Development of an Algorithm to Indicate the Right Moment of Plant Watering Using the Analysis of Plant Biomasses Based on Dahlia × hybrida
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Fertilization
2.3. Water Monitoring
2.4. Biometric Measurements
2.5. Evaluation of Substrate and Leaf Relative Water Content
2.6. Biochemical Analyses
2.7. Statistical Analysis
3. Results
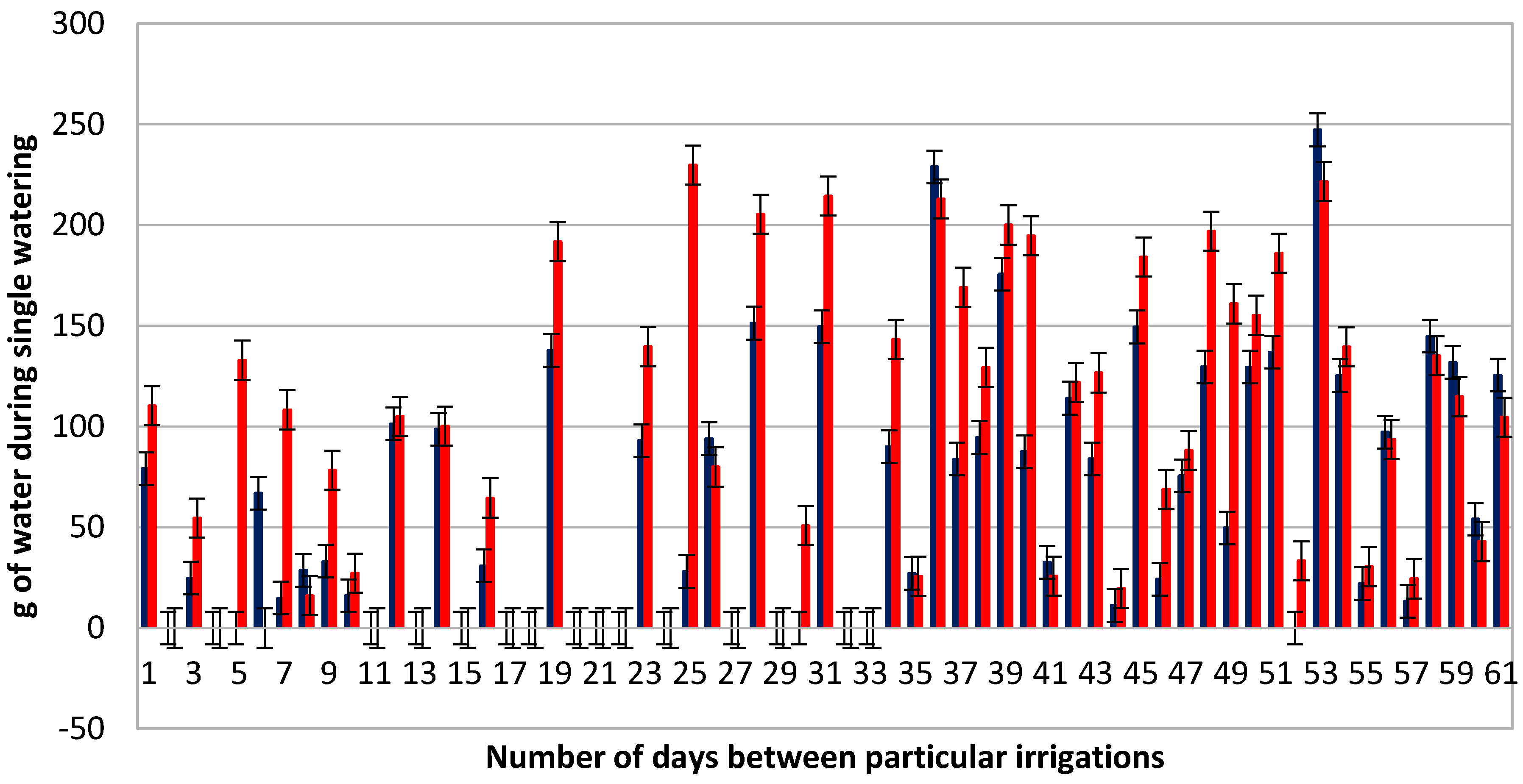
3.1. Average Use of Water during Plant Irrigation
3.2. Biometric Measurement
3.3. Biochemical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allaire-Leung, S.E.; Caron, J.; Parent, L.E. Changes in physical properties of peat substrates during plant growth. Can. J. Soil Sci. 1999, 79, 137–139. [Google Scholar] [CrossRef]
- Argo, W.R. Root medium physical properties. HortTechnology 1998, 8, 481–485. [Google Scholar] [CrossRef]
- Beeson, R.C. Determining plant-available water of woody ornamentals in containers in situ during production. HortScience 2007, 42, 1700–1704. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.S.; Altland, J.E. Container height and Douglas fir bark texture affect substrate physical properties. HortScience 2008, 43, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.; Bilderback, T.; Bir, D.; Doug, B.; Bailey, T. Water considerations for container production of plants. Nurs. Crop. Sci. 1999, 7799, 151. [Google Scholar]
- Pitton, B.J.L.; Hall, C.R.; Haver, D.L.; White, S.A.; Oki, L.R. A cost analysis for using recycled irrigation runoff water in container nursery production: A Southern California nursery case study. Irrig. Sci. 2018, 36, 217–226. [Google Scholar] [CrossRef]
- Guo, Y.; Starman, T.; Hall, C. Reducing substrate moisture content during greenhouse production of poinsettia improves postproduction quality and economic value. HortScience 2018, 53, 1006–1011. [Google Scholar] [CrossRef]
- Warner, L.A.; Lamm, A.J.; Beattie, P.; White, S.A.; Fisher, P.R. Identifying opportunities to promote water conservation practices among nursery and greenhouse growers. HortScience 2018, 53, 958–962. [Google Scholar] [CrossRef] [Green Version]
- Morvant, J.K.; Dole, J.M.; Cole, J.C. Irrigation frequency and system affect poinsettia growth, water use, and runoff. HortScience 1998, 33, 42–46. [Google Scholar]
- Stanghellini, C. Horticultural production in greenhouses: Efficient use of water. Acta Hortic. 2014, 1034, 25–32. [Google Scholar] [CrossRef]
- FAO. Global Aquaculture Production Statistics Database Updated to 2013: Summary Information; FAO: Rome, Italy, 2013. [Google Scholar]
- Kim, S.H.; Jeong, J.H.; Nackley, L.L. Photosynthetic and transpiration responses to light, CO2, temperature, and leaf senescence in garlic: Analysis and modeling. J. Am. Soc. Hortic. Sci. 2013, 138, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Lichtenberg, E.; Majsztrik, J.; Saavoss, M. Grower demand for sensor-controlled irrigation. Water Resour. Res. 2015, 51, 341–358. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Price, E.W.; King, D.M. Environmental benefits of wireless sensor-based irrigation networks: Case-study projections and potential adoption rates. HortTechnology 2013, 23, 783–793. [Google Scholar] [CrossRef]
- Nackley, L.L.; Vogt, K.A.; Kim, S.H. Arundo donax water use and photosynthetic responses to drought and elevated CO2. Agric. Water Manag. 2014, 136, 13–22. [Google Scholar] [CrossRef]
- Vereecken, H.; Huisman, J.A.; Bogena, H.; Vanderborght, J.; Vrugt, J.A.; Hopmans, J.W. On the value of soil moisture measurements in vadose zone hydrology. Water Resour. Res. 2008, 44, W00D06. [Google Scholar] [CrossRef] [Green Version]
- Nambuthiri, S.; Hagen, E.; Fulcher, A.; Geneve, R. Evaluating a physiological-based, on-demand irrigation system for container-grown woody plants with different water requirements. HortScience 2017, 52, 251–257. [Google Scholar] [CrossRef]
- Daniels, A.B.; Barnard, D.M.; Chapman, P.L.; Bauerle, W.L. Optimizing substrate moisture measurements in containerized nurseries. HortScience 2012, 47, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Karczewska, A.; Kabała, C. Metodyka Analiz Laboratoryjnych Gleb i Roślin; Uniwersytet Przyrodniczy we Wrocławiu, Instytut Nauk o Glebie i Ochrony Środowiska, Zakład Ochrony Środowiska: Wroclaw, Poland, 2008; p. 45. [Google Scholar]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Boyle, R.K.A.; McAinsh, M.; Dodd, I.C. Daily irrigation attenuates xylem abscisic acid concentration and increases leaf water potential of Pelargonium × hortorum compared with infrequent irrigation. Physiol. Plant. 2016, 158, 23–33. [Google Scholar] [CrossRef] [Green Version]
- BIO Intelligence Service. Water Saving Potential in Agriculture in Europe: Findings from the Existing Studies and Application to Case Studies; Final report prepared for European Commission DG ENV; BIO Intelligence Service: Paris, France, 2012. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, E.A.; Bailey-Serres, J.; And Weretilnyk, E. Responses to abiotic stresses. In Biochemistry and Molecular Biology of Plants; Gruissem, W., Buchnnan, B., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1249. [Google Scholar]
- Weng, Q.; Yang, S. Managing the adverse thermal effects of urban development in a densely populated Chinese city. J. Environ. Manag. 2004, 70, 145–156. [Google Scholar] [CrossRef]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- De Micco, V.; Aronne, G. Root structure of rumex scutatus growing on slopes. IAWA J. 2010, 31, 13–28. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Davies, W.J. Influence of soil drying on root development, water relations and leaf growth of Ceratonia siliqua L. Oecologia 1991, 88, 41–47. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. Research 2016, 5, F1000. [Google Scholar] [CrossRef]
- Auge, R.M.; Stodola, A.J.W.; Moore, J.L.; Klingeman, W.E.; Duan, X. Comparative dehydration tolerance of foliage of several ornamental crops. Sci. Hortic. 2003, 98, 511–516. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Sheng, H.; Liu, X.; Yao, Y.; Gong, C. Variations in internal water distribution and leaf anatomical structure in maize under persistently reduced soil water content and growth recovery after re-watering. Acta Physiol. Plant. 2015, 37, 263. [Google Scholar] [CrossRef]
- Barr, H.D.; Weatherley, P.E. A reexamination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of water stress on gas exchange, water relations and leaf structure in two ornamental shrubs in the Mediterranean area. Agronomy 2019, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Martignago, D.; Rico-Medina, A.; Blasco-Escámez, D.; Fontanet-Manzaneque, J.B.; Caño-Delgado, A.I. Drought Resistance by Engineering Plant Tissue-Specific Responses. Front. Plant Sci. 2019, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C.; Rouphael, Y. Biochemical, physiological, and molecular aspects of ornamental plants adaptation to deficit irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmed, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Fuzy, A.; Kovács, R.; Cseresnyés, I.; Parádil, I.; Szili-Kovács, T.; Kelemen, B.; Rajkai1, K.; Takács, T. Selection of plant physiological parameters to detect stress effects in pot experiments using principal component analysis. Acta Physiol. Plant. 2019, 41, 56. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Nadler, A.; Tyree, M.T. Substituting stem’s water content by electrical conductivity for monitoring water status changes. Soil Sci. Soc. Am. J. 2008, 72, 1006–1013. [Google Scholar] [CrossRef]
- Gurovich, L.; Hermosilla, P. Electric signaling in fruit trees in response to water applications and light-darkness conditions. J. Plant Phys. 2009, 166, 290–300. [Google Scholar] [CrossRef]
- Yu, L.; Tao, S.; Ren, Y.; Gao, W.; Liu, X.; Hu, Y.; Shamshiri, R.R. Comprehensive Evaluation of Soil Moisture Sensing Technology Applications Based on Analytic Hierarchy Process and Delphi. Agriculture 2021, 11, 1116. [Google Scholar] [CrossRef]
- Oyarce, P.; Gurovich, L. Evidence for the transmission of information through electric potentials in injured avocado trees. J. Plant Physiol. 2011, 168, 103–108. [Google Scholar] [CrossRef]
- Burnett, S.E.; van Iersel, M.W. Morphology and irrigation efficiency of Gaura lindheimeri grown with capacitance sensor-controlled irrigation. HortScience 2008, 43, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Garland, K.; Burnett, S.E.; Day, M.; van Iersel, M.W. Influence of substrate water content and daily light integral on photosynthesis, water use efficiency, and morphology of Heuchera americana. J. Am. Soc. Hortic. Sci. 2012, 137, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Nemali, K.S.; van Iersel, M.W. An automated system for controlling drought stress and irrigation in potted plants. Sci. Hortic. 2006, 110, 292–297. [Google Scholar] [CrossRef]
- van Iersel, M.W.; Nemali, K.S. Drought stress can produce small but not compact marigolds. HortScience 2004, 39, 1298–1301. [Google Scholar] [CrossRef]
- van Iersel, M.W.; Dove, S.; Kang, J.G.; Burnett, S.E. Growth and water use of petunia as affected by substrate water content and daily light integral. HortScience 2010, 45, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Oki, T.; Agata, Y.; Kanae, S.; Saruhashi, T.; Yang, D.; Musiake, K. Global assessment of current water resources using total runoff integrating pathways. Hydrol. Sci. J. 2001, 46, 983–995. [Google Scholar] [CrossRef] [Green Version]
- Bayer, A.; Mahbub, I.; Chappell, M.; Ruter, J.; van Iersel, M.W. Water use and growth of Hibiscus acetosella ‘Panama Red’ grown with a soil moisture sensor-controlled irrigation system. HortScience 2013, 48, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Cuellar-Ortiz, S.M.; De La Paz Arrieta-Montiel, M.; Acosta-Gallegos, J.; Covarrubias, A.A. Relationship between carbohydrate partitioning and drought resistance in common bean. Plant Cell Environ. 2008, 31, 1399–1409. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, S.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought resistant wheat. Crop. J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Hill, K.E.; Greg, A.C.; Guerin, C.; Hill, R.A.; Watling, J.R. Temperature influences stomatal density and maximum potential water loss through stomata of Dodonaea viscosa subsp. angustissima along a latitude gradient in southern Australia. Aust. J. Bot. 2014, 62, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Liu, S.; Ma, H.; Chen, J.; Shen, Q.; Ge, C.; Zhang, X.; Pang, C.; Zhao, X. The compensation effects of physiology and yield in cotton after drought stress. J. Plant Physiol. 2018, 224, 30–48. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—Not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biobiomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [Green Version]
- Rustioni, L.; Bianchi, D. Drought increases chlorophyll content in stems of Vitis interspecific hybrids. Theor. Exp. Plant Physiol. 2021, 33, 69–78. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Kunert, K.J.; Vorster, B.J.; Fenta, B.A.; Kibido, T.; Dionisio, G.; Foyer, C.H. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 2016, 7, 1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop. Prod. 2019, 140, 111–597. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Rich, S.M.; Watt, M. Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver. J. Exp. Bot. 2013, 64, 1193–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]



| Plant Height (cm) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 19.6 Ac * ± 0.09 | 24.7 Ab ± 0.31 | 30.87 Aa ± 0.36 | 25.05 A |
| Controlled watering | 18.2 Bc ± 0.10 | 19.6 Bb ± 0.17 | 28.28 Ba ± 0.22 | 22.04 B |
| mean | 18.93 c | 22.15 b | 29.58 a | |
| Number of branchings | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 5.8 Ab ± 0.09 | 6.7 Aab ± 0.19 | 7.1 Aa ± 0.23 | 6.5 A |
| Controlled watering | 5.6 Bb ± 0.23 | 6.0 Bab ± 0.21 | 6.6 Ba ± 0.21 | 6.1 B |
| mean | 5.7 b | 6.3 ab | 6.85 a | |
| Leaf area (mm2) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 2045.8 Ca ± 0.11 | 2160.8 Ba ± 0.06 | 2350.5 Aa ± 0.09 | 2185.7 A |
| Controlled watering | 1756.5 Bb ± 0.08 | 1759.6 Bb ±0.12 | 1856.4 Ba ± 0.08 | 1790.3 B |
| mean | 1901.15 b | 1960.2 ab | 2103.45 a | |
| Stomatal conductance (mmol·m−2·s−1) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 30.8 Bc ± 0.16 | 39.03 Ab ± 0.16 | 52.4 Ba ± 0.08 | 40.7 B |
| Controlled watering | 51.8 Ab ± 0.18 | 37.2 Bc ±0.21 | 57.8 Aa ± 0.10 | 48.9 A |
| mean | 41.3 b | 38.1 c | 55.1 a | |
| Fresh biomass of the plant (g) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 45.8 Ac ± 0.10 | 183.2 Ab ± 0.22 | 289.9 Aa ± 0.04 | 172.9 A |
| Controlled watering | 38.9 Bc ± 0.11 | 149.7 Bb ± 0.23 | 246.2 Ba ± 0.09 | 144.9 B |
| mean | 42.3 c | 166.5 b | 268.0 a | |
| Fresh biomass of the root ball (g) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 259.5 Bb ± 0.11 | 260.8 Bb ± 0.39 | 276.0 Aa ± 0.02 | 265.4 B |
| Controlled watering | 274.9 Ac ± 0.11 | 321.3 Ab ± 0.31 | 329.9 Aa ± 0.30 | 308.7 A |
| mean | 267.2 c | 291.0 b | 302.9 a | |
| Leaf Relative Water Content % | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 97.7 Aa * ± 0.02 | 93.2 Ab ± 0.06 | 90.3 Ac ± 0.07 | 93.7 A |
| Controlled watering | 82.3 Bb ± 0,08 | 57.8 Bc ± 0.06 | 87.3 Ba ± 0.10 | 75.8 B |
| mean | 90 a | 75.5 c | 88.8 b | |
| Substrate relative water content % | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 95.1 Aa ± 0.02 | 70.6 Ab ± 0.12 | 67.7 Bc ± 0.09 | 77.8 A |
| Controlled watering | 57.6 Bb ± 0.02 | 37.2 Bc ± 0.18 | 75.1 Aa ± 0.09 | 56.6 B |
| mean | 76.4 a | 53.9 c | 71.4 b | |
| Chorophyll a (mg·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 15.4 Aa * | 14.9 Ab | 6.7 Bc | 12.3 B |
| Controlled watering | 15.5 Aa | 15.0 Aa | 9.7 Ab | 13.4 A |
| mean | 15.5 a | 14.9 b | 8.3 c | |
| Chorophyll b (mg·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 5.9 Aa | 5.5 Aa | 2.2 Ab | 4.6 A |
| Controlled watering | 5.6 Aa | 5.4 Aab | 3.4 Ab | 4.8 A |
| mean | 5.8 a | 5.5 b | 2.8 c | |
| Chorophyll a + b (mg·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 21.4 Aa | 20.4 Ab | 8.9 Bc | 16.9 B |
| Controlled watering | 21.1 Aa | 20.5 Ab | 13.1 Ac | 18.2 A |
| mean | 21.2 a | 20.4 b | 11.1 c | |
| Carotenoids (µg·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 163.1 Bb | 234.6 Ba | 147.1 Bc | 181.6 B |
| Controlled watering | 170.1 Ac | 239.6 Aa | 190.2 Ab | 199.9 A |
| mean | 166.6 c | 237.1 a | 169.0 b | |
| Total Sugars Content (mg glucose·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 70.5 Ac * | 154.5 Aa | 148.5 Bb | 124.5 B |
| Controlled watering | 70.5 Ac | 150.5 Ab | 335.7 Aa | 185.6 A |
| mean | 70.5 c | 152.5 b | 242.1 a | |
| Reducing sugars (mg glucose·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 124.3 Ac | 147.7 Aa | 138.5 Bb | 136.8 B |
| Controlled watering | 122.8 Ab | 76.1 Bc | 337.5 Aa | 178.8 A |
| mean | 123.5 b | 111.9 c | 237.9 a | |
| Starch (mg·g−1 DW) | ||||
| D1(20 DAES) | D2(40 DAES) | D3(60 DAES) | mean | |
| Standard watering | 65.2 Ac | 87.1 Ab | 119.7 Aa | 90.67 A |
| Controlled watering | 59.1 Bb | 84.8 Ba | 77.0 Ba | 73.64 B |
| mean | 62.1 c | 85.9 b | 98.4 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejuk, A.; Bator, M.; Werno, A.; Karkoszka, L.; Kuźma, N.; Zaraś, E.; Budzynski, R. Development of an Algorithm to Indicate the Right Moment of Plant Watering Using the Analysis of Plant Biomasses Based on Dahlia × hybrida. Sustainability 2022, 14, 5165. https://doi.org/10.3390/su14095165
Jędrzejuk A, Bator M, Werno A, Karkoszka L, Kuźma N, Zaraś E, Budzynski R. Development of an Algorithm to Indicate the Right Moment of Plant Watering Using the Analysis of Plant Biomasses Based on Dahlia × hybrida. Sustainability. 2022; 14(9):5165. https://doi.org/10.3390/su14095165
Chicago/Turabian StyleJędrzejuk, Agata, Marcin Bator, Adrian Werno, Lukasz Karkoszka, Natalia Kuźma, Ewa Zaraś, and Robert Budzynski. 2022. "Development of an Algorithm to Indicate the Right Moment of Plant Watering Using the Analysis of Plant Biomasses Based on Dahlia × hybrida" Sustainability 14, no. 9: 5165. https://doi.org/10.3390/su14095165
APA StyleJędrzejuk, A., Bator, M., Werno, A., Karkoszka, L., Kuźma, N., Zaraś, E., & Budzynski, R. (2022). Development of an Algorithm to Indicate the Right Moment of Plant Watering Using the Analysis of Plant Biomasses Based on Dahlia × hybrida. Sustainability, 14(9), 5165. https://doi.org/10.3390/su14095165






