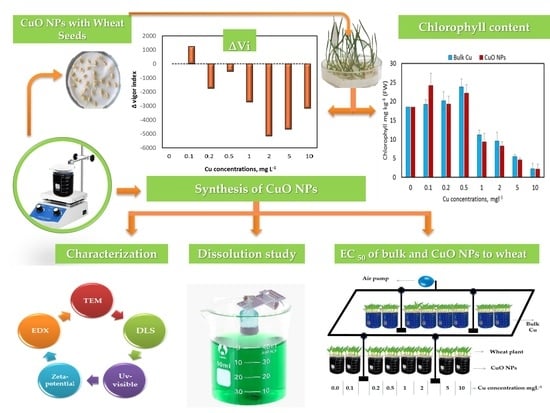
Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesize and Characterize Copper Oxide Nanoparticles
2.2. Stability and Copper Release Studies
2.3. Wheat Seed Germination and Root Growth Experiment
2.3.1. Wheat Seed Germination
2.3.2. Root Elongation Rate of Wheat and Toxicity Dose of Copper Forms
2.4. Plant Characteristics
2.5. Statistical Analysis
3. Results and Discussion
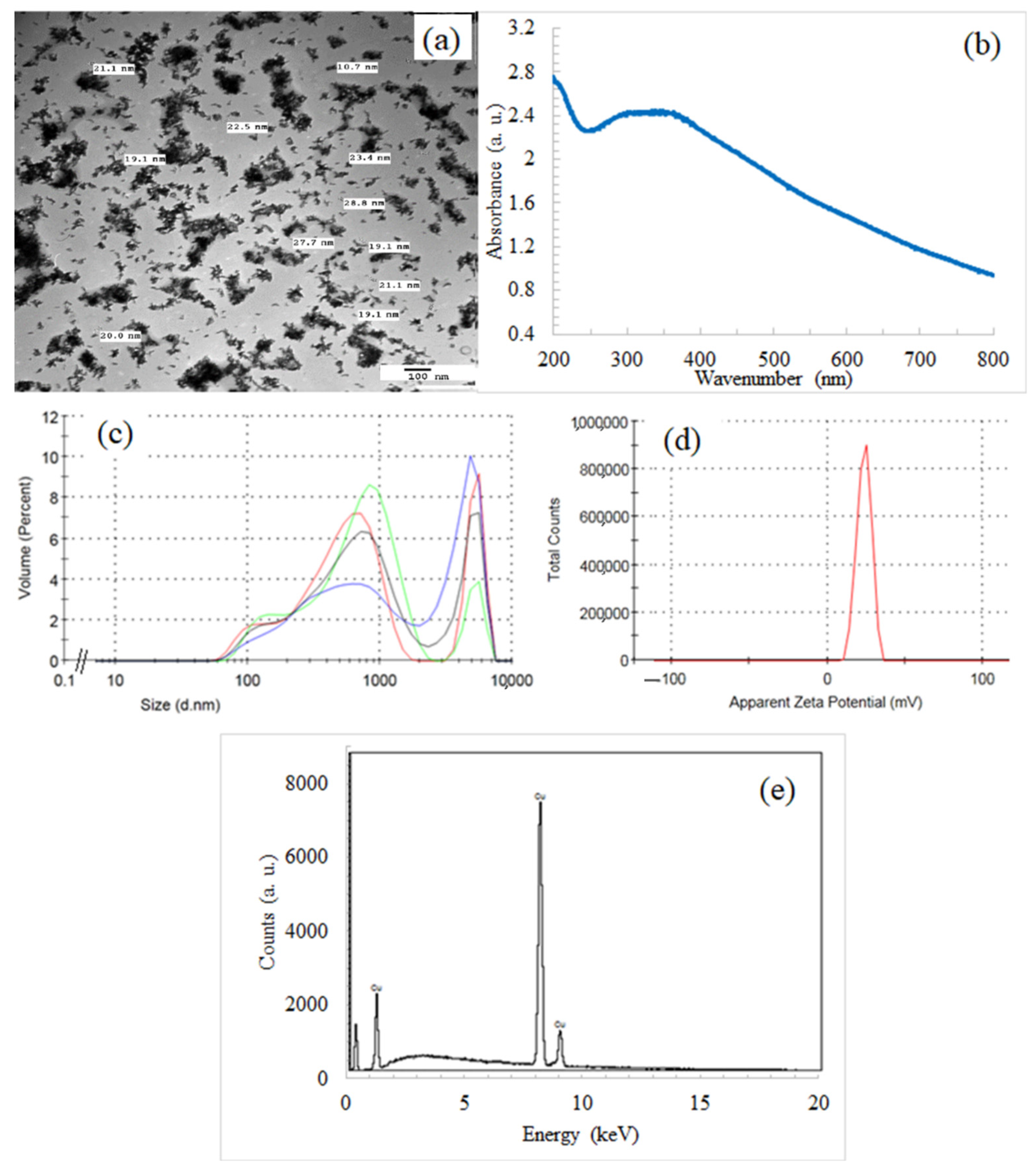
3.1. Characterization of CuO NPs
3.2. Copper Oxide Nanoparticle Dissolution Study
3.3. Effect of Copper Oxide Nanoparticles on Wheat Plant
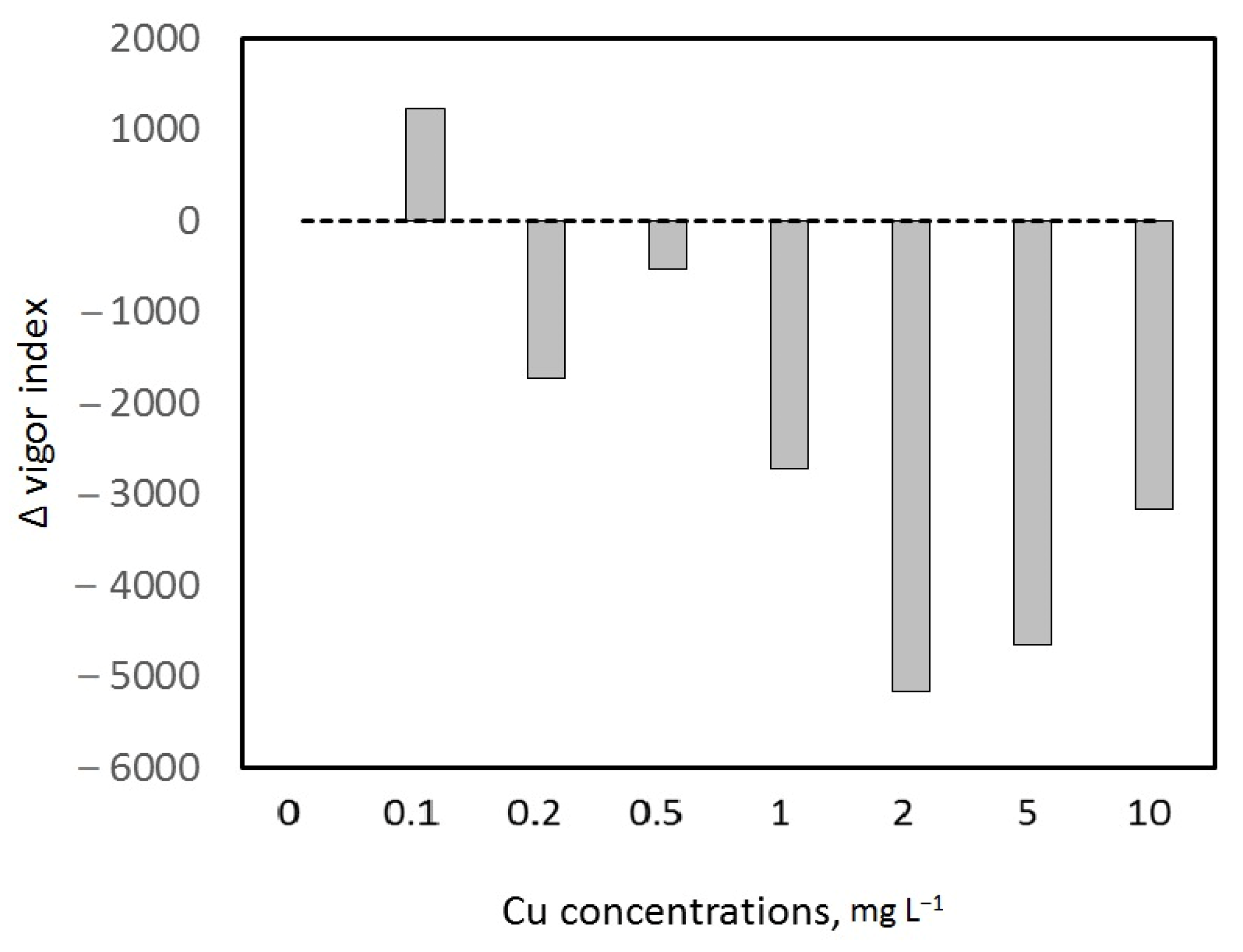
3.3.1. Wheat Germination Characteristics
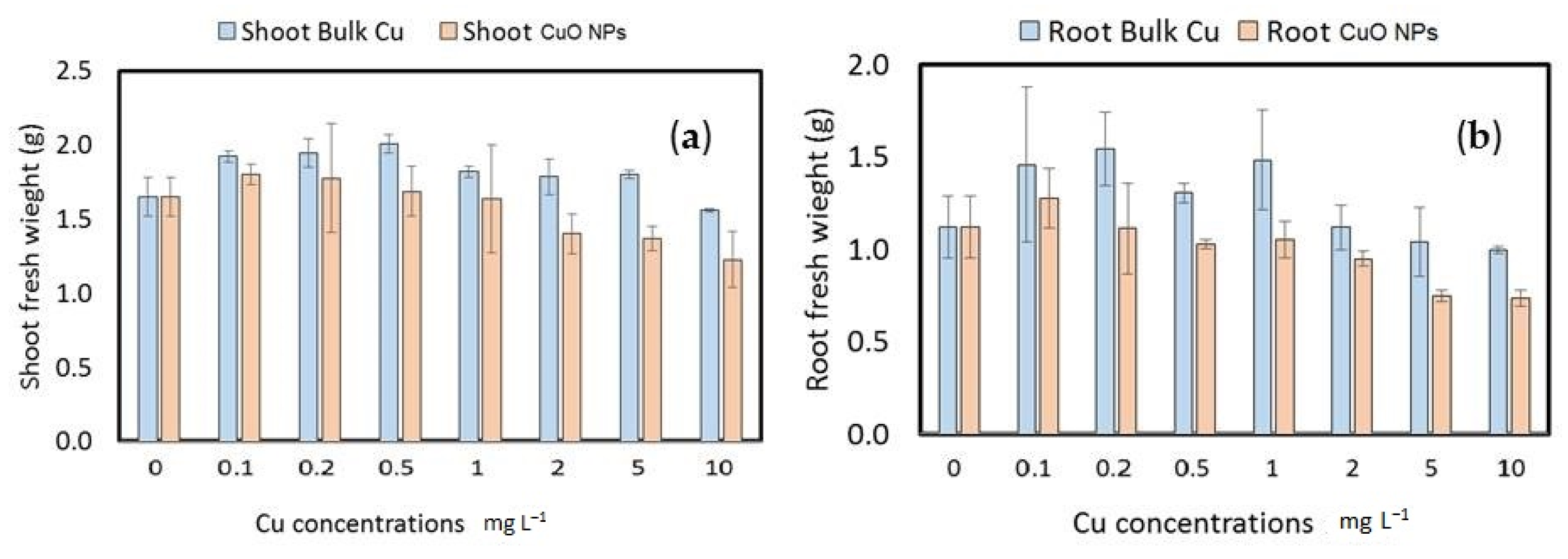
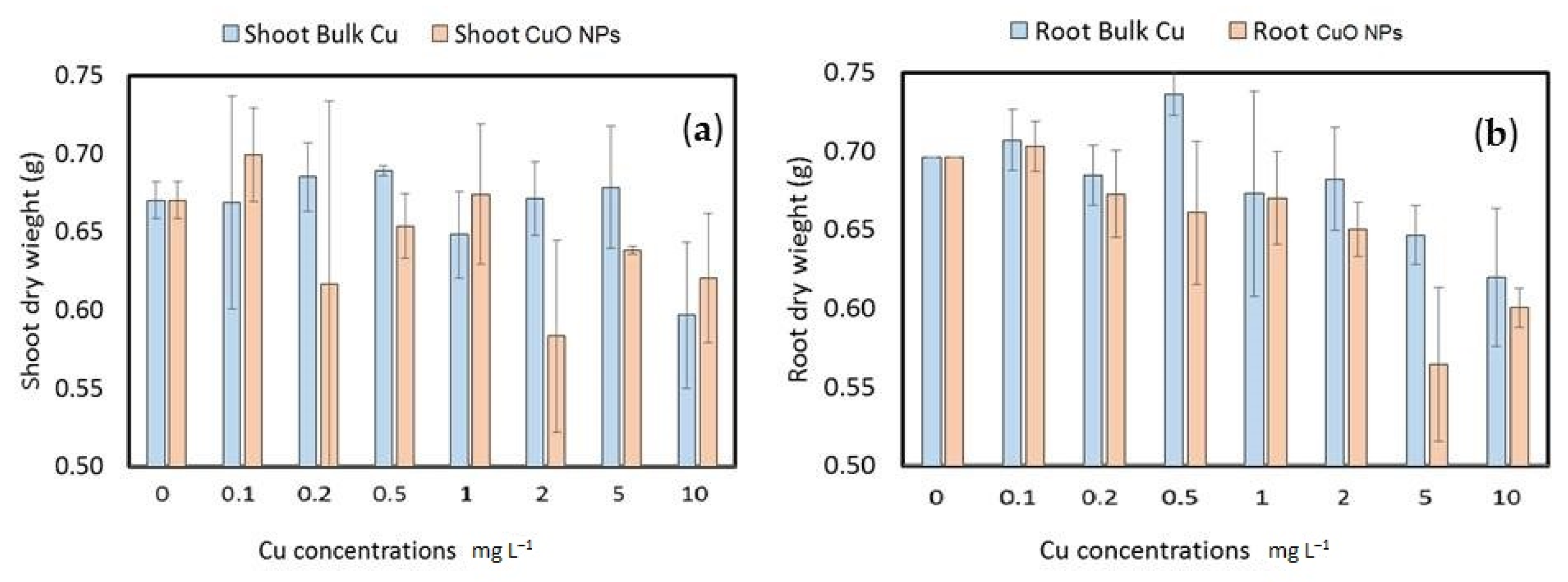
3.3.2. Wheat Seedling Fresh and Dry Weight
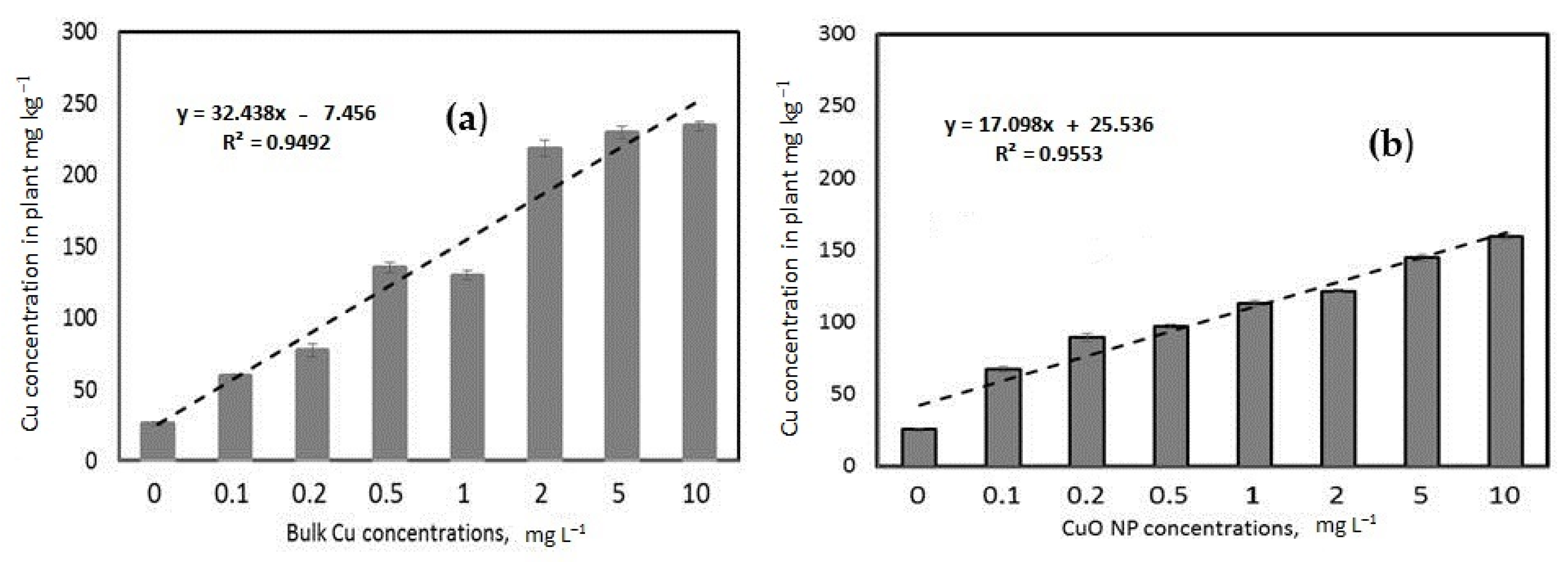
3.3.3. Copper Concentrations in Wheat Plant Tissue
3.3.4. Wheat Chlorophyll Content
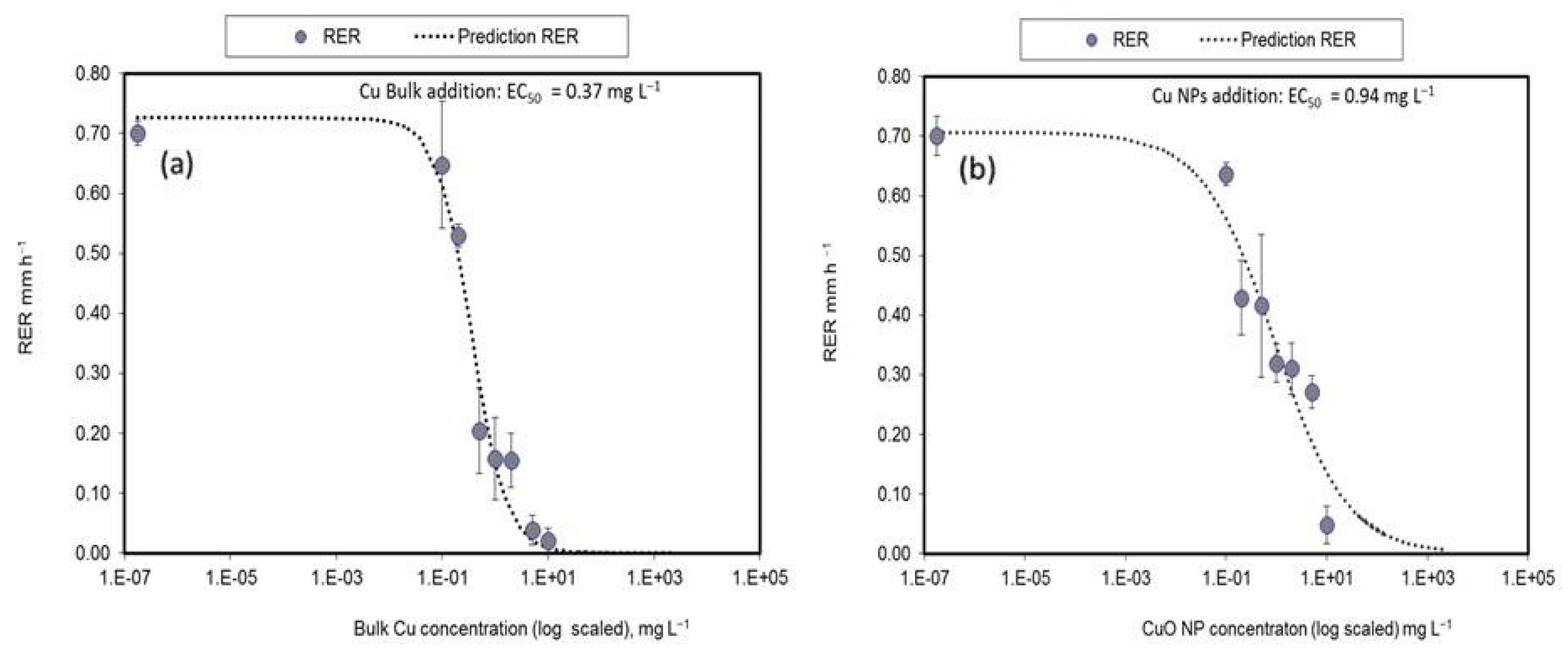
3.4. Copper Oxide Nanoparticles Toxicity to Wheat, EC50
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neme, K.; Nafady, A.; Uddin, S.; Tola, Y.B. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: Food security implication and challenges. Heliyon 2021, 7, e08539. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Asghar, M.; Habib, S.; Zaman, W.; Hussain, S.; Ali, H.; Saqib, S. Synthesis and characterization of microbial mediated cadmium oxide nanoparticles. Microsc. Res. Tech. 2020, 83, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Marina, P.E.; Ali, G.A.M.; See, L.M.; Teo, E.Y.L.; Ng, E.-P.; Chong, K.F. In situ growth of redox-active iron-centered nanoparticles on graphene sheets for specific capacitance enhancement. Arab. J. Chem. 2016, 12, 3883–3889. [Google Scholar] [CrossRef] [Green Version]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The recent progress on silver nanoparticles: Synthesis and electronic applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Alhasan, H.S.; Alahmadi, N.; Yasin, S.A.; Khalaf, M.Y.; Ali, G.A.M. Low-cost and eco-friendly hydroxyapatite nanoparticles derived from eggshell waste for cephalexin removal. Separations 2022, 9, 10. [Google Scholar] [CrossRef]
- Panagiotou, S.; Saha, S. Therapeutic benefits of nanoparticles in stroke. Front. Neurosci. 2015, 9, 182. [Google Scholar] [CrossRef]
- Ali, M.; Wang, X.; Haroon, U.; Chaudhary, H.J.; Kamal, A.; Ali, Q.; Saleem, M.H.; Usman, K.; Alatawi, A.; Ali, S.; et al. Antifungal activity of Zinc nitrate derived nano ZnO fungicide synthesized from Trachyspermum ammi to control fruit rot disease of grapefruit. Ecotoxicol. Environ. Saf. 2022, 233, 113311. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Habeeb, O.A.; Algarni, H.; Chong, K.F. CaO impregnated highly porous honeycomb activated carbon from agriculture waste: Symmetrical supercapacitor study. J. Mater. Sci. 2018, 54, 683–692. [Google Scholar] [CrossRef]
- Schreck, E.; Foucault, Y.; Sarret, G.; Sobanska, S.; Cécillon, L.; Castrec-Rouelle, M.; Uzu, G.; Dumat, C. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: Mechanisms involved for lead. Sci. Total Environ. 2012, 427, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Speciat. Bioavailab. 2018, 30, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.S. Nanotechnology in agriculture: Prospects and constraints. Nanotechnol. Sci. Appl. 2014, 7, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, K.S.; Gunasekaran, K.; Chinnamuthu, N.; Lakshmanan, A.; Rajkishore, S.K. (Eds.) Nanotechnology in Agriculture; New India Publishing Agency: New Delhi, India, 2015. [Google Scholar]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Ullah, F.; Nasar, M.Q.; Bahadur, S.; Irfan, M.M.; et al. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M. Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef] [Green Version]
- Khodakovskaya, M.V. Future Roadmap for Plant Nanotechnology. In Plant Nanotechnology; Kole, C., Kumar, D., Khodakovskaya, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 367–371. [Google Scholar]
- Ditta, A.; Arshad, M.; Ibrahim, M. Nanoparticles in sustainable agricultural crop production: Applications and perspectives. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 55–75. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Bacchu, M.S.; Ali, M.R.; Khan, M.Z.H. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Bayoumi, Y.; Eid, Y.; Elbasiouny, H.; Elbehiry, F.; Prokisch, J.; El-Ramady, H.; Ling, W. Can nanofertilizers mitigate multiple environmental stresses for higher crop productivity? Sustainability 2022, 14, 3480. [Google Scholar] [CrossRef]
- Tarafdar, J.; Raliya, R.; Rathore, I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J. Bionanosci. 2012, 6, 84–89. [Google Scholar] [CrossRef]
- Tarafdar, J.; Sharma, S.; Raliya, R. Nanotechnology: Interdisciplinary science of applications. Afr. J. Biotechnol. 2013, 12, 219–226. [Google Scholar]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13. [Google Scholar]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; Alnadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef]
- Perea Vélez, Y.S.; Carrillo-González, R.; González-Chávez, M.d.C.A. Interaction of metal nanoparticles–plants–microorganisms in agriculture and soil remediation. J. Nanopart. Res. 2021, 23, 206. [Google Scholar] [CrossRef]
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef] [Green Version]
- Kurczynska, E.; Sala, K.; Godel-Jedrychowska, K.; Milewska-Hendel, A. Nanoparticles-Plant interaction: What we know, where we are? Appl. Sci. 2021, 11, 5473. [Google Scholar] [CrossRef]
- Khan, M.R.; Adam, V.; Rizvi, T.F.; Zhang, B.; Ahamad, F.; Jośko, I.; Zhu, Y.; Yang, M.; Mao, C. Nanoparticle–Plant interactions: Two-way traffic. Small 2019, 15, 1901794. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Zhao, J.; Xu, L.; Xu, L.; Yu, X.; Wei, Y.; Xing, B. Interaction of CuO nanoparticles with plant cells: Internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environ. Sci. Nano 2018, 5, 2269–2281. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Croteau, M.N.; Luoma, S.N.; Boccaccini, A.R.; Valsami-Jones, E. Isotopically modified nanoparticles for enhanced detection in bioaccumulation studies. Environ. Sci. Technol. 2012, 46, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Tapeh-Esmaeil, E.; Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Synthesis of copper and copper oxide nanoparticles with different morphologies using aniline as reducing agent. Solid State Commun. 2021, 334–335, 114364. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper transporters and copper chaperones: Roles in cardiovascular physiology and disease. Am. J. Physiol. Cell Physiol. 2018, 315, C186–C201. [Google Scholar] [CrossRef] [PubMed]
- Printz, B.; Lutts, S.; Hausman, J.F.; Sergeant, K. Copper trafficking in plants and its implication on cell wall dynamics. Front. Plant Sci. 2016, 7, 601. [Google Scholar] [CrossRef] [Green Version]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, S.K.; Al Sharif, M.A. Copper (Cu) an essential redox-active transition metal in living system—A review article. Am. J. Anal. Chem. 2018, 9, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Panda, P.K.; Jha, E.; Pramanik, N.; Nisha, K.; Kumari, K.; Soni, N.; Mallick, M.A.; Verma, S.K. Molecular insight to in vitro biocompatibility of phytofabricated copper oxide nanoparticles with human embryonic kidney cells. Nanomedicine 2018, 13, 2415–2433. [Google Scholar] [CrossRef]
- Hemmati, S.; Ahmeda, A.; Salehabadi, Y.; Zangeneh, A.; Zangeneh, M.M. Synthesis, characterization, and evaluation of cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing effects of copper nanoparticles using the aqueous extract of Strawberry fruit and L-Ascorbic acid. Polyhedron 2020, 180, 114425. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic synthesis of copper oxide nanoparticles using Olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro study. Biotechnol. Prog. 2018, 34, 218–230. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper nanoparticles: Synthesis and characterization, physiology, toxicity and antimicrobial applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Rodhe, Y.; Skoglund, S.; Wallinder, I.O.; Potácová, Z.; Möller, L. Copper-based nanoparticles induce high toxicity in leukemic HL60 cells. Toxicol. In Vitro 2015, 29, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Singh, H.; Abhilash, P. The unseen impact of nanoparticles: More or less? Curr. Sci. 2014, 106, 350–352. [Google Scholar]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cai, Q.; Jiang, W.; Qu, X. Assessing impacts of a dam construction on benthic macroinvertebrate communities in a mountain stream. Fresenius Environ. Bull. 2013, 22, 103–110. [Google Scholar]
- Baqer, A.A.; Matori, K.A.; Al-Hada, N.M.; Kamari, H.M.; Shaari, A.H.; Saion, E.; Chyi, J.L.Y. Copper oxide nanoparticles synthesized by a heat treatment approach with structural, morphological and optical characteristics. J. Mater. Sci. Mater. Electron. 2018, 29, 1025–1033. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Liu, J.-F.; Geng, F.-L. Determination of metal oxide nanoparticles and their ionic counterparts in environmental waters by size exclusion chromatography coupled to ICP-MS. NanoImpact 2016, 1, 13–20. [Google Scholar] [CrossRef]
- Pereira, P.S.; van de Flierdt, T.; Hemming, S.R.; Hammond, S.J.; Kuhn, G.; Brachfeld, S.; Doherty, C.; Hillenbrand, C.-D. Geochemical fingerprints of glacially eroded bedrock from West Antarctica: Detrital thermochronology, radiogenic isotope systematics and trace element geochemistry in Late Holocene glacial-marine sediments. Earth-Sci. Rev. 2018, 182, 204–232. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.A.; Morris, E.C. Pseudoreplication in experimental designs for the manipulation of seed germination treatments. Austral Ecol. 2000, 25, 292–296. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.d.; Ferreira, W.R.; Mendes-Rodrigues, C. Calculating germination measurements and organizing spreadsheets. Braz. J. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Kopittke, P.M.; Kinraide, T.B.; Wang, P.; Blamey, F.P.C.; Reichman, S.M.; Menzies, N.W. Alleviation of Cu and Pb rhizotoxicities in cowpea (Vigna unguiculata) as related to ion activities at root-cell plasma membrane surface. Environ. Sci. Technol. 2011, 45, 4966–4973. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Rasband, W. ImageJ User Guide IJ 1.46r; Technical Report; National Institutes of Health: Bethesda, MA, USA, 2012.
- Marzouk, E.R.; Chenery, S.R.; Young, S.D. Predicting the solubility and lability of Zn, Cd, and Pb in soils from a minespoil-contaminated catchment by stable isotopic exchange. Geochim. Cosmochim. Acta 2013, 123, 1–16. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Van der Vliet, L.; Ritz, C. Statistics for analyzing ecotoxicity test data. In Encyclopedia of Aquatic Ecotoxicology; Springer: New York, NY, USA, 2013; pp. 1081–1096. [Google Scholar]
- Nabulo, G.; Black, C.; Young, S. Trace metal uptake by tropical vegetables grown on soil amended with urban sewage sludge. Environ. Pollut. 2011, 159, 368–376. [Google Scholar] [CrossRef]
- Shetaya, W.H.; Bailey, E.H.; Young, S.D.; Mohamed, E.F.; Antoniadis, V.; Rinklebe, J.; Shaheen, S.M.; Marzouk, E.R. Soil and plant contamination by potentially toxic and emerging elements and the associated human health risk in some Egyptian environments. Environ. Geochem. Health 2021. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Zhang, H.; Smith, J.A.; Oyanedel-Craver, V. The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Res. 2012, 46, 691–699. [Google Scholar] [CrossRef]
- Majedi, S.M.; Kelly, B.C.; Lee, H.K. Role of combinatorial environmental factors in the behavior and fate of ZnO nanoparticles in aqueous systems: A multiparametric analysis. J. Hazard. Mater. 2014, 264, 370–379. [Google Scholar] [CrossRef]
- Pettibone, J.M.; Adamcakova-Dodd, A.; Thorne, P.S.; O’Shaughnessy, P.T.; Weydert, J.A.; Grassian, V.H. Inflammatory response of mice following inhalation exposure to iron and copper nanoparticles. Nanotoxicology 2008, 2, 189–204. [Google Scholar] [CrossRef]
- Hejazy, M.; Koohi, M.K.; Bassiri Mohamad Pour, A.; Najafi, D. Toxicity of manufactured copper nanoparticles—A review. Nanomed. Res. J. 2018, 3, 1–9. [Google Scholar]
- Sekine, R.; Marzouk, E.; Khaksar, M.; Scheckel, K.G.; Stegemeier, J.P.; Lowry, G.V.; Donner, E.; Lombi, E. Ageing of dissolved copper and copper-based nanoparticles in five different soils: Short term kinetics vs long term fate. J. Environ. Qual. 2017, 46, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McShane, H.V.; Sunahara, G.I.; Whalen, J.K.; Hendershot, W.H. Differences in soil solution chemistry between soils amended with nanosized CuO or Cu reference materials: Implications for nanotoxicity tests. Environ. Sci. Technol. 2014, 48, 8135–8142. [Google Scholar] [CrossRef] [Green Version]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar]
- Kadu, P.J.; Kushare, S.S.; Thacker, D.D.; Gattani, S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm. Dev. Technol. 2011, 16, 65–74. [Google Scholar] [CrossRef]
- Servin, A.D.; Pagano, L.; Castillo-Michel, H.; De la Torre-Roche, R.; Hawthorne, J.; Hernandez-Viezcas, J.A.; Loredo-Portales, R.; Majumdar, S.; Gardea-Torresday, J.; Dhankher, O.P. Weathering in soil increases nanoparticle CuO bioaccumulation within a terrestrial food chain. Nanotoxicology 2017, 11, 98–111. [Google Scholar] [CrossRef]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Engineered nanoparticles and organic matter: A review of the state-of-the-art. Chemosphere 2015, 119, 608–619. [Google Scholar] [CrossRef]
- Wang, Z.; Von Dem Bussche, A.; Kabadi, P.K.; Kane, A.B.; Hurt, R.H. Biological and environmental transformations of copper-based nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef] [Green Version]
- Adam, N.; Schmitt, C.; Galceran, J.; Companys, E.; Vakurov, A.; Wallace, R.; Knapen, D.; Blust, R. The chronic toxicity of ZnO nanoparticles and ZnCl2 to Daphnia magna and the use of different methods to assess nanoparticle aggregation and dissolution. Nanotoxicology 2014, 8, 709–717. [Google Scholar]
- Miao, L.; Wang, C.; Hou, J.; Wang, P.; Ao, Y.; Li, Y.; Lv, B.; Yang, Y.; You, G.; Xu, Y. Enhanced stability and dissolution of CuO nanoparticles by extracellular polymeric substances in aqueous environment. J. Nanopart. Res. 2015, 17, 404. [Google Scholar] [CrossRef]
- Jośko, I.; Oleszczuk, P. Influence of soil type and environmental conditions on ZnO, TiO2 and Ni nanoparticles phytotoxicity. Chemosphere 2013, 92, 91–99. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Liu, D.; Zhao, G. Effects of NaCl and silicon on activities of antioxidative enzymes in roots, shoots and leaves of alfalfa. Afr. J. Biotechnol. 2011, 10, 545–549. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Khalaki, M.; Ghorbani, A.; Moameri, M. Effects of silica and silver nanoparticles on seed germination traits of Thymus kotschyanus in laboratory conditions. J. Rangel. Sci. 2016, 6, 221–231. [Google Scholar]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Biswas, A.K.; Tarafdar, J.C.; Rao, A.S. Effect of copper oxide nano particle on seed germination of selected crops. J. Agric. Sci. Technol. A 2012, 2, 815. [Google Scholar]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar]
- Bour, A.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Pinelli, E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. J. Hazard. Mater. 2015, 283, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water–insoluble nanoparticles. Environ. Toxicol. Chem. Internat. J. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Falco, W.F.; Queiroz, A.M.; Fernandes, J.; Botero, E.R.; Falcão, E.A.; Guimarães, F.E.G.; M’Peko, J.C.; Oliveira, S.L.; Colbeck, I.; Caires, A.R.L. Interaction between chlorophyll and silver nanoparticles: A close analysis of chlorophyll fluorescence quenching. J. Photochem. Photobiol. A Chem. 2015, 299, 203–209. [Google Scholar] [CrossRef]
- Moustakas, M.; Malea, P.; Haritonidou, K.; Sperdouli, I. Copper bioaccumulation, photosystem II functioning, and oxidative stress in the seagrass Cymodocea nodosa exposed to copper oxide nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 16007–16018. [Google Scholar] [CrossRef]
- Marzouk, E.R.; Ismai, S.A.; Hamza, A.F. Toxicity of Synthesized Copper Nanoparticles to Soybean: Deferential Effects of Nano and Bulk-Size Particles on Root Elongation Rate. In Proceedings of the 3rd International Conference on Sustainable Agriculture and Environment (3rd ICSAE), Warsaw, Poland, 26–28 September 2016. [Google Scholar]
- Zhang, Z.; Ke, M.; Qu, Q.; Peijnenburg, W.; Lu, T.; Zhang, Q.; Ye, Y.; Xu, P.; Du, B.; Sun, L. Impact of copper nanoparticles and ionic copper exposure on wheat (Triticum aestivum L.) root morphology and antioxidant response. Environ. Pollut. 2018, 239, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.-L.; Fang, T.; Dimkpa, C.O.; Britt, D.W.; McLean, J.E.; Jacobson, A.; Anderson, A.J. The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals 2015, 28, 101–112. [Google Scholar] [CrossRef]
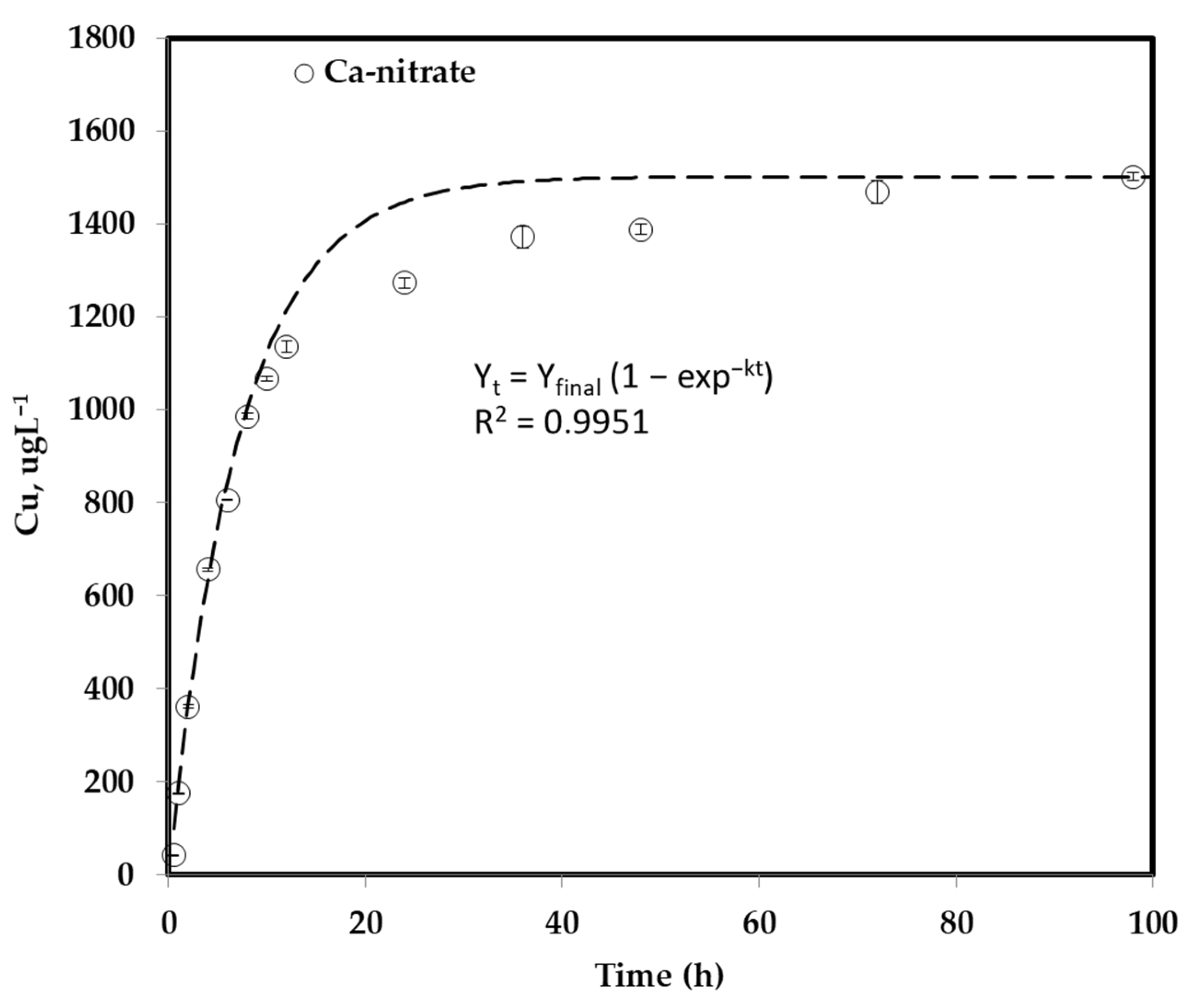





| Time (h) | Cu Concentration (µg L−1) | Standard Deviation |
|---|---|---|
| 0.5 | 42.7 | 0.259 |
| 1 | 176 | 1.130 |
| 2 | 362 | 4.580 |
| 4 | 656 | 2.680 |
| 6 | 807 | 0.682 |
| 8 | 986 | 5.300 |
| 10 | 1066 | 4.740 |
| 12 | 1136 | 12.500 |
| 24 | 1273 | 11.300 |
| 36 | 1372 | 24.000 |
| 48 | 1388 | 11.400 |
| 72 | 1469 | 24.000 |
| 98 | 1502 | 9.140 |
| Cu, mg L−1 | G (%) | t− (Day) | CVt (%) | v− (Day −1) |
|---|---|---|---|---|
| Bulk Cu | ||||
| 0.00 | 100 ± 0.0 a | 1.77 ± 0.028 bc | 31.5 ± 0.611 a | 0.57 ± 0.009 b |
| 0.10 | 100 ± 0.0 a | 1.50 ± 0.00 c | 36.7 ± 0.456 a | 0.67 ± 0.00 a |
| 0.20 | 100 ± 0.0 a | 1.81 ± 0.022 b | 31.9 ± 0.815 a | 0.55 ± 0.007 b |
| 0.50 | 100 ± 0.0 a | 1.93 ± 0.022 ab | 37.3 ± 985 a | 0.52 ± 0.005 bc |
| 1.00 | 100 ± 0.0 a | 2.00 ± 0.019 ab | 31.8 ± 1.06 a | 0.50 ± 0.007 bc |
| 2.00 | 100 ± 0.0 a | 2.04 ± 0.027 ab | 36.1 ± 0.56 a | 0.49 ± 0.004 bc |
| 5.00 | 100 ± 0.0 a | 2.02 ± 0.016 ab | 37.6 ± 0.824 a | 0.49 ± 0.003 bc |
| 10.0 | 100 ± 0.0 a | 2.19 ± 0.013 a | 36.3 ± 0.831 a | 0.46 ± 0.003 c |
| CuO NPs | ||||
| 0.00 | 100 ± 0.0 a | 1.77 ± 0.028 a | 31.5 ± 0.611 a | 0.57 ± 0.009 a |
| 0.10 | 100 ± 0.0 a | 1.54 ± 0.037 a | 34.1 ± 0.651 a | 0.66 ± 0.016 a |
| 0.20 | 100 ± 0.0 a | 1.71 ± 0.009 a | 33.3 ± 0.494 a | 0.58 ± 0.003 a |
| 0.50 | 100 ± 0.0 a | 1.67 ± 0.016 a | 34.9 ± 0.799 a | 0.60 ± 0.06 a |
| 1.00 | 100 ± 0.0 a | 1.73 ± 0.028 a | 30.5 ± 0.535 a | 0.58 ± 0.010 a |
| 2.00 | 100 ± 0.0 a | 1.71 ± 0.014 a | 34.2 ± 0.441 a | 0.59 ± 0.005 a |
| 5.00 | 100 ± 0.0 a | 1.92 ± 0.054 a | 50.6 ± 1.46 b | 0.53 ± 0.016 a |
| 10.0 | 96.7 ± 0.62 a | 1.81 ± 0.037 a | 51.1 ± 0.342 b | 0.56 ± 0.011 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.S.; Ali, G.A.M.; Hassanein, A.; Attia, A.M.; Marzouk, E.R. Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant. Sustainability 2022, 14, 4914. https://doi.org/10.3390/su14094914
Ibrahim AS, Ali GAM, Hassanein A, Attia AM, Marzouk ER. Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant. Sustainability. 2022; 14(9):4914. https://doi.org/10.3390/su14094914
Chicago/Turabian StyleIbrahim, Amany S., Gomaa A. M. Ali, Amro Hassanein, Ahmed M. Attia, and Ezzat R. Marzouk. 2022. "Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant" Sustainability 14, no. 9: 4914. https://doi.org/10.3390/su14094914
APA StyleIbrahim, A. S., Ali, G. A. M., Hassanein, A., Attia, A. M., & Marzouk, E. R. (2022). Toxicity and Uptake of CuO Nanoparticles: Evaluation of an Emerging Nanofertilizer on Wheat (Triticum aestivum L.) Plant. Sustainability, 14(9), 4914. https://doi.org/10.3390/su14094914