Performance Assessment of Three Latent Heat Storage Designs for a Solar Hot Water Tank
Abstract
1. Introduction
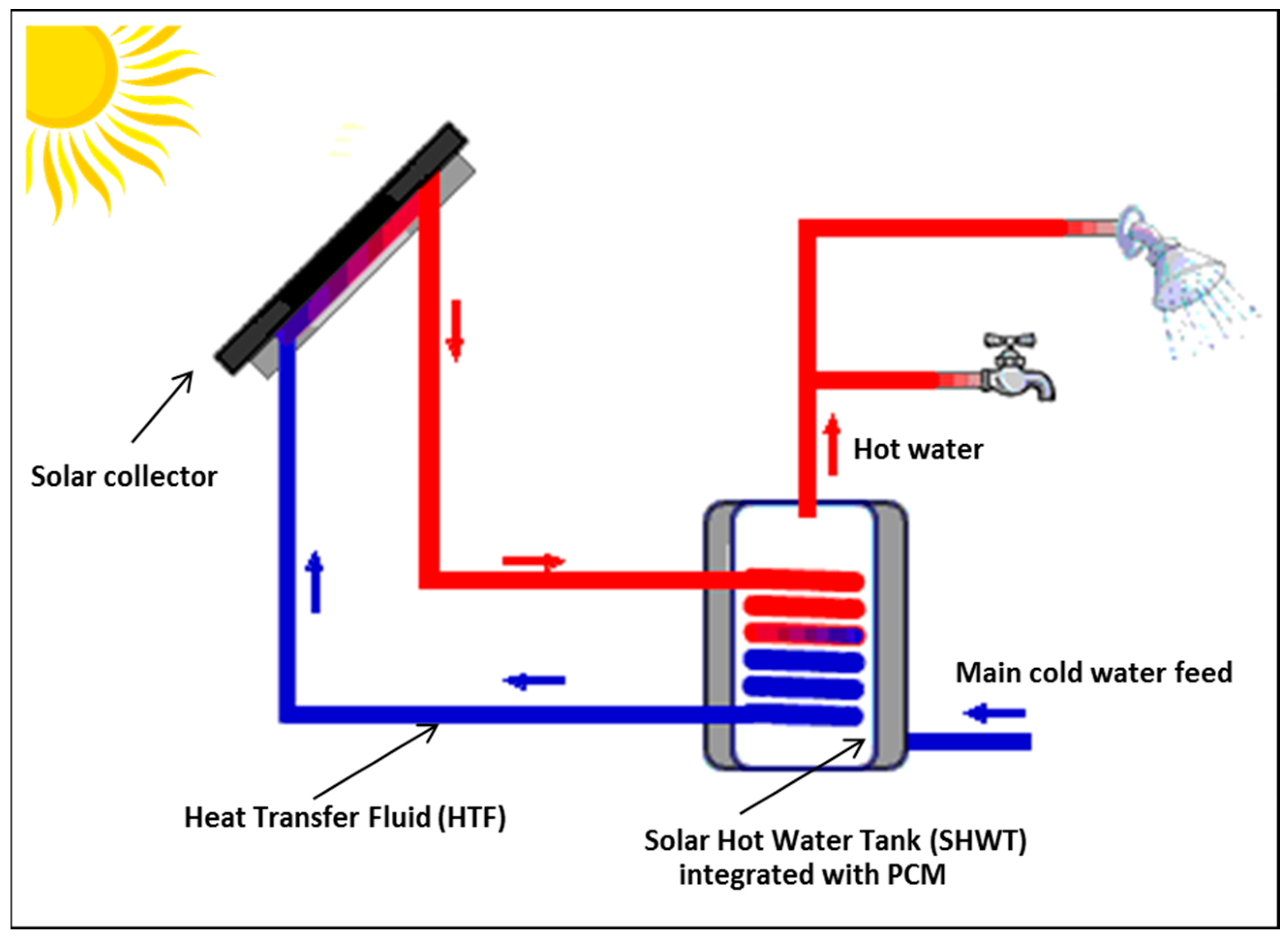
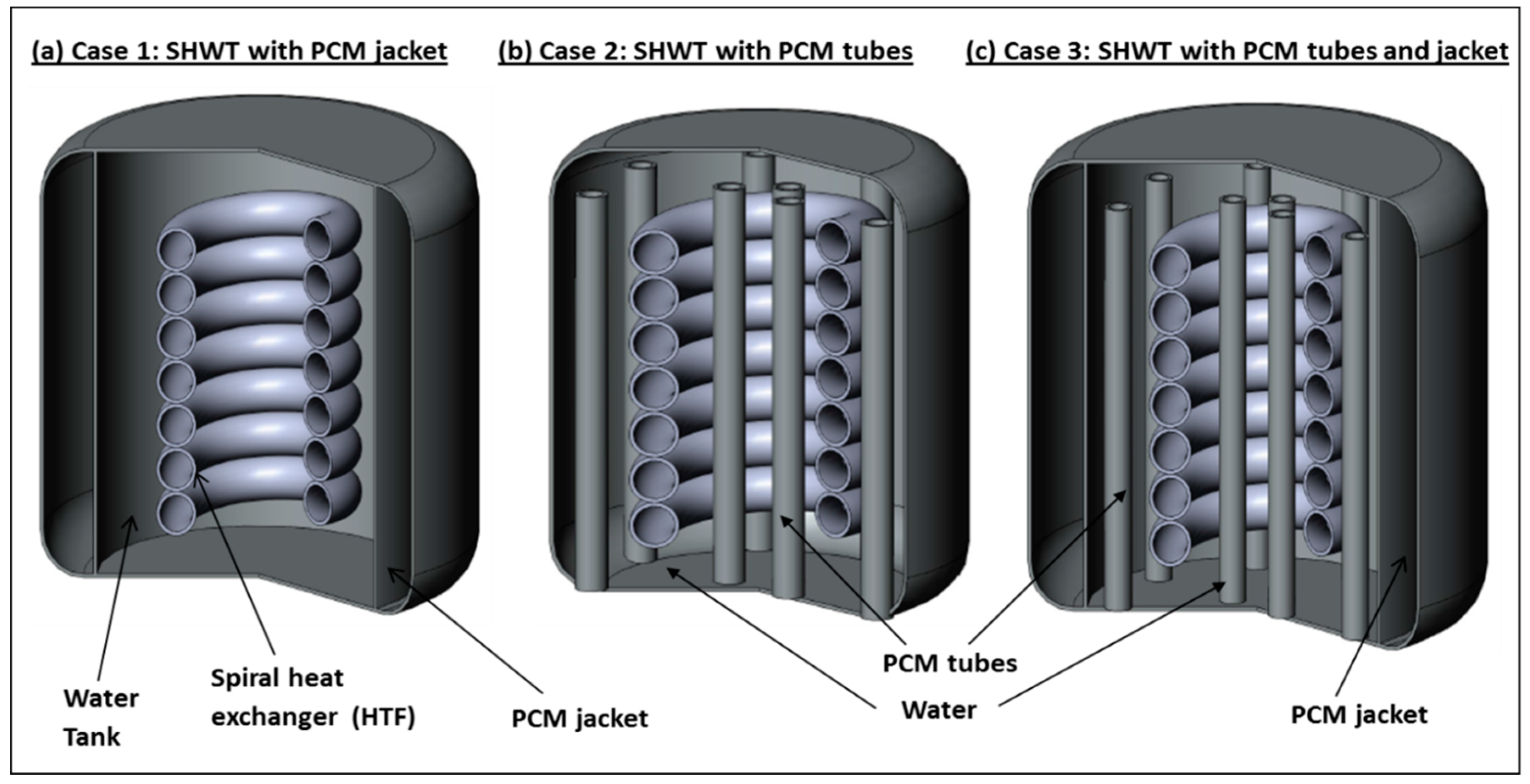
2. Description of the Three SHWT Designs
- (i)
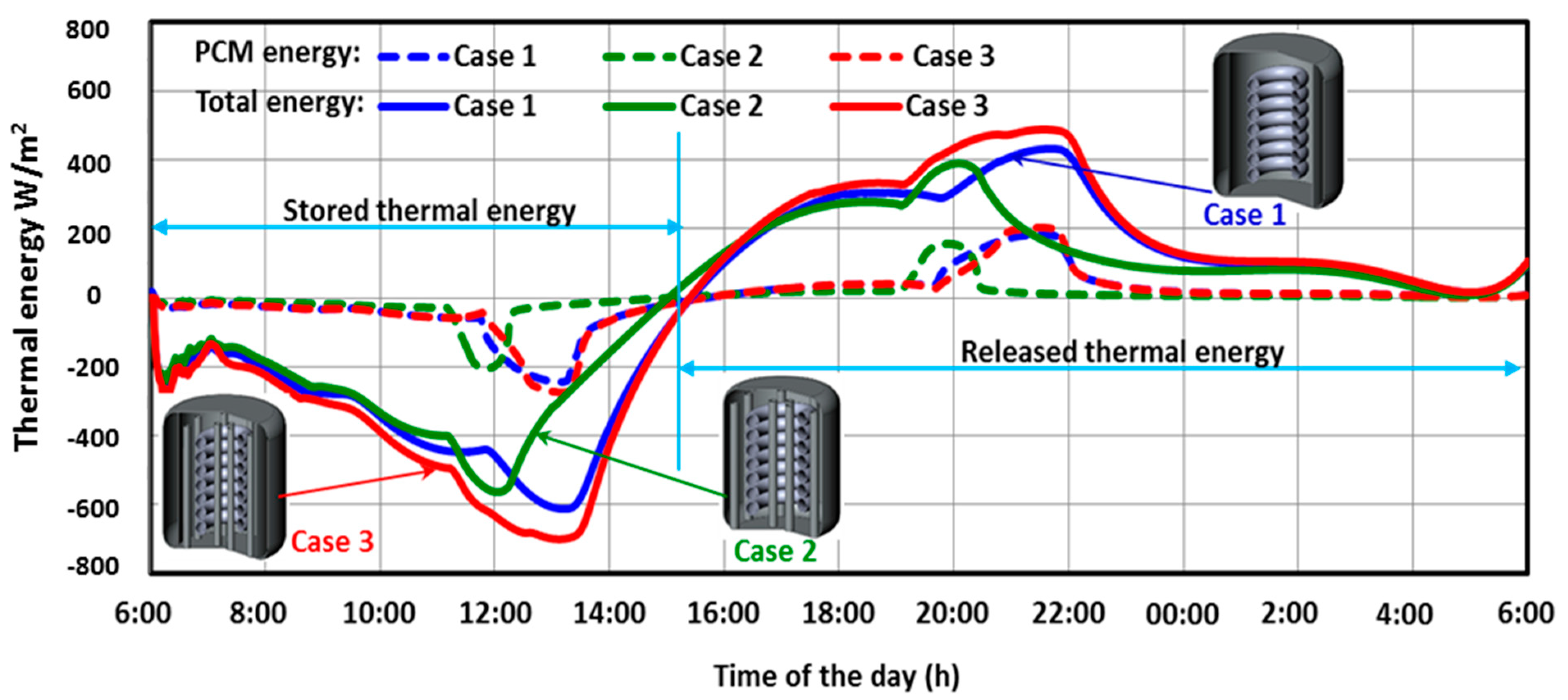
- Design 1: consists of a cylindrical tank which is integrated to a central spiral heat exchanger tube. The spiral tube is further connected to a solar thermal collector which converts the solar radiation into thermal energy. This thermal energy is then used to heat an HTF that passes through the spiral tube inside the water tank. The heat collected by the solar collector passes through a tube via an HTF to a spiral-type heat exchanger located in the SHWT. The HTF then gives up its calories to the domestic water before returning, once cooled, to the collector to be heated as long as the sunshine allows it. During bad weather, extra energy takes over. A heat accumulator, which is connected to the SHWT and PCM, is contained in an external jacket that stores the thermal energy while passing from a solid state to a liquid state. The PCM jacket changes phase when the solar collector begins to be overproduced. The energy is then restored when the need arises. This system thus makes it possible to increase the storage capacity without increasing the volume of the water tank. Its application is, therefore, effective in overcoming the intermittent solar energy utilization problem.
- (ii)
- Design 2: consists of a cylindrical tank integrated with a central spiral heat exchanger tube. The spiral tube is connected to a solar thermal collector. The SHWT is integrated with twelve encapsulated PCM tubes.
- (iii)
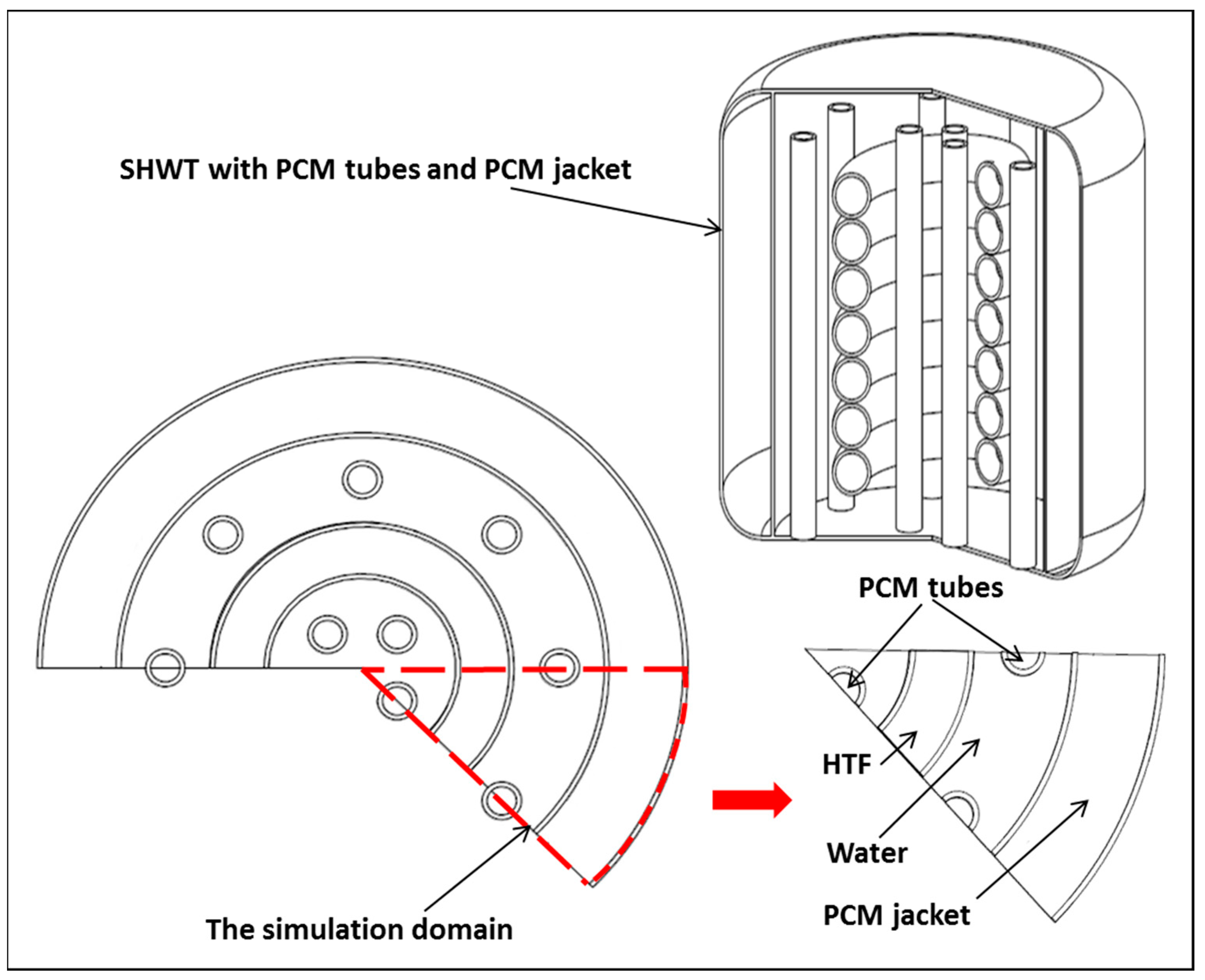
- Design 3: similar to design one and design two, the SHWT is equipped with an external PCM jacket and integrated with twelve encapsulated PCM tubes.
3. Mathematical Model
- (i)
- constant thermo-physical properties
- (ii)
- the wall’s thermal resistance is negligible.
- (iii)
- the fluid is incompressible fluid
3.1. Continuity Equation
3.2. Momentum Equation
3.3. Energy Equations
3.4. The HTF Temperature
3.5. The Storage Efficiency of the SHWT-PCM System
3.6. Initial and Boundary Conditions
- (i)
- The temperatures of the PCM, HTF, and water are uniform
- (ii)
- The external wall thickness of the SHWT-PCM system is thermally insulated,
- (iii)
- The HTF temperature is variable with time, as expected in Equation (7),
- (iv)
- The domain interface is thermally insulated.
4. Simulation Method and Model Validation
5. Results and Discussion
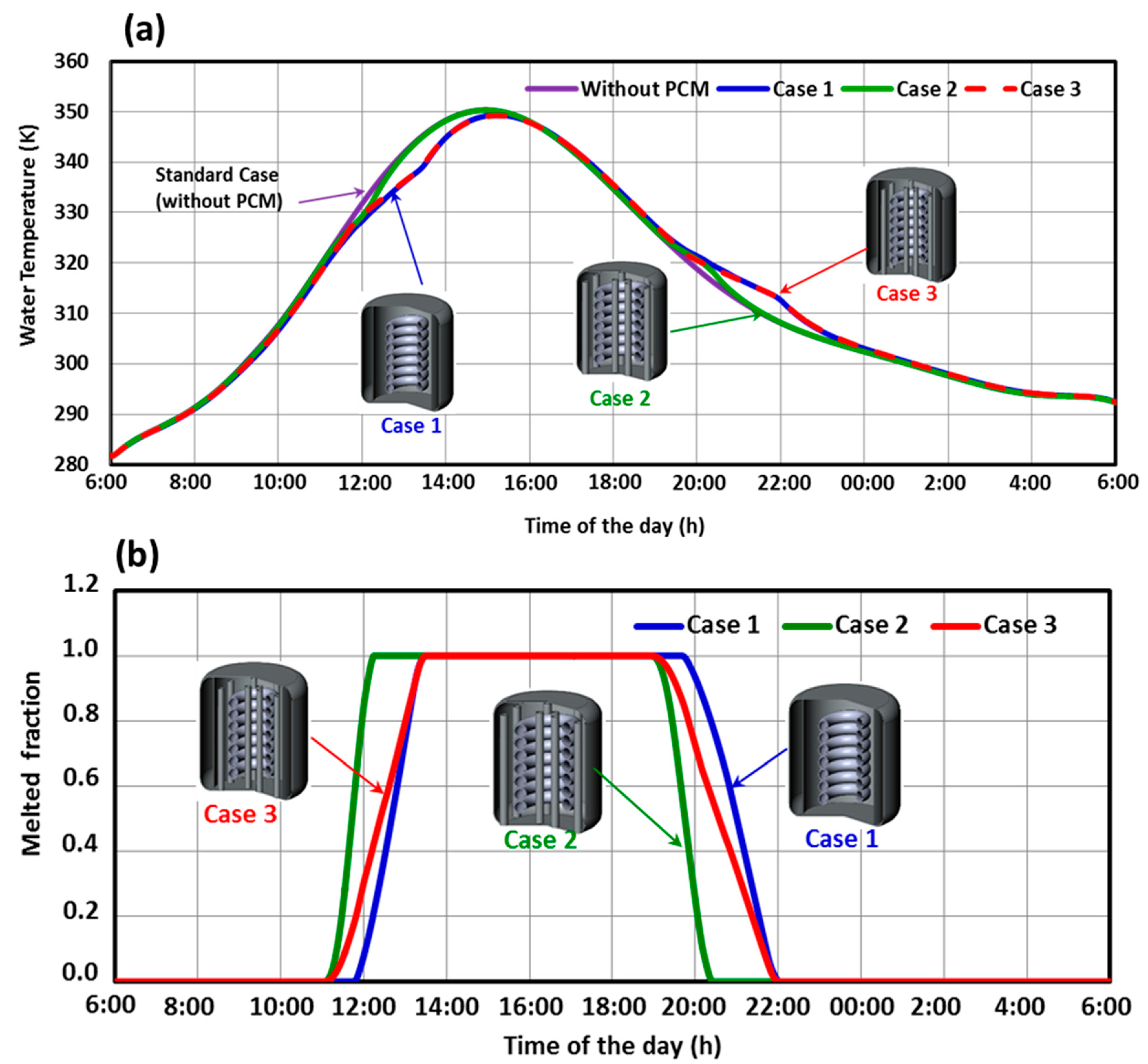
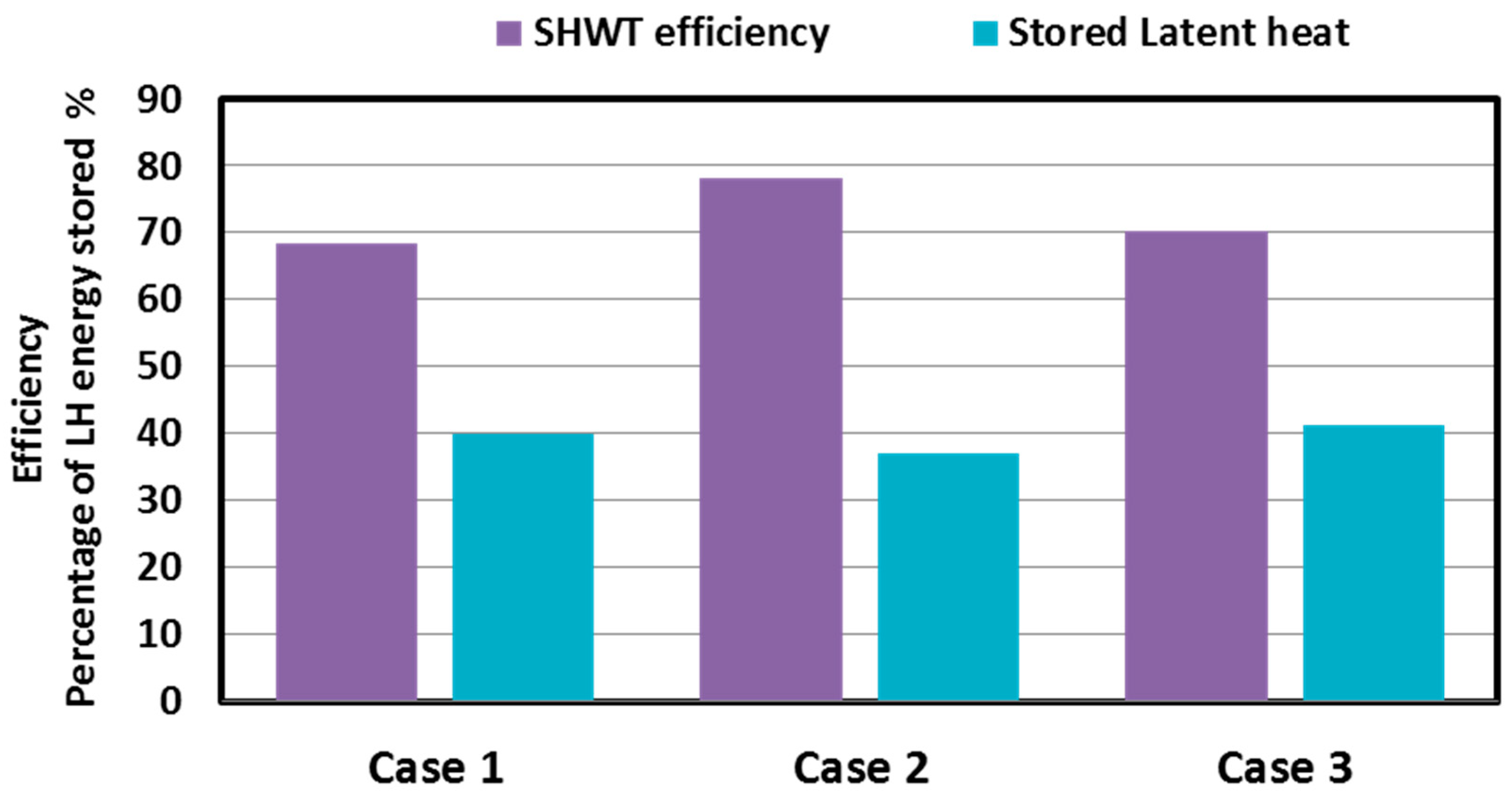
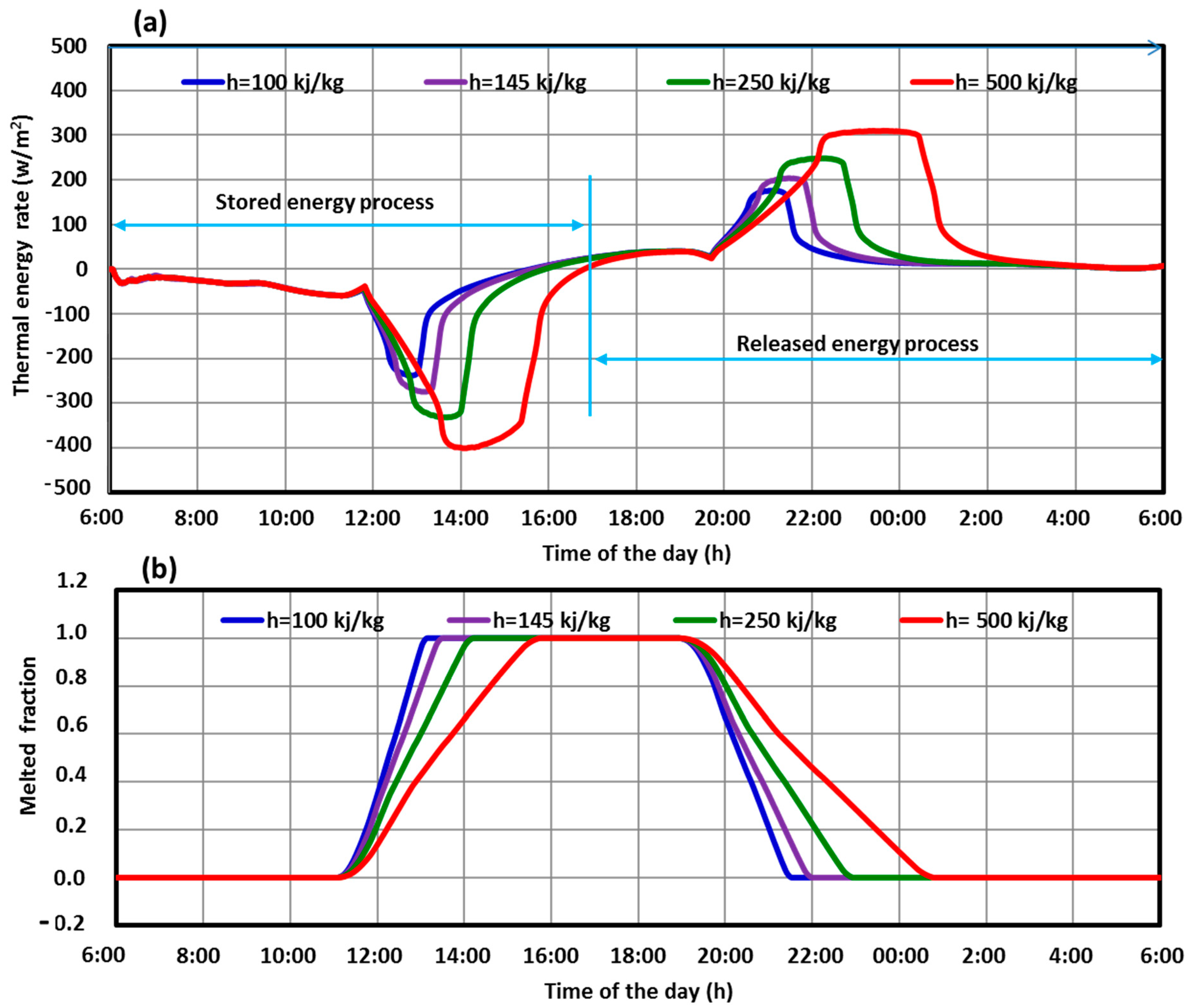
5.1. Performance of the Three SHWT-PCM Tank Designs
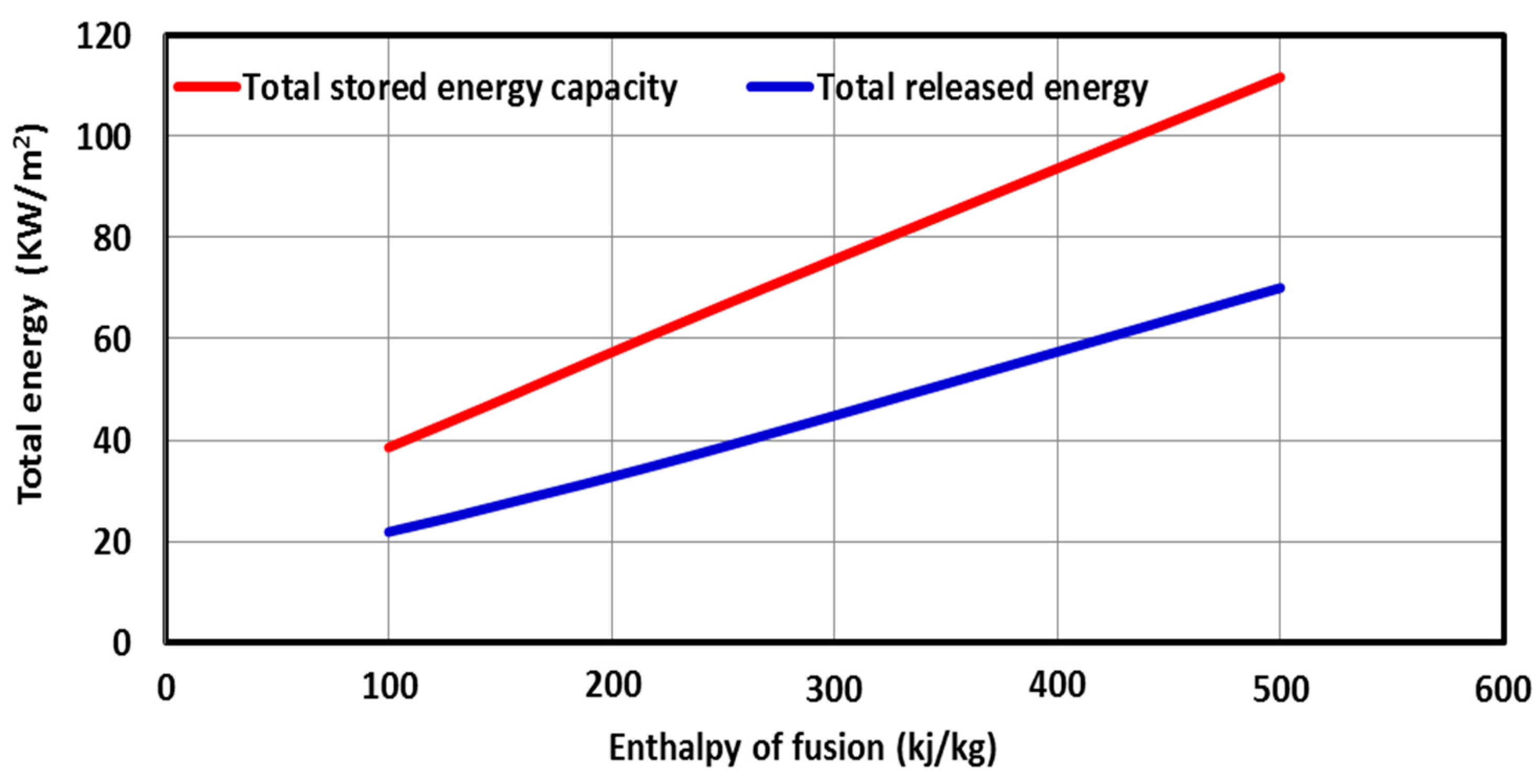
5.2. Effect of the PCM Type
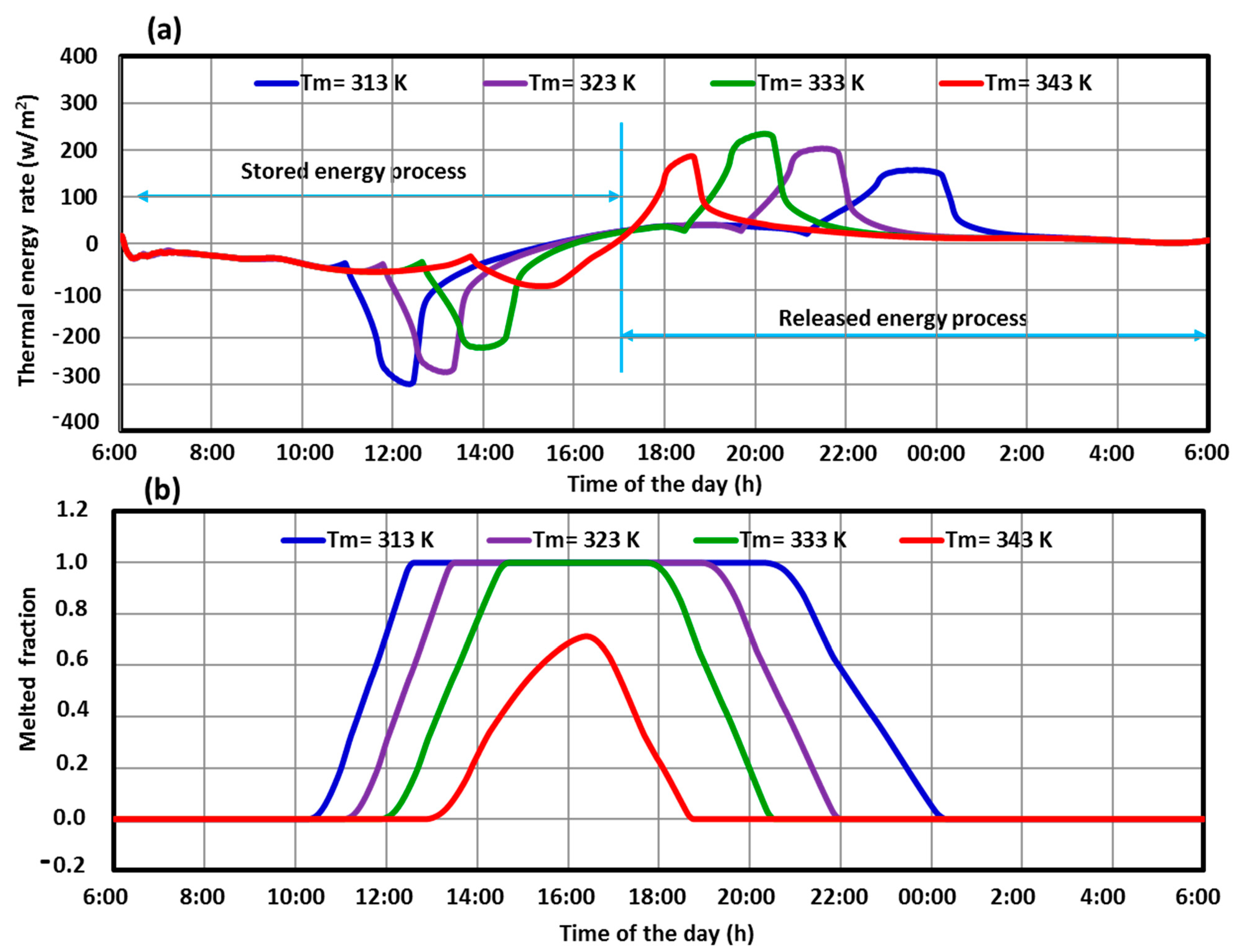
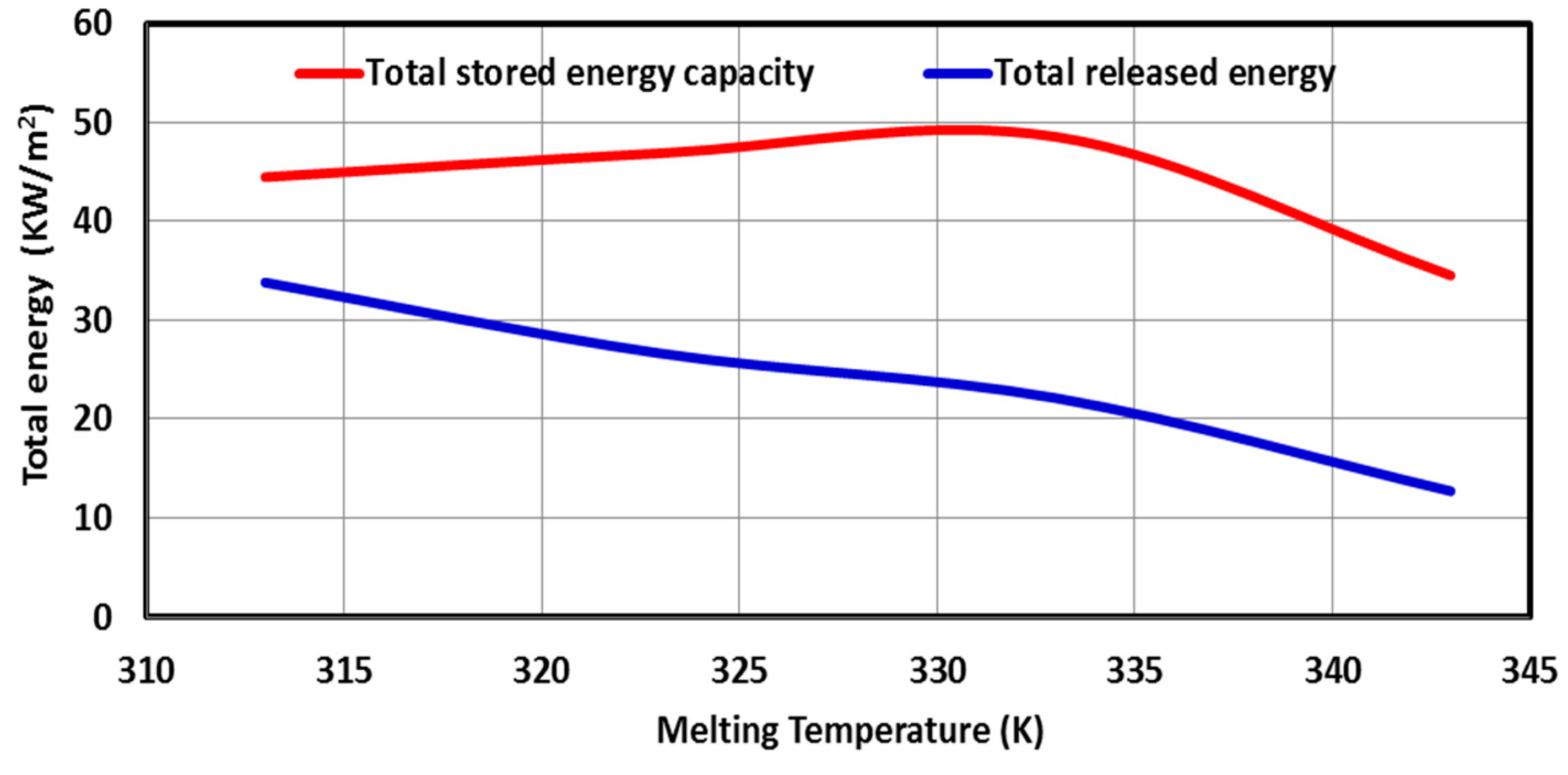
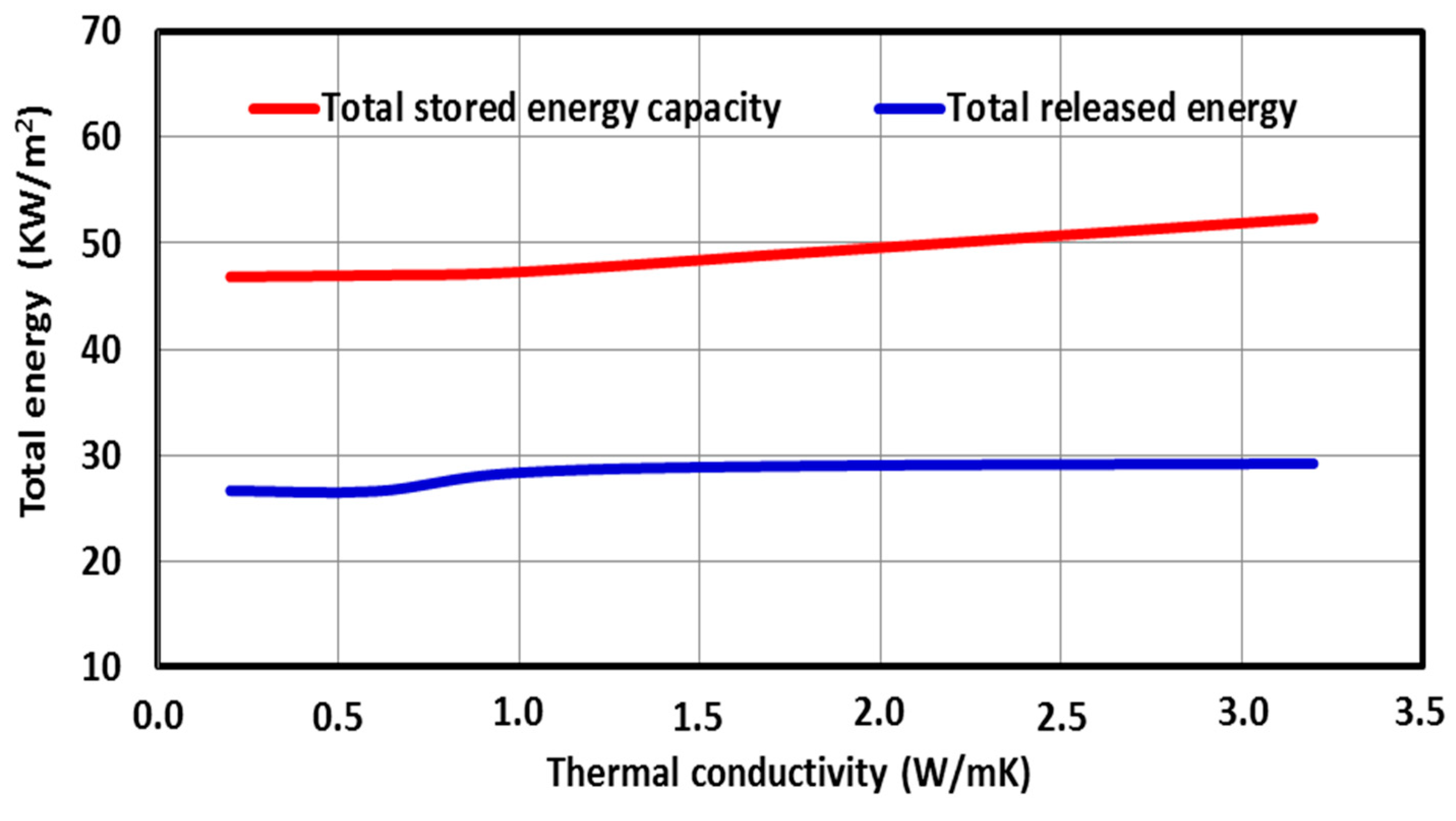
5.3. Effect of PCM Thermo-Physical Properties
6. Conclusions
- –
- The SHWT-PCM system’s design significantly affects the storage process.
- –
- With the proposed design (case 3), where the PCM tubes and the PCM jacket are integrated, a higher portion of the latent heat energy (41%) was stored, and the thermal storage efficiency was up to 70%.
- –
- The water temperature of the SHWT in case 3 was higher compared to another design. This was caused by the integration of the PCM jacket, which decreases thermal losses by increasing the heat storage and insulation properties of the PCM.
- –
- Both the latent heat and the thermal conductivity of the PCM must be as high as possible to quickly store a large amount of thermal energy on-peak period load, which is then shifted to the off-peak period.
- –
- An optimum PCM melting temperature can be selected for the better SHWT-PCM system’s thermal performance. The PCM melting temperature value is 333 K, favored when a more extended shifting period is needed.
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CP | heat capacity (kj/kg.K) |
| F | the melted fraction of PCM |
| the acceleration of gravity (m/s2) | |
| H | the enthalpy (kj/kg) |
| K | the thermal conductivity (W/m.K) |
| L | the latent heat (kj/kg) |
| P | pressure (Pa) |
| T | the temperature (K) |
| the velocity (m/s) | |
| the density (kg/m3) | |
| the dynamic viscosity (Pa s) | |
| Abbreviations: | |
| HTF | Heat Transfer Fluid |
| PCM | Phase Change Material |
| SHWT | Solar Hot Water Tank |
References
- Elarem, R.; Alqahtani, T.; Mellouli, S.; Askri, F.; Edacherian, A.; Vineet, T.; Badruddin, I.A.; Abdelmajid, J. A comprehensive review of heat transfer intensification methods for latent heat storage units. Energy Storage 2020, 3, e127. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Ibrahim, N.I.; Mahbubul, I.M.; Ali, H.M.; Saidur, R.; Al-Sulaiman, F.A. Evaluation of solar collector designs with integrated latent heat thermal energy storage: A review. Sol. Energy 2018, 166, 334–350. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Kürklü, A.; Özmerzi, A.; Bilgin, S. Thermal performance of a water-phase change material solar collector. Renew. Energy 2002, 26, 391–399. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Eames, P.; Griffiths, P. Thermal behaviour of integrated solar collector/storage unit with 65°C phase change material. Energy Convers. Manag. 2006, 47, 3611–3618. [Google Scholar] [CrossRef]
- Plantier, C. Etude Numérique et Expérimentale d’un Prototype de Chauffe- eau Solaire Equipé d’un Stockage à Chaleur Latente. Ph.D. Thesis, ESIGEC—Univérsité de Savoie, Chambéry, French, 2005. [Google Scholar]
- Haillot, D.; Goetz, V.; Py, X.; Abdelkarim, M.B. Integrated solar collector storage system based on phase change material and graphite composite. In Proceedings of the 2008, 1st International Congress on Heating, Cooling and Buildings, Lisbon, Portugal, 7–10 October 2008. [Google Scholar]
- Mettaweea, E.B.S.; Assassab, G.M.R. Experimental Study of a Compact PCM Solar Collector. Energy 2006, 31, 2958–2968. [Google Scholar] [CrossRef]
- Lacroix, M. Study of the Heat Transfer Behavior of Latent Heat Thermal Energy StorageUnit with a Finned Tube. Int. J. Heat Mass Transf. 1993, 36, 2083–2092. [Google Scholar] [CrossRef]
- Laouadi, A.; Lacroix, M. Thermal performance of a latent heat energy storage ventilated panel for electric load management. Int. J. Heat Mass Transf. 1999, 42, 275–286. [Google Scholar] [CrossRef]
- Bayomy, A.; Davies, S.; Saghir, Z. Domestic Hot Water Storage Tank Utilizing Phase Change Materials (PCMs): Numerical Approach. Energies 2019, 12, 2170. [Google Scholar] [CrossRef]
- Kılıçkap, S.; El, C.; Yıldız, E. Investigation of the Effect on the efficiency of phase change material placed in solar collector tank. Therm. Sci. Eng. Prog. 2018, 5, 25–31. [Google Scholar] [CrossRef]
- Harris Bernal, I.A.; James Rivas, A.M.; Ortega Del Rosario, M.D.L.A.; Saghir, M.Z. Redesign Methodology to Improve the Performance of a Thermal Energy Storage with Phase Change Materials: A Numerical Approach. Energies 2022, 15, 960. [Google Scholar] [CrossRef]
- Farid, M.M.; Husian, R.M. An electrical storage heater using the phase-change method of heat storage. Energy Convers. Mgmt 1990, 3, 219–230. [Google Scholar] [CrossRef]
- Hammadi, S.H. Study of solar water heating system with natural circulation in basrah. Al-Qadisiya J. Eng. Sci. 2009, 2, 3. [Google Scholar]
- Wu, W.; Dai, S.; Liu, Z.; Dou, Y.; Hua, J.; Li, M.; Wang, X.; Wang, X. Experimental study on the performance of a novel solar water heating system with and without PCM Sol. Energy 2018, 171, 604–612. [Google Scholar]
- Algarni, S.; Mellouli, S.; Alqahtani, T.; Almutairi, K.; Khan, A.; Anqi, A. Experimental investigation of an evacuated tube solar collector incorporating nano-enhanced PCM as a thermal booster. Appl. Therm. Eng. 2020, 180, 115831. [Google Scholar] [CrossRef]
- Xue, H.S. Experimental investigation of a domestic solar water heater with solar collector coupled phase-change energy storage. Renew. Energy 2016, 86, 257–261. [Google Scholar] [CrossRef]
- Saraswat, A.; Bhattacharjee, R.; Verma, A.; Das, M.K.; Khandekar, S. Investigation of diffusional transport of heat and its enhancement in phase-change thermal energy storage systems. Appl. Therm. Eng. 2017, 111, 1611–1621. [Google Scholar] [CrossRef]
- Bouhal, T.; El Rhafiki, T.; Kousksou, T.; Jamil, A.; Zeraouli, Y. PCM addition inside solar water heaters: Numerical comparative approach. J. Energy Storage 2018, 19, 232–246. [Google Scholar] [CrossRef]
- Koca, A.; Oztop, H.F.; Koyun, T.; Varol, Y. Energy and exergy analysis of a latent heat storage system with phase change material for a solar collector. Renew. Energy 2008, 33, 567–574. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Solar water heaters with phase change material thermal energy storage medium: A review. Renew. Sustain. Energy Rev. 2009, 13, 2119–2125. [Google Scholar] [CrossRef]
- Hooshmand, A.; Zahmatkesh, I.; Karami, M.; Delfani, S. Exergy analysis of a nanofluid–based direct absorption solar collector (DASC) occupied by porous foam. Chall. Nano Micro Scale Sci. Technol. 2021, 9, 1–11. [Google Scholar]
- Nemati, H.; Javanmardi, M.J. Exergy optimization of domestic solar cylindrical-parabolic cooker. J. Renew. Sustain. Energy 2012, 4, 063134. [Google Scholar] [CrossRef]



| 0 | a1 | a2 | a3 | a4 | a5 | a6 | a7 | a8 | a9 |
|---|---|---|---|---|---|---|---|---|---|
| 281.316 | 0.0025 | −3.881 × 10−7 | 4.60162 × 10−11 | −1.907 × 10−15 | 3.050 × 10−20 | −3.307 × 10−26 | −4.699 × 10−30 | 5.27 × 10−35 | −1.814 × 10−40 |
| Proprieties | PCM1 | PCM2 | PCM3 | PCM4 |
|---|---|---|---|---|
| Melting point (°C) | 50 | 40 | 44 | 64 |
| Enthalpy of fusion (kj/kg) | 145 | 174 | 190 | 250 |
| Density (kg/m) | 1412 | 900 | 930 | 880 |
| Heat capacity (kj/kg.K) | 2.4 | 2.5 | 2.1 | 2 |
| Thermal conductivity (W/m.K) | 0.2 | 0,21 | 0.21 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakri, W.; Mellouli, S.; Fageehi, Y. Performance Assessment of Three Latent Heat Storage Designs for a Solar Hot Water Tank. Sustainability 2023, 15, 640. https://doi.org/10.3390/su15010640
Zakri W, Mellouli S, Fageehi Y. Performance Assessment of Three Latent Heat Storage Designs for a Solar Hot Water Tank. Sustainability. 2023; 15(1):640. https://doi.org/10.3390/su15010640
Chicago/Turabian StyleZakri, Waleed, Sofiene Mellouli, and Yahya Fageehi. 2023. "Performance Assessment of Three Latent Heat Storage Designs for a Solar Hot Water Tank" Sustainability 15, no. 1: 640. https://doi.org/10.3390/su15010640
APA StyleZakri, W., Mellouli, S., & Fageehi, Y. (2023). Performance Assessment of Three Latent Heat Storage Designs for a Solar Hot Water Tank. Sustainability, 15(1), 640. https://doi.org/10.3390/su15010640






