Unlocking the Land Capability and Soil Suitability of Makuleke Farm for Sustainable Banana Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and History
2.2. Field Soil Survey and Classification
2.3. Collection of Soil Samples in the Field
2.4. Preparation and Laboratory Analysis of Soil Physicochemical Properties
2.5. Derivation of Land Capability and Soil Suitability Classes
2.6. Generation of Soil Form, Land Capability and Soil Suitability Maps
3. Results
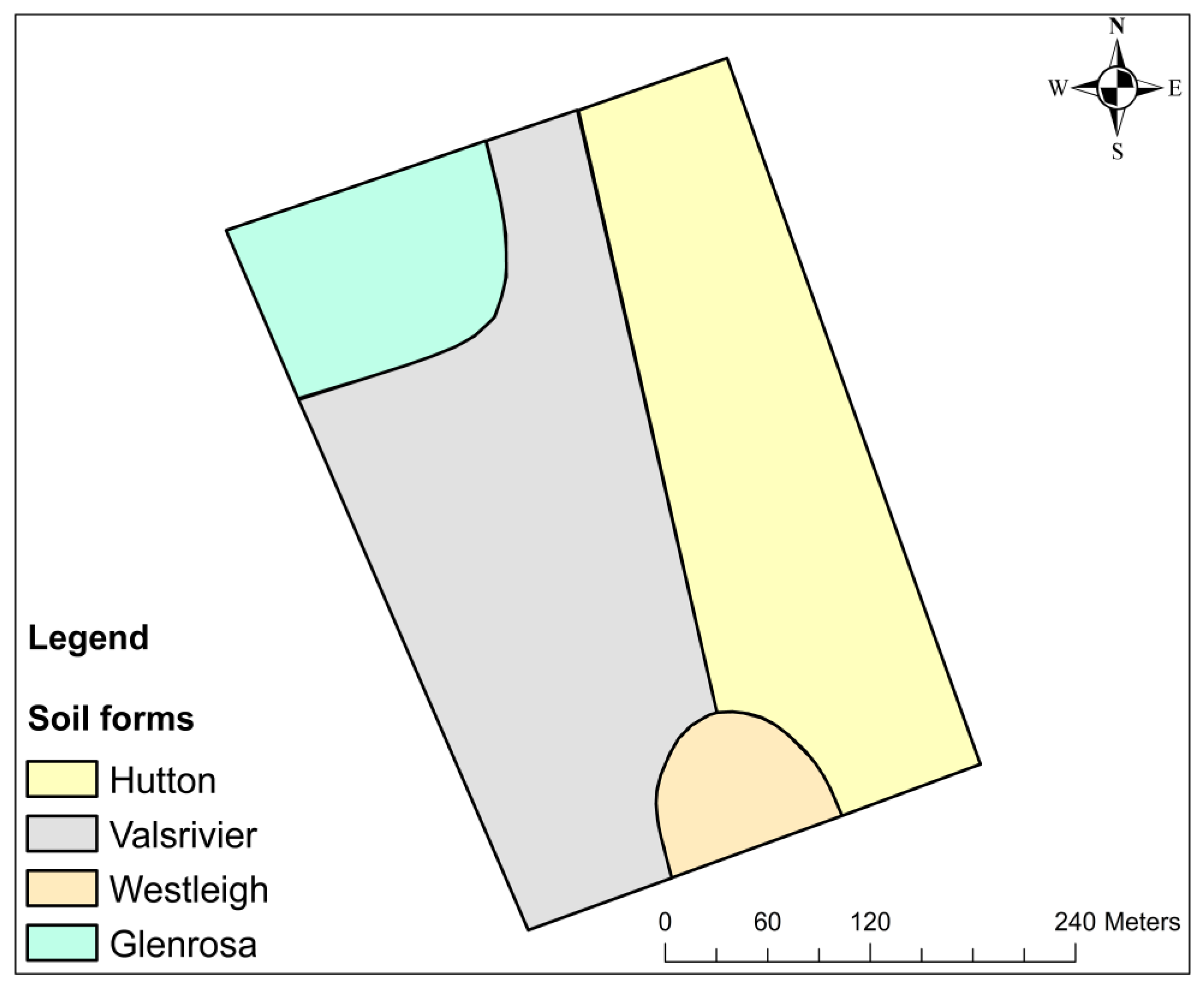
3.1. Pedological and Morphological Characteristics of the Soils Underlying the 12 ha Banana Plantation
3.2. Chemical Properties of the Soils across the 12 ha Banana Plantation
3.3. Land Capability Classification for Arable Farming
3.4. Soil Site Suitability for Banana Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giller, K.E. The food security conundrum of sub-Saharan Africa. Glob. Food Sec. 2020, 26, 100431. [Google Scholar] [CrossRef]
- Pienaar, L.; Traub, L. Understanding the smallholder farmer in South Africa: Towards a sustainable livelihoods classification. In Proceedings of the International Association of Agricultural Economists, Milan, Italy, 9–14 August 2015. [Google Scholar]
- Adekunle, O.O. An investigation of challenges facing home gardening farmers in South Africa: A case study of three villages in Nkonkonbe municipality, Eastern Cape Province. J. Agric. Sci. 2014, 6, 102–109. [Google Scholar] [CrossRef]
- Quandt, A.; Herrick, J.; Peacock, G.; Salley, S.; Buni, A.; Mkalawa, C.C.; Neff, J. A standardized land capability classification system for land evaluation using mobile phone technology. J. Soil Water Conserv. 2020, 75, 579–589. [Google Scholar] [CrossRef]
- Nciizah, A.D.; Mupambwa, H.A.; Nyambo, P.; Muchara, B. From Soil to Fork: Can Sustainable Intensification Guarantee Food Security for Smallholder Farmers? In Food Security for African Smallholder Farmers; Springer: Singapore, 2022; pp. 27–46. [Google Scholar]
- Barnard, R.O.; van der Merwe, A.J.; de Villers, M.C.; van der Merwe, G.M.E.; Mulibana, N.E. Problem soils (including degraded soils): Extent, present use, management and rehabilitation in South Africa. In Proceedings of the 2nd MADS-SEA (Management of degraded soils in Southern and East Africa) Network Meeting, Agricultural Research Council Institute for Soil, Climate and Water, Pretoria, South Africa, 6 July 2000. [Google Scholar]
- Nziguheba, G.; Adewopo, J.; Masso, C.; Nabahungu, N.L.; Six, J.; Sseguya, H.; Taulya, G.; Vanlauwe, B. Assessment of sustainable land use: Linking land management practices to sustainable land use indicators. Int. J. Agric. Sustain. 2022, 20, 265–288. [Google Scholar] [CrossRef]
- Scholes, R.J.; Biggs, R. Ecosystem Services in Southern Africa: A Regional Assessment; Millenium Ecosystem Assessment; Council for Scientific and Industrial Research: Pretoria, South Africa, 2004. [Google Scholar]
- Manderson, A.; Palmer, A. Soil information for agricultural decision making: A New Zealand perspective. Soil Use Man. 2006, 22, 393–400. [Google Scholar] [CrossRef]
- Abd-Elmabod, S.K.; Bakr, N.; Muñoz-Rojas, M.; Pereira, P.; Zhang, Z.; Cerdà, A.; Jordán, A.; Mansour, H.; De la Rosa, D.; Jones, L. Assessment of soil suitability for improvement of soil factors and agricultural management. Sustainability 2009, 11, 1588. [Google Scholar] [CrossRef]
- Munialo, S.; Dahlin, A.S.; Onyango, M.C.; Oluoch-Kosura, W.; Marstorp, H.; Öborn, I. Soil and management-related factors contributing to maize yield gaps in western Kenya. J. Food Energy Secur. 2020, 9, 189–204. [Google Scholar] [CrossRef]
- Olivaries, B.O.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.B.; Gómez, J.A. Correlation of banana productivity levels and soil morphological properties using regularized optimal scaling regression. CATENA 2021, 208, 105–118. [Google Scholar]
- Dobarco, M.R.; McBratney, A.; Minasny, B.; Malone, B. A framework to assess changes in soil condition and capability over large areas. Soil Secur. 2021, 4, 100011. [Google Scholar] [CrossRef]
- De Feudis, M.; Falsone, G.; Gherardi, M.; Speranza, M.; Vianello, G.; Antisari, L.V. GIS-based soil maps as tools to evaluate land capability and suitability in a coastal reclaimed area (Ravenna, northern Italy). Int. Soil Water Conserv. Res. 2021, 9, 167–179. [Google Scholar] [CrossRef]
- Naidu, L.G.K.; Ramamurthy, V.; Challa, O.; Hegde, R.; Krishnan, P. Manual on Soil-Site Suitability Criteria for Major Crops; NBSS publication 129; NBSS: Nagpur, India, 2006. [Google Scholar]
- AbdelRahman, M.A.E.; Natarajan, A.; Hegde, R. Assessment of land suitability and capability by integrating remote sensing and GIS for agriculture in Chamarajanagar district, Karnataka, India. Egypt. J. Remote Sens. Space Sci. 2016, 19, 125–141. [Google Scholar] [CrossRef]
- Amara, D.M.K.; Nadaf, S.A.; Saidu, D.H.; Vonu, O.S.; Musa, R.M.; Kamanda, P.J.; Sawyerr, P.A.; Mboma, J.C.; Mansaray, S.D.; Sannoh, M.A. Studies on Land Resource Inventory for Agricultural Land Use Planning in Northern Transition Zone of India through Remote Sensing and GIS Techniques. J. Geographic Infor. Sys. 2021, 13, 710–728. [Google Scholar] [CrossRef]
- Hilton-Baber, B.; Berger, L.R. Prime Origins Guide to Exploring Kruger, 2nd ed.; Prime Origin (Pty) Ltd.: Cape Town, South Africa, 2007. [Google Scholar]
- Kock, A. Diatom Diversity and Response to Water Quality within the Makuleke Wetlands and Lake Sibaya. Master’s Thesis, The Potchefstroom Campus of the North-West University, Potchefstroom, South Africa, 2017. [Google Scholar]
- United States. Department of Agriculture. Soil Survey Division, and United states. Division of Soil Survey. In Soil Survey Manual (No. 18); US Department of Agriculture: Washington, DC, USA, 1993. [Google Scholar]
- Soil Classification Working Group. Soil Classification: A Natural and Anthropogenic System for South Africa; Agricultural Research Council, Institute for Soil, Climate and Water (ARC-ISCW): Pretoria, South Africa, 2018. [Google Scholar]
- Smith, B. The Farming Handbook; UKZN-Press: Pietermaritzburg, South Africa, 2006. [Google Scholar]
- Bouyoucos, G.J. Hydrometer Method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of Different Method for Determining Soil Organic Matter and a Proposed Modification of the Chromic Acid Titration Method. J. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Manson, A.D.; Bainbridge, S.H.; Thibaud, G.R. Methods Used for the Analysis of Soils and Plant Material by Analytical Services at Cedara; KwaZulu-Natal Department of Agriculture and Rural Development: KwaDukuza, South Africa, 2020. [Google Scholar]
- Murphy, J.; Riley, J.R. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Food and Agriculture Organizations of the United States, FAO. A Framework for land evaluation. In Soils Bulletin 3; FAO: Rome, Italy, 1976; Volume 72. [Google Scholar]
- Food and Agriculture Organizations of the United States, FAO. Guidelines in land evaluation for rain fed agriculture. In Soils Bulletin; Food and agricultural organization: Rome, Italy, 1983; Volume 52, pp. 210–237. [Google Scholar]
- Sys, C.; Van Ranst, E.; Debaveye, J.; Beenaert, F. Land evaluation part III. Crop Requir. 1993, 1, 3. [Google Scholar]
- Food and Agriculture Organizations of the United States, FAO. Soil Fertility Degradation and Restoration under Banana Cultivation; Ekona Banana Estate of CDC. Soil resources project. Technical report No. 19; FAO: Rome, Italy, 1983. [Google Scholar]
- Balasubramanian, A. Soil Morphology; University of Mysore: Mysuru, India, 2017. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; Inter-national Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Fey, M. Soils of South Africa; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Baxter, S. Guidelines for soil description. Experimental Agric. 2007, 43, 63–264. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils (Global Edition); Pearson: Harlow, UK, 2017. [Google Scholar]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of clay minerals on some soil fertility attributes: A review. Open J. Soil Sci. 2019, 9, 155–188. [Google Scholar] [CrossRef]
- Food and Agriculture Organizations of the United States, FAO. Banana. 2018. Available online: http://www.fao.org/land-water/databases-and-software/crop information/banana/en/ (accessed on 23 June 2022).
- White, R.E. Principles and Practice of Soil Science: The Soil as a Natural Resource, 4th ed.; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Pearson Education: Noida, India, 2016. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 15, 216–227. [Google Scholar] [CrossRef]
- Fullen, M.A.; Catt, J.A. Soil Management: Problems and Solutions; Routledge: Abingdon-on-Thames, UK, 2014. [Google Scholar]
- Jackson, R.S. 5—Site selection and climate. In Food Science and Technology, Wine Science, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 239–269. [Google Scholar]
- Ploetz, R.C. Panama disease: A classic and destructive disease of banana. Plant Health Progress. 2000, 1, 10. [Google Scholar] [CrossRef]
- Orr, R.; Nelson, P.N. Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Applied Soil Eco. 2018, 132, 20–33. [Google Scholar] [CrossRef]
- Domínguez, J.; Negrín, M.A.; Rodríguez, C.M. Aggregate water-stability, particle-size and soil solution properties in conducive and suppressive soils to Fusarium wilt of banana from Canary Islands (Spain). Soil Biol. Biochem. 2001, 33, 449–455. [Google Scholar] [CrossRef]
- Moreno-Roblero, M.D.J.; Pineda-Pineda, J.; Colinas-León, M.T.; Sahagún-Castellanos, J. Oxygen in the root zone and its effect on plants. Revista Mexicana De Ciencias Agríc. 2020, 11, 931–943. [Google Scholar]
- Bhattarai, S.P.; Midmore, D.J.; Pendergast, L. Yield, water-use efficiencies and root distribution of soybean, chickpea and pumpkin under different subsurface drip irrigation depths and oxygation treatments in vertisols. Irrig. Sci. 2008, 26, 439–450. [Google Scholar] [CrossRef]
- Maestre, V.J.F.; Martínez, A.V. Effects of drip irrigation systems on the recovery of dissolved oxygen from hypoxic water. Science Direct. Agric. Water Management. 2010, 97, 1806–1812. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Adatte, T.; Verrecchia, E.P. A cascading influence of calcium carbonate on the biogeochemistry and pedogenic trajectories of subalpine soils, Switzerland. Geoderma 2020, 361, 114–135. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E. The chemistry of soil organic matter. In Soil Physical Chemistry; CRC Press: Boca Raton, FL, USA, 2018; pp. 239–370. [Google Scholar]
- Stott, D.E.; Kennedy, A.C.; Cambardella, C.A. Impact of soil organisms and organic matter on soil structure. In Soil Quality and Soil Erosion; CRC Press: Boca Raton, FL, USA, 2018; pp. 57–73. [Google Scholar]

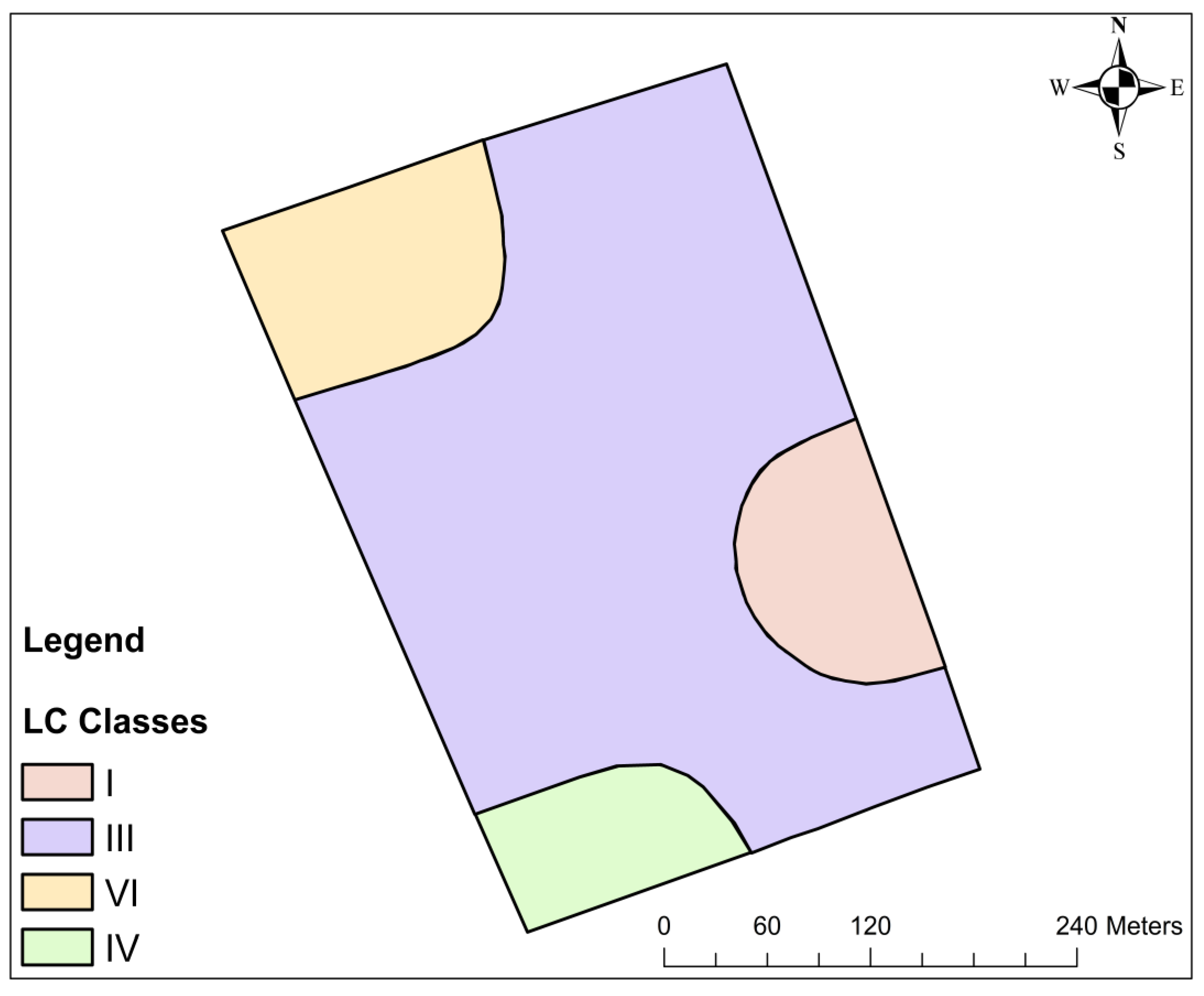

| Soil Site Characteristics | Class, Degree of Limitation and Rating Scale | |||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | N1 | N2 | ||
| Climatic Regime (c) | ||||||
| Mean temperature in growing season (°C) | 26–33 | 34–36; 24–25 | 37–38 | >38 | ||
| Topography (t) | ||||||
| Slope (%) | 0–2 | 2–4 | 4–8 | 8–16 | - | >16 |
| Wetness (w) | ||||||
| Drainage | Good | Well drained | Moderately drained | Poorly drained | Very poorly drained | |
| Physical soil characteristics (s) | ||||||
| Texture/structure. | L, Cl, Scl, Sil | Sicl, Sc, C (<45%) | C (>45%), Lic, sl | Is, s | ||
| Soil depth (M) | >1.25 | 1.25–0.75 | 0.5–0.75 | <0.5 | ||
| Soil fertility characteristics (f) | ||||||
| Base saturation (%) | >50 | 50–35 | 35–20 | <20 | - | - |
| Sum of basic cations (cmol (+)/kg soil) | >6.5 | 6.5–4 | 4–2.8 | - | - | - |
| pH | 6.0–5.4 | 5.4–5.0 | 5.0–4.8 | 4.8–4.1 | <4.1 | - |
| Organic carbon (%) | >2.4 | 2.4–1.5 | 1.5–0.8 | <0.8 | - | - |
| Transect No. | Pit No. | Topsoil Name | Colour (Topsoil) | Subsoil Name | Colour (Subsoil) | TSD (m) | ERD (mm) | Soil Form | Permeability (s) | Slope (%) | Terrain Unit | Particle Size Distribution | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay (%) | Silt (%) | Sand (%) | Texture Class | ||||||||||||
| 1 | 1 | Orthic A | 10R 2.5/1 Reddish black | Pedocutanic B | 2.5YR 3/2 Dusky Red | 1.5 | 200–300 | Valsrivier | 1–3 | 0–3 | Footslope | 19 | 26 | 55 | Sandy loam |
| 2 | Orthic A | 5YR 3/4 Dark Reddish Brown | Soft Plinthic B | 2.5YR 4/6 Red | 1.02 | 0–200 | Westleigh | 1–3 | 0–3 | Footslope | 29 | 25 | 46 | Sandy clay loam | |
| 3 | Orthic A | 5YR 3/4 Dark Reddish Brown | Red Apedal B | 5YR 3/4 Dark Reddish Brown | 1.35 | 200–300 | Hutton | 1–3 | 0–3 | Footslope | 41 | 33 | 26 | Clay | |
| 2 | 1 | Orthic A | 7.5YR 3/4 Dark Brown | Pedocutanic B | 7.5YR 3/3 Dark Brown | 1.32 | 300–500 | Valsrivier | 4–8 | 0–3 | Footslope | 25 | 27 | 48 | Sandy clay loam |
| 2 | Orthic A | 7.5YR 3/4 Dark Brown | Pedocutanic B | 2.5YR 4/4 Reddish Brown | 3.01 | 200–300 | Valsrivier | 4–8 | 0–3 | Footslope | 41 | 33 | 26 | Clay | |
| 3 | Orthic A | 10R 3/3 Dusky Red | Red Apedal B | 5YR 4/6 Yellowish Red | 1.16 | 200–300 | Hutton | 4–8 | 0–3 | Footslope | 39 | 32 | 29 | Clay loam | |
| 3 | 1 | Orthic A | 5YR 3/3 Dark Reddish Brown | Pedocutanic B | 10R 3/3 Dusky Red | 0.907 | 200–500 | Valsrivier | 1–3 | 4–8 | Middleslope | 29 | 27 | 44 | Clay loam |
| 2 | Orthic A | 5YR 3/3 Dark Reddish Brown | Pedocutanic B | 2.5YR 3/3 Dark Reddish Brown | 1.35 | 0–200 | Valsrivier | 1–3 | 4–8 | Middleslope | 33 | 33 | 34 | Clay loam | |
| 3 | Orthic A | 5YR 3/4 Dark Reddish Brown | Red Apedal B | 2.5YR 3/4 Dark Reddish Brown | 1.12 | 200–300 | Hutton | 1–3 | 4–8 | Middleslope | 33 | 31 | 36 | Clay loam | |
| 4 | 1 | Orthic A | 5YR 3/3 Dark Reddish Brown | Lithocutanic B | 2.5YR 4/4 Reddish Brown | 1.2 | 0–200 | Glenrosa | 1–3 | 4–8 | Middleslope | 21 | 17 | 62 | Sandy clay loam |
| 2 | Orthic A | 5YR 3/3 Dark Reddish Brown | Pedocutanic B | 5YR 3/4 Dark Reddish Brown | 1.3 | 0–200 | Valsrivier | 1–3 | 4–8 | Middleslope | 25 | 33 | 42 | Loam | |
| 3 | Orthic A | 7.5YR 3/3 Dark Brown | Red Apedal B | 5YR 3/4 Dark Reddish Brown | 1.1 | 200–300 | Hutton | 1–3 | 4–8 | Middleslope | 39 | 32 | 29 | Clay loam | |
| Transect No. | Pit No. | Soil Form | Slope % | Terrain Unit | P | K | Ca | Mg | pH | OC | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | (KCl) | % | |||||||||
| 1 | 1 | Valsrivier | 0–3 | FS | 32 | 147 | 2662 | 573 | 5.2 | 1.6 | 0.04 |
| 2 | Westleigh | 0–3 | FS | 19 | 157 | 1355 | 340 | 5.22 | 1.4 | 0.08 | |
| 3 | Hutton | 0–3 | FS | 12 | 82 | 1649 | 429 | 5.31 | 0.5 | 0.03 | |
| 2 | 1 | Valsrivier | 0–3 | FS | 24 | 174 | 1843 | 751 | 5.06 | 1.3 | 0.03 |
| 2 | Valsrivier | 0–3 | FS | 31 | 305 | 2025 | 406 | 4.97 | 1.5 | 0.06 | |
| 3 | Hutton | 0–3 | FS | 31 | 175 | 1539 | 375 | 5.05 | 1.6 | 0.09 | |
| 3 | 1 | Valsrivier | 4–8 | MS | 21 | 106 | 1790 | 498 | 5.42 | 0.9 | 0.03 |
| 2 | Valsrivier | 4–8 | MS | 18 | 112 | 1725 | 425 | 5.04 | 1.7 | 0.04 | |
| 3 | Hutton | 4–8 | MS | 23 | 206 | 1805 | 501 | 5.27 | 1.2 | 0.03 | |
| 4 | 1 | Glenrosa | 4–8 | MS | 8 | 175 | 1212 | 327 | 4.62 | 1.1 | 0.05 |
| 2 | Valsrivier | 4–8 | MS | 37 | 254 | 2424 | 361 | 5.25 | 1.7 | 0.08 | |
| 3 | Hutton | 4–8 | MS | 29 | 147 | 1862 | 477 | 5.33 | 1.2 | 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swafo, S.M.; Dlamini, P.E. Unlocking the Land Capability and Soil Suitability of Makuleke Farm for Sustainable Banana Production. Sustainability 2023, 15, 453. https://doi.org/10.3390/su15010453
Swafo SM, Dlamini PE. Unlocking the Land Capability and Soil Suitability of Makuleke Farm for Sustainable Banana Production. Sustainability. 2023; 15(1):453. https://doi.org/10.3390/su15010453
Chicago/Turabian StyleSwafo, Seome Michael, and Phesheya Eugine Dlamini. 2023. "Unlocking the Land Capability and Soil Suitability of Makuleke Farm for Sustainable Banana Production" Sustainability 15, no. 1: 453. https://doi.org/10.3390/su15010453
APA StyleSwafo, S. M., & Dlamini, P. E. (2023). Unlocking the Land Capability and Soil Suitability of Makuleke Farm for Sustainable Banana Production. Sustainability, 15(1), 453. https://doi.org/10.3390/su15010453






