Influence of Magnesium Oxide on Carbonation of Cement Paste Containing Limestone and Metakaolin
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Compressive Strength
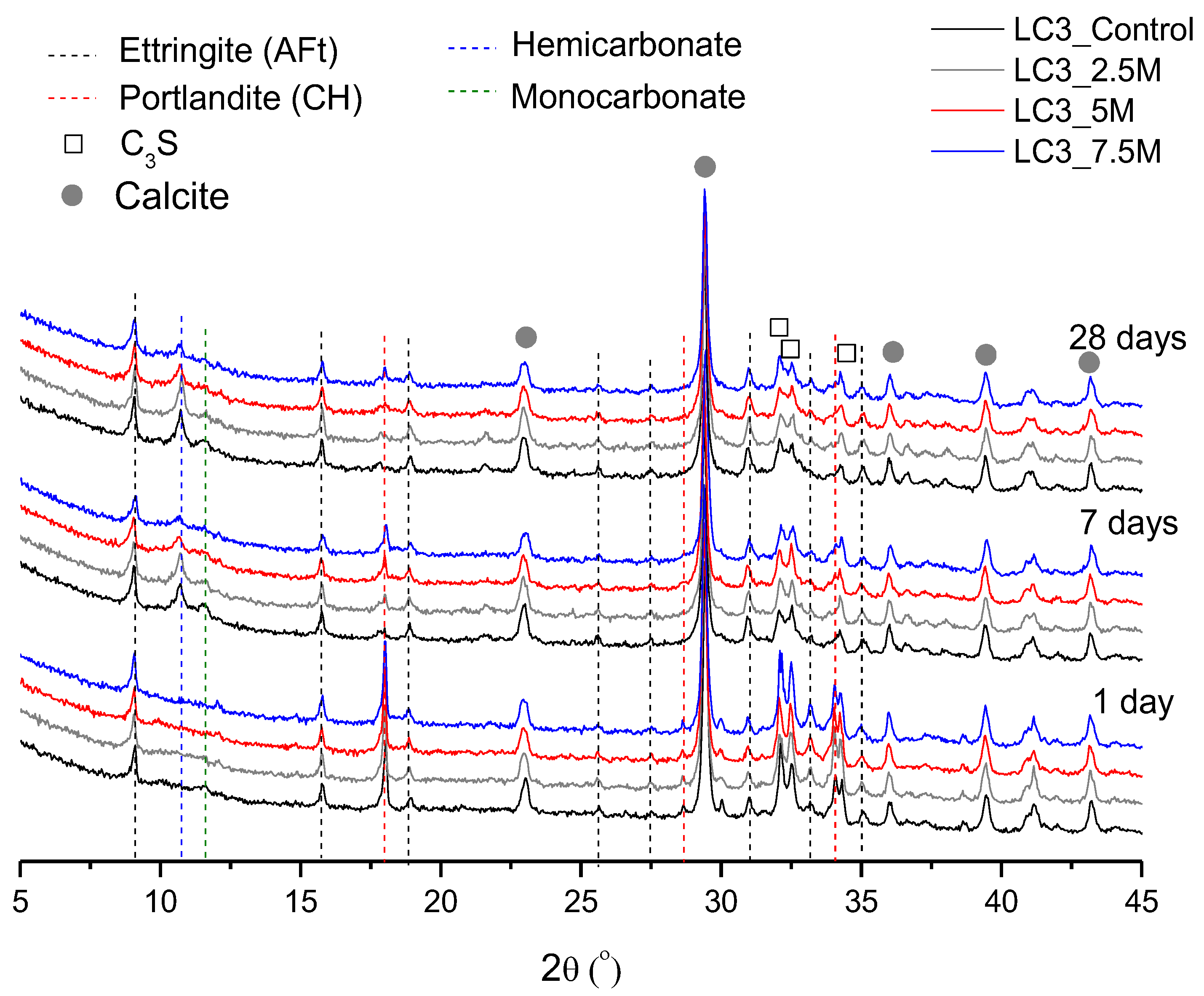
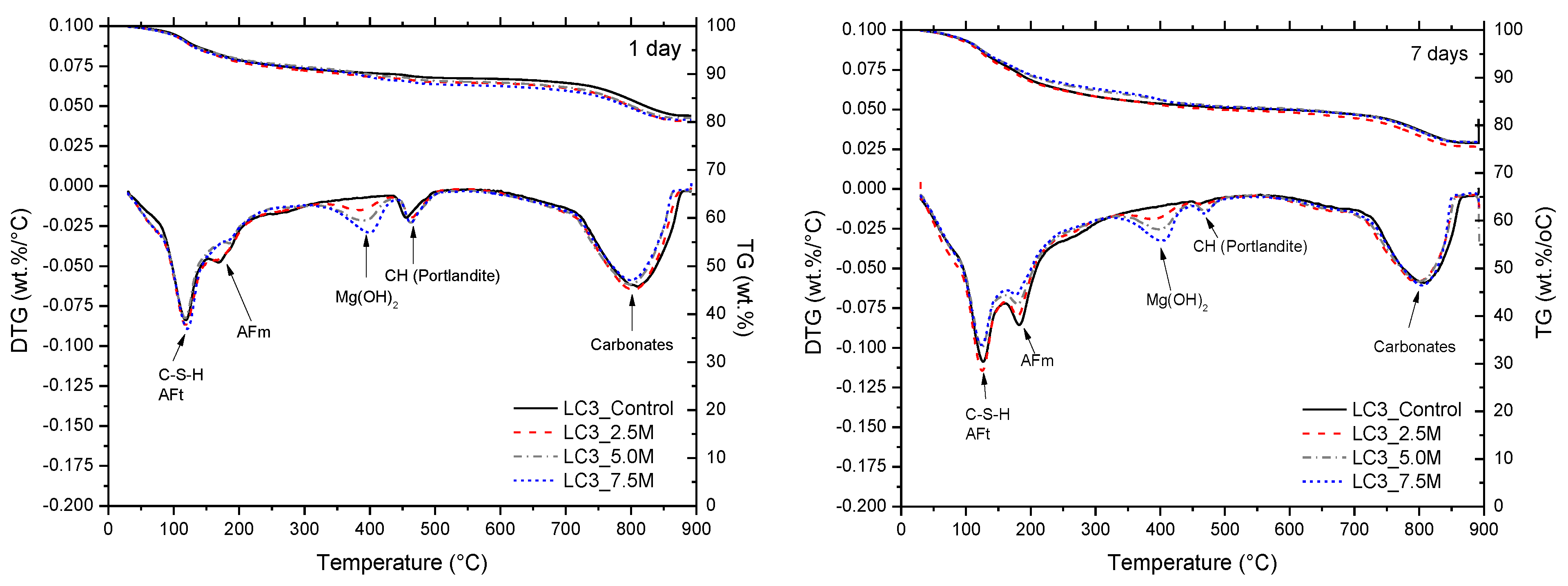
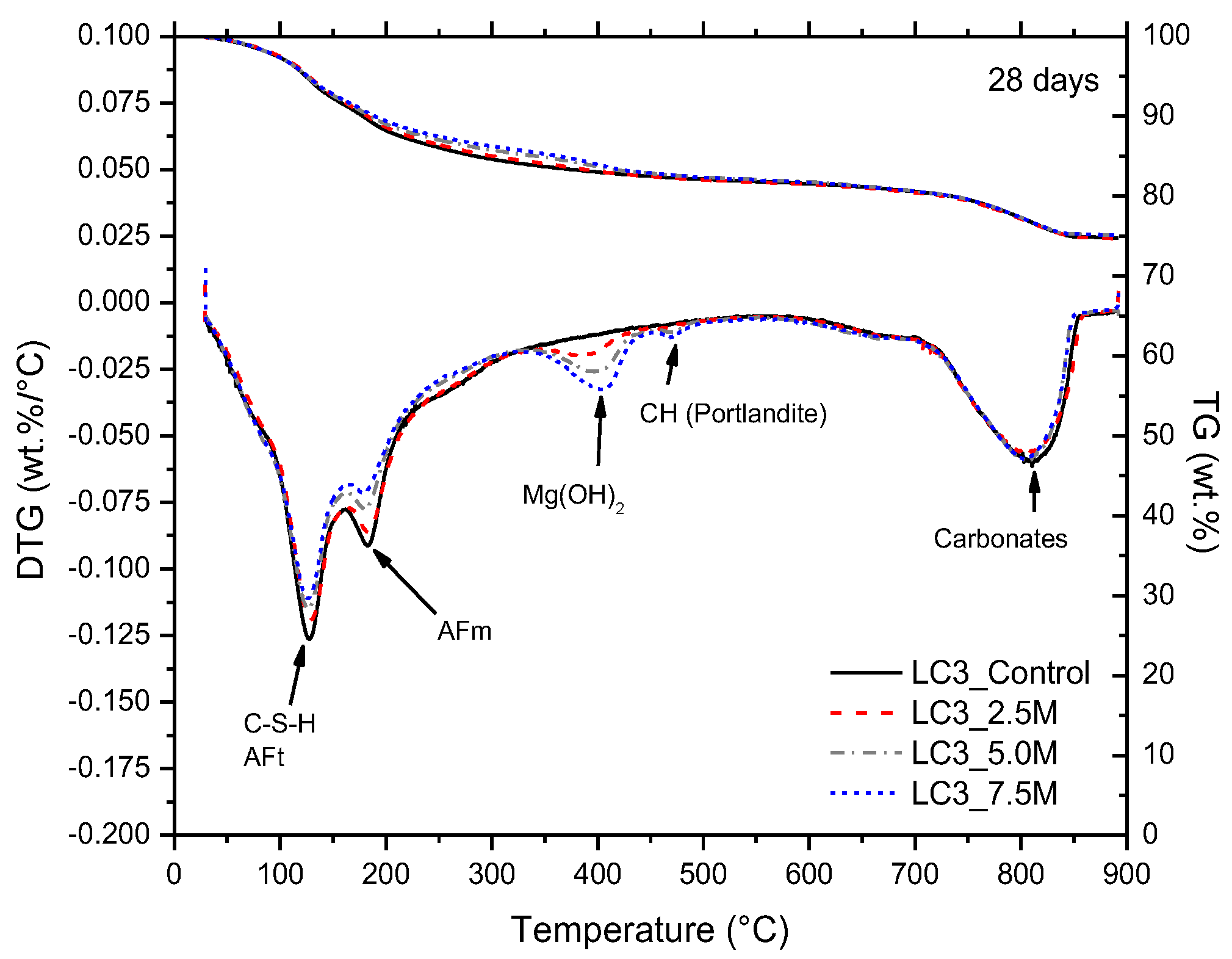
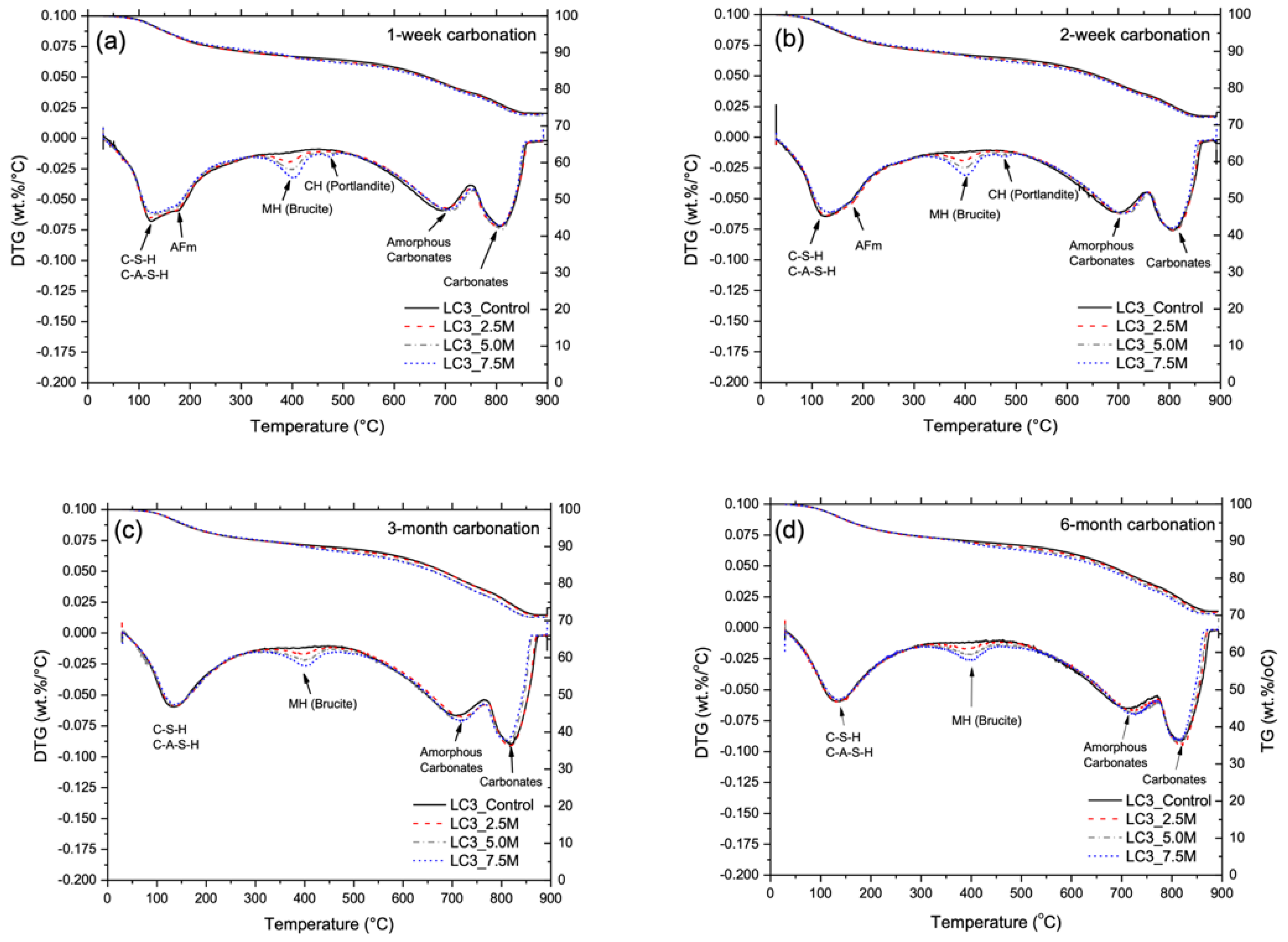
3.2. Phase and Molecular Analysis
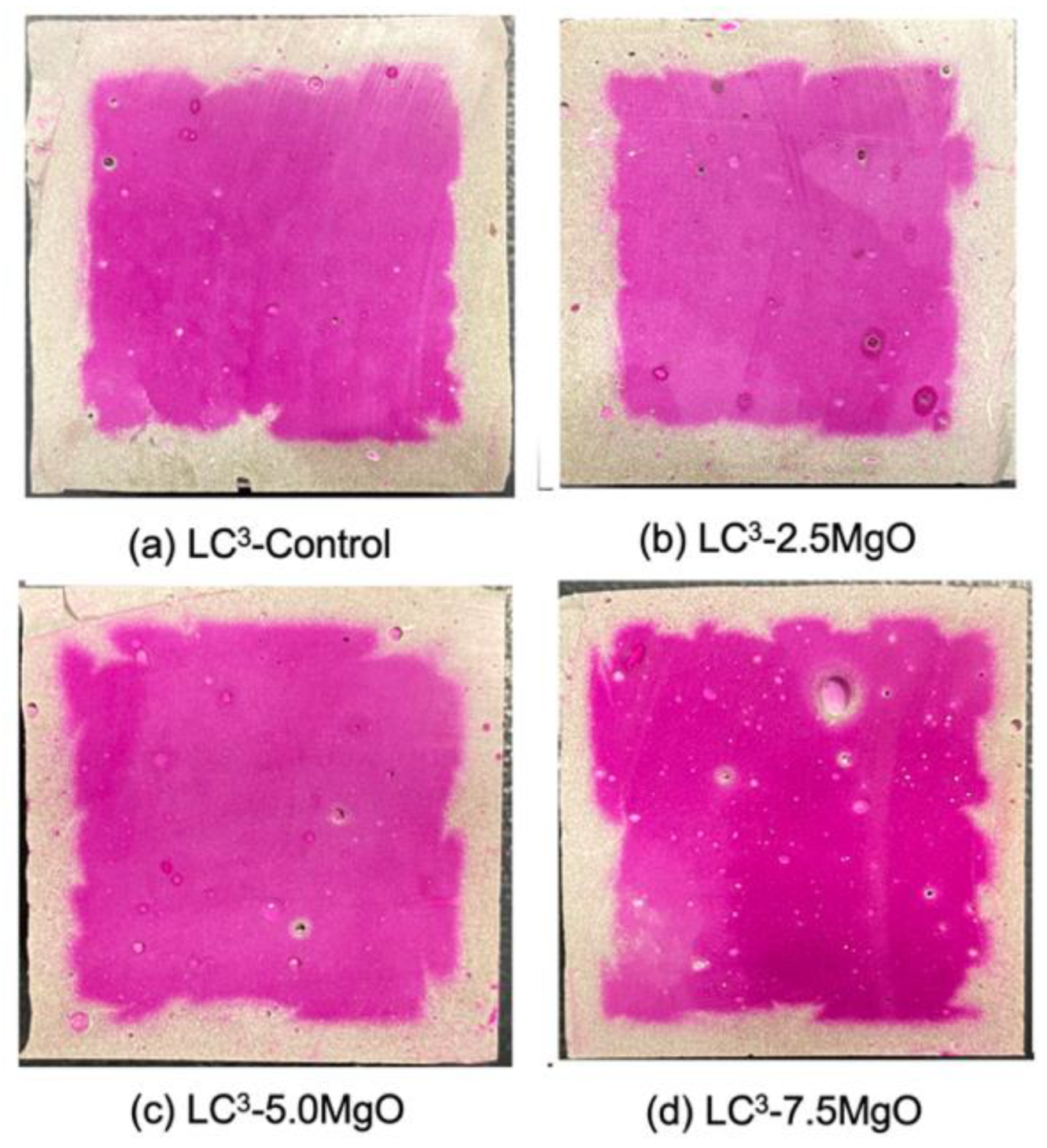
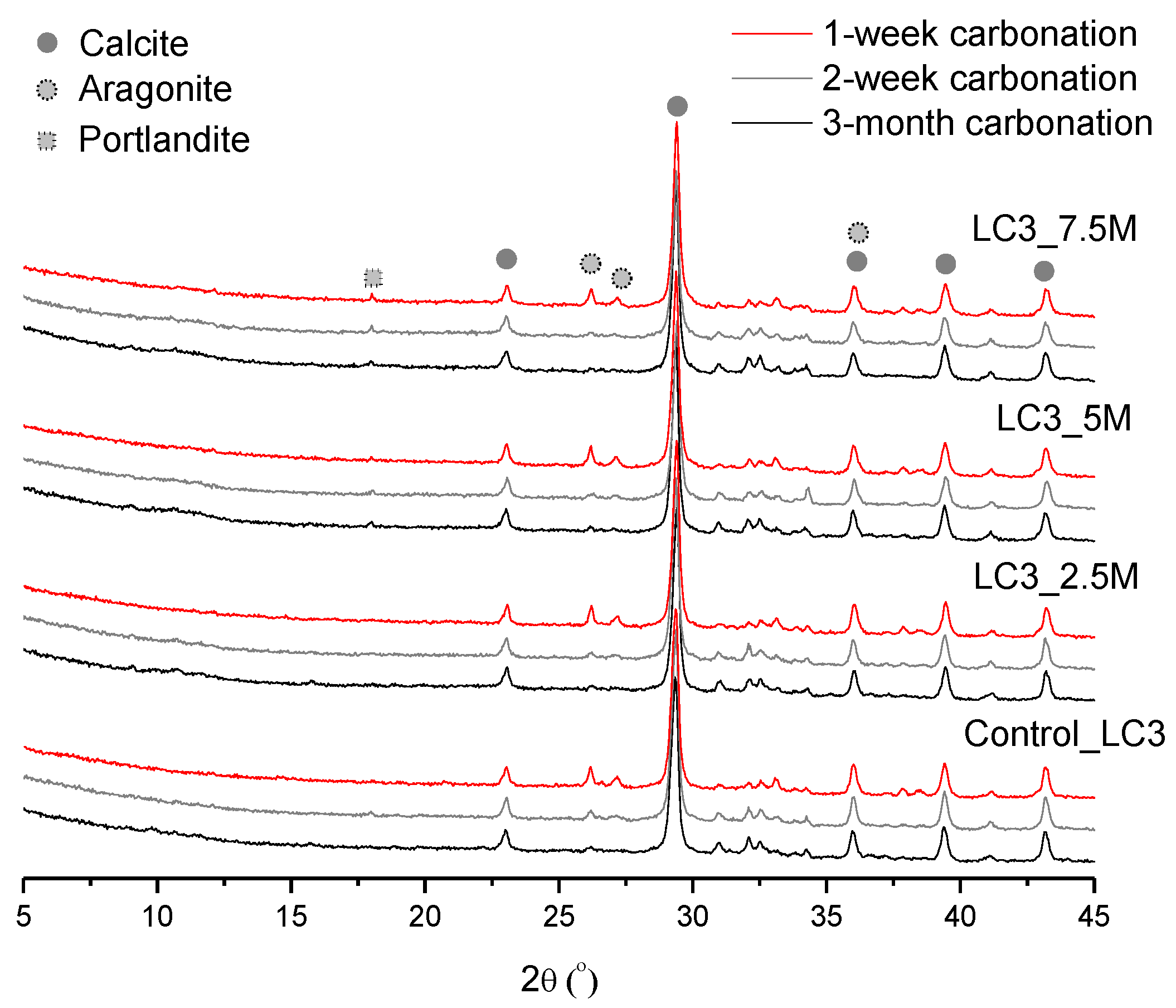
3.3. Carbonation Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Cai, R.; Tian, Z.; Ye, H.; He, Z.; Tang, S. The role of metakaolin in pore structure evolution of Portland cement pastes revealed by an impedance approach. Cem. Concr. Compos. 2021, 119, 103999. [Google Scholar] [CrossRef]
- Hossain, M.U.; Cai, R.; Ng, S.T.; Xuan, D.; Ye, H. Sustainable natural pozzolana concrete–A comparative study on its environmental performance against concretes with other industrial by-products. Constr. Build. Mater. 2021, 270, 121429. [Google Scholar] [CrossRef]
- Ng, S.T.; Hossain, M.U.; Liu, J.-C.; Xuan, D.; Ye, H.; Abdulla, S.J. Designing sustainable concrete mixes with potentially alternative binder systems: Multicriteria decision making process. J. Build. Eng. 2022, 45, 103587. [Google Scholar]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Milad, A.; Ali, A.S.B.; Babalghaith, A.M.; Memon, Z.A.; Mashaan, N.S.; Arafa, S.; Md. Yusoff, N.I. Utilisation of waste-based geopolymer in asphalt pavement modification and construction—A review. Sustainability 2021, 13, 3330. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Tironi, A.; Scian, A.N.; Irassar, E.F. Blended cements with limestone filler and kaolinitic calcined clay: Filler and pozzolanic effects. J. Mater. Civ. Eng. 2017, 29, 4017116. [Google Scholar] [CrossRef]
- Soroka, I.; Setter, N. The effect of fillers on strength of cement mortars. Cem. Concr. Res. 1977, 7, 449–456. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Nguyen, Q.D.; Castel, A. Performance of limestone calcined clay blended cement-based concrete against carbonation. Adv. Cem. Res. 2020, 32, 481–491. [Google Scholar] [CrossRef]
- Ye, H. Self-assembly of corrosion inhibitors-intercalated layered double hydroxides (LDHs) in cementitious systems. Appl. Clay Sci. 2022, 216, 106380. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Castel, A. Reinforcement corrosion in limestone flash calcined clay cement-based concrete. Cem. Concr. Res. 2020, 132, 106051. [Google Scholar] [CrossRef]
- Díaz, E.; González, R.; Rocha, D.; Alujas, A.; Martirena, F. Carbonation of concrete with low carbon cement LC3 exposed to different environmental conditions. In Calcined Clays Sustainable Concrete; Springer: Berlin/Heidelberg, Germany, 2018; pp. 141–146. [Google Scholar]
- Shi, Z.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Li, R.; Ye, H. Influence of Alkalis on Natural Carbonation of Limestone Calcined Clay Cement Pastes. Sustainability 2021, 13, 12833. [Google Scholar] [CrossRef]
- Ye, H. Mitigation of Drying and Carbonation Shrinkage of Cement Paste using Magnesia. J. Adv. Concr. Technol. 2018, 16, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Lee, H.-K. Unlocking the role of MgO in the carbonation of alkali-activated slag cement. Inorg. Chem. Front. 2018, 5, 1661–1670. [Google Scholar] [CrossRef]
- Ye, H.; Cai, R.; Tian, Z. Natural carbonation-induced phase and molecular evolution of alkali-activated slag: Effect of activator composition and curing temperature. Constr. Build. Mater. 2020, 248, 118726. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Factors influencing the sulfate balance in pure phase C3S/C3A systems. Cem. Concr. Res. 2020, 133, 106085. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; de Brito, J. Magnesia (MgO) production and characterization, and its influence on the performance of cementitious materials: A review. Materials 2020, 13, 4752. [Google Scholar] [CrossRef] [PubMed]
- Zunino, F.; Scrivener, K. The reaction between metakaolin and limestone and its effect in porosity refinement and mechanical properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- McQueen, N.; Kelemen, P.; Dipple, G.; Renforth, P.; Wilcox, J. Ambient weathering of magnesium oxide for CO2 removal from air. Nat. Commun. 2020, 11, 3299. [Google Scholar] [CrossRef]
- Fagerlund, J.; Highfield, J.; Zevenhoven, R. Kinetics studies on wet and dry gas–solid carbonation of MgO and Mg(OH)2 for CO2 sequestration. RSC Adv. 2012, 2, 10380–10393. [Google Scholar] [CrossRef]

| Mixture ID | OPC (%) | MK (%) | LS (%) | Anhydrite CaSO4 (%) | MgO (%) | Water/Powder Ratio |
|---|---|---|---|---|---|---|
| LC3_Control | 52.5 | 30.0 | 15.0 | 2.5 | - | 0.4 |
| LC3_2.5M | 52.5 | 30.0 | 15.0 | 2.5 | 2.5 | |
| LC3_5M | 52.5 | 30.0 | 15.0 | 2.5 | 5.0 | |
| LC3_7.5M | 52.5 | 30.0 | 15.0 | 2.5 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Jin, Y. Influence of Magnesium Oxide on Carbonation of Cement Paste Containing Limestone and Metakaolin. Sustainability 2022, 14, 5627. https://doi.org/10.3390/su14095627
Jiang T, Jin Y. Influence of Magnesium Oxide on Carbonation of Cement Paste Containing Limestone and Metakaolin. Sustainability. 2022; 14(9):5627. https://doi.org/10.3390/su14095627
Chicago/Turabian StyleJiang, Tao, and Ying Jin. 2022. "Influence of Magnesium Oxide on Carbonation of Cement Paste Containing Limestone and Metakaolin" Sustainability 14, no. 9: 5627. https://doi.org/10.3390/su14095627
APA StyleJiang, T., & Jin, Y. (2022). Influence of Magnesium Oxide on Carbonation of Cement Paste Containing Limestone and Metakaolin. Sustainability, 14(9), 5627. https://doi.org/10.3390/su14095627






