Effects of Increasing Salinity by Drip Irrigation on Total Grain Weight Show High Yield Potential of Putative Salt-Tolerant Mutagenized Wheat Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Field and Weather Conditions at the Salt Farm Texel
2.3. Phenotypic Measurements
2.4. Data Analysis
3. Results
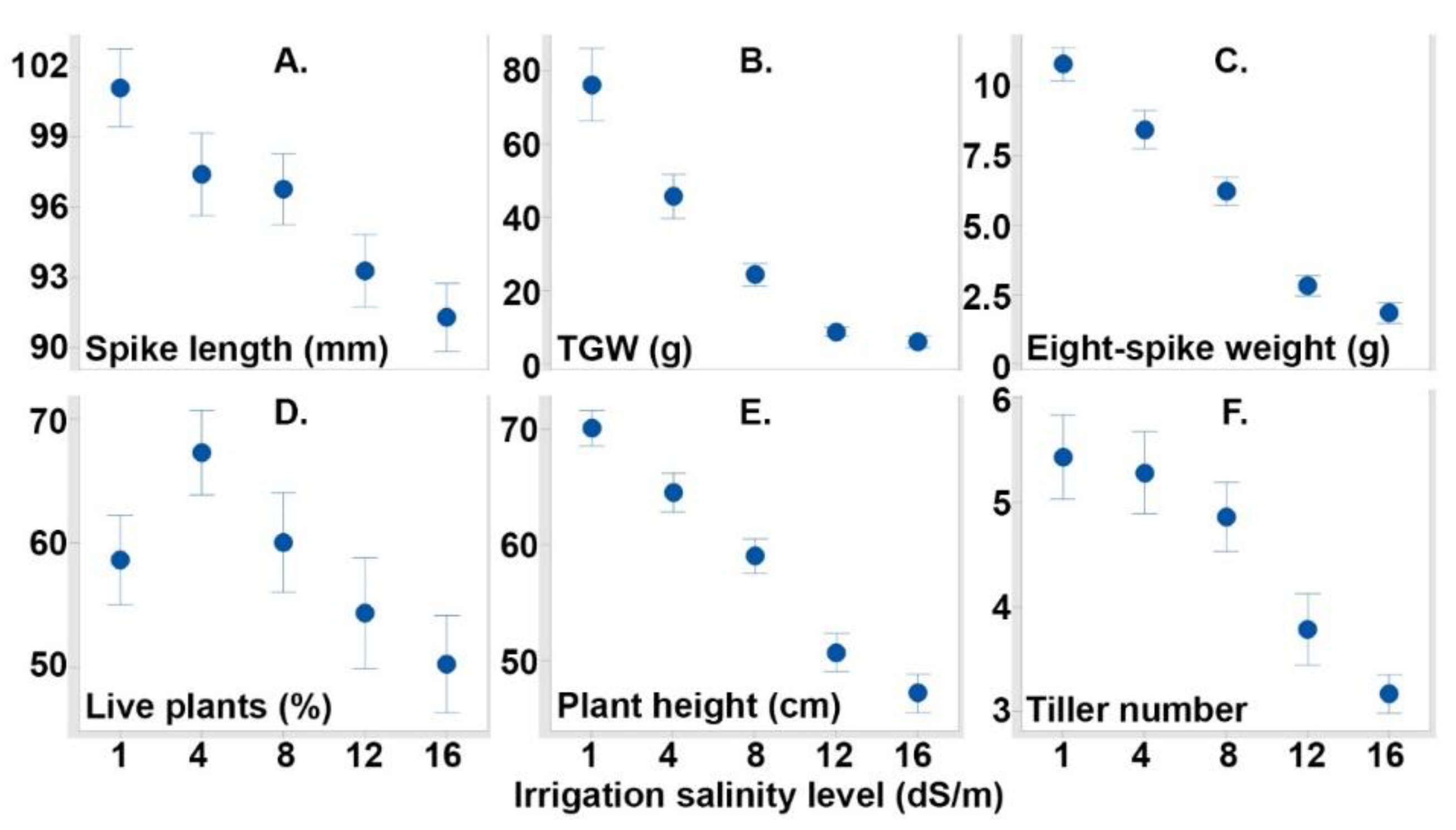
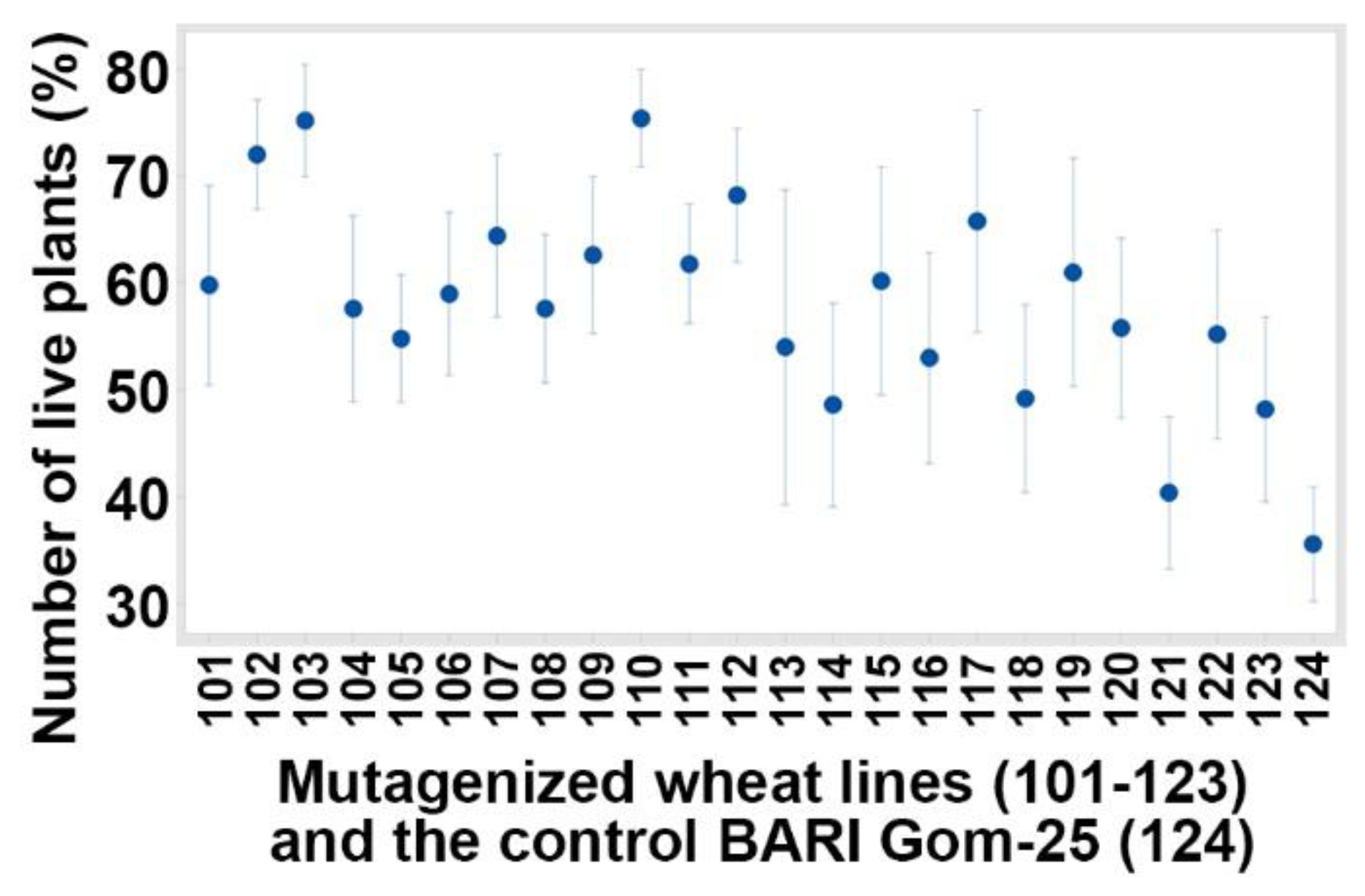
3.1. Evaluated Variables
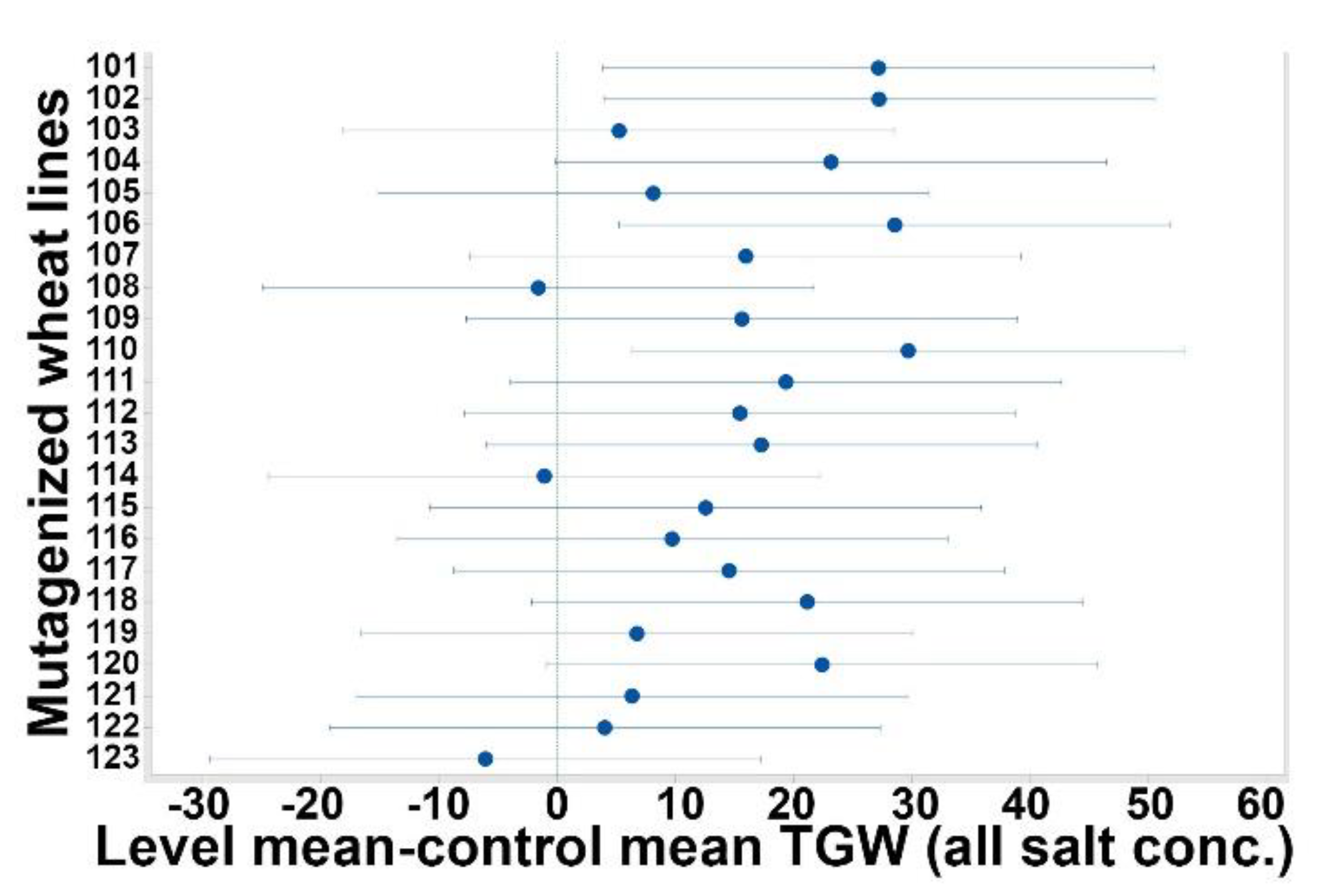
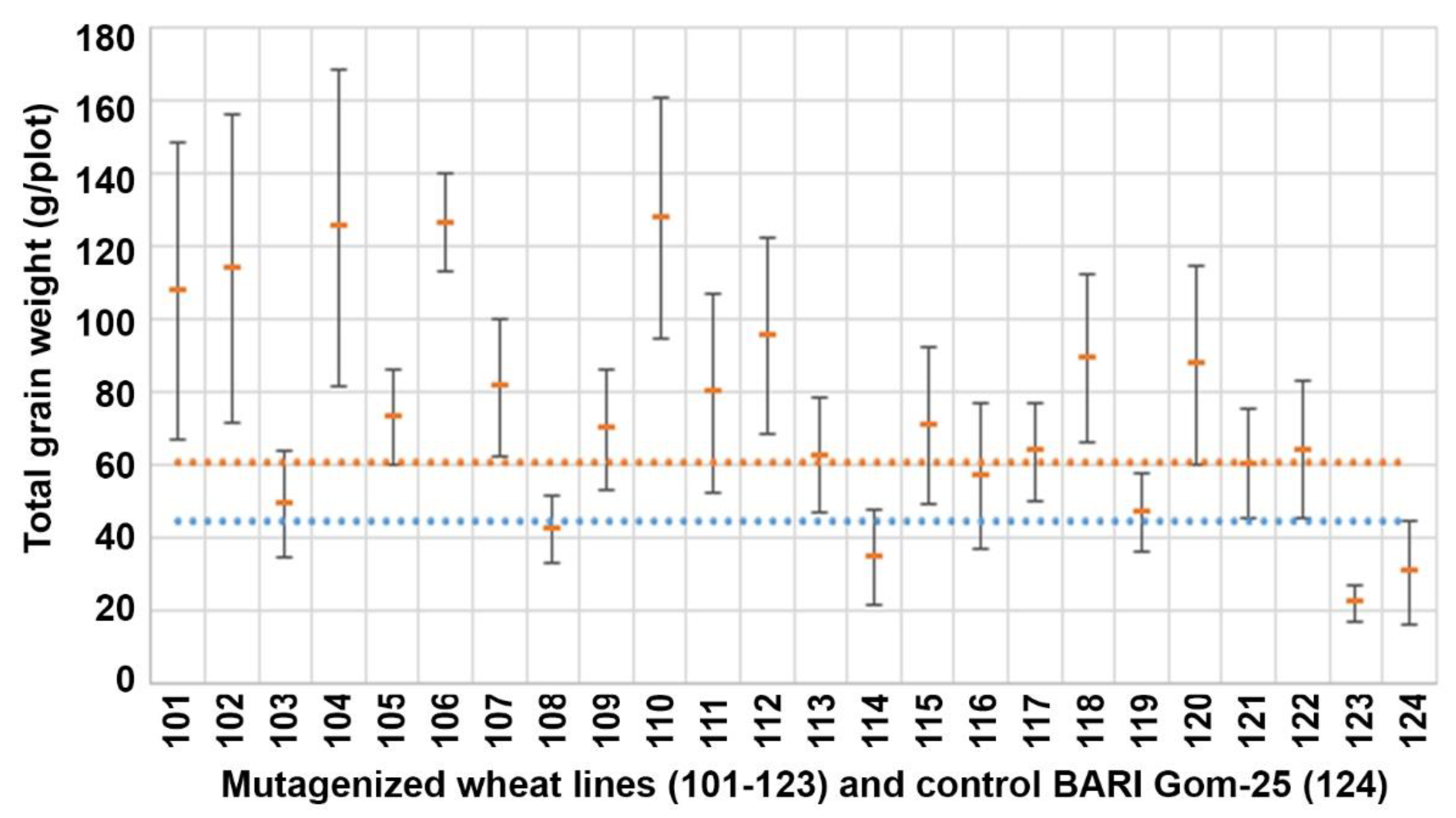
3.2. Total Grain Weight (TGW)
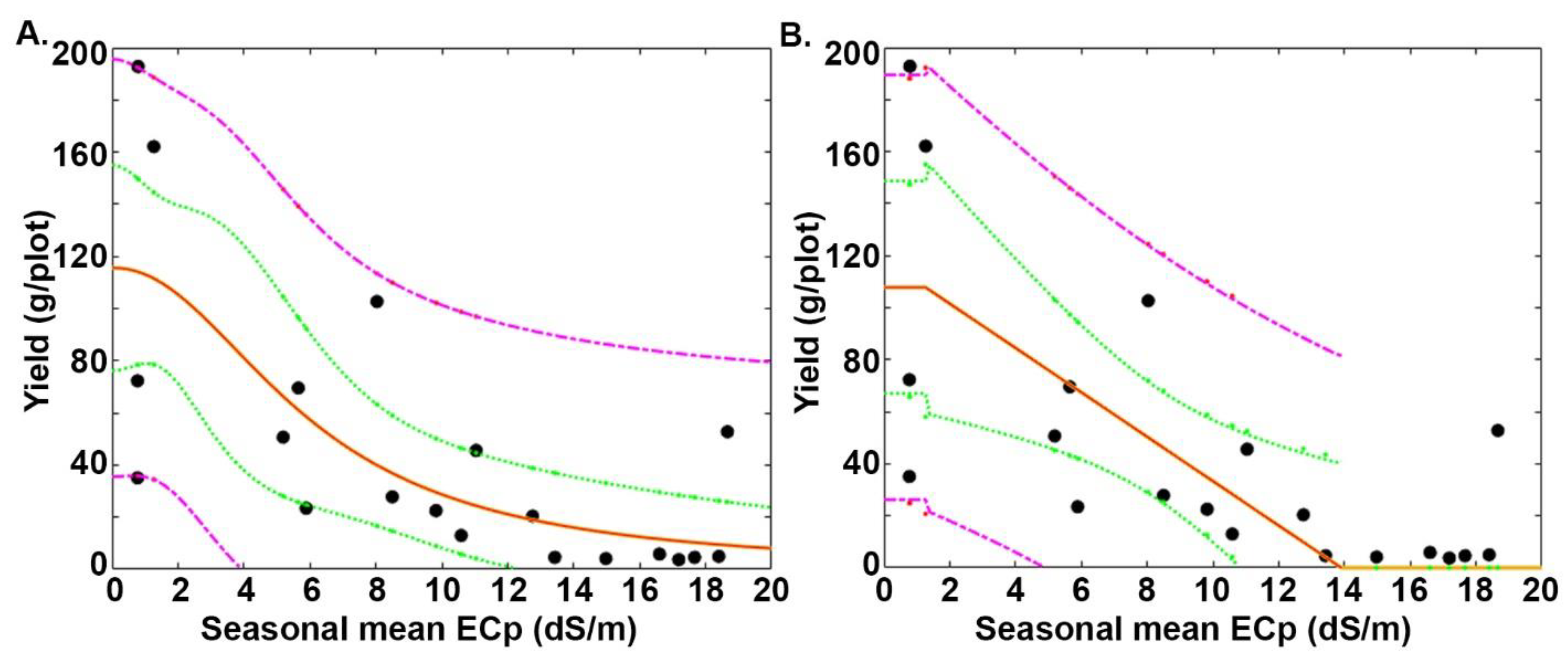
3.3. Relative Performance on the Mutagenized Lines under Saline Conditions According to Two Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.E.; Hassan, W.; Al-Suhaibani, N.A.; Refay, Y.; Abdella, K.A. Comparative performance of multivariable agro-physiological parameters for detecting salt tolerance of wheat cultivars under simulated saline field growing conditions. Front. Plant Sci. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabot, P.; Tondel, F. A Regional View of Wheat Markets and Food Security in Central Asia: With a Focus on Afghanistan and Tajikistan; Famine Early Warning Systems Network FEWS NET; United States Agency for International Development: Washington, DC, USA, 2011. Available online: https://documents.wfp.org/stellent/groups/public/documents/ena/wfp238576.pdf (accessed on 15 March 2022).
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Al-Taey, D.; Al-Naely, I.; Kshash, B. A study on the effects of water quality, cultivars, organic and chemical fertilizers on potato (Solanum tuberosum L.) growth and yield to calculate the economic feasibility. Bulg. J. Agric. Sci. 2019, 25, 1239–1245. [Google Scholar]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Thomas, D.S.G.; Middleton, N.J. Salinization: New perspectives on a major desertification issue. J. Arid Environ. 1993, 24, 95–105. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.; Yeo, A. Breeding for salinity resistance in crop plants: Where next? Funct. Plant Biol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Yeo, A. Molecular biology of salt tolerance in the context of whole-plant physiology. J. Exp. Bot. 1998, 49, 915–929. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, H.; Ullah, S.; Iqbal, M.; Bilal, H.M.; Shah, G.M.; Ahmad, S.; Zakir, A.; Ditta, A.; Farooqi, M.A.; Ahmad, I. Salinity types and level-based effects on the growth, physiology and nutrient contents of maize (Zea mays). Ital. J. Agron. 2019, 14, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Fortmeier, R.; Schubert, S. Salt tolerance of maize (Zea mays L.): The role of sodium exclusion. Plant Cell Environ. 1995, 18, 1041–1047. [Google Scholar] [CrossRef]
- Abbas, G.; Saqib, M.; Rafique, Q.; Rahman, M.; Javaid, A.; Anwar-ul-Haq, M.; Nasim, M. Effect of salinity on grain yield and grain quality of wheat (Triticum aestivum L.). Pak. J. Agric. Res. 2013, 50, 185–189. [Google Scholar]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [Green Version]
- Maas, E.V.; Grieve, C.M. Spike and leaf development of salt stressed wheat. Crop Sci. 1990, 30, 1309–1313. [Google Scholar] [CrossRef]
- Khatun, S.; Flowers, T.J. Effects of salinity on seed set in rice. Plant Cell Environ. 1995, 18, 61–67. [Google Scholar] [CrossRef]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. In Agricultural Drainage; American Society of Agronomy Inc.: Madison, WI, USA, 1999; Volume 38, pp. 55–108. [Google Scholar] [CrossRef]
- FAO. Crop Salt Tolerance Data; FAO: Rome, Italy, 1985; Available online: https://www.fao.org/3/y4263e/y4263e0e.htm (accessed on 15 March 2022).
- Shahzad, M.; Saqib, Z.A.; Hafeez, F.; Bilal, M.; Khan, S.A.; Asad, S.A.; Akhtar, J. Growth-related changes in wheat (Triticum aestivum L.) genotypes grown under salinity stress. J. Plant Nutr. 2015, 39, 1257–1265. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [Green Version]
- Genc, Y.; Mcdonald, G.K.; Tester, M. Reassessment of tissue Na+concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ. 2007, 30, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Flowers, T.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Dadshani, S.; Sharma, R.C.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Multi-dimensional evaluation of response to salt stress in wheat. PLoS ONE 2019, 14, e0222659. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance–current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Van Genuchten, M.T.; Hoffman, G. Analysis of crop production. In Soil Salinity Under Irrigation—Process and Management; Shainberg, I., Shalhevet, J., Eds.; Springer: Berlin, Germany, 1984; pp. 258–271. [Google Scholar]
- van Straten, G.; de Vos, A.C.; Rozema, J.; Bruning, B.; van Bodegom, P.M. An improved methodology to evaluate crop salt tolerance from field trials. Agric. Water Manag. 2019, 213, 375–387. [Google Scholar] [CrossRef]
- Lethin, J.; Shakil, S.S.M.; Hassan, S.; Sirijovski, N.; Töpel, M.; Olsson, O.; Aronsson, H. Development and characterization of an EMS-mutagenized wheat population and identification of salt-tolerant wheat lines. BMC Plant Biol. 2020, 20, 18. [Google Scholar] [CrossRef] [Green Version]
- Bruning, B.; van Logtestijn, R.; Broekman, R.; De Vos, A.; González, A.P.; Rozema, J. Growth and nitrogen fixation of legumes at increased salinity under field conditions: Implications for the use of green manures in saline environments. AoB Plants 2015, 7, plv010. [Google Scholar] [CrossRef]
- de Vos, A.; Bruning, B.; van Straten, G.; Oosterbaan, R.; Rozema, J.; van Bodegom, P. Crop Salt Tolerance under Controlled Field Conditions in The Netherlands, Based on Trials Conducted at Salt Farm Texel; Salt Farm Texel: Den Burg, The Netherlands, 2016; Available online: https://edepot.wur.nl/409817 (accessed on 15 March 2022).
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Minitab 19 Statistical Software, version 19. Computer Software. Minitab, Inc: State College, PA, USA, 2019.
- Muhammad, H.; Shemana, M.; Nihar, S.; Shamsun, B. Evaluation of wheat cultivars for salinity tolerance at seedling stage based on morphological and molecular markers. J. Bangladesh Agric. Univ. 2020, 18, 234–244. [Google Scholar] [CrossRef]
- Haque, S.A. Salinity problems and crop production in coastal regions of Bangladesh. Pak. J.Bot. 2006, 38, 1359–1365. [Google Scholar]
- Rasel, H.M.; Hasan, M.R.; Ahmed, B.; Miah, M.S.U. Investigation of soil and water salinity, its effect on crop production and adaptation strategy. Int. J. Water Resour. Environ. Eng. 2013, 5, 475–481. [Google Scholar]
- Clarke, D.; Williams, S.; Jahiruddin, M.; Parks, K.; Salehin, M. Projections of on-farm salinity in coastal Bangladesh. Environ. Sci. Process. Impacts 2015, 17, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Janda, T.; Darko, É.; Shehata, S.; Kovács, V.; Pál, M.; Szalai, G. Salt acclimation processes in wheat. Plant Physiol. Biochem. 2016, 101, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Geleta, B.; Atak, M.; Baenziger, P.S.; Nelson, L.A.; Baltenesperger, D.D.; Eskridge, K.M.; Shipman, M.J.; Shelton, D.R. Seeding rate and genotype effect on agronomic performance and end-use quality of winter wheat. Crop Sci. 2002, 42, 827–832. [Google Scholar]
- Valério, I.P.; de Carvalho, F.I.F.; de Oliveira, A.C.; Benin, G.; de Souza, V.Q.; Machado, A.D.A.; Bertan, I.; Busato, C.C.; da Silveira, G.; Fonseca, D.A.R. Seeding density in wheat genotypes as a function of tillering potential. Sci. Agric. 2009, 66, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Bastos, L.M.; Carciochi, W.; Lollato, R.P.; Jaenisch, B.R.; Rezende, C.R.; Schwalbert, R.; Prasad, P.V.; Zhang, G.; Fritz, A.K.; Foster, C.; et al. Winter wheat yield response to plant density as a function of yield environment and tillering potential: A review and field studies. Front. Plant Sci. 2020, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Lloveras, J.; Manent, J.; Viudas, J.; López, A.; Santiveri, P. Seeding rate influence on yield and yield components of irrigated winter wheat in a mediterranean climate. Agron. J. 2004, 96, 1258–1265. [Google Scholar] [CrossRef]
- Whaley, J.M.; Sparkes, D.L.; Foulkes, M.J.; Spink, J.H.; Semere, T.; Scott, R.K. The physiological response of winter wheat to reductions in plant density. Ann. Appl. Biol. 2000, 137, 165–177. [Google Scholar] [CrossRef]
- Dahlke, B.J.; Oplinger, E.S.; Gaska, J.M.; Martinka, M.J. Influence of planting date and seeding rate on winter wheat grain yield and yield components. J. Prod. Agric. 1993, 6, 408–414. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengasamy, P. Salt-Affected Soils in Australia; Grains Research and Development Corporation: Canberra, Australia, 2016; pp. 1–64. [Google Scholar]
- Hoffmann, G.R. Genetic effects of dimethyl sulfate, diethyl sulfate, and related compounds. Mutat. Res. Genet. Toxicol. 1980, 75, 63–129. [Google Scholar] [CrossRef]
- Walker, G.R. Effectiveness of Current Farming Systems in the Control of Dryland Salinity; Walker, G.R., Gilfedder, M., Williams, J., Eds.; CSIRO Land and Water CSIRO Land and Water: Canberra, Australia, 1999. [Google Scholar]
- COAG Standing Council on Environment and Water. In Australia’s Native Vegetation Framework; Australian Government, Department of Sustainability, Environment, Water, Population and Communities: Canberra, Australia, 2012; ISBN 978-1-921733-57-4.
- Naveed, M.; Ditta, A.; Ahmad, M.; Mustafa, A.; Ahmad, Z.; Conde-Cid, M.; Tahir, S.; Shah, S.A.A.; Abrar, M.M.; Fahad, S. Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environ. Sci. Pollut. Res. 2021, 28, 45629–45645. [Google Scholar] [CrossRef]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N.; Xu, M. Calcium-enriched animal manure alleviates the adverse effects of salt stress on growth, physiology and nutrients homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [Green Version]

| Source | Amount | N | P2O5 | K2O | CaO | S | MgO | Organic Matter |
|---|---|---|---|---|---|---|---|---|
| Compost | 20 | 14 | 21 | 48 | 16 | 3840 | ||
| Manure | 12 | 44 | 17 | 72 | 2 | 26 | 1824 | |
| Monterra | 1 | 90 | 10 | 40 | 20 | 30 | 3 | 750 |
| Orgevit | 1 | 40 | 32 | 25 | 90 | 10 | 10 | 650 |
| Total | 188 | 80 | 185 | 110 | 42 | 55 | 7064 |
| Variables | Salt Conc | Spike Length | TGW | Eight-Spike Grain Weight | Live Number | Plant Height |
|---|---|---|---|---|---|---|
| Spike length | −0.392 ** | |||||
| TGW | −0.662 ** | 0.360 ** | ||||
| Eight-spike grain weight | −0.786 ** | 0.522 ** | 0.716 ** | |||
| Live number | −0.220 ** | 0.074 | 0.327 ** | 0.209 ** | ||
| Plant height | −0.727 ** | 0.476 ** | 0.644 ** | 0.692 ** | 0.256 ** | |
| Tiller number | −0.457 ** | 0.200 ** | 0.519 ** | 0.392 ** | 0.085 | 0.468 ** |
| Maas–Hoffman Model | Van Genuchten–Hoffman Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Line No. | ECpthr (dS m−1) | S (g/plot)/(dS m−1) | Y0 (g/plot) | S% (dS m−1) | ECp50 (dS m−1) | ECp90 (dS m−1) | ECp50 (dS m−1) | p (−) | Y0 (g/plot) | ECp90 (dS m−1) |
| 101 | 1.3 | −8.5 | 107.8 | 7.9 | 7.6 | 2.5 | 6.0 | 2.1 | 115.6 | 2.1 |
| 102 | 0.8 | −9.2 | 114.0 | 8.1 | 7.0 | 2.0 | 4.7 | 1.7 | 127.9 | 1.3 |
| 103 | 1.3 | −3.4 | 49.1 | 6.9 | 8.6 | 2.7 | 6.2 | 1.7 | 54.9 | 1.7 |
| 104 | 0.8 | −15.6 | 125.2 | 12.4 | 4.8 | 1.6 | 1.8 | 1.3 | 182.4 | 0.3 |
| 105 | 1.3 | −6.9 | 73.0 | 9.4 | 6.6 | 2.3 | 6.1 | 3.6 | 75.0 | 3.3 |
| 106 | 0.7 | −10.9 | 126.4 | 8.6 | 6.5 | 1.9 | 5.2 | 2.2 | 133.2 | 2.0 |
| 107 | 1.3 | −6.6 | 81.3 | 8.1 | 7.4 | 2.5 | 5.9 | 2.3 | 87.7 | 2.2 |
| 108 | 1.3 | −3.6 | 42.1 | 8.4 | 7.2 | 2.5 | 5.9 | 2.3 | 44.8 | 2.3 |
| 109 | 0.7 | −3.8 | 69.7 | 5.5 | 9.8 | 2.5 | 4.4 | 1.5 | 92.4 | 1.0 |
| 110 | 0.8 | −11.1 | 128.1 | 8.7 | 6.6 | 1.9 | 4.4 | 1.9 | 144.3 | 1.4 |
| 111 | 1.3 | −5.1 | 79.6 | 6.4 | 9.1 | 2.8 | 7.5 | 2.3 | 83.2 | 2.9 |
| 112 | 0.8 | −10.9 | 95.3 | 11.4 | 5.2 | 1.7 | 3.4 | 1.6 | 111.9 | 0.9 |
| 113 | 7.6 | −16.8 | 62.6 | 26.8 | 9.4 | 8.0 | 9.3 | 6.6 | 65.1 | 6.7 |
| 114 | 5.5 | −5.1 | 34.5 | 14.7 | 8.9 | 6.2 | 9.0 | 6.8 | 34.5 | 6.5 |
| 115 | 5.0 | −12.2 | 70.7 | 17.3 | 7.9 | 5.6 | 7.4 | 6.5 | 73.1 | 5.2 |
| 116 | 4.0 | −5.7 | 56.7 | 10.1 | 9.0 | 5.0 | 8.7 | 4.1 | 56.7 | 5.1 |
| 117 | 5.5 | −9.3 | 63.5 | 14.7 | 8.9 | 6.2 | 8.8 | 5.3 | 66.1 | 5.8 |
| 118 | 2.6 | −8.2 | 89.1 | 9.2 | 8.0 | 3.7 | 7.7 | 3.9 | 88.5 | 4.4 |
| 119 | 5.6 | −5.7 | 47.0 | 12.1 | 9.7 | 6.4 | 9.8 | 6.3 | 46.6 | 6.9 |
| 120 | 3.5 | −8.8 | 87.4 | 10.1 | 8.5 | 4.5 | 7.9 | 4.0 | 88.3 | 4.6 |
| 121 | 0.6 | −4.6 | 60.1 | 7.6 | 7.2 | 2.0 | 2.8 | 1.2 | 86.2 | 0.4 |
| 122 | 0.4 | −5.7 | 64.1 | 8.8 | 6.1 | 1.6 | 2.0 | 1.2 | 96.5 | 0.3 |
| 123 | 5.5 | −2.4 | 22.0 | 11.0 | 10.0 | 6.4 | 9.5 | 5.6 | 22.7 | 6.4 |
| Control | 5.5 | −2.7 | 30.4 | 8.8 | 11.2 | 6.6 | 11.0 | 4.8 | 30.7 | 6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, H.; Bruning, B.; Van Straten, G.; Almås, Å.R.; Lethin, J.; Naik, N.; Hassan, S.; Olsson, O.; Aronsson, H. Effects of Increasing Salinity by Drip Irrigation on Total Grain Weight Show High Yield Potential of Putative Salt-Tolerant Mutagenized Wheat Lines. Sustainability 2022, 14, 5061. https://doi.org/10.3390/su14095061
Mousavi H, Bruning B, Van Straten G, Almås ÅR, Lethin J, Naik N, Hassan S, Olsson O, Aronsson H. Effects of Increasing Salinity by Drip Irrigation on Total Grain Weight Show High Yield Potential of Putative Salt-Tolerant Mutagenized Wheat Lines. Sustainability. 2022; 14(9):5061. https://doi.org/10.3390/su14095061
Chicago/Turabian StyleMousavi, Hesam, Bas Bruning, Gerrit Van Straten, Åsgeir R. Almås, Johanna Lethin, Nupur Naik, Sameer Hassan, Olof Olsson, and Henrik Aronsson. 2022. "Effects of Increasing Salinity by Drip Irrigation on Total Grain Weight Show High Yield Potential of Putative Salt-Tolerant Mutagenized Wheat Lines" Sustainability 14, no. 9: 5061. https://doi.org/10.3390/su14095061
APA StyleMousavi, H., Bruning, B., Van Straten, G., Almås, Å. R., Lethin, J., Naik, N., Hassan, S., Olsson, O., & Aronsson, H. (2022). Effects of Increasing Salinity by Drip Irrigation on Total Grain Weight Show High Yield Potential of Putative Salt-Tolerant Mutagenized Wheat Lines. Sustainability, 14(9), 5061. https://doi.org/10.3390/su14095061







