Multi-Objective Optimization of Integrated Solar-Driven CO2 Capture System for an Industrial Building
Abstract
1. Introduction
2. Materials and Methods
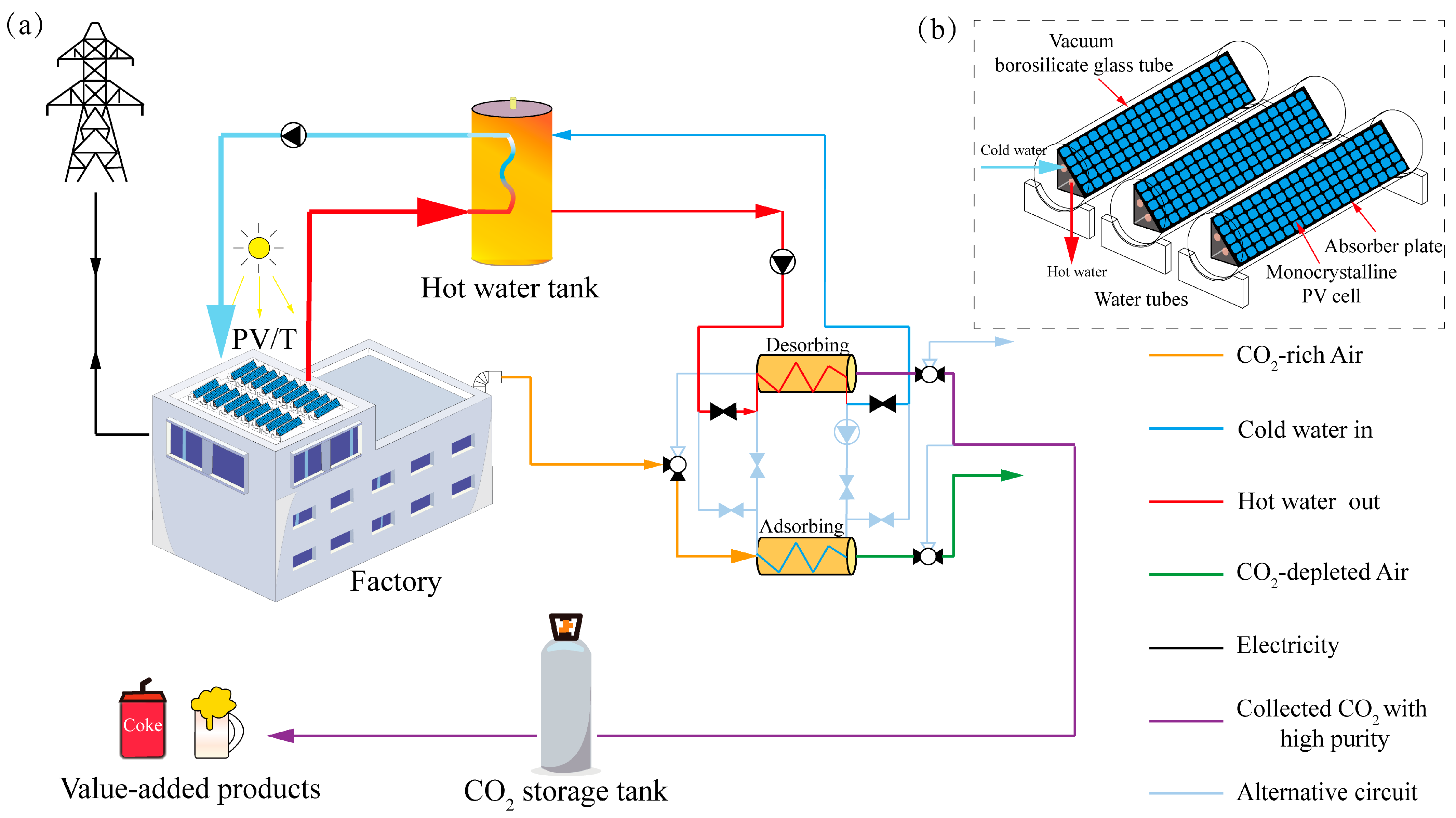
2.1. The Overall System
2.2. Technical Objectives
2.3. Economic Objective
3. Optimization Framework
4. Results and Discussion
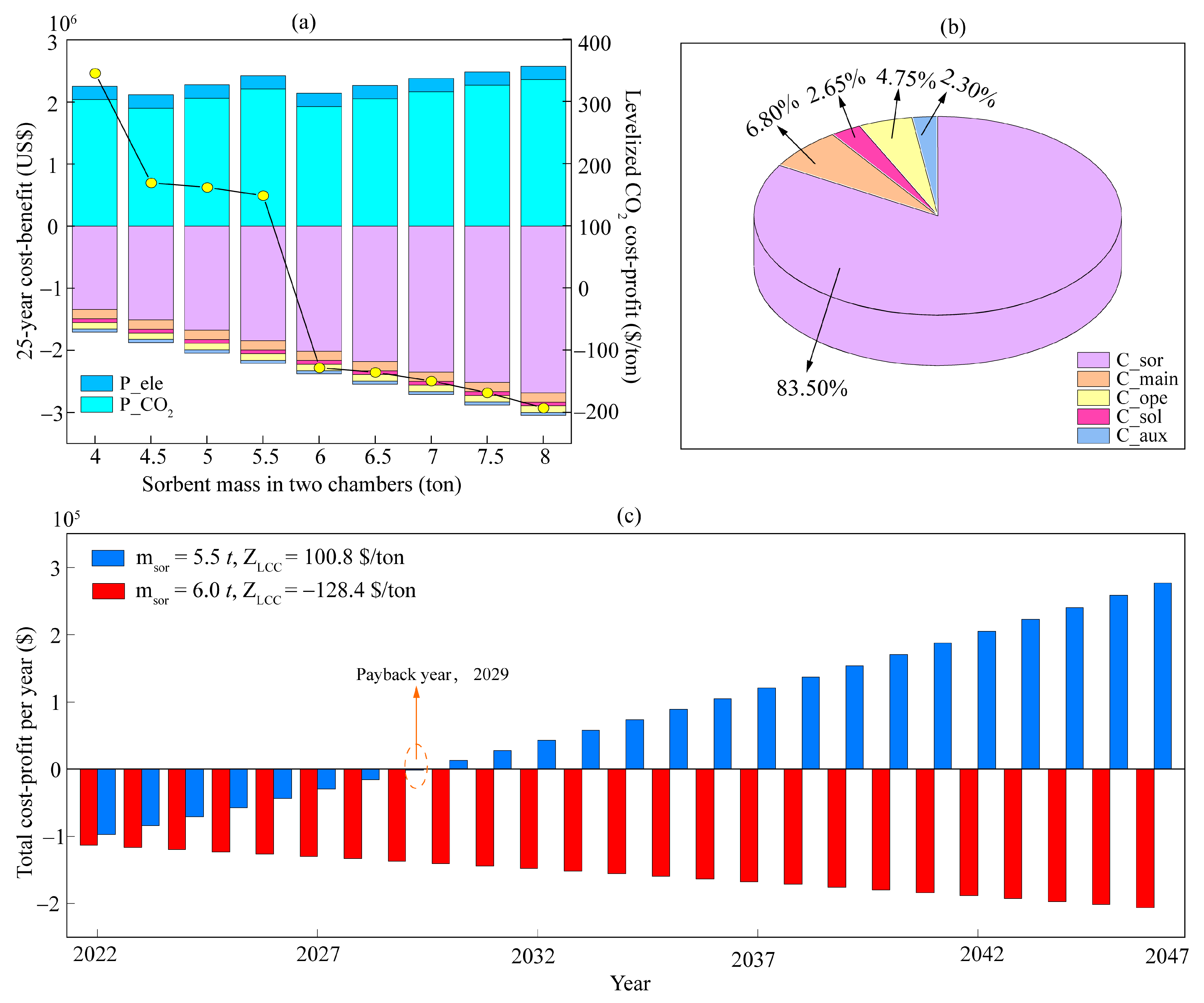
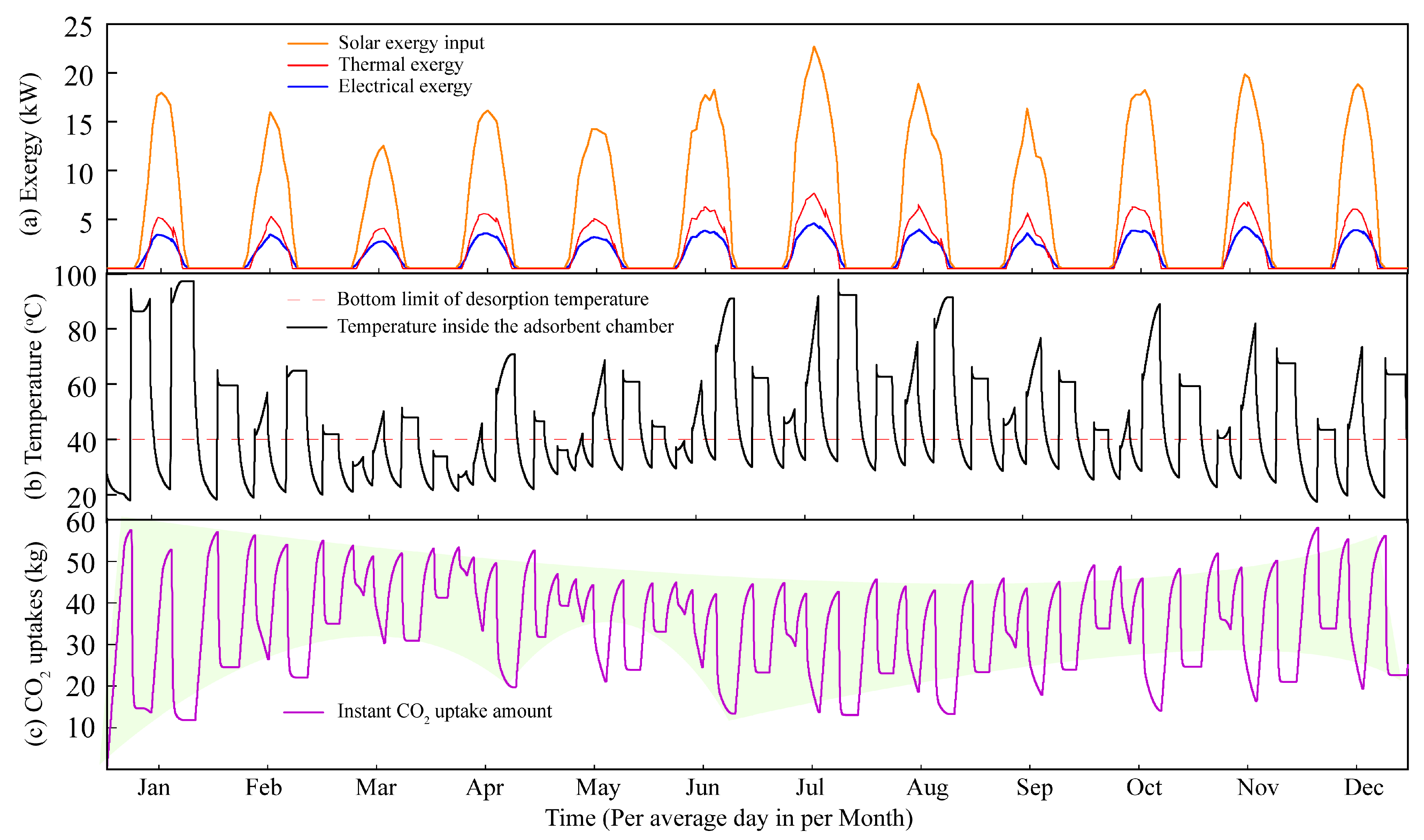
4.1. Optimization Based on the Energy-Balance Principle
4.2. Optimization Based on the Mass-Balance Principle
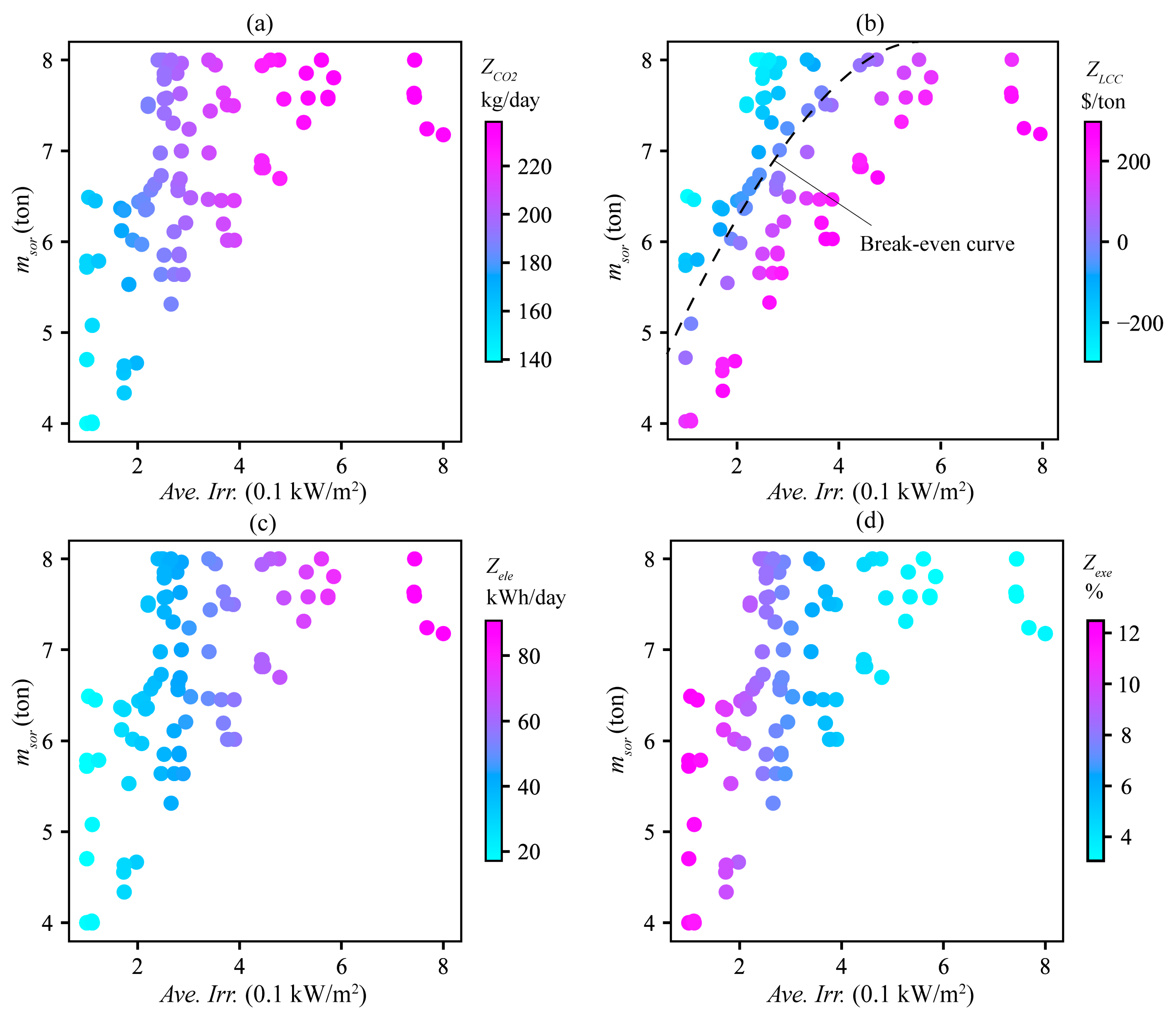
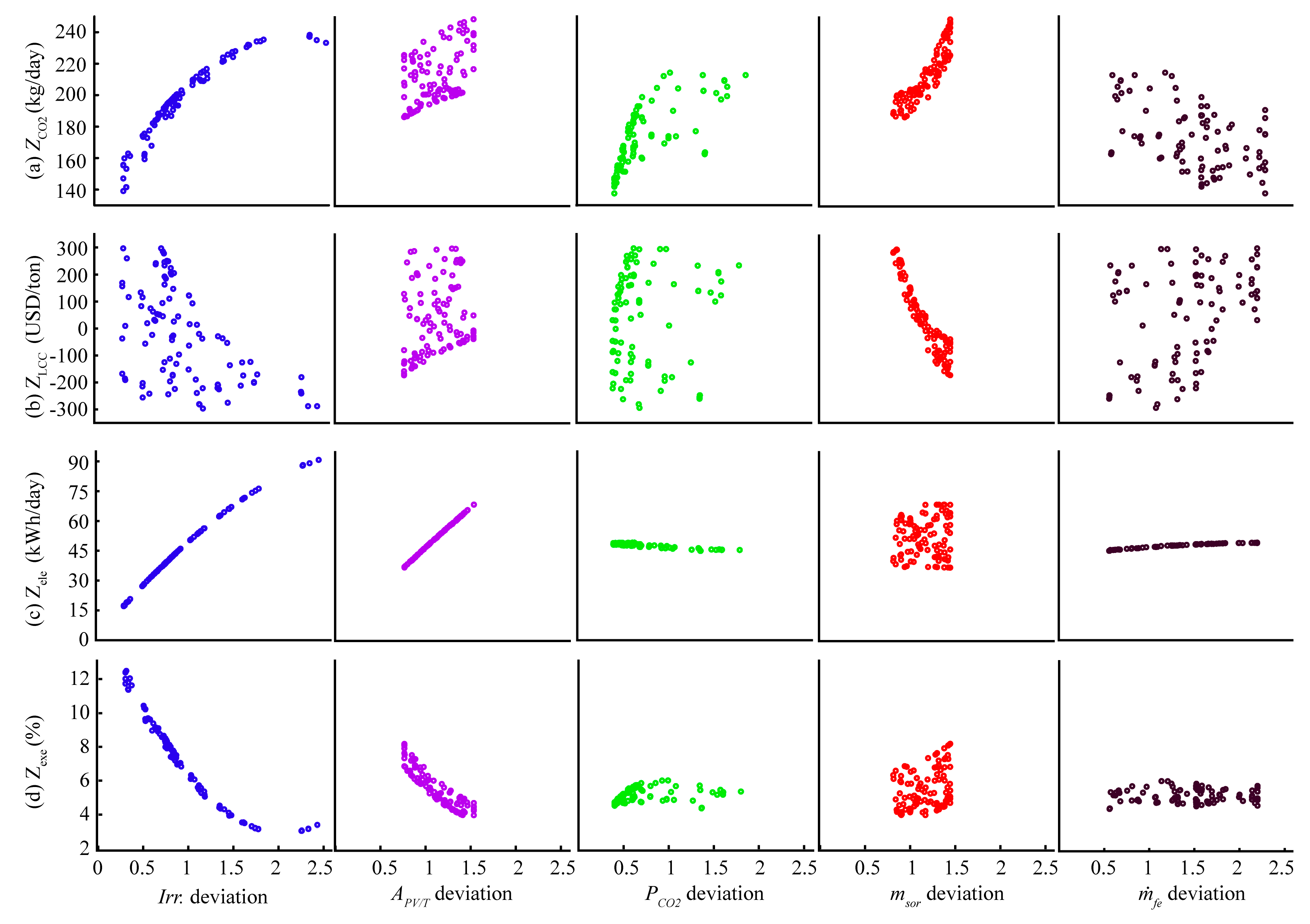
4.3. Sensitivity Analysis
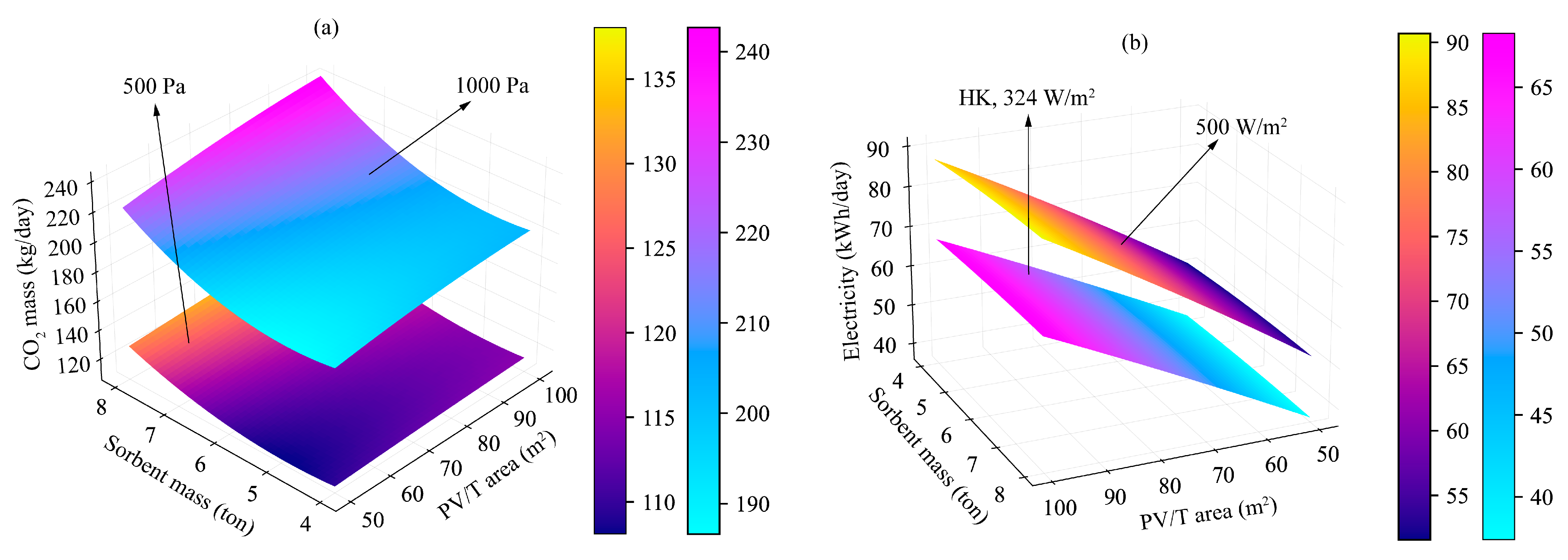
4.4. Scenarios Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| DAC | Direct air capture of CO2 |
| CCA | Carbon capture by adsorption |
| LDF | Linear driving force |
| RH | Relative humidity |
| Net present value | |
| Variables | |
| The area of the rooftop evacuated thermal collector (m2) | |
| The contact area of water pipes and the adsorbent (m2) | |
| The contact area between feed air and the adsorbent (m2) | |
| Specific heat capacity of water at constant pressure (J/(kg K)) | |
| The maintenance cost ($/year) | |
| The operation cost ($/year) | |
| Specific heat capacity of solid adsorbent (J/(kg K)) | |
| The specific heat capacity of different gas species (J/(kg K)) | |
| The total heat demand for CO2 regeneration (J) | |
| The magnitude of the incident radiation (Wh/m2) | |
| The convective heat transfer coefficient of air | |
| The convective heat transfer coefficient of water | |
| The sorbent mass (kg or ton) | |
| The mass flow rate of the feed air (kg/s) | |
| The molar number of inlet/outlet gas species (mol) | |
| The profits from selling collected CO2 ($/year) | |
| The profits from selling generated electricity ($/year) | |
| The partial pressure of CO2 in the feed air (Pa) | |
| Consumed regeneration heat (W) | |
| Solar input (W) | |
| Heat collected by flowing water (W) | |
| Radiative heat loss (W) | |
| Convective heat transfer from the flue gas during adsorption (W) | |
| Isosteric heat from adsorption/desorption reaction (W) | |
| Instantaneous chamber temperature (°C) | |
| Desorption temperature (°C) | |
| The given adsorption temperature (°C) | |
| Ambient temperature (°C) | |
| The inlet/outlet water temperature of the solar thermal collector (°C) | |
| The surface temperature of PV/T (°C) | |
| The water temperature inside the buffer tank (°C) | |
| Minimum work requirement for CO2 capture(W) | |
| X | Average solar input exergy |
| Objective 1: Collected CO2 mass (kg/day) | |
| Objective 2: Levelized CO2 cost-profit ($/ton) | |
| Objective 3: Generated electricity (kWh/day) | |
| Objective 4: Exergy efficiency of the entire system |
References
- Capstick, S.; Thierry, A.; Cox, E.; Berglund, O.; Westlake, S.; Steinberger, J.K. Preparing for a post-net-zero world. Nat. Clim. Chang. 2022, 12, 773–774. [Google Scholar] [CrossRef]
- Hannah Ritchie, M.R. Natural Disasters Kill Tens of Thousands Each Year. Available online: https://ourworldindata.org/natural-disasters#natural-disasters-kill-tens-of-thousands-each-year (accessed on 19 September 2022).
- IEA. Global Energy Review: CO2 Emissions in 2021; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Project, G.C. Annual CO2 Emissions Worldwide from 1940 to 2020 (in Billion Metric Tons). Available online: https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed on 19 September 2022).
- Kintisch, E. Amazon rainforest ability to soak up carbon dioxide is falling. Science 2015. [Google Scholar] [CrossRef]
- Chen, S. The inequality impacts of the carbon tax in China. Humanit. Soc. Sci. Commun. 2022, 9, 277. [Google Scholar] [CrossRef]
- Inman, M. Natural gas stands to get a boost from carbon tax. Nature 2015. [Google Scholar] [CrossRef]
- News, T.W. Wanted a fair carbon tax. Nature 2018, 564, 161. [Google Scholar]
- IEA. Transforming Industry Through CCUS; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Roussanaly, S.; Berghout, N.; Fout, T.; Garcia, M.; Gardarsdottir, S.; Nazir, S.M.; Ramirez, A.; Rubin, E.S. Towards improved cost evaluation of Carbon Capture and Storage from industry. Int. J. Greenh. Gas Control. 2021, 106, 103263. [Google Scholar] [CrossRef]
- Rao, M.; Fernandes, A.; Pronk, P.; Aravind, P.V. Design, modelling and techno-economic analysis of a solid oxide fuel cell-gas turbine system with CO2 capture fueled by gases from steel industry. Appl. Therm. Eng. 2019, 148, 1258–1270. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Zhang, Z.; Manovic, V. Inherent potential of steelmaking to contribute to decarbonisation targets via industrial carbon capture and storage. Nat. Commun. 2018, 9, 4422. [Google Scholar] [CrossRef]
- Hao, Z.; Barecka, M.H.; Lapkin, A.A. Accelerating net zero from the perspective of optimizing a carbon capture and utilization system. Energy Environ. Sci. 2022, 15, 2139–2153. [Google Scholar] [CrossRef]
- International Energy Agency. Net Zero by 2050 a Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Hesami, M.; Naderi, R.; Tohidfar, M. Modeling and Optimizing Medium Composition for Shoot Regeneration of Chrysanthemum via Radial Basis Function-Non-dominated Sorting Genetic Algorithm-II (RBF-NSGAII). Sci. Rep. 2019, 9, 18237. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Zhang, X.; Cui, G.; Sun, J. An Improved Non-dominated Sorting Genetic Algorithm-II (INSGA-II) applied to the design of DNA codewords. Math. Comput. Simul. 2018, 151, 131–139. [Google Scholar] [CrossRef]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T.A.M.T. A fast and elitist multiobjective genetic algorithm NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Pal, P.; Sharma, R.P.; Tripathi, S.; Kumar, C.; Ramesh, D. Genetic algorithm optimized node deployment in IEEE 802.15.4 potato and wheat crop monitoring infrastructure. Sci. Rep. 2021, 11, 8231. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.A.; Pereira, H.A.; Chaves, D.A.R.; Almeida, R.C. Evolutionary Multiobjective Strategy for Regenerator Placement in Elastic Optical Networks. IEEE Trans. Commun. 2018, 66, 3583–3596. [Google Scholar] [CrossRef]
- Sharifi, M.R.; Akbarifard, S.; Qaderi, K.; Madadi, M.R. A new optimization algorithm to solve multi-objective problems. Sci. Rep. 2021, 11, 20326. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Pini, R. Efficient Bayesian Optimization of Industrial-Scale Pressure-Vacuum Swing Adsorption Processes for CO2 Capture. Ind. Eng. Chem. Res. 2022, 61, 13650–13668. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Zhang, D.; Tang, Z.; Li, W. A comparative study of multi-objective optimization with ANN-based VPSA model for CO2 capture from dry flue gas. J. Environ. Chem. Eng. 2022, 10, 108031. [Google Scholar] [CrossRef]
- Pai, K.N.; Prasad, V.; Rajendran, A. Practically Achievable Process Performance Limits for Pressure-Vacuum Swing Adsorption-Based Postcombustion CO2 Capture. ACS Sustain. Chem. Eng. 2021, 9, 3838–3849. [Google Scholar] [CrossRef]
- Hao, Z.; Caspari, A.; Schweidtmann, A.M.; Vaupel, Y.; Lapkin, A.A.; Mhamdi, A. Efficient hybrid multiobjective optimization of pressure swing adsorption. Chem. Eng. J. 2021, 423, 130248. [Google Scholar] [CrossRef]
- Zhang, F.-Y.; Feng, Y.-Q.; He, Z.-X.; Xu, J.-W.; Zhang, Q.; Xu, K.-J. Thermo-economic optimization of biomass-fired organic Rankine cycles combined heat and power system coupled CO2 capture with a rated power of 30 kW. Energy 2022, 254, 124433. [Google Scholar] [CrossRef]
- Breyer, C.; Fasihi, M.; Bajamundi, C.; Creutzig, F. Direct Air Capture of CO2: A Key Technology for Ambitious Climate Change Mitigation. Joule 2019, 3, 2053–2057. [Google Scholar] [CrossRef]
- Climeworks. Climeworks Begins Operations of Orca, the World’s Largest Direct Air Capture and CO2 Storage Plant. Available online: https://climeworks.com/news/climeworks-launches-orca (accessed on 19 September 2022).
- Thermostat, G. The GT Solution. 2021. Available online: https://globalthermostat.com/ (accessed on 19 September 2022).
- Announcing Project Bison: A 5 Megaton DAC Carbon Removal Project in Wyoming. Available online: https://www.carboncapture.com/project-bison (accessed on 17 October 2022).
- Niaz, H.; Shams, M.H.; Liu, J.J.; You, F. Mining bitcoins with carbon capture and renewable energy for carbon neutrality across states in the USA. Energy Environ. Sci. 2022, 15, 3551–3570. [Google Scholar] [CrossRef]
- Prajapati, A.; Sartape, R.; Galante, M.T.; Xie, J.; Leung, S.L.; Bessa, I.; Andrade, M.H.S.; Somich, R.T.; Rebouças, M.V.; Hutras, G.T.; et al. Fully-integrated electrochemical system that captures CO2 from flue gas to produce value-added chemicals at ambient conditions. Energy Environ. Sci. 2022, 15, 5105–5117. [Google Scholar] [CrossRef]
- Bustillos, S.; Alturki, A.; Prentice, D.; La Plante, E.C.; Rogers, M.; Keller, M.; Ragipani, R.; Wang, B.; Sant, G.; Simonetti, D.A. Implementation of Ion Exchange Processes for Carbon Dioxide Mineralization Using Industrial Waste Streams. Front. Energy Res. 2020, 8, 610392. [Google Scholar] [CrossRef]
- Bustillos, S.; Prentice, D.; La Plante, E.C.; Wang, B.; Sant, G.; Simonetti, D. Process Simulations Reveal the Carbon Dioxide Removal Potential of a Process That Mineralizes Industrial Waste Streams via an Ion Exchange-Based Regenerable pH Swing. ACS Sustain. Chem. Eng. 2022, 10, 6255–6264. [Google Scholar] [CrossRef]
- CO2Meter.com. Why is Monitoring Ammonia and Carbon Dioxide in Poultry Farms Important? Available online: https://www.co2meter.com/blogs/news/ammonia-meter-poultry-farms (accessed on 1 October 2022).
- EnergyPlus. Weather Data. Available online: https://energyplus.net/weather (accessed on 14 September 2022).
- Lux, D. Does CO2 Harm Your Body? Available online: https://medium.com/swlh/does-co2-harm-your-body-d20b4a0d03c1 (accessed on 14 September 2022).
- SunPower, Corporation. SunPower® X-Series Comercial Solar Panel X21-470-COM. Available online: https://us.sunpower.com/sites/default/files/sunpower-x-series-commercial-solar-panels-x21-470-com-datasheet-524935-revb_1.pdf (accessed on 14 September 2022).
- Energy, N. Virtu PVT Product. Available online: https://www.nakedenergy.co.uk/products (accessed on 14 September 2022).
- Shen, Y.; Kwan, T.H.; Yao, Q. Combined microgrid power production and carbon dioxide capture by waste heat cascade utilization of the solar driven Organic Rankine cycle. Energy Convers. Manag. 2021, 236, 114034. [Google Scholar] [CrossRef]
- Tech, W.L.B. Zeolite 13X Product. Available online: https://www.casmart.com.cn/product-details/page/300028576/501822071 (accessed on 14 September 2022).
- Alados-Arboledas, L.; Vida, J.; Olmo, F.J. The estimation of thermal atmospheric radiation under cloudy conditions. Int. J. Climatol. 1995, 15, 107–116. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, H. Achieving reduced emission and enhanced air quality by designing a solar-driven indoor CO2 capture system. J. Clean. Prod. 2022, 379, 134869. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Ben-Mansour, R.; Habib, M.A. Enhancement of adsorption carbon capture capacity of 13X with optimal incorporation of carbon nanotubes. Int. J. Energy Environ. Eng. 2017, 8, 219–230. [Google Scholar] [CrossRef]
- Lei, M.; Vallieres, C.; Grevillot, G.; Latifi, M.A. Thermal Swing Adsorption Process for Carbon Dioxide Capture and Recovery: Modeling, Simulation, Parameters Estimability, and Identification. Ind. Eng. Chem. Res. 2013, 52, 7526–7533. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Qasem, N.A.A. An efficient temperature swing adsorption (TSA) process for separating CO2 from CO2/N2 mixture using Mg-MOF-74. Energy Convers. Manag. 2018, 156, 10–24. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, S.; Liu, Y.; Zhao, Q.; He, J.; Zhao, L. Carbon pump: Fundamental theory and applications. Energy 2017, 119, 1131–1143. [Google Scholar] [CrossRef]
- Holdren, J.M.M.W.X.J.P. Carbon Capture, Utilization and Storage: Technologies and Costs in the U.S. Context. Available online: https://www.belfercenter.org/publication/carbon-capture-utilization-and-storage-technologies-and-costs-us-context (accessed on 9 November 2022).
- Keith, D.W.; Holmes, G.; Angelo, D.S.; Heidel, K. A Process for Capturing CO2 from the atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- van der Giesen, C.; Meinrenken, C.J.; Kleijn, R.; Sprecher, B.; Lackner, K.S.; Kramer, G.J. A Life Cycle Assessment Case Study of Coal-Fired Electricity Generation with Humidity Swing Direct Air Capture of CO2 versus MEA-Based Postcombustion Capture. Environ. Sci. Technol. 2017, 51, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Alibaba. Price for Solar Photovoltaic/Thermal Collectors. Available online: https://detail.tmall.com/item_o.htm?spm=a230r.1.14.16.1b4f357byYV6L4&id=685179266851&ns=1&abbucket=15 (accessed on 3 November 2022).
- Alibaba. Price for High Purity 13X HP Zeolite Molecular Sieve. Available online: https://www.alibaba.com/product-detail/13x-Zeolite-XINTAO-High-Purity-13X_1600200876872.html?spm=a2700.7735675.0.0.2deb5b21xmWA4U&s=p (accessed on 14 September 2022).
- Su, F.; Lu, C. CO2 capture from gas stream by zeolite 13X using a dual-column temperature/vacuum swing adsorption. Energy Environ. Sci. 2012, 5, 1573–1594. [Google Scholar] [CrossRef]
- Prices, G.P. Electricity Prices. Available online: https://www.globalpetrolprices.com/electricity_prices/ (accessed on 3 November 2022).
- HK Electric Investments Limited. Feed-in Tariff Scheme. Available online: https://www.hkelectric.com/en/smart-power-services/feed-in-tariff-scheme (accessed on 3 November 2022).
- Alibaba. Liquid Gas Carbon Dioxide Price. Available online: https://www.alibaba.com/product-detail/Best-Price-Purity-99-995-Liquid_1600465210806.html?spm=a2700.7724857.0.0.1d1b79721R06Hn (accessed on 14 November 2022).
- Richardson, L. How Long do Solar Panels Last? Solar Panel Lifespan Explained. Available online: https://news.energysage.com/how-long-do-solar-panels-last/#:~:text=The%20industry%20standard%20for%20a,below%20what%20the%20manufacturer%20projected. (accessed on 3 November 2022).
- Shen, Y.; Hocksun Kwan, T.; Yang, H. Parametric and global seasonal analysis of a hybrid PV/T-CCA system for combined CO2 capture and power generation. Appl. Energy 2022, 311, 118681. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Kim, J.T.; Suh, J.K.; Lee, J.M.; Lee, C.H. Adsorption Equilibria of CO2 on Zeolite 13X and Zeolite X Activated Carbon Composite. J. Chem. Eng. Data 2002, 47, 1237–1242. [Google Scholar] [CrossRef]
- Kwan, T.H.; Wu, X.; Yao, Q. Thermoelectric device multi-objective optimization using a simultaneous TEG and TEC characterization. Energy Convers. Manag. 2018, 168, 85–97. [Google Scholar] [CrossRef]
- Fu, D.; Davis, M.E. Carbon dioxide capture with zeotype materials. Chem. Soc. Rev. 2022, 51, 9340–9370. [Google Scholar] [CrossRef]
- Sutherland, B.R. Pricing CO2 Direct Air Capture. Joule 2019, 3, 1571–1573. [Google Scholar] [CrossRef]
- Azarabadi, H.; Lackner, K.S. A sorbent-focused techno-economic analysis of direct air capture. Appl. Energy 2019, 250, 959–975. [Google Scholar] [CrossRef]
- Kitamura, H.; Ishigaki, Y.; Kuriyama, T.; Moritake, T. CO2 concentration visualization for COVID-19 infection prevention in concert halls. Environ. Occup. Health Pract. 2021, 3. [Google Scholar] [CrossRef]
- Langefeld, B. Climate Protection in the Manufacturing Sector: Challenges and Solutions. Available online: https://www.rolandberger.com/en/Insights/Publications/Climate-protection-in-the-manufacturing-sector-Challenges-and-solutions.html (accessed on 1 October 2022).






| Item | Cost $ per Unit | Amount per Year | Total $ |
|---|---|---|---|
| Running cost | 0.6 | 5833 kWh | −105,220 |
| Maintenance | 2500 | Two times | −150,315 |
| PV/T | 500 | 50 m2 | −25,000 |
| Zeolite 13X | 6200 | 9.9 ton | −1,845,268 |
| Installation | −35,000 | 1 | −35,000 |
| Tank | 600 | 1 | −600 |
| Fan | 1,00 | 10 | −1000 |
| Pipe | 1,00 | 10 | −1000 |
| Valve | 40 | 10 | −400 |
| Others | 20,000 | 1 | −20,000 |
| Pump | 3500 | 2 | −7000 |
| Heat Exchanger | 20,000 | 1 | −20,000 |
| Sell CO2 | 1000 | 74.7 ton | 2,245,848 |
| Sell electricity | 0.38 | 13,413.6 kWh | 153,225.2 |
| NPV | 188,270.0 $ | ||
| Total CO2 | 1867.617 ton | ||
| Specific NPV | 100.8 $/ton |
| Variables | Description | Lowest Bound | Highest Bound | Units |
|---|---|---|---|---|
| 100 | 800 | W/m2 | ||
| 50 | 100 | m2 | ||
| 4 | 8 | tons | ||
| 400 | 1800 | Pa | ||
| 0.1 | 0.55 | kg/s | ||
| Objectives | Description | |||
| Captured CO2 mass in one day | ||||
| Levelized cost of CO2 based on a 25-year lifespan | ||||
| Generated electricity amount in one day | ||||
| Average exergy efficiency in one day | ||||
| Parameter | |||||
|---|---|---|---|---|---|
| Base value | 324 W/m2 | 65 m2 | 5.5 ton | 1000 Pa | 0.25 kg/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Yang, H. Multi-Objective Optimization of Integrated Solar-Driven CO2 Capture System for an Industrial Building. Sustainability 2023, 15, 526. https://doi.org/10.3390/su15010526
Shen Y, Yang H. Multi-Objective Optimization of Integrated Solar-Driven CO2 Capture System for an Industrial Building. Sustainability. 2023; 15(1):526. https://doi.org/10.3390/su15010526
Chicago/Turabian StyleShen, Yongting, and Hongxing Yang. 2023. "Multi-Objective Optimization of Integrated Solar-Driven CO2 Capture System for an Industrial Building" Sustainability 15, no. 1: 526. https://doi.org/10.3390/su15010526
APA StyleShen, Y., & Yang, H. (2023). Multi-Objective Optimization of Integrated Solar-Driven CO2 Capture System for an Industrial Building. Sustainability, 15(1), 526. https://doi.org/10.3390/su15010526






