Abstract
Every year, more than 50 million metric tons of apples are produced, and apple pomace is frequently discarded as waste in the food industry. Apple pomace, a byproduct of apple juice and cider production, is used as a sustainable raw material to make valuable products such as nutraceuticals and pectin. Apple pomace contains a substantial amount of antioxidant compounds, which have been related to several health advantages. Therefore, valuable components extracted from this byproduct may be used in the food and pharmaceutical industries. The common and new technologies to obtain valuable products from apple pomace which has come from production of apple juice or cider. Especially, emphasis of new and green technique is very important and will contribute the literature. Therefore, this review discussed apple processing, pectin as a bioactive compound, the extraction methods, current applications of apple pomace byproducts, and future studies on its potential uses in food.
1. Introduction
With the inefficient usage of raw materials and the rise in waste production, waste management has shifted from emission reduction or mitigation to a more practical method. Waste materials are still seen as valuable commodities used as raw materials, which is a need for sustainable growth. The value of limiting natural resource consumption to achieve a balanced standard is related to a decrease in greenhouse gas emissions, which have a detrimental effect on climate change. Agroindustry wastes are significant on a global scale because they are linked to greenhouse gas pollution from processing, use, disposal and can result in natural resource depletion as a result of an ever-increasing global population [1,2]. Under the biorefinery model, concepts such as “circular economy” and “cradle to cradle” aim to create a world in which wastes from specific industrial processes can be used as sustainable raw materials for other products and commodities. These concepts would raise both critical economic and environmental issues that can prevent the use of non-renewable resources [3].
Vegetable and fruit factories generate vast volumes of waste that impact landfills, producing approximately 80% sugars and hemicellulose, 9% cellulose, and 5% lignin. They produce methane and leachates and have high chemical and biochemical oxygen demands since they are biodegradable, leading to increased recovery costs. The agricultural wastes such as peanut skin and banana peels have been valorized in our group since 2017, moving towards a “zero waste” economy to obtain products and substances that, due to their nature, will interact with traditional ones, reducing emissions and contributing to healthier material and commodity output. For example, peanut skin as peanut butter wastes have been extracted by supercritical carbon dioxide as green technology to recover catechin, antioxidant, and flavonoid compounds [4,5]. Banana peels as banana chips waste are also extracted to obtain using a renewable technology called subcritical water extraction [6].
In 2019, the world’s apple harvest totaled more than 70 million metric tons, with Asia accounting for more than half of it, as shown in Figure 1 [7]. Around 75% of apples may be turned into jelly, while the remainder, classified as apple pomace, comprises around 20–30% dried matter and is mainly used as animal feed or manure. Since apple pomace is produced in large amounts and includes a large percentage of water, it presents storage issues and necessitates prompt care to avoid putrefaction.
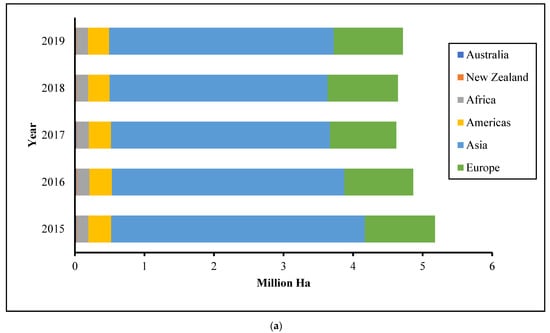
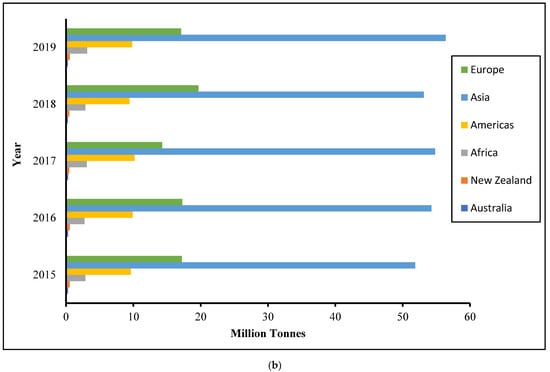
Figure 1.
Apple world plantation (a) and production (b) by years (http://www.fao.org/faostat/en/#data/QC (accessed on 4 May 2020)).
The processing of waste into value-added goods, which reduces the waste amount, is a feasible alternative for the world. The pomace has been traditionally used as animal feed material for cows, buffalo, sheep, and goats [8,9,10]. The apple pomace also gives the high antioxidant capacity, antimicrobial and anti-viral properties [11,12,13,14]. It is a promising source with potential applications in the nutraceutical and pharmaceutical industries.
Formerly, the conventional extraction consisted of the maceration and Soxhlet associated with organic frequently used in the extraction of apple pomace. Nevertheless, the ‘green extraction’ of plant material is widely revealed as a challenge to the conventional extraction method. Demanding green extraction has aimed to increase the yield at a lower cost. Since no organic solvents are used, the formation of toxic residue is reduced [15,16,17,18].
Thus, significant advances have been developed in green extraction technologies such as the microwave-assisted extraction, subcritical water extraction, and supercritical carbon dioxide (SC-CO2) [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. The conventional method had proven to comprise higher yield, yet the SWE and SC-CO2 approached could be alerted regardless of long-term effect, especially to our environment and health issues. Therefore, this review comprehensively highlights apple pomace, reviews its current extractions of this byproduct, and finally proposes a trend for future studies on apple pomace.
2. Apple Production
Apple is one of the most commonly grown tree fruits and is the domesticated tree Malus domestica (family Rosaceae). The apple plantation is one of the world’s largest, spanning over 5.3 million hectares and reaching up to a height of 15 m with climacteric fruit. It is expected to be the most common fruit, accounting for 16.8% of global fruit production, followed by orange, accounting for 11.4%. In the last five years, apple demand has increased from 50 million tons to 53 million tons [7]. The market is due to apple products such as chips, ice cream, jelly, and cake. Furthermore, rising apple demand has been aided by demographic growth, as well as a rise in planted area and productivity. Figure 1 shows that the Asian region produced the most apples in 2019, with 56.34 million tons [7].
3. Pectin
In 1790, a pioneer discovered the complex polysaccharides of pectin in fruit juices [36]. Pectin is one of the compounds in apple pomace that is of concern for the last decade, the majority of study on pectin has been based on this molecule. Pectin roots are found above the mid layer of lamella and primary cell walls. According to the American Chemical Society, pectin is a complex compounds including of colloidal carbohydrate derivatives [37]. In pectin, the methylated ester of 1,4-linked galacturonic acid (GA) attaches to the galactose, arabinose, and xylose [38]. Extensively, a portion of the GA backbone’s C-6 carboxyl units is esterified with methoxyl groups or live as uronic acid salts [39]. Additionally, the GA could have been methylated to various degrees based on the source plant.
4. Antioxidants
Plants are known to have a variety of natural antioxidants that preserve their physical and metabolic integrity. Many of these plant extracts promise in lowering the ageing of skin by limiting the metabolism of oxidation. Antioxidants are proven to occur in phytochemicals containing phenol. This indicates that antioxidants can inhibit the interaction of free radicals with other molecules in the body, reducing DNA damage and long-term health repercussions stable [35,40,41,42,43,44]. Free radicals are unstable compounds that have lost an electron. Commonly, Vitamin C, vitamin E, anthocyanin and catechin are widely utilized in foods and cosmetics because of their strong antioxidant activity. The apple pomace also gives the significant antioxidant activity including phenolic compounds [45]. Therefore, the apple pomace can be valorized to high end products for health and wellness.
5. Extraction Methods
5.1. Soxhlet Extraction
5.1.1. Principles of Soxhlet Extraction
Von Soxhlet invented a novel extraction method in 1879, that has been used leaching technology for a long time [46]. In reality, Soxhlet extraction has been a standard procedure for more than a century. The benefits and drawbacks of Soxhlet extraction have been exploited to generate a range of changes aimed at alleviating or reducing the latter while maintaining or even enhancing the former. The majority of the described modifications over the previous several decades have been focused on bringing Soxhlet closer to that of more modern solid sample preparation procedures, such as using auxiliary energies to minimize leaching durations and automating the extraction assembly [47].
This method also offers several appealing features. The sample is continually brought into touch with new parts of the extractant, allowing the transfer equilibrium to be displaced. Moreover, the system maintains a reasonably high temperature due to heat transferred from the distillation flask to the extraction cavity. As a result, no filtering is required after leaching, and sample throughput can be increased by running multiple simultaneous extractions in parallel, which is possible by the low cost of the essential equipment. Aside from that, this method is requiring fewer minor procedures, could extract more sample mass, and appears to be free of matrix effects. Several established methods, including a sample preparation step based on Soxhlet extraction, have been previously reported [48,49,50].
5.1.2. Apple Pomace Extraction by Soxhlet Extraction
Water was proved to be an excellent solvent in the Soxhlet extraction with high antioxidant activity from apple pomace [51]. Apple pomace also contains feruloyl quinic acid. The extraction process could be shortened in half if using environmentally safe organic solvents. However, water is ineffective for extracting quercetin mono-glycosides. There are no significant differences between the optimal ethanolic and acetonic extracts from apple pomace to achieve the highest antioxidant activity measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH) [52]. In this case, acetone was chosen over ethanol due to the lower optimum extraction temperature (25 °C), which resulted in lower energy costs.
Furthermore, since acetone has a lower boiling point than ethanol, it will require fewer resources to evaporate acetone before final product processing. apple pomace extracts were tested for phenolic profiles, antioxidant properties, and antiviral activity against herpes simplex virus (HSV) [14]. Acetone yielded the most phenolic compounds. Although antioxidant activity was weakly associated with phenol concentration, the extraction process impacted the phenolic composition. Quercetin glycosides were the most significant class of polyphenols studied, followed by dihydrochalcones. As a result, apple pomace extracts inhibited HSV in Vero cells by more than 50% at non-cytotoxic concentrations.
5.2. Microwave-Assisted Extraction (MAE)
5.2.1. Principles of MAE
Microwaves have frequencies ranging from 300 MHz to 300 GHz [53]. In modern science, microwaves are used primarily for two purposes: transmission and energy vectors. The microwave involves the direct impact of waves on materials, converting some of the absorbed electromagnetic energy to heat energy. Microwaves consist of two perpendicular oscillating fields: electric and magnetic fields [53]. Unlike the conventional conduction–convection technique, a significant amount of heat energy is lost to the surroundings. Heating in MAE is targeted and selective, with almost no heat wasted on the environment as it is conducted in a closed system. Compared to Soxhlet, this innovative heating process may drastically shorten extraction time (i.e., <30 min) [54]. Microwave heating is based on its contact with polar materials/solvents, determined by two phenomena: ionic conduction and dipole rotation [53].
Ionic conduction is the electrophoretic movement of ions under the influence of a changing electric field. The solution’s resistance to ion movement causes friction, which causes the liquid to heat up. Dipole rotation realigns the molecule’s dipoles with a rapidly changing electric field. Heating is only affected by the frequency of 2450 MHz. The electric component of the wave changes 4.9 × 104 times per second [55]. The solvent molecules will try to align themselves with the electric field to stay in phase. Nonetheless, as the electrical particles of the wave change rapidly, the molecules fail to realign and begin vibrating, hence generating heat through frictional force. Since a frequency higher than 2450 MHz changes the electrical particles significantly faster, the solvent molecules do not have enough time to align with the external field; hence, there is no heating.
However, as the frequency is less than 2450 MHz, the electrical component changes significantly slower, giving the molecules enough time to align with the electric field and evade heating. Based on the preceding methods, only dielectric materials or liquids with persistent dipoles are heated by microwave. A dissipation factor (Ɵ) could determine how efficiently various solvents heat up under microwave. It measures the solvent’s capacity to absorb microwave energy and transfer it as heat to the surrounding molecules [56].
Although most extractions are done with dried plant material, plant cells retain minute microscopic moisture residues that serve as a target for microwave heating. When the microwave effect heats the water within the plant cell, it evaporates, putting enormous pressure on the cell wall and causing swelling, straining, and eventually rupturing it. This process allows the active components in the ruptured cells to leak out into the surrounding solvent, increasing the output of phytoconstituents [57].
This phenomenon may be amplified if the plant matrix is treated with solvents with higher heating efficiency under the microwave. In 1 to 2 min, microwave radiation can hydrolyze the ether bonds of cellulose, the primary component of plant cell walls, and convert it to soluble fractions. During MAE, the high temperature accelerates cellulose dehydration at the cell wall and lowers its mechanical strength, hence ease the solvent to reach components within the cell [58].
Nevertheless, microwave treatment will affect cell structure if there is a sudden increase in temperature and internal pressure. The chemical substance within the cell rapidly exudes into the surrounding solvents and breaks the cell. Since the MAE mechanism involves exposing the analytes to the solvent via cell rupture, this technique differs from heat-reflux extraction. Heat-reflux extraction relies on a series of permeation and solubilization processes to extract the analytes from the matrix. A study reported that microwave treatment caused destructive changes in the plant tissue of fresh orange peel when observed using scanning electron micrographs (SEM) [59]. The changes in the plant tissue, however, had significantly increased the yield of extractable pectin. Moreover, dissolved ion migration enhances solvent penetration into the matrix, allowing compounds to be released more quickly. MAE has also been shown to aid in extracting essential oils from plant materials by facilitating the desorption of chemicals of interest from the plant matrix [46].
Due to continuous heating of free water molecules in the gland and vascular systems, this process has caused the cell wall a significant swelling before ruptured, thus enabling essential oil to flow towards the organic solvent [60]. The dielectric susceptibility of both the liquid and the solid plant matrix substantially influences microwave radiation. Therefore, water is more favorable solvent to extract the apple pomace due to high polar compounds, where is nontoxic and green solvent [61]. This method is efficient in preventing the deterioration of thermolabile components. Although microwave energy has a massive heating capacity, this technology has only recently gained popularity in analytical labs.
5.2.2. Apple Pomace Extraction by MAE
A study had reported pectin extraction from apple pomace using MAE [56]. They have used response surface methodology (RSM) to maximize the impact of extraction processing parameters on pectin yield. Extraction time (minutes), solid-liquid ratio, pH of HCl solution, and microwave power were four independent variables (W) studied. The optimum conditions were calculated, and mathematical models were used to plot three-dimensional response surfaces.
Based on the F-test and p-value, the pH of the HCl solution and extraction time significantly affected the reaction value. The quadratic of microwave power also had a significant impact, as did the interactions of pH and solid-liquid ratio. Based on performance, energy savings, and experiment viability, the optimal conditions for pectin extraction were 20.8 min of extraction time, a solid-liquid ratio of 0.069, pH 1.01, and microwave power of 499.4 W. The MAE application in the dried apple pomace extraction resulted in a significant reduction in the extraction period. The optimum expected pectin yield was 0.315 g from 2 g dried apple pomace. Compared to conventional extraction methods, MAE of phenolics from apple pomace has significant potential [62]. MAE reduce the time of extraction and consumption of solvent and higher yields. The optimal extraction conditions were discovered to be independent of apple diversity, allowing them to extract phenolics from other apple cultivars’ pomace. These value-added extracts can be used to extend commodity shelf life (as an alternative to synthetic antioxidants), as dietary supplements, and as functional food additives. The pomace had a maximum total phenolics content (TPC) of 15.8 mg GAE/g under optimal extraction conditions of 735 W, 149 s and 10.3 mL of ethanol/g dry sample. With the DPPH of 77.1%. The extracts also contained the primary polyphenols phloridzin, quercetin, chlorogenic acid, and caffeic acid.
MAE also was used to remove polyphenolic compounds from apple pomace in the presence of ethanol and water as solvents ([63]. The values were compared to Soxhlet extraction and maceration. The study used several experimental conditions, including solvent form, microwave power, solvent to sample ratio, and extraction period. Their finding showed that as microwave power increases, extraction yields decreased (from 90 to 360 W). Ethanol-water was an excellent solvent for MAE (65:35 ratio). For a higher water share in the extraction, a 35:65 (ethanol-water) ratio may be used to achieve 90% recovery in a shorter extraction period. In their study, the best solvent-to-sample ratio was 20:1. MAE was significantly quicker than maceration and Soxhlet methods to extract polyphenolic compounds.
Meanwhile, RSM proved that MAE could effectively extract polyphenols from industrial waste to maximize yield [64]. The effect of solvent to raw material (g/mL) ratio, microwave strength, ethanol concentration, and extraction time on polyphenol yield were studied. The best conditions were microwave power of 650.4 W, extraction time of 53.7 s, the ethanol concentration of 62.10%, and solvent to a raw material ratio of 22.9:1. The result. The primary polyphenols found in apple pomace were procyanidin B2, (-)-having the highest concentration of 219.4 mg/kg.
5.3. Ultrasound-Asissted Extraction (UAE)
5.3.1. Principles of UAE
Based on the effects of sonic cavitation, the UAE is also utilized to search for bioactive chemicals from apple pomace. Ultrasonic wave propagation allows more solvent to penetrate the sample matrix, increasing contact between the sample and the solvent (or reagent) and mass transfer rates. Furthermore, since it stimulates the shattering of biological cell walls, this approach effectively extracts chemicals from living organisms. This method allows simultaneous extractions, small amounts of solvent, shorter working times, and increased extract yield and quality. UAE is also less expensive, faster, and more adaptable than previous procedures since it can utilize different solvents with different polarities. UAE, on the other hand, has several drawbacks, including issues with combining different instruments and automation [65,66]. Polysaccharide extraction from the microalgae Spirulina maxima was evaluated by Gharibzahedi, et al. [67]. The discovery showed that compared to traditional extraction methods such as hot water or 80% ethanol, the extraction yield of UAE was increased by at least 25% to 30%. A study has discovered that ultrasonic frequency is more effective than extraction period in increasing extraction yields to obtain S. maxima extracts, which are particularly important due to their anticancer potential [68]. UAE extracts were also less cytotoxic than extracts obtained through traditional solvent and hydrothermal methods. Furthermore, irradiation caused matrix fragmentation, and ultrasound increased matrix hydration [69]. The researchers discovered that sonicated samples outperformed non-sonicated samples in terms of extraction index. We looked at various experiments to better explain and demonstrate the impact of ultrasound on a vegetal matrix during the UAE. They proved that ultrasound extraction ranging from 20 to 25 kHz works by different separate or combination processes, such as detexturation, separation, capillarity, degradation, or sonoporation.
5.3.2. Apple Pomace Extraction by UAE
UAE is considered a simpler and more successful alternative than the conventional extraction to separate xyloglucan from apple pomace [70]. Apple pomace samples were extracted using indirect sonication in an ultrasonic cleaning bath. The UAE was compared to the usual approach in the xyloglucan synthesis and was shown to be three times faster than the conventional method. The effects of the liquid-solid ratio, KOH concentration, and UAE time on xyloglucan production from apple pomace were investigated. The most significant effect was the liquid-solid ratio. The best combination was found to be a 34.4:1 (v/w) liquid-solid ratio, a KOH concentration of 3.3 M, and a 2.5-h UAE period.
Apple pomace contains a high concentration of exploitable polyphenols by UAE [13]. The optimal conditions for polyphenols water-extraction were 40 °C, 40 min, and 0.764 W/cm2. TPC obtained by the UAE was 30% higher than that obtained by Soxhlet extraction (555 and 420 mg/100 g, respectively). Ultrasonic extracts also had higher antioxidant activity. Large-scale testing of the ultrasonic procedure revealed a potential industrial application. The UAE was also used to extract pectin from the apple pomace [71]. They investigated the impact on extraction yield, galacturonic acid content, and esterification degree. Under optimized conditions, the maximum output was obtained at 100% amplitude, a solid-liquid ratio of 1:10 g/mL, pH of 1.8, and 30 min. The chemical composition, thermal behavior, rheological properties, and morphological structure of pectin produced under ideal extraction conditions were compared to commercial citrus and apple pectin.
Fourier-transform infrared spectroscopy (FTIR) analysis of ultrasonic-extracted pectin confirmed the high degree of esterification and revealed similarities to commercial citrus and apple pectin samples (88.52%). The thermal behavior of UAE pectin was influenced by its narrower molecular weight distribution and ordered molecular organization. The morphological structure and galacturonic acid concentration, on the other hand, were found to affect the rheological parameters (high viscosity, G0, and G1) of this sample. The R2 between the viscosity and galacturonic acid concentration showed a significant positive association.
5.4. Pressurized Liquid Extraction (PLE)
5.4.1. Principles of PLE
Pressurized liquid extraction uses solvents at high temperatures, above their boiling point and below their critical point. The pressure is needed to maintain the solvent in the liquid form. In this section, this review will discuss the primary criteria for selecting optimal operational parameters from a theoretical standpoint. The essential concepts of PLE for solid samples are also included. Because commercial equipment has limitations, the solution to deal with liquid samples is to convert them in the solids form using absorbent or adsorbent.
The extraction of analytes from semisolid and solid samples can be described in five stages [72]:
- Moistening the sample with extraction solvent;
- Chemical desorption from the matrix (including or excluding chemical bond breakdown);
- Compound dispersion out of the matrix;
- Diffusion through the matrix’s closest solvent layer to reach the bulk solvent;
- Diffusion through the closest solvent layer surrounding the matrix to reach the bulk solvent eventually.
Both kinetic and thermodynamic properties influence the extraction efficiency. As a result, three interconnected factors affect extraction efficiency; matrix effect, mass transfer, and solubility. The other properties in PLE are constrained by various factors such as the flow rate, pressure, temperature, and duration of the extraction. The temperature during the extraction is one of the most significant factors affecting the efficiency and selectivity of PLE. High temperatures could be used to improve extraction efficiency to prevent van der Waals forces, hydrogen bonding, and dipole attraction from disrupting analyte-sample matrix connections [73]. Thermal energy may help overcome cohesive (molecule–molecule) and adhesive contacts between different molecules, i.e., the analyte and the sample matrix, by lowering the activation energy required for the desorption process.
Furthermore, increasing the temperature reduces the surface tension of the solvent, solutes, and matrix, which improves solvent wetting in the sample. Reduced solvent surface tension facilitates the formation of solvent cavities, allowing analytes to dissolve in the solvent more quickly [74]. As the temperature rises, the viscosity of a liquid solvent decreases, allowing it to penetrate deeper into the matrix particle and facilitate the extraction process [75]. Finally, using a higher temperature solvent improves the molecule’s diffusion rate, or mass transfer in the solvent, allowing for faster extractions, especially in diffusion-controlled samples. However, at higher temperatures, the number of co-extracted analytes may be significantly larger, implying lower extraction selectivity.
The primary benefit of using pressure during extraction is that we can use a temperature above the boiling point while the solvent remains liquid. High pressure at high temperatures, combined with low solvent surface tension, allows the solvent to reach and extract the analyte from the matrix pore. Using pressure during extraction may cause matrix disturbance, which improves the mass transfer of analyte from the sample to the solvent. High-pressure extraction prevents air bubbles in the matrix from preventing the solvent from reaching the analyte. Under these conditions, the analyte’s solubility and desorption kinetics from the sample matrix could improve [76]. However, as has been demonstrated for essential oil extraction from various herbal plants, the impact of pressure on the recovery of most compounds is usually negligible [77,78]. It has been proposed that instead of organic solvents, additives such as micellar media could be used in PLE to extract organic contaminants from liquid and environmental samples [79].
5.4.2. Apple Pomace Extraction by PLE
PLE is a green extraction method that has the potential to increase bioactive component extraction rates. PLE was used to extract antioxidants and polyphenols from industrially manufactured apple pomace at two different temperature ranges: 160 to 193 °C and 75 to 125 °C. Three distinct polyphenol groups, TPC, and antioxidant activity (DPPH radical scavenging test) were assessed. The temperatures ranging from 75 to 125 °C were recommended to obtain maximum antioxidant activity. Compared to conventional solid-liquid extraction, PLE increased antioxidant activity by 2.4 times, implying that the method could be a viable alternative to standard antioxidant extraction procedures [80].
It was discovered that the combination of PLE and solid-phase extraction could be used to separate phenolic compounds from apple pomace [45]. The first-stage water volume (0–120 mL), temperature (60–80 °C), solid-phase extraction adsorbent (Sepra, Isolute, Strata X, and Oasis), and activation/elution solvent (methanol and ethanol) were investigated. The temperature did not affect recovery, but phlorizin and a quercetin derivative showed significant differences. The results demonstrated that ethanol, rather than methanol, could be used as an activation, extraction, and elution solvent. this method produced higher or comparable yields of acids (2.85 mg/g) and flavonoids (0.97 mg/g) than Soxhlet extraction using green solvents (ethanol, water, or a small quantity of reusable methanol).
5.5. Subcritical Water Extraction (SWE)
5.5.1. Principles of SWE
Subcritical water is heated water at a pressure sufficient to keep it in a liquid form at a temperature (100 to 374 °C) and a pressure (1 to 22.1 MPa) [81]. As temperature rises, the dielectric constant, viscosity, and surface tension decrease. Therefore, the diffusivity of water can be enhanced. At a certain temperature level, an optimal pressure could be applied to keep the water liquid [82]. SWE has the advantage of allowing the dielectric constant to be adjusted over a broad temperature and pressure range [83]. SWE also contributes to mass transport via the diffusion and convection processes [84]. However, the subcritical water energy could disrupt the interaction between adhesive (solute-matrix) and cohesive (solute-solute) interactions if lowering the activation energy required for the desorption process [85]. Several water properties change as temperature and pressure change; for example, the polarity of subcritical water decreases as temperature rises. Consequently, it is possible to distinguish between polar, medium-polar, low-polar, and non-polar substances.
Subcritical water has high diffusivity, low viscosity, and low surface tension under high-temperature conditions. Increased vapor pressure and rapid thermal degradation of the target chemicals. The nature of subcritical water changes as the temperature rises, and the water’s property shifts from polar to non-polar. Less polar substances dissolve more quickly in water [86]. The increased flow rate not only shortens the time the compounds spend in high-temperature water but also significantly improves extraction efficiency. It should be noted that a high extractant flow rate may result in significant dilution of the extracts, necessitating a second concentration step once the extraction is complete. If the primary factors limiting the extraction kinetics within the pores of the sample matrix are resolution and diffusion, rising the flow rate will not improve compound extraction yield [87].
5.5.2. Apple Pomace Extraction by SWE
SWE was previously reported to extract pectin from apple pomace to study the influence of extraction temperature on pectin characteristics and had a high yield, i.e., 16.68% [88]. The extraction temperature affected pectin’s endothermic property. Based on differential scanning calorimetry studies, the exothermic property of pectin was primarily influenced by its components and raw material. In a rheological study, pectin solutions were shear-thinning and tended to be more elastic. Furthermore, the Extraction duration, temperature, and solvent to solid ratio were the variables that greatly influenced the apple pomace recovery by SWE [56]. temperature of 100 °C, 37 min and a solvent to solid ratio of 100 mL/g are optimum condition to extract the antioxidants The phenolic content of apple pomace was 8341 mg/kg dry matter under optimal conditions, implying that organic solvents are safer alternatives to extract antioxidants. The optimum conditions of 140 °C extraction temperature, 5 min extraction time, and an Solid/Water ratio of 1:14 to extract pectic polysaccharides from apple pomace were also established [56]. The extracted pectic polysaccharides had lower GA and protein content than commercial pectin but higher ash and neutral sugar content.
5.6. Supercritical Carbon Dioxide (SC-CO2) Extraction
5.6.1. Principles of SC-CO2 Extraction
This method provides a green recovery extract with the high purity of the solute. Its low-temperature solubility offers high amounts of specific/bioactive compounds and can be adjusted based on temperature and pressure conditions [89]. The flaw of this technique is that it can only be used to extract non-polar compounds. One of the development methods for removing polar compounds is ethanol/methanol as a modifier into SC-CO2 [90].
The dehydration/drying of raw materials in SC-CO2 obtained to recover phytochemical compounds has sparked considerable interest, particularly in industrial processes. The high-water content of seeds has a significant impact on the extraction efficiency of lipophilic nutraceuticals using SC-CO2. The presence of water in raw materials impedes the fluid flow of CO2 through the seeds, reducing surface contact between solutes and solvents [91]. The presence of water in seeds also influences restrictor or valve clogging caused by ice formation. As a result, water and extracts may be hard to separate [92].
Hot air, oven, freeze, convective, microwave, and osmotic drying methods were evaluated. These processes will affect drying kinetics and the chemical properties of moisture, fibers, proteins, lipids, and isoprenoids. Oven drying is the most commonly utilized dehydration method due to its low cost [93]. Extraction of thermolabile compounds and oxidizable substrates such as lipids and carotenoids, on the other hand, is ineffective. The color, chemical composition, and nutritional value of the dried product may be altered by high temperatures [94]. Color changes during heat processing could be an indicator of bioactive degradation. The particle size of the dried sample also affects the recovery of interest compounds. Because of the higher surface-to-volume ratio of smaller particles and the shorter path length to the bulk phase, smaller particle sizes will increase the amount of extracted compounds [95].
The solute’s solubility are affected by temperature and pressure. The higher the temperature at constant pressure, the lower the density, and thus its solvating power, but it increases the vapor pressure of compounds [96]. Furthermore, at a constant temperature, the density increases with increasing pressure, improving the solubility of the interest compounds [97]. The SC-CO2 extraction process has a limitation in compound interest. It can extract fatty acids, terpenes, and other non-polar compounds. However, extracting flavonoids and tannins, which are polar compounds, from the seeds is difficult. As a result, modifying SC-CO2 extraction is required to overcome this limitation. Depending on the molecular polarity of the modifier, the addition of a polar co-solvent or modifier to CO2 can increase the solvent power and selectivity and thus increase the solute solubility in supercritical fluid [98]. The modifier’s swelling effect on the matrix will increase the contact surface area between the matrix and the supercritical fluid [99]. Ethanol is a preferable modifier due to its polarity. It is a polar solvent with the ability to extract polar compounds. Furthermore, ethanol is available in the food-grade form, making it safe for human consumption.
5.6.2. Apple Pomace Extraction by SC-CO2
A SC-CO2 was used to investigate the possibility of recovering phenolic compounds and antioxidants from apple pomace [100]. The antioxidant activity of freeze-dried apple pomace using SC-CO2 at 30 MPa and 45 °C for two hours with ethanol (5%) as a co-solvent yielded 5.63 mg/g of extract. However, Soxhlet (2.05 mg/g of extract) and boiling water maceration (1.14 mg/g of extract) achieved a higher value. The amount of several phenolic components in SC-CO2 extract was also validated using high-performance liquid chromatography coupled with diode-array detection and electrospray ionization tandem mass spectrometry (HPLC-DAD-MS). This study is one of the first to look into the effects of SC-CO2 on antioxidant extraction from apple pomace.
Chromatographic analysis proved that the pomace contains a high concentration of the most prevalent compounds, such as ursolic acid, oleanolic acid, and sitosterol [101]. The extraction parameters were determined and the extraction rate was greatly influenced by CO2 pressure and temperature. From their study, the optimal extraction conditions were 80 °C and 30 MPa. Chrastil’s equation was used to describe the effects of process variables on analyte solubility. Although the equation was designed for binary systems, the coefficients of determination obtained were adequate. The observed differences were primarily due to changes in analyte solubility, with mass transfer coefficients were ignored in this situation. Furthermore, as the flow rate increased, so did the extraction rate. By increasing the flow rate, the process could be sped up significantly, though this was associated with a slight increase in the mass of CO2 required for extraction.
6. Future Perspectives and Conclusions
Waste management has changed from emission reduction or mitigation to a more practical strategy. Waste products are still considered viable materials utilized as raw resources, which is a need for long-term development. The necessity of restricting natural resources to achieve a balanced norm is associated with reducing greenhouse gas emissions, contributing to climate change. Agro-industrial wastes have become one of the global concerns due to their processing, usage, and disposal. Consequently, this has led to a natural resource depletion for the world’s growing population. Meanwhile, the concepts such as “cradle to cradle” and “circular economy” are recently used in the biorefinery model. These models aim to utilize and treat waste from specific industrial processes as a sustainable raw material, focusing on the economic and environmental aspects that could minimize the use of non-renewable resources.
Since apple pomace is produced in enormous quantities and contains a high proportion of water, it has storage issues that must be addressed immediately to prevent putrefaction. Processing garbage into value-added products could reduce the waste and become a viable option for the global community. Pomace has long been utilized as an animal feed source for cows, buffaloes, sheep, and goats. Its potent antioxidant capacity, antibacterial and antiviral properties are also beneficial and could be used in future extraction processes, particularly in the nutraceutical and pharmaceutical industries. Previously, traditional extraction techniques have included maceration and Soxhlet, both of which were commonly employed to extract apple pomace. However, “green extraction” of plant material has overcome the traditional extraction method. Green extraction has been demanded to enhance yield at a lower cost. This technique also could prevent the generation of harmful residue if the organic solvent is not used. Therefore, significant advances in green extraction technology have been made, including MAE, SC-CO2, and SWE. The summary of factors and responses of conventional and modern extraction technology for apple pomace valorization are shown in Table 1.

Table 1.
Factors and responses of conventional and modern extraction technology for apple pomace valorization.
Pectin is a polymer found naturally in the primary walls of non-woody plant cells and could be obtained from apple pomace. It is widely used, particularly in the food industry as a hydrocolloid, to absorb water and shape gels at low concentrations. The applications also have expanded to other sectors with various purposes [102]. Pectin applications are associated with some of the structural and functional properties of polysaccharides caused by the extraction process. Currently, the pectin extraction method is well-established. The phenolic compounds also are the most interest compounds extracted from the apple pomace by different methods. Antioxidants are proven to occur in phytochemicals containing phenol. This indicates that antioxidants can inhibit the interaction of free radicals with other molecules in the body, reducing DNA damage and long-term health repercussions. Free radicals are unstable compounds that have lost an electron.
Nonetheless, to meet rising demand, an improvement should enhance the process’s efficiency, predictability, and repeatability of product quality. As highlighted in this review, microwave, ultrasound, subcritical water, and SC-CO2 are among the effective and reliable novel strategies investigated for incorporating the pectin extraction method, with varying degrees of performance. Although these methods are quantitatively and qualitatively appropriate for laboratory use, their conventional industrial application is hampered by a lack of understanding. The drawback is that it is not applicable at scale-up, as a continuous method is preferred.
Finally, while this may not be the case for more prominent niche chemical/ingredient manufacturers, some of these innovations are incredibly expensive for new and small manufacturers. However, careful optimization of the process parameters of the latest methods is required. It won’t be long before market players employ one or more of these methods for producing specialized pectin, most probably using microwave heating for rapid mass transfer. Aside from the previously mentioned industrially developed apple pomace, several research institutes and laboratories also have focused on identifying and utilizing apple pomace as raw materials for pectin processing. Furthermore, research on pectin/bioactive compounds recovery from apple pomace using green technology is scarce, such as UAE, SWE, and SC-CO2. However, conventional pectin extraction methods, such as Soxhlet extraction and MAE, were commonly used. Therefore, there is a technological gap in extracting pectin/bioactive compounds using green technologies.
Author Contributions
Conceptualization, N.R.P.; methodology, A.H.A.A.; software, N.R.P.; validation, D.N.R.; writing—original draft preparation, A.H.A.A.; writing—review and editing, I.V., I.H., A.T.; funding, I.H. and N.R.P.; visualization, N.R.P.; supervision, M.A.C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia and Department General Education, Faculty of Resilence, Rabdan Academy, Abu Dhabi, United Arab Emirates.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
The authors would like to acknowledge the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia for supporting this work and Department General Education, Faculty of Resilence, Rabdan Academy, Abu Dhabi, United Arab Emirates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yates, M.; Gomez, M.R.; Martin-Luengo, M.A.; Ibañez, V.Z.; Serrano, A.M.M. Multivalorization of apple pomace towards materials and chemicals. Waste to wealth. J. Clean. Prod. 2017, 143, 847–853. [Google Scholar] [CrossRef]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; bin Ujang, Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Machmudah, S.; Yunus, M.A.C. Solubility of catechin and epicatechin from Arachis Hypogea skins wastes by using supercritical carbon dioxide-ethanol and its optimization. J. Food Meas. Charact. 2021, 15, 2031–2038. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Machmudah, S.; Shalleh, L.M.; Che Yunus, M.A. Recovery and solubility of flavonoid and phenolic contents from Arachis Hypogea in supercritical carbon dioxide assisted by ethanol as cosolvent. J. Food Process. Preserv. 2020, 44, e14768. [Google Scholar] [CrossRef]
- Rasidek, N.A.M.; Nordin, M.F.M.; Tokuyama, H.; Nagatsu, Y.; Mili, N.; Zaini, A.S.; Idham, Z.; Yunus, M.A.C. Subcritical water-based pectin from banana peels (Musa Paradisiaca Cv. Tanduk) as a natural gelation agent. Mater. Today Proc. 2021, 47, 1329–1335. [Google Scholar]
- FAO. Production of Apple. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 May 2020).
- Joshi, V.K.; Sandhu, D. Preparation and evaluation of an animal feed byproduct produced by solid-state fermentation of apple pomace. Bioresour. Technol. 1996, 56, 251–255. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D. Utilization of pomace from apple processing industries: A review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef]
- Alibes, X.; Munoz, F.; Rodriguez, J. Feeding value of apple pomace silage for sheep. Anim. Feed Sci. Technol. 1984, 11, 189–197. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Pingret, D.; Fabiano-Tixier, A.-S.; Le Bourvellec, C.; Renard, C.M.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef]
- Mandana, B.; Russly, A.; Farah, S.; Noranizan, M.; Zaidul, I.; Ali, G. Antioxidant activity of winter melon (Benincasa hispida) seeds using conventional Soxhlet extraction technique. Int. Food Res. J. 2012, 19, 229–234. [Google Scholar]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Salgın, U.; Salgın, S.; Ekici, D.D.; UludaĿ, G. Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. J. Supercrit. Fluids 2016, 118, 194–202. [Google Scholar] [CrossRef]
- Hasmida, M.; Liza, M.; Nur Syukriah, A.; Harisun, Y.; Mohd Azizi, C.; Fadzilah Adibah, A. Total Phenolic Content and Antioxidant Activity of Quercus infectoria Galls Using Supercritical CO2 Extraction Technique and Its Comparison with Soxhlet Extraction. Pertanika J. Sci. Technol. 2015, 23, 287–295. [Google Scholar]
- Sodeifian, G.; Saadati Ardestani, N.; Sajadian, S.A.; Ghorbandoost, S. Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: Experimental, response surface and grey wolf optimization methodology. J. Supercrit. Fluids 2016, 114, 55–63. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak-Błaszczyk, K.; Sójka, M.; Kosmala, M.; Dobrzyńska-Inger, A.; Rój, E. Changes of bioactive components in berry seed oils during supercritical CO2 extraction. J. Food Process. Preserv. 2018, 42, e13368. [Google Scholar] [CrossRef]
- Al-Rawi, S.S.; Ibrahim, A.H.; Majid, A.S.A.; Majid, A.M.A.; Ab Kadir, M.O. Comparison of yields and quality of nutmeg butter obtained by extraction of nutmeg rind by soxhlet and supercritical carbon dioxide (SC-CO2). J. Food Eng. 2013, 119, 595–601. [Google Scholar] [CrossRef]
- De Melo, M.; Şen, A.; Silvestre, A.J.; Pereira, H.; Silva, C.M. Experimental and modeling study of supercritical CO2 extraction of Quercus cerris cork: Influence of ethanol and particle size on extraction kinetics and selectivity to friedelin. Sep. Purif. Technol. 2017, 187, 34–45. [Google Scholar] [CrossRef]
- Pour Hosseini, S.R.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Chai, Y.H.; Uemura, Y.; Loh, S.K. Extraction of palm kernel shell derived pyrolysis oil by supercritical carbon dioxide: Evaluation and modeling of phenol solubility. Biomass Bioenergy 2018, 116, 106–112. [Google Scholar] [CrossRef]
- Asghari, J.; Ondruschka, B.; Mazaheritehrani, M. Extraction of bioactive chemical compounds from the medicinal Asian plants by microwave irradiation. J. Med. Plants Res. 2011, 5, 495–506. [Google Scholar]
- Taheri, S.; Brodie, G.; Gupta, D. Fluidisation of lentil seeds during microwave drying and disinfection could prevent detrimental impacts on their chemical and biochemical characteristics. LWT 2020, 129, 109534. [Google Scholar] [CrossRef]
- Shah, S.; Gani, A.; Ahmad, M.; Shah, A.; Gani, A.; Masoodi, F. In vitro antioxidant and antiproliferative activity of microwave-extracted green tea and black tea (Camellia sinensis): A comparative study. NutraFoods 2015, 14, 207–215. [Google Scholar] [CrossRef]
- Li, Z.; Huang, D.; Tang, Z.; Deng, C. Microwave-assisted extraction followed by CE for determination of catechin and epicatechin in green tea. J. Sep. Sci. 2010, 33, 1079–1084. [Google Scholar] [CrossRef]
- Yunus, M.; Hasan, M.; Othman, N.; Mohd-Septapar, S.; Ahmad-Zaini, M.; Idham, Z.; Zhari, S. Effect of particle size on the oil yield and catechin compound using accelerated solvent extraction. J. Teknol. Sci. Eng. 2013, 60, 21–25. [Google Scholar]
- Saim, N.a.; Dean, J.R.; Abdullah, M.P.; Zakaria, Z. Extraction of polycyclic aromatic hydrocarbons from contaminated soil using Soxhlet extraction, pressurised and atmospheric microwave-assisted extraction, supercritical fluid extraction and accelerated solvent extraction. J. Chromatogr. A 1997, 791, 361–366. [Google Scholar] [CrossRef]
- Sarip, M.S.M.; Yamashita, Y.; Morad, N.A.; Yunus, M.A.C.; Aziz, M.K.A. Modeling and Optimization of the Hot Compressed Water Extraction of Palm Oil Using Artificial Neural Network. J. Chem. Eng. Jpn. 2016, 49, 614–621. [Google Scholar] [CrossRef]
- Hans, N.; Naik, S.N.; Malik, A. Platform Molecules from Algae by Using Supercritical CO2 and Subcritical Water Extraction. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 229–243. [Google Scholar]
- Kim, J.-W.; Nagaoka, T.; Ishida, Y.; Hasegawa, T.; Kitagawa, K.; Lee, S.-C. Subcritical water extraction of nutraceutical compounds from citrus pomaces. Sep. Sci. Technol. 2009, 44, 2598–2608. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Rizkiyah, D.N.; Jusoh, W.M.S.W.; Idham, Z.; Putra, N.R.; Che Yunus, M.A. Investigation of Phenolic, Flavonoid and Antioxidant Recovery and Solubility from Roselle Using Supercritical Carbon Dioxide: Experimental and Modelling. J. Food Process. Preserv. 2022, 46, e16670. [Google Scholar] [CrossRef]
- Kertesz, Z.I. The Pectic Substances; Interscience Publishers: New York, NY, USA, 1951; Volume 1. [Google Scholar]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2020, 254, 117324. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefining 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Salma, M.; Jahan, N.; Islam, M.; Hoque, M. Extraction of Pectin from lemon peel: Technology development. J. Chem. Eng. 2012, 27, 25–30. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: Optimization and modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Putra, N.R.; Wibobo, A.G.; Machmudah, S.; Winardi, S. Recovery of valuable compounds from palm-pressed fiber by using supercritical CO2 assisted by ethanol: Modeling and optimization. Sep. Sci. Technol. 2020, 55, 3126–3139. [Google Scholar] [CrossRef]
- Idham, Z.; Putra, N.R.; Aziz, A.H.A.; Zaini, A.S.; Rasidek, N.A.M.; Mili, N.; Yunus, M.A.C. Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J. CO2 Util. 2022, 56, 101839. [Google Scholar] [CrossRef]
- Idham, Z.; Putra, N.R.; Nasir, H.; Yian, L.N.; Idrus, N.F.M.; Yunus, M.A.C. Extraction and Solubility Modeling of Anthocyanins Rich Extract from Hibiscus sabdariffa L. using Supercritical Carbon Dioxide. Malays. J. Fundam. Appl. Sci. 2021, 17, 720–730. [Google Scholar] [CrossRef]
- da Silva, L.C.; Souza, M.C.; Sumere, B.R.; Silva, L.G.; da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.; Rostagno, M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- de Castro, M.D.L.; Valcárcel, M.; Tena, M.T. Analytical Supercritical Fluid Extraction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Guerin, T. The extraction of aged polycyclic aromatic hydrocarbon (PAH) residues from a clay soil using sonication and a Soxhlet procedure: A comparative study. J. Environ. Monit. 1999, 1, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, C.; Rodriguez-Saona, L. Application of NIR and MIR spectroscopy in quality control of potato chips. J. Food Compost. Anal. 2009, 22, 596–605. [Google Scholar] [CrossRef]
- Wu, T.R.; Wang, H.L.; Jiang, S.W.; Liu, D.D.; Wei, F. Optimization of Extraction of Tannins from Banana Peel Using Response Surface Methodology. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2014; pp. 566–571. [Google Scholar]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012, 135, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Brunton, N. The optimisation of solid–liquid extraction of antioxidants from apple pomace by response surface methodology. J. Food Eng. 2010, 96, 134–140. [Google Scholar] [CrossRef]
- Letellier, M.; Budzinski, H.; Charrier, L.; Capes, S.; Dorthe, A. Optimization by factorial design of focused microwave assisted extraction of polycyclic aromatic hydrocarbons from marine sediment. Fresenius J. Anal. Chem. 1999, 364, 228–237. [Google Scholar] [CrossRef]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Zuloaga, O.; Etxebarria, N.; Fernández, L.A.; Madariaga, J.M. Optimisation and comparison of microwave-assisted extraction and Soxhlet extraction for the determination of polychlorinated biphenyls in soil samples using an experimental design approach. Talanta 1999, 50, 345–357. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Latha, C. Microwave-assisted extraction of embelin from Embelia ribes. Biotechnol. Lett. 2007, 29, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- García-Ayuso, L.; Velasco, J.; Dobarganes, M.; De Castro, M.L. Determination of the oil content of seeds by focused microwave-assisted soxhlet extraction. Chromatographia 2000, 52, 103–108. [Google Scholar] [CrossRef]
- Moirangthem, K.; Ramakrishna, P.; Amer, M.H.; Tucker, G.A. Bioactivity and anthocyanin content of microwave-assisted subcritical water extracts of Manipur black rice (Chakhao) bran and straw. Future Foods 2021, 3, 100030. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Martín-González, M.S.; Hirst, P.; Ballard, T.S. Optimizing Microwave-Assisted Extraction of Phenolic Antioxidants from R ed D elicious and J onathan Apple Pomace. J. Food Process Eng. 2015, 38, 571–582. [Google Scholar] [CrossRef]
- Rezaei, S.; Rezaei, K.; Haghighi, M.; Labbafi, M. Solvent and solvent to sample ratio as main parameters in the microwave-assisted extraction of polyphenolic compounds from apple pomace. Food Sci. Biotechnol. 2013, 22, 1–6. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Ishak, N.A.; Razak, N.A.A.; Dek, M.S.P.; Baharuddin, A.S. Production of High Tannin Content and Antioxidant Activity Extract from an Unripe Peel of Musa acuminata (Cavendish) Using Ultrasound-Assisted Extraction (UAE). BioResources 2020, 15, 1877–1893. [Google Scholar]
- De la Guardia, M.; Armenta, S. Origins of green analytical chemistry. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 57, pp. 1–23. [Google Scholar]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-H.; Ahn, J.; Kang, D.-H.; Lee, H.-Y. The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochemistry 2001, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Tian, H.; Li, Q.; Cai, T.; Du, W. Ultrasound-assisted extraction of xyloglucan from apple pomace. Ultrason. Sonochem. 2006, 13, 511–516. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Ultrasound-assisted extraction of pectin from Malus domestica ‘Fălticeni’apple pomace. Processes 2019, 7, 488. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of extraction. In Handbook of Sample Preparation; John Wiley and Sons: Hoboken, NJ, USA, 2010; pp. 1–24. [Google Scholar]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Möckel, H.; Welter, G.; Melzer, H. Correlation between reversed-phase retention and solute molecular surface type and area: I. Theoretical outlines and retention of various hydrocarbon classes. J. Chromatogr. A 1987, 388, 255–266. [Google Scholar] [CrossRef]
- Green, D.W.; Southard, M.Z. Perry’s Chemical Engineers’ Handbook; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Richter, B.; Ezzell, J.; Knowles, D.; Hoefler, F.; Mattulat, A.; Scheutwinkel, M.; Waddell, D.; Gobran, T.; Khurana, V. Extraction of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans from environmental samples using accelerated solvent extraction (ASE). Chemosphere 1997, 34, 975–987. [Google Scholar] [CrossRef]
- Deng, C.; Ji, J.; Wang, X.; Zhang, X. Development of pressurized hot water extraction followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry for determination of ligustilides in Ligusticum chuanxiong and Angelica sinensis. J. Sep. Sci. 2005, 28, 1237–1243. [Google Scholar] [CrossRef]
- Choi, M.P.; Chan, K.K.; Leung, H.W.; Huie, C.W. Pressurized liquid extraction of active ingredients (ginsenosides) from medicinal plants using non-ionic surfactant solutions. J. Chromatogr. A 2003, 983, 153–162. [Google Scholar] [CrossRef]
- Ong, E.S.; Cheong, J.S.H.; Goh, D. Pressurized hot water extraction of bioactive or marker compounds in botanicals and medicinal plant materials. J. Chromatogr. A 2006, 1112, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Brunton, N. The optimization of extraction of antioxidants from apple pomace by pressurized liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef] [PubMed]
- Klinchongkon, K.; Khuwijitjaru, P.; Adachi, S. Properties of subcritical water-hydrolyzed passion fruit (Passiflora edulis) pectin. Food Hydrocoll. 2018, 74, 72–77. [Google Scholar] [CrossRef]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Munir, M.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Budrat, P.; Shotipruk, A. Enhanced recovery of phenolic compounds from bitter melon (Momordica charantia) by subcritical water extraction. Sep. Purif. Technol. 2009, 66, 125–129. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Putra, N.R.; Che Yunus, M.A.; Machmudah, S. Solubility model of arachis hypogea skin oil by modified supercritical carbon dioxide. Sep. Sci. Technol. 2019, 54, 731–740. [Google Scholar] [CrossRef]
- Radfar, S.; Ghoreishi, S.M. Experimental extraction of L-Carnitine from oyster mushroom with supercritical carbon dioxide and methanol as co-solvent: Modeling and optimization. J. Supercrit. Fluids 2018, 140, 207–217. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Ganjloo, A.; Taip, F.S.; Adzahan, N.M.; Sarker, M.Z.I. Characterization of Valuable Compounds from Winter Melon (Benincasa hispida (Thunb.) Cogn.) Seeds Using Supercritical Carbon Dioxide Extraction Combined with Pressure Swing Technique. Food Bioprocess Technol. 2016, 9, 396–406. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; Yian, L.N.; Yunus, M.A.C.; Muhamad, I.-I.; Zaini, M.A.A. Extraction of Rubber (Hevea brasiliensis) Seeds Oil Using Supercritical Carbon Dioxide. J. Biobased Mater. Bioenergy 2013, 7, 213–218. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Nunes, S.; Martínez-Blázquez, J.A.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Effect of high hydrostatic pressure and drying methods on phenolic compounds profile of jabuticaba (Myrciaria jaboticaba) peel and seed. Food Chem. 2020, 309, 125794. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef]
- Ekinci, M.S.; Gürü, M. Extraction of oil and β-sitosterol from peach (Prunus persica) seeds using supercritical carbon dioxide. J. Supercrit. Fluids 2014, 92, 319–323. [Google Scholar] [CrossRef]
- Xie, L.; Cahoon, E.; Zhang, Y.; Ciftci, O.N. Extraction of astaxanthin from engineered Camelina sativa seed using ethanol-modified supercritical carbon dioxide. J. Supercrit. Fluids 2019, 143, 171–178. [Google Scholar] [CrossRef]
- Natolino, A.; Da Porto, C. Supercritical carbon dioxide extraction of pomegranate (Punica granatum L.) seed oil: Kinetic modelling and solubility evaluation. J. Supercrit. Fluids 2019, 151, 30–39. [Google Scholar] [CrossRef]
- Martins, M.A.; Domańska, U.; Schröder, B.; Coutinho, J.A.; Pinho, S.o.P. Selection of ionic liquids to be used as separation agents for terpenes and terpenoids. ACS Sustain. Chem. Eng. 2016, 4, 548–556. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus p luvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of triterpenic acids and phytosterols from apple pomace with supercritical carbon dioxide: Impact of process parameters, modelling of kinetics, and scaling-up study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro. Food Ind. Hi-Tech 2016, 27, 17–20. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).