Abstract
In this study, we investigated the seasonal phytoplankton community and phytoplankton functional types (PFTs) in the vicinity of Dokdo Island, located in the East/Japan Sea, in 2019. With strong seasonal winds, the water column was well mixed in winter. In spring and autumn, the upper mixed layer depth (MLD) was relatively deep, and the Subsurface Chlorophyll Maximum (SCM) formed in the middle layer. Small phytoplankton were dominant in the summer, which is a time of high water temperatures and strong stratification associated with a shallower MLD. Based on CHEMTAX analysis, in spring, the high phytoplankton biomass was mainly derived from cyanobacteria, diatoms, and dinoflagellates. In summer, >73.2% of the surface biomass was comprised of cyanobacteria. In autumn, pelagophytes accounted for the highest proportion of the biomass. The fraction of microphytoplankton (fmicro) was highest in winter and spring, whereas the fraction of nanophytoplankton (fnano) was highest in autumn and summer. A high fraction of picophytoplankton (fpico) was evident in the surface layers in summer. Values for both the photoprotection index (PI) and the ratio of photoprotective carotenoids (PPC) to photosynthetic carotenoids (PSC) indicate that this study area had high primary productivity in 2019. In order to predict long-term changes in marine food webs due to climate change, it is important to evaluate the size and composition of phytoplankton.
1. Introduction
Phytoplankton are a fundamental component of marine food webs and are responsible for primary productivity in marine ecosystems []. Seasonal variation in phytoplankton is influenced by environmental factors and biological top-down control effects [,]. Over recent decades, there has been a significant increase in the occurrence of harmful algal blooms in coastal and oceanic waters which have adversely affected biodiversity, an indicator of the health of an ecosystem []. Continuous monitoring of phytoplankton and their links to environmental factors is important to provide a better understanding of potential long-term variation in fishery resources related to shifts in the marine ecosystem caused by climate change [].
The East Sea (Japan Sea) is a typical mid-latitude, marginal, semi-closed sea in the northwestern Pacific Ocean that is located between the Eurasian continent and Japan. The average water depth of the East Sea is 1700 m, but it has deep basins exceeding 2500 m in depth, including the Japan Basin in the southwest, the Yamato Basin in the southeast, and the Ulleung Basin (UB) in the southeast. The East Sea has a well-defined Sub-polar Front (SPF) at approximately 37–40° N [,]. This front is created between a warm water mass from the East Korea Warm Current (EKWC), which branches from the Tsushima Warm Current (TWC), and a cold-water mass from the North Korea Cold Current (NKCC), which branches from the Liman Current. The exact location of this front varies seasonally, as the gradient of the surface water temperature is strong in summer and weak in winter [,].
Dokdo Island is relatively small and located in the northeast region of the UB in SPF. Since Dokdo Island is uninhabited, there are minimal anthropogenic influences, and it has relatively pristine natural marine resources. However, few reported marine ecology studies have been conducted in this region since it is difficult to access by ship, especially during harsh weather conditions in the autumn and winter. The area is also highly regulated and requires a permit for entry [,,]. This region is influenced by the TWC, NKCC, upwelling [], warm eddies [], and the SPF []. Baek et al. [] demonstrated that phytoplankton blooms have occurred in the East Sea in spring after episodic windstorm events associated with the ‘small island effect’ in the vicinity of the Ulleung and Dokdo islands. The dynamic geological and environmental characteristics of the region allow for wide biodiversity, high primary production, and commercially valued fisheries [,].
Studies on the composition of phytoplankton communities have typically been performed using optical microscopy, which requires a high level of taxonomic skill and is very time consuming. However, pico-and nanosized phytoplankton are difficult to identify using optical microscope analyses, which often result in errors or omissions in species identification [,]. Although molecular analysis of 16S and 18S parts has been performed recently, this is still expensive and time-intensive, whereas chemotaxonomic methods based on pigment analysis using HPLC can readily detect and estimate relative contributions of small phytoplankton [,,], and biomarker pigments can be used to rapidly quantify specific phytoplankton groups []. A widely used software that uses marker pigment ratios to determine the relative contributions of phytoplankton groups to the total biomass is CHEMTAX (CHEMical TAXonomy) [,,].
Due to the development of analytical technologies such as NGS (next-generation sequencing), FCM (flow cytometry), and pigment analysis, high proportions of small-sized phytoplankton have recently been reported []. In addition, the composition and size of phytoplankton are changing due to climate change, so it is important to use environmental indicators to predict ecological shifts []. These studies have been intensively conducted in the Atlantic, Baltic, and Arctic Oceans [,,,]. There have been few studies in the East Sea, and recent studies have mainly been conducted in the East China Sea [,,]. The East Sea is also susceptible to global climate change, and an anticipated effect is elevated water temperatures, so it is critical to gain a baseline understanding of general marine ecology and biogeochemical processes [].
In this study, we describe the spatial–temporal distribution of phytoplankton based on pigment analysis and investigate the relationships among functional groups of phytoplankton based on pigmentary indices in waters adjacent to Dokdo Island. This study aims to provide insight to enhance the understanding of the bioecological features of these complex offshore waters. Shifts in small phytoplankton dynamics will be useful to predict changes in marine food structure due to climate change.
2. Materials and Methods
We conducted four surveys throughout the year, in winter (27 February 2019), spring (3 June 2019), summer (31 August 2019), and autumn (23 October 2019), at five stations in the vicinity of Dokdo Island (Figure 1). Vertical profiles of temperature, salinity, and chlorophyll a (calculated from fluorescence) were measured concurrently using a conductivity-temperature-depth profiler (SBE 911 plus CTD; SeaBird Inc., Bellevue, WA, USA) mounted on a rosette sampler aboard the R/V Eardo and R/V Jangmok II. We collected water samples at the surface, the Subsurface Chlorophyll Maximum layer (SCM), and the bottom. As each depth varies with season and station, sampling depths were located between 0 and 70 m and were plotted by dots on all contour plots.
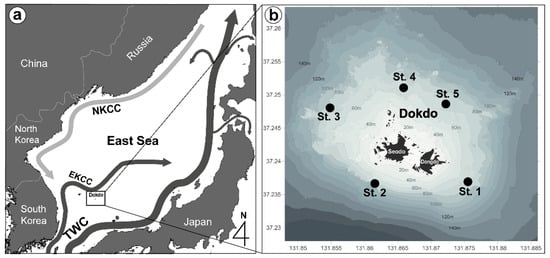
Figure 1.
Map of the study area schematically showing the main ocean currents (a) and locations of sampling stations around Dokdo Island in the East Sea (b).
For the pigment and nutrient analysis, a 4 L water sample from each station was filtered (47 mm GF/F; Whatman, Middlesex, UK). The filtered samples were placed in acid-cleaned polyethylene bottles and stored at −20 °C until pigment analysis. For the nutrient analysis, filtered water samples were fixed using HgCl2 and stored at −20 °C until further analysis. The concentrations of nutrients (ammonia, nitrate, nitrite, phosphate, and silicate) were measured using an auto-analyzer (QuikChem 8000; Lachat Instruments, Loveland, CO, USA). Data were calibrated using Reference Materials for Nutrients in Seawater (RMNS; KANSO Technos Co., Ltd., Osaka, Japan), and the level of precision was verified using RMNS.
Phytoplankton pigments were analyzed using a HPLC (LC-10A system; Shimadzu Co., Kyoto, Japan) fitted with a Waters C8 column (Waters Corporation, Milford, MA, USA), as proposed by Zapata et al. []. Standard pigments purchased from Sigma Chemicals and DHI (Hørsholm, Denmark) were used to identify peaks and calibrate concentrations (Table 1).

Table 1.
Abbreviations, full names, formula, and selected taxonomic designations for chlorophylls, carotenoids, pigment combinations, and indices.
Based on the pigments measured using HPLC, the composition of phytoplankton was estimated using the CHEMTAX program []. In MATLAB (The MathWorks, Inc., Natick, MA, USA), CHEMTAX uses factor analysis and a descent algorithm to determine the proportions of phytoplankton classes fitted to the total pigment concentration based on a cellular pigment ratio for each algal group [,]. The diagnostic biomarker pigments used included chl-a, fucoxanthin, 19′-hexanoyloxy-fucoxanthin, 19′-butanoyloxy-fucoxanthin, peridinin, prasinoxanthin, lutein, alloxanthin, zeaxanthin, violaxanthin, neoxanthin, and chlorophyll-b. Since the purpose of this study was to understand the characteristics of seasonal changes, the analysis was conducted by grouping by season. The initial pigment to chl-a ratio was based on the modified results of Lee et al. [] using various algae groups collected around the Korean Peninsula (Tables S1 and S2).
To assess the changing contributions of chlorophylls and carotenoids to the total pigment pool, pigment indices were derived in accordance with Barlow et al. []. The carotenoid pigments were converted into phytoplankton functional types (PFTs), including photosynthetic carotenoids (PSC) and photo-protective carotenoids (PPC). The index on size fraction was calculated using formulas proposed by Brewin et al. [] and Hirata et al. [] (Table 1). The upper mixed layer depth (MLD) was calculated from temperature and salinity derived density profiles using a density difference criterion (∆σ = 0.125 kg m−3) [].
All statistical analyses were conducted by using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). We used nonparametric Kruskal–Wallis tests with post-hoc Dunn’s tests to evaluate statistically significant differences between seasons and layers. For all tests, p < 0.01 was considered significant. The impacts of the measured environmental factors for all depths and seasons on phytoplankton pigments and groups were investigated with CCA (Canonical correspondence analysis) using CANOCO (Canoco 4.5 software package, Biometris, Wageningen, The Netherlands).
3. Results
3.1. Hydrological Parameters
The seasonal distribution of temperature showed characteristics typical of temperate waters. The water temperature ranged from 11.76 to 11.79 °C in winter, 12.7 to 18.3 °C in spring, 7.24 to 25.4 °C in summer, and 6.08 to 19.2 °C in autumn (Figure 2). In winter, the water column was well mixed and, consequently the temperatures was homogeneous throughout the water column. In spring and autumn, the surface mixed layer was relatively deep, whereas during the summer, strong stratification with a shallow mixed layer formed, and the water temperature difference between the surface and bottom was as great as 18.1 °C.
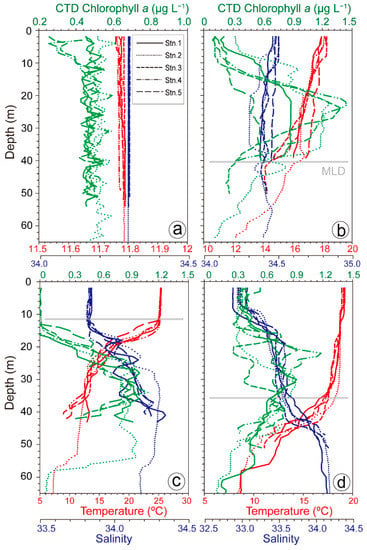
Figure 2.
Vertical profiles of the water temperature (red), salinity (blue), and CTD chlorophyll a (green) at each station during winter (a), spring (b), summer (c), and autumn (d). Gray lines indicate the MLD.
The salinity was similar throughout the water column at 34.3. In spring, the salinity ranged from 34.3 to 34.5. In summer and autumn, the surface salinity averaged 33.8 and 33.0, respectively, and was slightly lower than in other seasons. The vertical distribution of chl-a calculated by CTD was very low in winter (0.25 to 0.97 µg L−1). In spring, the SCM layer formed at approximately 25 m, and the maximum chl-a value in this layer was 1.44 µg L−1. In summer, the chl-a value was extremely low from the surface to a depth of 15 m but reached a maximum level of 1.09 µg L−1 at a depth of about 10 m. In autumn, the chl-a level was relatively high (an average of 0.72 µg L−1 and a maximum of 1.18 µg L−1) over a wide depth range (approximately 20–40 m), but no clear SCM layer was observed. The MLD, known to be associated with the formation of SCM in the East Sea, was observed in all seasons except winter. The MLD formed at the deepest depth of 41 m in spring, followed by 36 m in autumn, and 12 m in summer with strong thermal stratification.
3.2. Nutrients
During the study period, the nitrate + nitrite concentration ranged from 0.01 to 14.61 μM. In winter, the average concentration was 8.25 μM, which was relatively high (Figure 3). High nutrient concentrations were only observed in the bottom layer in other seasons (spring, summer, autumn). Regardless of the season, the nutrient concentrations at the bottom were similar. The ammonium concentration varied from 0.04 to 3.83 μM. The ammonium level was somewhat higher in winter but, overall, was very low during the other seasons. The phosphate concentration ranged from 0.05 to 0.92 μM, and its distribution pattern was almost similar to that of nitrate + nitrite. The silicate concentration remained relatively high (ranged from 2.96 to 14.49 μM), despite the study sites being in open waters, which are usually oligotrophic.
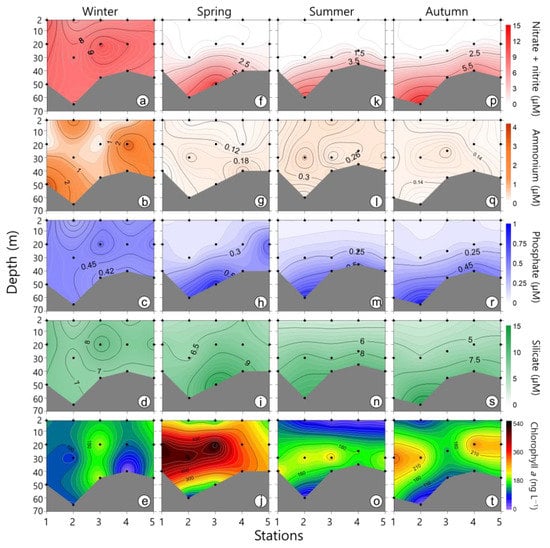
Figure 3.
Contour plots showing the vertical distribution of nutrients and chlorophyll a in winter (a–e), spring (f–j), summer (k–o), and autumn (p–t). Dots indicate water sampling depths.
3.3. Phytoplankton Pigments
The major pigments identified over the four seasons were chl-a, fucoxanthin, peridinin, 19′-hex-fucoxanthin, chl-b, zeaxanthin, 19′-but-fucoxanthin, alloxanthin, and prasinoxanthin (Figure 3, Figure 4 and Figure 5). Among the major pigments, the concentration of chl-a was the highest, particularly in the SCM layer during spring and then autumn. The concentration of peridinin (the maker pigment for dinoflagellates; Table 1) was high in spring and was clearly correlated with the chl-a concentration (r = 0.86, p < 0.001). The peridinin concentration in the SCM layer reached 125 ng L−1. In addition, the concentration of 19′-but-fucoxanthin was significantly correlated with the concentrations of neoxanthin (r = 0.80, p < 0.001) and 19′-hex-fucoxanthin (r = 0.88, p < 0.001). The 19′-but-fucoxanthin concentration was high in autumn (max. 40.84 ng L−1). The fucoxanthin concentration (the marker pigment for diatoms) showed extremely high levels in spring. The prasinoxanthin concentration (the major pigment in prasinophytes) was significantly correlated with the concentrations of neoxanthin (r = 0.92, p < 0.001) and 19′-hex-fucoxanthin (r = 0.83, p < 0.001). The seasonal distribution of the prasinoxanthin concentration was similar to that of chl-a. The maximum concentration of prasinoxanthin was similar in spring, summer, and autumn. The alloxanthin (the major pigment in cryptophytes) concentration remained high in the SCM layer in summer, with an average of 23.1 ng L−1. The zeaxanthin (the maker pigment for cyanobacteria) concentration was high in the surface layer in spring and in the SCM layer in summer. The concentration of chl-b (the major pigment in chlorophytes) reached a maximum of 85.1 ng L−1 in the middle layer during autumn.
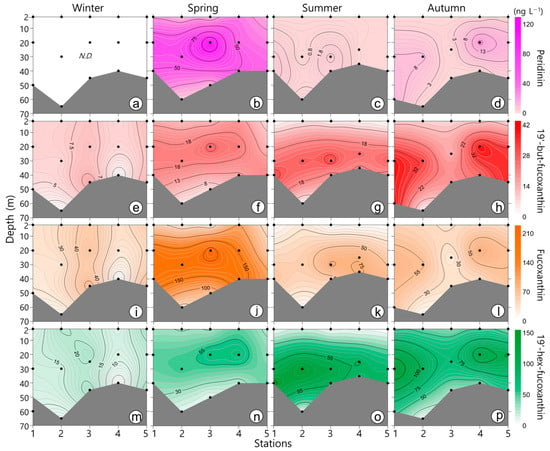
Figure 4.
Contour plots showing the vertical distribution of the pigments peridinin, 19′-but-fucoxanthin, fucoxanthin, and 19′-hex-fucoxanthin during winter (a–d), spring (e–h), (i–l) summer, and autumn (m–p). Dots indicate water sampling depths. N.D.: not detected. Dots indicate water sampling depths.
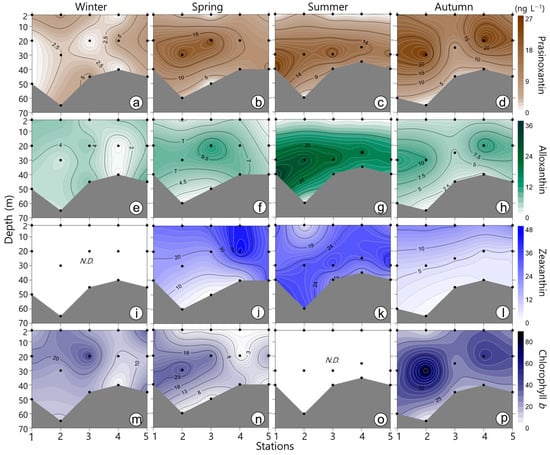
Figure 5.
Contour plots showing the vertical distribution of the pigments prasinoxanthin, alloxanthin, zeaxanthin, and chlorophyll b during winter (a–d), spring (e–h), summer (i–l), and autumn (m–p). Dots indicate water sampling depths. N.D.: not detected. Dots indicate water sampling depths.
3.4. CHEMTAX Community Analysis
The results of the CHEMTAX analysis showed the relative contributions of various classes in the phytoplankton community to the total chl-a concentration (Figure 6). In winter, the proportion of diatoms was high (50.4%), followed by cryptophytes (17.4%) and then prasinophytes (14.0%). The water column was well mixed, and there were no differences in phytoplankton pigments between stations or layers. In spring, the chl-a concentration was relatively high in the SCM layer. Diatoms were present in high proportions throughout the entire water column, averaging 35.5%. However, the proportion of cyanobacteria was higher (34.4%) than that of diatoms (21.5%) in the surface layer but declined in the middle and lower layers. Meanwhile, dinoflagellates accounted for 13.4% of the biomass. In summer, the chl-a concentration was considerably low in the surface layer. Cyanobacteria accounted for 73.2% of the biomass at the surface, while proportions in the middle (12.7%) and bottom (9.05%) layers were low. Cryptophytes comprised 35.0% and 23.9% in the SCM and bottom layers, respectively. In autumn, all phytoplankton groups had similar proportions, except for dinoflagellates and chlorophyte, which were present in low proportions in the surface layer. The proportions of pelagophytes (25.8%), prymnesiophytes (24.0%), and cryptophytes (20.8%) were high in the SCM layer. In the bottom layer, the distribution was similar to that of the SCM layer, and the proportion of pelagophytes was the highest, with an average of 27.6%.
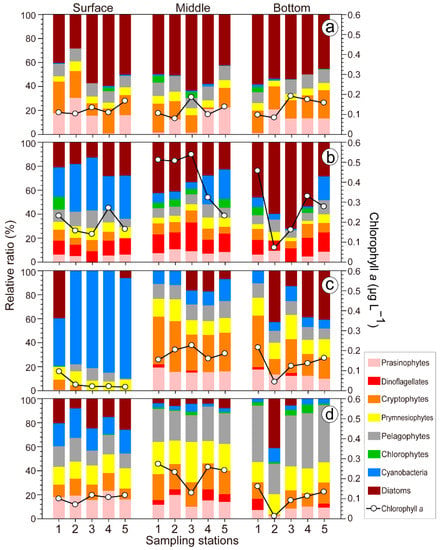
Figure 6.
The contribution of each taxonomic group to the total phytoplankton biomass, calculated using CHEMTAX, and total chlorophyll a. Winter (a), spring (b), summer (c), and autumn (d).
3.5. Canonical Correspondence Analysis of Phytoplankton and Environmental Parameters
The canonical correspondence analysis (CCA) revealed the relationships of pigments and the phytoplankton community composition to environmental variables. Based on the pigment results (Figure 7a), the first two axes can explain 79.6% of the relationship. For the CHEMTAX phytoplankton composition (Figure 7b), the first two axes represented 90.1% of the variation. Nutrients and water temperature showed a strong negative correlation, implying that the nutrient concentrations were maintained at high levels, even in relatively low temperatures in winter, when the water column was well mixed. Additionally, in other seasons, nutrients were present in high concentrations in the lower layers where the water temperature was low. There was a high correlation between the phosphate and nitrate + nitrite concentrations. The nutrients and water temperature led the trend along the second axis of the CCA, implying that the stations were distributed vertically. However, in autumn, a horizontal distribution was shown according to salinity rather than nutrients. With respect to pigments, the peridinin, chl-b, and β-car concentrations showed significant seasonal variation, but other pigments showed relatively minimal seasonal variation. Chlorophytes, dinoflagellates, and diatoms were dominant in spring, and their occurrences were closely correlated. In addition, they showed a horizontal distribution along the first axis, and phytoplankton groups were identified as being relatively affected by the seasons.
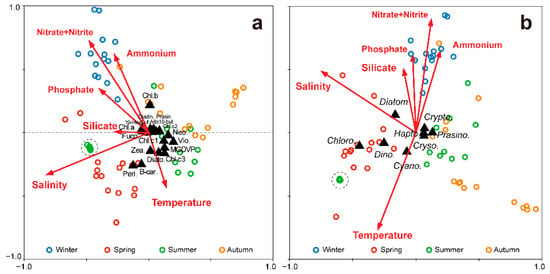
Figure 7.
Canonical correspondence analysis of the relationships of the pigments (a) and the dominant phytoplankton groups (b) both with environmental factors in the vicinity of Dokdo Island. Round dots indicate stations, and each color indicates a season (Blue: winter, Red: spring, Green: summer, Yellow: autumn). Triangles indicate phytoplankton groups and pigments. Dotted circles indicate stations in the surface water during summer.
Cyanobacteria dominated in summer, and their occurrence was correlated with high water temperatures and low nutrient concentrations. On the other hand, only the summer surface layer had different environmental properties, indicated by the dotted circle (Figure 7), where the water temperature was high and the nutrient concentrations were extremely low.
3.6. Pigment Indices and PFGs
Pigment indices can provide physiological information about the phytoplankton community and nutrient conditions. The photoprotection index (PI) remained low in winter and spring. In relative terms, it increased in summer (Figure 8), particularly in the surface (average, 0.71) and bottom (average, 0.39) layers. In autumn, the PI was high in the surface layer and relatively low in the bottom layer. The PPC:PSC ratio was low (<1), except for at several stations in summer (Figure 9). The phytoplankton size structure using diagnostic pigments was as follows: the size fractions of microphytoplankton (fmicro) were high in winter and spring, whereas the size fractions of nanophytoplankton (fnano) were high in autumn and summer. The highest value for the size fractions of picophytoplankton (fpico) was found in the surface euphotic layer in summer (Table 2).
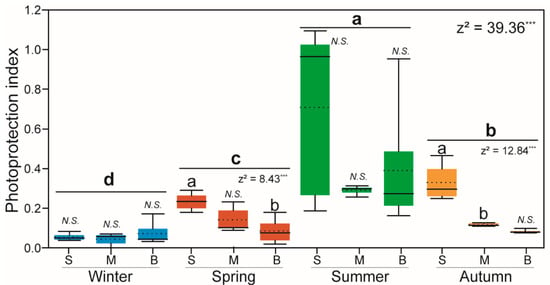
Figure 8.
Box plots of the photoprotection index (PI) for all water layers and seasons. The results were analyzed using Kruskal–Wallis tests with post-hoc DUNN’S tests. Bold letters (a–d) indicate significant differences between seasons, and normal letters (a, b) indicate differences between depths; ***: p < 0.001; N.S.: Not significant.
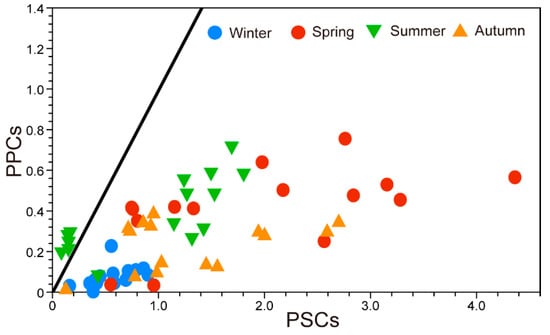
Figure 9.
Relationships among the concentrations of photoprotective carotenoids (PPC) and photosynthetic carotenoids (PSC) around Dokdo Island. The thick line indicates a PPC:PSC ratio of 1.

Table 2.
The size fractions of phytoplankton. Integrated values were derived from diagnostic pigments. Fractions exceeding 0.35 are marked in bold type.
4. Discussion
4.1. Environmental Factors
It is known that coastal waters are typically nutrient rich and that the nutrient concentration gradually declines further offshore [,]. The East Sea has relatively high productivity in spite of its oligotrophic waters, because of complex water mixing processes related to seasonal ocean-atmosphere interactions and the ocean current systems (including the TWC, NKCC, and eddies). Many studies are still being conducted to explain the high productivity in the East Sea, involving factors such as the supply of other nutrients (i.e., dissolved organic nitrate) or the influence of currents [,,,]. Therefore, we investigated the relationship between the seasonal oceanographic characteristics and the structure of the pigment-based phytoplankton community in the waters near Dokdo, which is located in the middle of the East Sea but has a low water depth.
The oceanographic characteristics for each season were found to be distinct. In winter, the whole water column is well mixed by prevailing winds, and thus high nutrient levels were maintained throughout the entire water column. A weak thermocline was evident in spring, characterized by moderate nutrient levels (surface: N = 2–5 µM; P = 0.2–0.5 µM; Si = 4–10 µM), even in the upper euphotic layer. The SCM was formed above the MLD at about 36 m in spring, and a relatively large biomass was observed. Phytoplankton is thought to grow in spring using nutrients supplied during the winter season. According to the results of nutrient addition experiments in the area adjacent to the study area, when nitrate was added, small-sized diatoms reacted significantly in spring []. In summer, there was a strongly stratified MLD at about 13 m. An extremely low biomass (mainly composed of cyanobacteria) was observed in the high-temperature water mass above the MLD, and a rather high biomass was observed below the MLD. In autumn, the MLD and SCM showed similar characteristics as in spring. Generally, SCM forms under the MLD in the East Sea, but that relationship varied with each season in the waters surrounding Dokdo Island []. Kim et al. [] demonstrated that, because of the topographic features, there are complex water masses in the vicinity of Dokdo Island.
In autumn and summer, the salinity remained at a relatively low level in the surface layer, even though there was no significant rainfall or freshwater inflow, especially in autumn. Rho et al. [] demonstrated that surface water with a salinity of <33.8 in the East Sea results from the introduction of Tsushima Warm Water, including Changjiang Diluted Water, which originates from the East China Sea. The seasonal width and strength of the TWC in the East Sea is strong in autumn but weak in spring, depending on the intensity of the southerly winds []. Therefore, the low salinity of waters around Dokdo Island in autumn may be related to the strong TWC and the influence of Changjiang Diluted Water. However, further studies are needed to determine whether this current causes changes in MLD and/or the phytoplankton composition.
4.2. Seasonal Phytoplankton Dynamics
Studies of phytoplankton in the East Sea have focused primarily on diatoms and dinoflagellates, and few have reported on species like prasinophytes, prymnesiophytes, pelagophytes, and cyanobacteria [,,]. Studies on seasonal variation have mainly focused on biomass, which greatly increases in spring despite the area being in the open ocean []. In addition, picosized plankton have been detected more often in the East Sea [].
Our pigment analysis showed that the chl-a concentration was high in spring, especially in the SCM layer (Figure 2b and Figure 3j). Among the accessory pigments detected, the concentrations of peridinin and fucoxanthin were very high. In general, the spring bloom was mainly composed of diatoms, and large contributions from cyanobacteria and dinoflagellates were also found (Figure 6). Diatom blooms are common in spring in the East Sea. According to a previous study that focused on the microscopic analysis of microsized phytoplankton, the predominant phytoplankton at the same time was the diatom Chaetoceros spp., and dinoflagellate Prorocentrum obtusidens (P. donghaiense) was most abundant in spring around the waters of Dokdo. In addition, various dinoflagellates, such as Gyrodinium spirale, P. triestinum, and Katodinium spp., were also found to be present in a microscopic analysis []. Specifically, P. donghaiense, which was observed to have a high biomass in spring, is a eurythermal and euryhaline species that causes red tides in the East China Sea and Korean coastal waters from early spring, coinciding with times of high nutrient concentrations [,,]. The phytoplankton population in the southeast Korean coastal waters is able to spread into the East Sea by water currents, and this is critical for maintaining high productivity in the UB area of the East Sea [,].
Cyanobacteria, which were detected in high proportions in the surface waters in spring, were detected in particularly high proportions in surface waters in summer when the level of productivity was extremely low. Despite the low productivity in summer, which resulted from a high light intensity, high water temperature, and nutrient depletion, we found a high concentration of zeaxanthin (a marker pigment for cyanobacteria). Although zeaxanthin is also present in green algae, we did not detect chl-b (indicator for green algae); therefore, we were able to conclude the presence of cyanobacteria. It is well known that cyanobacteria in oligotrophic waters contribute to high primary production in these areas, but they are difficult to detect using microscopy, and their presence needs to be confirmed using flow cytometry or pigment analysis. Kim et al. [] reported the distribution of cyanobacteria in the East Sea for the first time. They reported that cyanobacteria (confirmed by zeaxanthin) account for 20–60% of the phytoplankton community in DIN-limited surface water of the East Sea during spring, consistent with our results. Among cyanobacteria, Synechococcus can survive and adapt to most ocean conditions, but Prochlorococcus is detected only in oligotrophic warm waters []. The presence of prochlorophytes, including Prochlorococcus, can be confirmed by the detection of divinyl chl-a. In our results, no significant concentration of divinyl chl-a was detected, even in summer. Divinyl chl-a has often been detected, along with zeaxanthin, in surveys from other years in similar areas. However, it is presumed that the cyanobacteria characterized only by zeaxanthin without divinyl chl-a in this investigation are Synechococcus.
The highest concentrations of alloxanthin were detected in the SCM and bottom waters in summer. Alloxanthin, which is a marker pigment for cryptophytes, was detected throughout the water column in all seasons and was present at high concentrations (up to 36 ng L−1) in the SCM and bottom waters in summer. Cryptophytes have been reported in the open ocean and inland lakes and are tolerant to oligotrophic conditions [,]. In addition, as cryptophytes are able to dominate under low biomass and low competition conditions, the present area is a suitable habitat for these phytoplankton in summer.
Prasinophytes comprised moderate proportions of the community in all water layers in winter (14.0%) and autumn (13.9%) but were also present in spring and summer. Although the presence of prasinophytes can be confirmed by the combined detection of neoxanthin, prasinoxanthin, violaxanthin, and lutein, only prasinoxanthin can be used as a marker pigment for prasinophyceae. Furuya and Marumo [] reported that the prasinophyte Micromonas prevails among delicate flagellate in Kuroshio waters. Although the presence of prasinophyceae has recently reported in the East China Sea, studies of its abundance and ecophysiology have been limited because of difficulties with species identification, isolation, and culture.
Prymnesiophytes and pelagophytes comprised 19.2% and 27.6% of the community, respectively, in autumn, and were detected by the marker pigment 19′-hex-fucoxanthin. Pelagophytes can be distinguished from prymnesiophytes because they have both 19′-but-fucoxanthin and fucoxanthin. Pelagophytes are abundant picosized phytoplankton found within the open ocean and are the causative algae of harmful brown tide in estuaries [,]. Despite their ubiquitous distribution in marine ecosystems, the physiological capabilities of pelagophytes remain poorly understood. They have been reported at high densities together with Synechococcus in low-salinity and nutrient-rich waters in the East China Sea affected by China Coastal Waters []. The present study reports the occurrence of significant amounts of nanoplankton and picoplankton, which were not detected by conventional analysis methods in the East Sea. This highlights that useful information on the presence of phytoplankton based on HPLC pigments can contribute to understanding the seasonal distribution of phytoplankton populations in the complex shallow offshore waters of Dokdo Island.
4.3. Phytoplankton Functional Groups
It is known that pigment indices can indicate the physiological condition of phytoplankton communities in relation to environmental and trophic conditions. Diagnostic pigments can be derived from marker pigment combinations, which roughly correspond to the biomass proportions of pico-, nano-, and microphytoplankton [,]. Among these, the fpico was high in the surface layers in summer and was characterized by strong stratification. The intense sunlight at the surface negatively affected photosynthesis activity and the growth of phytoplankton. In addition, the depletion of inorganic nutrients in the surface layers led to a high fpico, because large phytoplankton require high nutrient levels []. The fpico was high in the mid-bottom layers in summer and throughout the water column in autumn. The fmicro was highest in spring, especially in the bottom layer, followed by winter. Although the fnano and fmicro have been reported to be generally high in stratified waters [,], this result shows that the fpico can also be high under relatively low nutrient conditions when few picoplankton competitors are present. Long-term and continuous monitoring is required, because changes in the phytoplankton size composition can cause shifts in the marine food chain and reduce the fisheries resources.
The PI is an indicator of the phytoplankton community response to changes in habitat conditions, including transparency, light intensity, and stratification []. High PI values are mostly found under stressful environmental conditions including oligotrophic conditions and intense radiation, whereas low PI values are generally associated with highly productive waters [,,]. In the present study, the PI was low, except in summer at the surface and in the bottom layer. It was relatively high in the surface layer in autumn as well. A high value represents a dynamic water column and oligotrophic conditions. The chl-a and nutrient concentrations were negatively correlated with the PI values (p < 0.05). Moreno et al. [] reported high chl-a and low PI values on the continental shelf, which is similar to the findings of our study. Additionally, the PI value varied from 0.37 to 0.71 in Palk Bay []. In the present study, the PI values were generally low, indicating that the sampling sites have relatively high primary productivity, even though the island is located in offshore oligotrophic waters. Further research is needed to determine whether the short-term supply of nutrients in winter can cause these results.
The PPC:PSC ratio can provide an estimate of the physiological conditions of the phytoplankton community []. Functionally, a dominance of PSC is considered to indicate productive conditions. In contrast, the dominance of PPC is associated with less productive ecosystems [,]. The ratio was high at most stations in each season but not in the surface layer in summer. Barlow et al. [] and Vijayan et al. [] demonstrated that the PPC:PSC ratio indicates the phytoplankton status, and it has a high relationship with intensive light, which forces phytoplankton to increase the concentration of PPC. In addition, the PPC:PSC ratio increases under high irradiance and low nutrient conditions []. For tropical waters, Barlow et al. [] and Moreno et al. [] reported that change in the proportion of PPC is a good indicator of the response of cells to photo-oxidative damage from strong ultraviolet radiation. The low ratio in the present study was caused by high PSC values, which have mainly been reported as being associated with highly productive waters, including coastal and estuarine areas [,]. Although this study area is geographically an open ocean, our index results of a four-time snapshot survey imply that this region is highly productive. These various indicators could be used as a marker to represent and compare the ocean condition. Many studies are being attempted, along with in-situ pigment research, to identify the active state of phytoplankton, primary productivity, and the phytoplankton community using remote sensing techniques, and further studies on pigment indices through satellites are to be expected.
5. Conclusions
The seasonal dynamics of phytoplankton in the vicinity of Dokdo Island in 2019 were conceptually summarized using the CTD and CHEMTAX data observed in the present work (Figure 10).
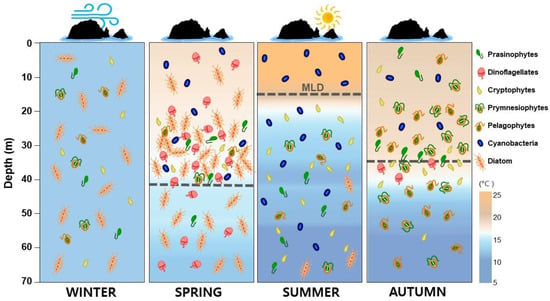
Figure 10.
Schematic representation of the observed seasonal phytoplankton community distributional patterns (CHEMTAX) and water conditions (CTD data) in the vicinity of Dokdo Island during 2019.
The waters around Dokdo Island have complex environmental characteristics because of the influence of various ocean currents, the mixing associated with the shallow depth, and the nutrients being supplied from the bottom layer. The water column was well mixed by seasonal winds in winter. In spring and autumn, the MLD was around 40 m deep, and in summer it was shallow at 13 m. There were significant seasonal fluctuations in the phytoplankton community based on the pigment analysis. In winter, diatoms represented by fucoxanthin as a marker pigment, accounted for a high proportion. In spring, cyanobacteria were dominant in the surface layer, while diatoms were dominant in the SCM and bottom layers. Additionally, some dinoflagellates (represented by peridinin) appeared in all waters in spring. A high proportion of cyanobacteria (represented by zeaxanthin) was evident in the surface layer in summer. In contrast, high proportions of prasinophytes and pelagophytes, which are small in size, were evident in autumn. For size classification using pigments, the fraction of microphytoplankton was high in winter and spring, while the nanophytoplankton proportion was very high in summer and autumn. In particular, the fraction of picophytoplankton was high in the surface layer in summer. The results of using indices suggest that our sampling site region has relatively high primary productivity. Therefore, seasonal studies of phytoplankton using pigment analysis could contribute to gaining a better understanding of the dynamics of small phytoplankton in complex offshore waters. Investigation of the size and composition of these phytoplankton is very important for predicting changes in aquatic ecosystems due to climate change in the long term. Since this study is a short-term survey conducted by season for one year, the limitation is that it is unable to represent the general ecological characteristics of the East Sea. Continuous research is needed to understand long-term changes in marine ecology.
Supplementary Materials
The following supporting information can be downloaded from https://www.mdpi.com/article/10.3390/su14095306/s1, Table S1: Initial pigment ratios used for the CHEMTAX analysis. Peri: Peridinin; But: 19′-But-fucoxanthin; Fuco: Fucoxanthin; Hex: 19′-Hex-fucoxanthin; Neo: Neoxanthin; Pras: Prasinoxanthin; Viol: Violaxanthin; Allo: Alloxanthin; Lut: Lutein; Zea: Zeaxanthin; Chl b: Chlorophyll b.; Table S2: The final output pigment ratio matrix results from the best fit of CHEMTAX for each season. Peri: Peridinin; But: 19′-But-fucoxanthin; Fuco: Fucoxanthin; Hex: 19′-Hex-fucoxanthin; Neo: Neoxanthin; Pras: Prasinoxanthin; Viol: Violaxanthin; Allo: Alloxanthin; Lut: Lutein; Zea: Zeaxanthin; Chl b: Chlorophyll b.
Author Contributions
Conceptualization, M.L.; Data curation, Y.-B.K.; Funding acquisition, C.-H.P. and S.-H.B.; Investigation, M.L. and Y.-B.K.; Project administration, C.-H.P.; Supervision, S.-H.B.; Writing—original draft, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Institute of Ocean Science and Technology (PEA0012) and Ministry of Oceans and Fisheries (PG52911).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Legendre, L.; Rassoulzadegan, F. Food-web mediated export of biogenic carbon in oceans: Hydrodynamic control. Mar. Ecol. Prog. Ser. 1996, 145, 179–193. [Google Scholar] [CrossRef]
- Doney, S.C. Plankton in a warmer world. Nature 2006, 444, 695–696. [Google Scholar] [CrossRef]
- Morán, X.A.G.; López-Urrutia, Á.; Calvo-Díaz, A.; Li, W.K. Increasing importance of small phytoplankton in a warmer ocean. Glob. Change Biol. 2010, 16, 1137–1144. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, D.; Kim, Y.O.; Son, M.; Kim, Y.J.; Lee, M.; Park, B.S. Seasonal changes in abiotic environmental conditions in the Busan coastal region (South Korea) due to the Nakdong River in 2013 and effect of these changes on phytoplankton communities. Cont. Shelf Res. 2019, 175, 116–126. [Google Scholar] [CrossRef]
- Malin, G.; Turner, S.M.; Liss, P.S. Sulfur: The plankton/climate connection. J. Phycol. 1992, 28, 590–597. [Google Scholar] [CrossRef]
- Isobe, A.; Isoda, Y. Circulation in the Japan Basin, the northern part of the Japan Sea. J. Oceanogr. 1997, 53, 373–382. [Google Scholar]
- Kim, T.; Yoon, J.-H. Seasonal variation of upper layer circulation in the northern part of the East/Japan Sea. Cont. Shelf Res. 2010, 30, 1283–1301. [Google Scholar] [CrossRef]
- Park, K.-A.; Ullman, D.S.; Kim, K.; Chung, J.Y.; Kim, K.-R. Spatial and temporal variability of satellite-observed Subpolar Front in the East/Japan Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 453–470. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.H.; Kim, Y.-B.; Park, C.H.; Shin, K.; Baek, S.H. Specific oceanographic characteristics and phytoplankton responses influencing the primary production around the Ulleung Basin area in spring. Acta Oceanol. Sin. 2020, 39, 107–122. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, G. Factors controlling the C: N: P stoichiometry of dissolved organic matter in the N-limited, cyanobacteria-dominated East/Japan Sea. J. Mar. Syst. 2013, 115, 1–9. [Google Scholar] [CrossRef]
- Song, S.J.; Park, J.; Ryu, J.; Rho, H.S.; Kim, W.; Khim, J.S. Biodiversity hotspot for marine invertebrates around the Dokdo, East Sea, Korea: Ecological checklist revisited. Mar. Pollut. Bull. 2017, 119, 162–170. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, M.; Kim, Y.B. Spring phytoplankton community response to an episodic windstorm event in oligotrophic waters offshore from the Ulleungdo and Dokdo islands, Korea. J. Sea Res. 2018, 132, 1–14. [Google Scholar] [CrossRef]
- Yoo, S.; Park, J. Why is the southwest the most productive region of the East Sea/Sea of Japan? J. Mar. Syst. 2009, 78, 301–315. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kim, K.-H.; Shim, J.-H.; Yoo, S.-J. The effect of anticyclonic eddy on nutrients and chlorophyll during spring and summer in the Ulleung Basin, East Sea. Sea 2007, 12, 280–286. [Google Scholar]
- Kwak, J.H.; Hwang, J.; Choy, E.J.; Park, H.J.; Kang, D.-J.; Lee, T.; Chang, K.-I.; Kim, K.-R.; Kang, C.-K. High primary productivity and f-ratio in summer in the Ulleung basin of the East/Japan Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 79, 74–85. [Google Scholar] [CrossRef]
- Lee, H.W.; Hong, B.K.; Sohn, M.H.; Chun, Y.Y.; Lee, D.W.; Choi, Y.M.; Hwang, K.S. Seasonal variation in species composition of fish collected by trammel net around Dokdo, East Sea of Korea. Korean J. Fish Aquat. Sci. 2010, 43, 693–704. [Google Scholar]
- Tester, P.A.; Geesey, M.E.; Guo, C.; Paerl, H.W.; Millie, D.F. Evaluating phytoplankton dynamics in the Newport River estuary (North Carolina, USA) by HPLC-derived pigment profiles. Mar. Ecol. Prog. Ser. 1995, 124, 237–245. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S.; Mantoura, R. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Unesco Publishing: Paris, France, 2005. [Google Scholar]
- Barlow, R.; Kyewalyanga, M.; Sessions, H.; Van den Berg, M.; Morris, T. Phytoplankton pigments, functional types, and absorption properties in the Delagoa and Natal Bights of the Agulhas ecosystem. Estuar. Coast. Shelf Sci. 2008, 80, 201–211. [Google Scholar] [CrossRef]
- Mendes, C.R.B.; Kerr, R.; Tavano, V.M.; Cavalheiro, F.A.; Garcia, C.A.E.; Dessai, D.R.G.; Anilkumar, N. Cross-front phytoplankton pigments and chemotaxonomic groups in the Indian sector of the Southern Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 118, 221–232. [Google Scholar] [CrossRef]
- Mackey, M.; Mackey, D.; Higgins, H.; Wright, S. CHEMTAX-a program for estimating class abundances from chemical markers: Application to HPLC measurements of phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef]
- Wright, S.W.; van den Enden, R.L.; Pearce, I.; Davidson, A.T.; Scott, F.J.; Westwood, K.J. Phytoplankton community structure and stocks in the Southern Ocean (30–80 E) determined by CHEMTAX analysis of HPLC pigment signatures. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 758–778. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Marañón, E.; Behrenfeld, M.J.; González, N.; Mouriño, B.; Zubkov, M.V. High variability of primary production in oligotrophic waters of the Atlantic Ocean: Uncoupling from phytoplankton biomass and size structure. Mar. Ecol. Prog. Ser. 2003, 257, 1–11. [Google Scholar] [CrossRef]
- Klauschies, T.; Bauer, B.; Aberle-Malzahn, N.; Sommer, U.; Gaedke, U. Climate change effects on phytoplankton depend on cell size and food web structure. Mar. Biol. 2012, 159, 2455–2478. [Google Scholar] [CrossRef]
- Terrado, R.; Scarcella, K.; Thaler, M.; Vincent, W.F.; Lovejoy, C. Small phytoplankton in Arctic seas: Vulnerability to climate change. Biodiversity 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Bolaños, L.M.; Karp-Boss, L.; Choi, C.J.; Worden, A.Z.; Graff, J.R.; Haëntjens, N.; Chase, A.P.; Panna, A.D.; Gaube, P.; Morison, F.; et al. Small phytoplankton dominate western North Atlantic biomass. ISME J. 2020, 14, 1663–1674. [Google Scholar] [CrossRef]
- Choi, D.H.; Noh, J.H.; Shim, J. Seasonal changes in picocyanobacterial diversity as revealed by pyrosequencing in temperate waters of the East China Sea and the East Sea. Aquat. Microb. Ecol. 2013, 71, 75–90. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Zheng, L.; Song, S.; Chen, B.; Huang, B. Seasonal and spatial patterns of picophytoplankton growth, grazing and distribution in the East China Sea. Biogeosciences 2014, 11, 1847–1862. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.K.; Youn, S.; Roh, S. Influence of the physical forcing of different water masses on the spatial and temporal distributions of picophytoplankton in the northern East China Sea. Cont. Shelf Res. 2014, 88, 216–227. [Google Scholar] [CrossRef]
- Chang, K.I.; Zhang, C.I.; Park, C.; Kang, D.J.; Ju, S.J.; Lee, S.H.; Wimbush, M. Oceanography of the East Sea (Japan Sea); Springer International Publishing: Cham, Switzerland, 2016; p. 460. [Google Scholar]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Riegman, R.; Kraay, G. Phytoplankton community structure derived from HPLC analysis of pigments in the Faroe-Shetland Channel during summer 1999: The distribution of taxonomic groups in relation to physical/chemical conditions in the photic zone. J. Plankton Res. 2001, 23, 191–205. [Google Scholar] [CrossRef]
- Lee, Y.W.; Park, M.O.; Im, Y.S.; Kim, S.S.; Kang, C.K. Application of photosynthetic pigment analysis using a HPLC and CHEMTAX program to studies of phytoplankton community composition. Sea 2011, 16, 117–124. [Google Scholar] [CrossRef]
- Barlow, R.; Stuart, V.; Lutz, V.; Sessions, H.; Sathyendranath, S.; Platt, T.; Kyewalyanga, M.; Clementson, L.; Fukasawa, M.; Watanabe, S. Seasonal pigment patterns of surface phytoplankton in the subtropical southern hemisphere. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1687–1703. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; Hardman-Mountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Modell. 2010, 221, 1472–1483. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface Chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef]
- Levitus, S. Climatological Atlas of the World Ocean; National Oceanic and Atmospheric Administration (NOAA): Washington, DC, USA, 1982; 173p.
- Wasmund, N.; Andrushaitis, A.; Łysiak-Pastuszak, E.; Müller-Karulis, B.; Nausch, G.; Neumann, T.; Ojaveer, H.; Olenina, I.; Postel, L.; Witek, Z. Trophic status of the south-eastern Baltic Sea: A comparison of coastal and open areas. Estuar. Coast. Shelf Sci. 2001, 53, 849–864. [Google Scholar] [CrossRef]
- Kim, K.; Chung, J. On the salinity-minimum and dissolved oxygen-maximum layer in the East Sea (Sea of Japan). Elsevier Oceanogr. Ser. 1984, 39, 55–65. [Google Scholar]
- Yang, H.-S.; Kim, S.-S.; Kang, C.-G.; Cho, K.-D. A study on sea water and ocean current in the sea adjacent to Korea Peninsula-III. Chemical characteristics of water masses in the polar front area of the central Korean East Sea. Korean J. Fish Aquat. Sci. 1991, 24, 185–192. [Google Scholar]
- Kim, H.-S.; Jung, M.-M.; Lee, J.-B. The Korean Peninsula warming based on appearance trend of tropical dinoflagellate species, genus. Ornithocercus. Sea 2008, 13, 303–307. [Google Scholar]
- Baek, S.-H.; Lee, M.; Kim, Y.-B. Growth and community response of phytoplankton by N, P and Fe nutrient addition in around water of Ulleungdo and Dokdo in East Sea. J. Korea Acad.-Ind. Coop. Soc. 2016, 17, 186–195. [Google Scholar]
- Son, Y.T.; Chang, K.I.; Yoon, S.T.; Rho, T.; Kwak, J.H.; Kang, C.K.; Kim, K.R. A newly observed physical cause of the onset of the subsurface spring phytoplankton bloom in the southwestern East Sea/Sea of Japan. Biogeosciences 2014, 11, 1319–1329. [Google Scholar] [CrossRef]
- Rho, T.-K.; Kim, Y.-B.; Park, J.-I.; Lee, Y.-W.; Im, D.-H.; Kang, D.-J.; Lee, T.-S.; Yoon, S.-T.; Kim, T.-H.; Kwak, J.-H. Plankton community response to physico-chemical forcing in the Ulleung Basin, East Sea during summer 2008. Ocean Polar Res. 2010, 32, 269–289. [Google Scholar] [CrossRef][Green Version]
- Bing-xian, G. Patterns and structures of the currents in Bohai, Huanghai and East China Seas. In Oceanology of China Seas; Springer: Cham, Switzerland, 1994; pp. 17–26. [Google Scholar]
- Kim, T.-H.; Lee, Y.-W.; Kim, G. Hydrographically mediated patterns of photosynthetic pigments in the East/Japan Sea: Low N: P ratios and cyanobacterial dominance. J. Mar. Syst. 2010, 82, 72–79. [Google Scholar] [CrossRef]
- Choi, D.H.; An, S.M.; Choi, Y.R.; Roh, H.S.; Noh, J.H. Seasonal variation of picocyanobacterial community composition in seawaters around Dokdo, Korea. Sea 2015, 20, 192–198. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, H.C. Suppression and enhancement of the spring bloom in the southwestern East Sea/Japan Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1093–1111. [Google Scholar] [CrossRef]
- Choi, J.K.; Noh, J.H.; Orlova, T.; Park, M.-O.; Lee, S.H.; Park, Y.-J.; Son, S.; Stonik, I.; Choi, D.H. Phytoplankton and primary production. In Oceanography of the East Sea (Japan Sea); Springer: Cham, Switzerland, 2016; pp. 217–245. [Google Scholar]
- Lee, M.; Kim, Y.-B.; Kang, J.H.; Park, C.H.; Baek, S.H. Seasonal distribution of phytoplankton and environmental factors in the offshore waters of Dokdo: Comparison between 2018 and 2019. Korean J. Environ. Biol. 2020, 38, 47–60. [Google Scholar] [CrossRef]
- Xu, N.; Duan, S.; Li, A.; Zhang, C.; Cai, Z.; Hu, Z. Effects of temperature, salinity and irradiance on the growth of the harmful dinoflagellate Prorocentrum donghaiense Lu. Harmful Algae 2010, 9, 13–17. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lim, A.S.; Lee, K.; Lee, M.J.; Seong, K.A.; Kang, N.S.; Jang, S.H.; Lee, K.H.; Lee, S.Y.; Kim, M.O. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: I—Temporal variations in three-dimensional distributions of red-tide organisms and environmental factors. Algae 2017, 32, 101–130. [Google Scholar] [CrossRef]
- Lim, Y.K.; Baek, S.H. Seasonal distributional characteristics of phytoplankton adjacent to the oyster farming area of Hansan-Geoje Island. Korean J. Environ. Biol. 2018, 36, 647–658. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, W.; Son, S.H.; Ahn, J.-H.; Park, Y.-J. Remote quantification of Cochlodinium polykrikoides blooms occurring in the East Sea using geostationary ocean color imager (GOCI). Harmful Algae 2018, 73, 129–137. [Google Scholar] [CrossRef]
- Islabão, C.; Mendes, C.; Detoni, A.; Odebrecht, C. Phytoplankton community structure in relation to hydrographic features along a coast-to-offshore transect on the SW Atlantic Continental Shelf. Cont. Shelf Res. 2017, 151, 30–39. [Google Scholar] [CrossRef]
- Ilmavirta, V. Phytoflagellates and their ecology in Finnish brown-water lakes. In Flagellates in Freshwater Ecosystems; Springer: Cham, Switzerland, 1988; pp. 255–270. [Google Scholar]
- Viličić, D.; Terzić, S.; Ahel, M.; Burić, Z.; Jasprica, N.; Carić, M.; Mihalić, K.C.; Olujić, G. Phytoplankton abundance and pigment biomarkers in the oligotrophic, eastern Adriatic estuary. Environ. Monit. Assess. 2008, 142, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Marumo, R. The structure of the phytoplankton community in the subsurface chlorophyll maxima in the western North Pacific Ocean. J. Plankton Res. 1983, 5, 393–406. [Google Scholar] [CrossRef]
- Andersen, R.A.; Bidigare, R.R.; Keller, M.D.; Latasa, M. A comparison of HPLC pigment signatures and electron microscopic observations for oligotrophic waters of the North Atlantic and Pacific Oceans. Deep Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 517–537. [Google Scholar] [CrossRef]
- Kang, Y.; Harke, M.J.; Berry, D.L.; Collier, J.L.; Wilhelm, S.W.; Dyhrman, S.T.; Gobler, C.J. Transcriptomic Responses of Four Pelagophytes to Nutrient (N, P) and Light Stress. Front. Mar. Sci. 2021, 8, 223. [Google Scholar] [CrossRef]
- Park, M.-O.; Kang, S.-W.; Lee, C.-I.; Choi, T.-S.; Lantoine, F. Structure of the phytoplanktonic communities in Jeju Strait and northern East China Sea and dinoflagellate blooms in spring 2004: Analysis of photosynthetic pigments. Sea 2008, 13, 27–41. [Google Scholar]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Oceans 2006, 111, 1–23. [Google Scholar] [CrossRef]
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.; Falkowski, P.G. Scaling-up from nutrient physiology to the size-structure of phytoplankton communities. J. Plankton Res. 2006, 28, 459–471. [Google Scholar] [CrossRef]
- Margalef, R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Moreno, D.V.; Marrero, J.P.; Morales, J.; García, C.L.; Úbeda, M.V.; Rueda, M.; Llinás, O. Phytoplankton functional community structure in Argentinian continental shelf determined by HPLC pigment signatures. Estuar. Coast. Shelf Sci. 2012, 100, 72–81. [Google Scholar] [CrossRef]
- Ras, J.; Claustre, H.; Uitz, J. Spatial variability of phytoplankton pigment distributions in the Subtropical South Pacific Ocean: Comparison between in situ and predicted data. Biogeosciences 2008, 5, 353–369. [Google Scholar] [CrossRef]
- Madhu, N.; Ullas, N.; Ashwini, R.; Meenu, P.; Rehitha, T.; Lallu, K. Characterization of phytoplankton pigments and functional community structure in the Gulf of Mannar and the Palk Bay using HPLC-CHEMTAX analysis. Cont. Shelf Res. 2014, 80, 79–90. [Google Scholar] [CrossRef]
- Gibb, S.; Barlow, R.; Cummings, D.; Rees, N.; Trees, C.; Holligan, P.; Suggett, D. Surface phytoplankton pigment distributions in the Atlantic Ocean: An assessment of basin scale variability between 50 N and 50 S. Prog. Oceanogr. 2000, 45, 339–368. [Google Scholar] [CrossRef]
- Vijayan, A.K.; Yoshikawa, T.; Watanabe, S.; Sasaki, H.; Matsumoto, K.; Saito, S.-I.; Takeda, S.; Furuya, K. Influence of non-photosynthetic pigments on light absorption and quantum yield of photosynthesis in the western equatorial Pacific and the subarctic North Pacific. J. Oceanogr. 2009, 65, 245–258. [Google Scholar] [CrossRef]
- Chai, C.; Jiang, T.; Cen, J.; Ge, W.; Lu, S. Phytoplankton pigments and functional community structure in relation to environmental factors in the Pearl River Estuary. Oceanologia 2016, 58, 201–211. [Google Scholar] [CrossRef]
- Lee, M.; Won, N.-I.; Baek, S.H. Comparison of HPLC pigment analysis and microscopy in phytoplankton assessment in the Seomjin River Estuary, Korea. Sustainability 2020, 12, 1675. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).