.; Bian, Y.; Wang, F.; Xu, M.; Ni, N.; Yang, X.; Gu, C.; Jiang, X. Dynamic Effects of Biochar on the Bacterial Community Structure in Soil Contaminated with Polycyclic Aromatic Hydrocarbons. J. Agric. Food Chem. 2017, 65, 6789–6796. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Effects of Biochar and Poultry Manure on Soil Characteristics and the Yield of Radish. Sci. Hortic. 2019, 243, 457–463. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil Biochemical Activities and the Geometric Mean of Enzyme Activities after Application of Sewage Sludge and Sewage Sludge Biochar to Soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Sarwar, T.; Shahid, M.; Natasha; Khalid, S.; Shah, A.H.; Ahmad, N.; Naeem, M.A.; Ul Haq, Z.; Murtaza, B.; Bakhat, H.F. Quantification and Risk Assessment of Heavy Metal Build-up in Soil–Plant System after Irrigation with Untreated City Wastewater in Vehari, Pakistan. Environ. Geochem. Health 2020, 42, 4281–4297. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, S.; Shimko, J.; Dengler, R.W. Mine Drainage: Treatment Technologies and Rare Earth Elements. Water Environ. Res. 2019, 91, 1061–1068. [Google Scholar] [CrossRef]
- Tyler, G. Rare Earth Elements in Soil and Plant Systems—A Review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a Potential Strategy for Remediation of Contaminated Mining Soils: Mechanisms, Applications, and Future Perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef]
- Jia, X.; Yuan, W.; Ju, X. Short Report: Effects of Biochar Addition on Manure Composting and Associated N2O Emissions. J. Sustain. Bioenergy Syst. 2015, 5, 56–61. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Ding, K.; Yang, Y.; Qiu, R. Effects of Organic Amendments and Biochar on Ecological Remediation of Ionic Rare Earth Mine Tailing. Acta Sci. Circumstantiae 2018, 38, 4769–4778. (In Chinese) [Google Scholar]
- Deng, X.; Chi, R.; Xiao, C.; Zhang, Z.; Liu, X.; Hu, J. The Intensified Effect of Nitrogen Removal Properties Using Pseudomonas Fulva K3 and MgBC for the Weathered Crust Rare Earth Wastewater Treatment. Physicochem. Probl. Miner. Process. 2021, 57, 84–96. [Google Scholar] [CrossRef]
- Haider, G.; Joseph, S.; Steffens, D.; Müller, C.; Taherymoosavi, S.; Mitchell, D.; Kammann, C.I. Mineral Nitrogen Captured in Field-Aged Biochar Is Plant-Available. Sci. Rep. 2020, 10, 13816. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of Biochar Amendment on Sorption and Leaching of Nitrate, Ammonium, and Phosphate in a Sandy Soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Anu, N.; Giri Nandagopal, M.S.; Selvaraju, N. Relevance of Isotherm Models in Biosorption of Pollutants by Agricultural Byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, Behaviour, and Environmental and Human Health Risks of High-Technology Rare Earth Elements as Emerging Contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- Serra-Ventura, J.; Vidal, M.; Rigol, A. Examining Samarium Sorption in Biochars and Carbon-Rich Materials for Water Remediation: Batch vs. Continuous-Flow Methods. Chemosphere 2022, 287, 132138. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of Scandium and Neodymium on Biochar Derived after Low-Temperature Pyrolysis of Sawdust. Minerals 2017, 7, 200. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Majdańska, M.; Fila, D. Sorption of Lanthanide Ions on Biochar Composites. J. Rare Earths 2018, 36, 1212–1220. [Google Scholar] [CrossRef]
- Jin, S.; Hu, Z.; Huang, Y.; Pan, H.; Hu, Y. Effects of Rice Straw, Rice Straw Ash, and Bone Charcoal on Uptake and Accumulation of Rare Earth Elements in Rice Plants. Bioresources 2018, 13, 8593–8613. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Xu, Z.; Yu, Z. The Potential Use of Straw-Derived Biochar as the Adsorbent for La(III) and Nd(III) Removal in Aqueous Solutions. Environ. Sci. Pollut. Res. 2021, 28, 47024–47034. [Google Scholar] [CrossRef]
- Liu, W.-S.; Chen, Y.-Y.; Huot, H.; Liu, C.; Guo, M.-N.; Qiu, R.-L.; Morel, J.L.; Tang, Y.-T. Phytoextraction of Rare Earth Elements from Ion-Adsorption Mine Tailings by Phytolacca Americana: Effects of Organic Material and Biochar Amendment. J. Clean. Prod. 2020, 275, 122959. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, N.; Li, Y.; Wu, P.; Dang, Z.; Ke, Y. Efficient Recovery of Rare Earth Elements from Discarded NdFeB Magnets. Process Saf. Environ. Prot. 2019, 124, 317–325. [Google Scholar] [CrossRef]
- You, H.; Zhang, Y.; Li, W.; Li, Y.; Ma, Y.; Feng, X. Removal of NO3-N in Alkaline Rare Earth Industry Effluent Using Modified Coconut Shell Biochar. Water Sci. Technol. 2019, 80, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Hadjittofi, L.; Charalambous, S.; Pashalidis, I. Removal of Trivalent Samarium from Aqueous Solutions by Activated Biochar Derived from Cactus Fibres. J. Rare Earths 2016, 34, 99–104. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. An Overview of Rare-Earth Recovery by Ion-Exchange Leaching from Ion-Adsorption Clays of Various Origins. Mineral. Mag. 2016, 80, 63–76. [Google Scholar] [CrossRef]
- Pourret, O.; Houben, D. Characterization of Metal Binding Sites onto Biochar Using Rare Earth Elements as a Fingerprint. Heliyon 2018, 4, e00543. [Google Scholar] [CrossRef] [PubMed]
- Royer-Lavallée, A.; Neculita, C.M.; Coudert, L. Removal and Potential Recovery of Rare Earth Elements from Mine Water. J. Ind. Eng. Chem. 2020, 89, 47–57. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.; Wilhelmi, B.; Duncan, J.R.; Burgess, J.E. Biosorption of Precious Metals. Biotechnol. Adv. 2007, 25, 264–271. [Google Scholar] [CrossRef]
- Costa, N.O.; Botelho, N.F.; Garnier, J. Concentration of Rare Earth Elements in the Faixa Placha Tin Deposit, Pedra Branca A-Type Granitic Massif, Central Brazil, and Its Potential for Ion-Adsorption-Type REE-Y Mineralization. Ore Geol. Rev. 2020, 123, 103606. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Lian, F.; Liu, X.; Gao, M.; Li, H.; Qiu, W.; Song, Z. Effects of Fe-Mn-Ce Oxide–Modified Biochar on As Accumulation, Morphology, and Quality of Rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2020, 27, 18196–18207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Gao, M.; Song, Z. Effect of Fe–Mn–Ce Modified Biochar Composite on Microbial Diversity and Properties of Arsenic-Contaminated Paddy Soils. Chemosphere 2020, 250, 126249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lu, H.-H.; Liu, Y.-X.; Yang, S.-M. Ammonium Citrate-Modified Biochar: An Adsorbent for La(III) Ions from Aqueous Solution. J. Environ. Manag. 2016, 509, 550–563. [Google Scholar] [CrossRef]
- Bao, D.; Li, Z.; Tang, R.; Wan, C.; Zhang, C.; Tan, X.; Liu, X. Metal-Modified Sludge-Based Biochar Enhance Catalytic Capacity: Characteristics and Mechanism. J. Environ. Manag. 2021, 284, 112113. [Google Scholar] [CrossRef]
- Hu, B.; Tang, Y.; Wang, X.; Wu, L.; Nong, J.; Yang, X.; Guo, J. Cobalt-Gadolinium Modified Biochar as an Adsorbent for Antibiotics in Single and Binary Systems. Microchem. J. 2021, 166, 106235. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; He, C.; Lyu, W.; Zhang, W.; Yan, W.; Yang, L. Development of Rare Earth Element Doped Magnetic Biochars with Enhanced Phosphate Adsorption Performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 236–243. [Google Scholar] [CrossRef]
- Liu, J.; Ren, S.; Cao, J.; Tsang, D.C.W.; Beiyuan, J.; Peng, Y.; Fang, F.; She, J.; Yin, M.; Shen, N.; et al. Highly Efficient Removal of Thallium in Wastewater by MnFe2O4-Biochar Composite. J. Hazard. Mater 2021, 401, 123311. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Dorris, A. Low-Temperature Acoustic-Based Activation of Biochar for Enhanced Removal of Heavy Metals. J. Water Process Eng. 2020, 34, 101166. [Google Scholar] [CrossRef]
- Soudek, P.; Rodriguez Valseca, I.M.; Petrová, Š.; Song, J.; Vaněk, T. Characteristics of Different Types of Biochar and Effects on the Toxicity of Heavy Metals to Germinating Sorghum Seeds. J. Geochem. Explor. 2017, 182, 157–165. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Condron, L.M.; Sherlock, R.R.; Anderson, C.R.; Craigie, R.A. Biochar Incorporation into Pasture Soil Suppresses in Situ Nitrous Oxide Emissions from Ruminant Urine Patches. J. Environ. Qual. 2011, 40, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and Negative Carbon Mineralization Priming Effects among a Variety of Biochar-Amended Soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of Polyacrylamide, Biopolymer and Biochar on the Decomposition of 14C-Labelled Maize Residues and on Their Stabilization in Soil Aggregates: Biochar and Biopolymer Effects on Litter Decomposition and C Stabilization. Eur. J. Soil Sci. 2013, 64, 488–499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).