Modelling and Environmental Profile Associated with the Valorization of Wheat Straw as Carbon Source in the Biotechnological Production of Manganese Peroxidase
Abstract
:1. Introduction
2. Materials and Methods
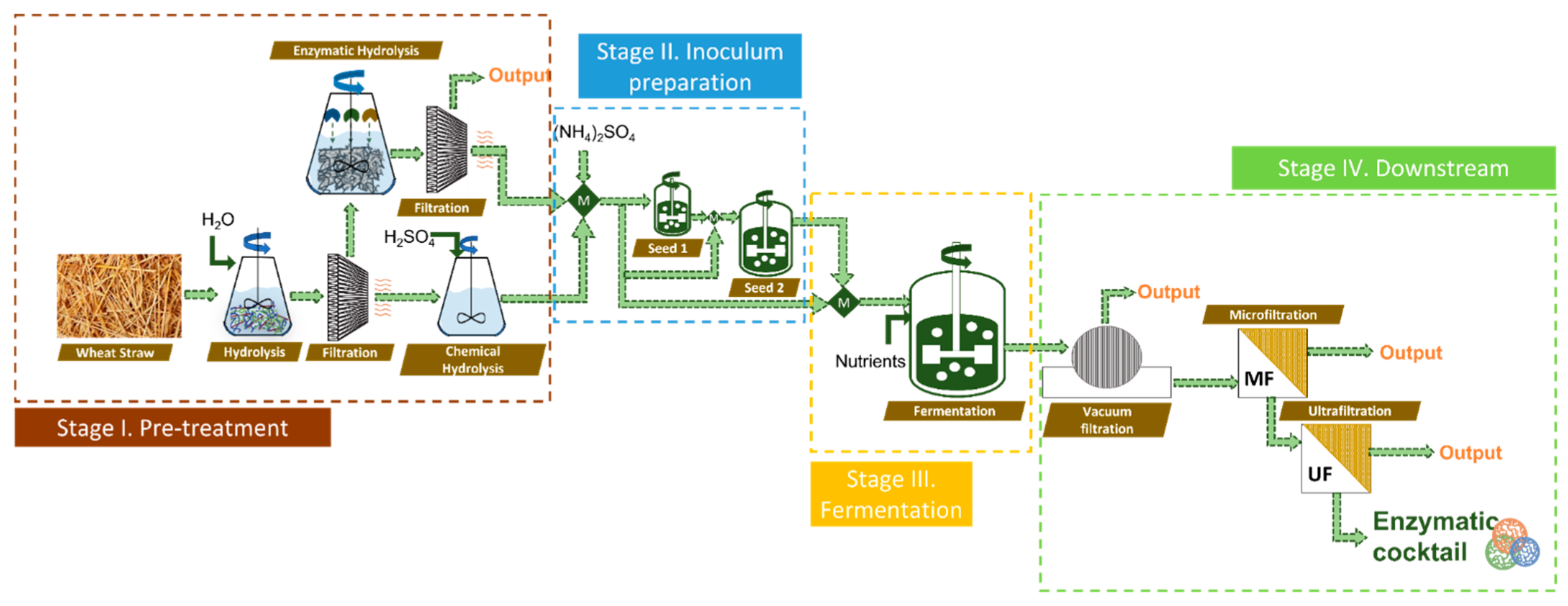
2.1. Process Description
2.1.1. Pretreatment of Wheat Straw (I)
2.1.2. Inoculum Preparation (II)
2.1.3. Fermentation Section (III)
2.1.4. Downstream Section (IV)
2.2. Life Cycle Assessment Methodology
2.2.1. Goal and Scope Definition
2.2.2. Life Cycle Inventories
2.2.3. Impact Evaluation
2.2.4. Interpretation of Results
3. Results and Discussion
3.1. Design and Simulation Results
3.2. Global Environmental Assessment
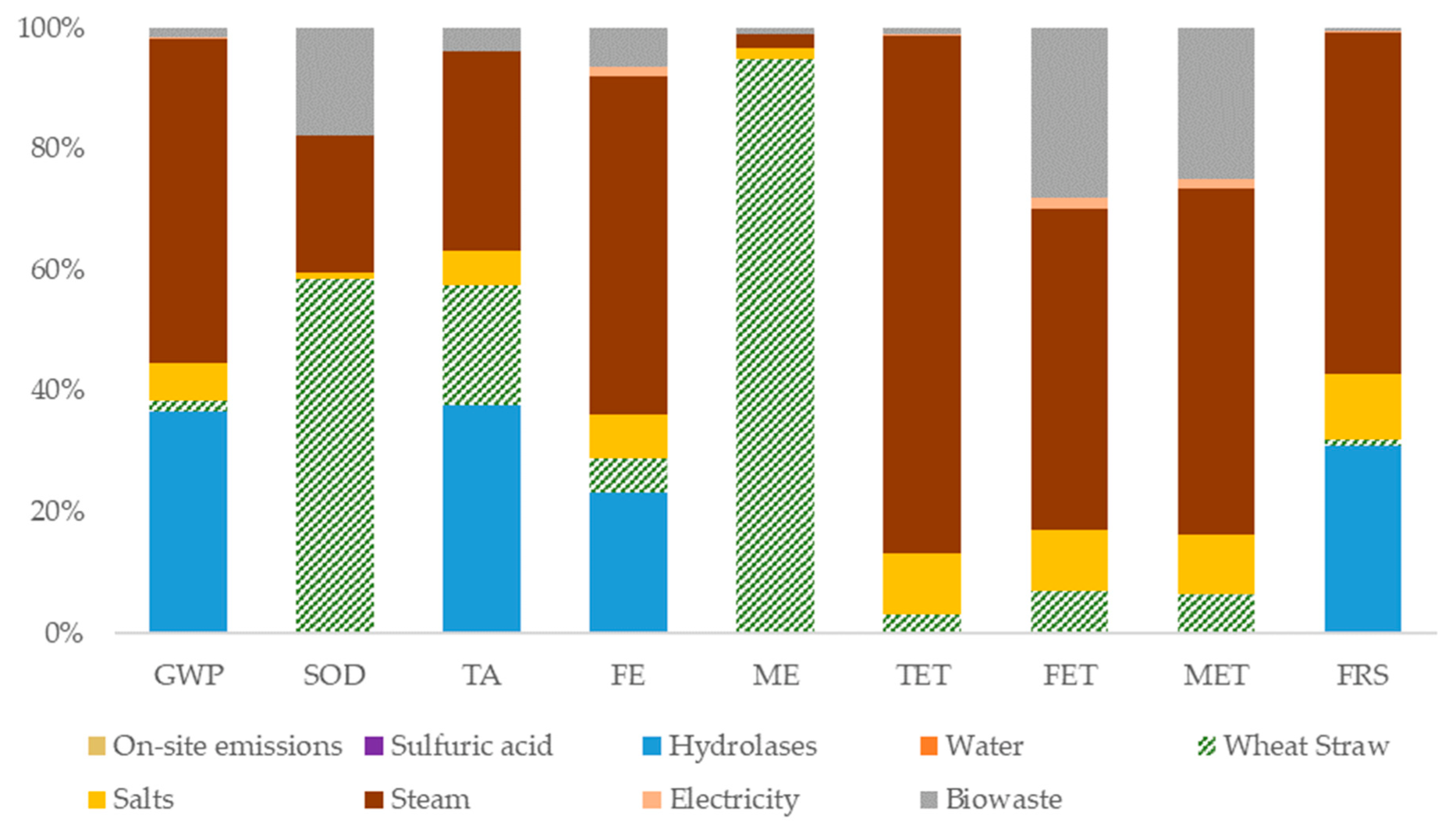
3.3. Environmental Assessment for Stage I Pretreatment
3.4. Environmental Assessment for Stage III Fermentation
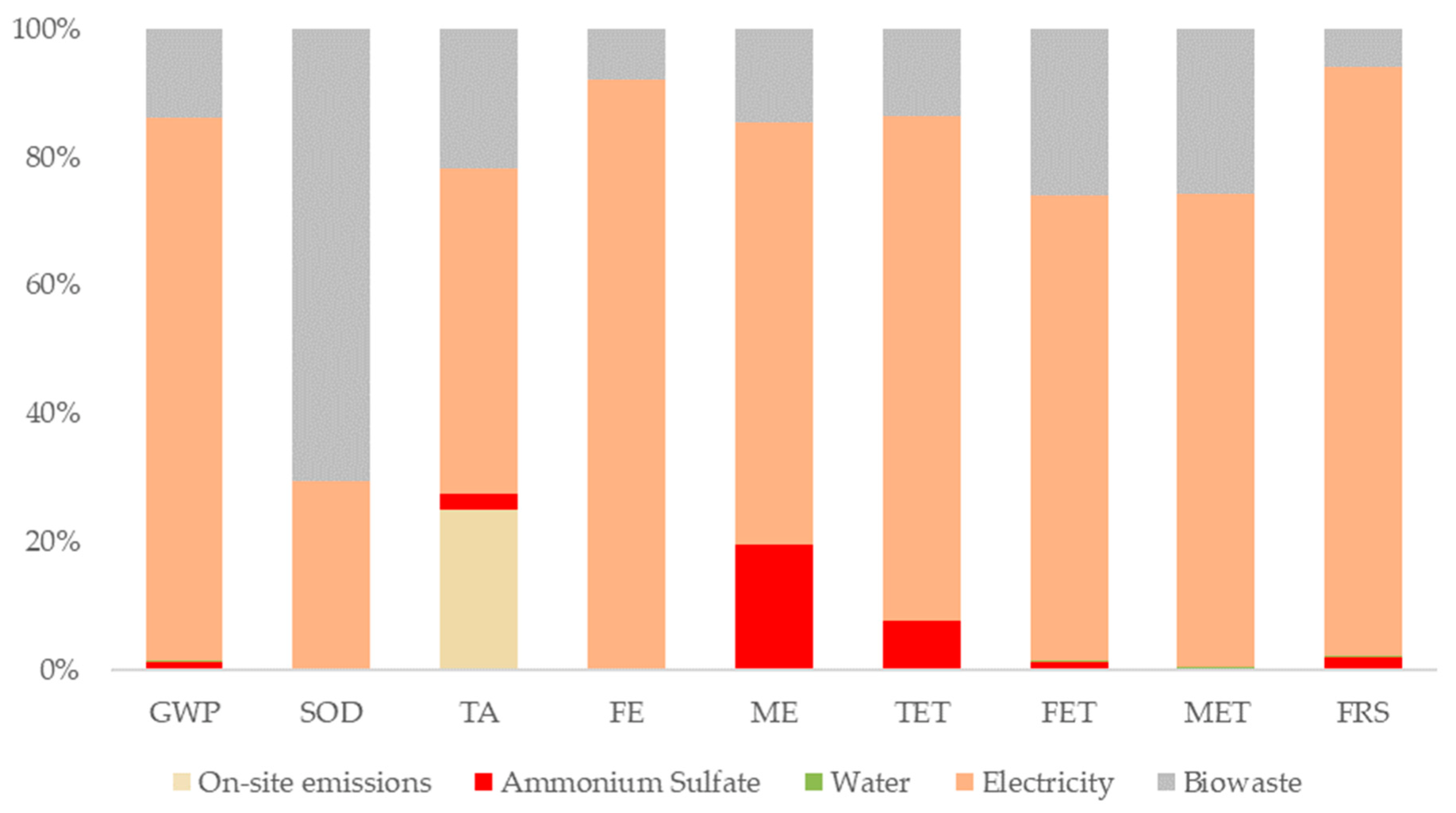
3.5. Environmental Assessment for Stages II and IV
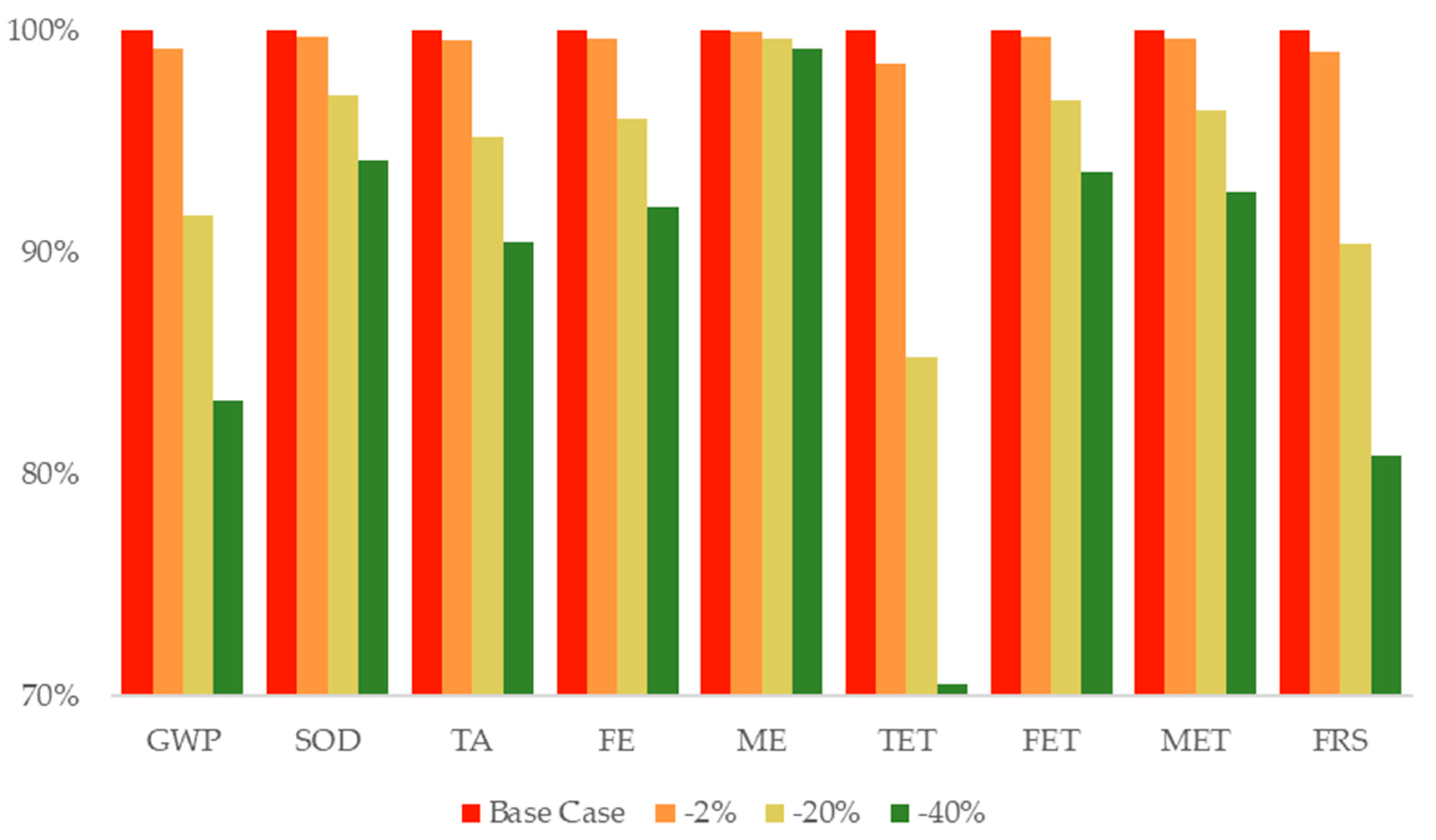
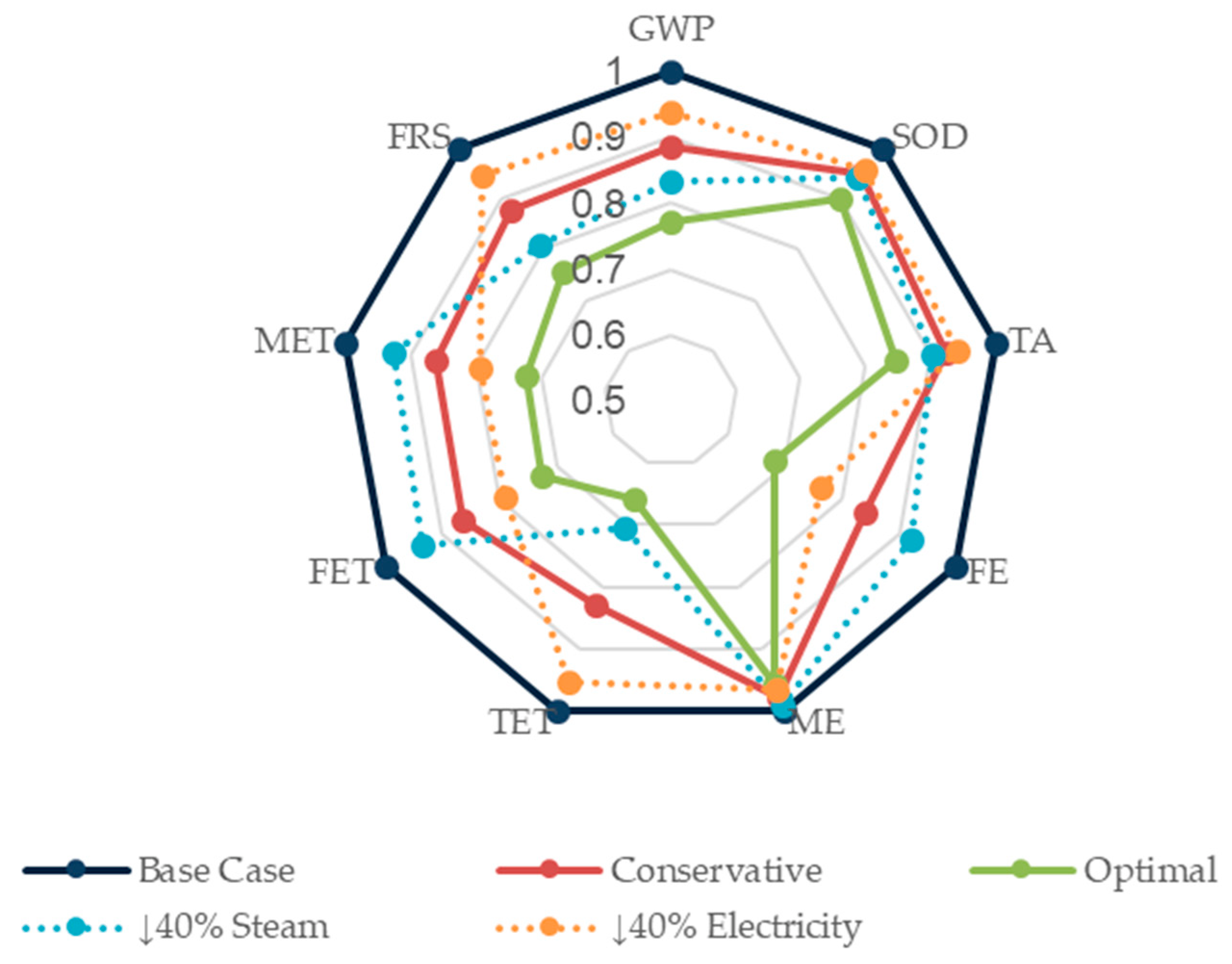
3.6. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.D.B.; Santos, V.E.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Awoyale, A.A.; Lokhat, D. Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production. Sci. Rep. 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Chang, C.; Zhang, L.; Cui, S.; Luo, X.; Hu, S.; Qin, Y.; Li, Y. Conversion of Lignocellulosic Biomass Into Platform Chemicals for Biobased Polyurethane Application. Adv. Bioenergy 2018, 3, 161–213. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Romero-Izquierdo, A.G.; Gómez-Castro, F.I.; Hernández, S. Production processes from lignocellulosic feedstock. Prod. Process. Renew. Aviat. Fuel 2021, 129–169. [Google Scholar] [CrossRef]
- Merklein, K.; Fong, S.S.; Deng, Y. Biomass Utilization. Biotechnol. Biofuel Prod. Optim. 2016, 291–324. [Google Scholar] [CrossRef]
- Salim, I.; González-García, S.; Feijoo, G.; Moreira, M.T. Assessing the environmental sustainability of glucose from wheat as a fermentation feedstock. J. Environ. Manag. 2019, 247, 323–332. [Google Scholar] [CrossRef]
- Aro, N.; Pakula, T.; Penttilä, M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol. Rev. 2005, 29, 719–739. [Google Scholar] [CrossRef] [Green Version]
- Sahay, S. Deconstruction of lignocelluloses: Potential biological approaches. Handb. Biofuels 2022, 207–232. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of lignocellulosic biomass. Lignocellul. Biomass Liq. Biofuels 2020, 1–15. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [Green Version]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Prommuak, C. Net Energy Analysis and Techno-Economic Assessment of Co-Production of Bioethanol and Biogas from Cellulosic Biomass. Ferment 2021, 7, 229. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Teixeira, R.S.; Silva, A.S.; Moutta, R.O.; Ferreira-Leitão, V.S.; Barros, R.R.; Ferrara, M.A.; Bon, E.P. Biomass pretreatment: A critical choice for biomass utilization via biotechnological routes. BMC Proc. 2014, 8, O34. [Google Scholar] [CrossRef] [Green Version]
- Cantero, D.; Jara, R.; Navarrete, A.; Pelaz, L.; Queiroz, J.; Rodríguez-Rojo, S.; Cocero, M.J. Pretreatment Processes of Biomass for Biorefineries: Current Status. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 289–310. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Zhang, Q.; Liu, Z.Y.; Li, F.L.; Lu, M.; Fang, X.C. Emerging technologies for the pretreatment of lignocellulosic materials for bio-based products. Appl. Microbiol. Biotechnol. 2020, 104, 455–473. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Fleurence, J.; Bergé, J.P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shi, Y.; Kong, W.; Wei, J.; Song, W.; Wang, S. Improving enzymatic hydrolysis of lignocellulosic biomass by bio-coordinated physicochemical pretreatment—A review. Energy Rep. 2022, 8, 696–709. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Ramnath, L.; Sithole, B.; Govinden, R. Classification of lipolytic enzymes and their biotechnological applications in the pulping industry. Can. J. Microbiol. 2017, 63, 179–192. [Google Scholar] [CrossRef]
- Meghwanshi, G.K.; Kaur, N.; Verma, S.; Dabi, N.K.; Vashishtha, A.; Charan, P.D.; Purohit, P.; Bhandari, H.S.; Bhojak, N.; Kumar, R. Enzymes for pharmaceutical and therapeutic applications. Biotechnol. Appl. Biochem. 2020, 67, 586–601. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Overview of Immobilized Enzymes’ Applications in Pharmaceutical, Chemical, and Food Industry. Methods Mol. Biol. 2020, 2100, 27–63. [Google Scholar] [CrossRef]
- Xu, H.; Guo, M.Y.; Gao, Y.H.; Bai, X.H.; Zhou, X.W. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol. 2017, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Li, W.; Duan, Z.; Zhang, Y.; Jia, R. Genome sequence of the white-rot fungus Irpex lacteus F17, a type strain of lignin degrader fungus. Stand. Genom. Sci. 2017, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Sun, X.; Huang, H.; Bai, Y.; Wang, Y.; Luo, H.; Yao, B.; Zhang, X.; Su, X. Oxidation of a non-phenolic lignin model compound by two Irpex lacteus manganese peroxidases: Evidence for implication of carboxylate and radicals. Biotechnol. Biofuels 2017, 10, 103. [Google Scholar] [CrossRef]
- Xin, L.; YuXian, Q.; Cun, Y. Optimization of Irpex lacteus culture conditions for manganese peroxidase production and dye decolorization ability of the enzyme. Mycosystema 2018, 37, 1233–1242. [Google Scholar]
- Yang, S.; Yang, J.; Wang, T.; Li, L.; Yu, S.; Jia, R.; Chen, P. Construction of a combined enzyme system of graphene oxide and manganese peroxidase for efficient oxidation of aromatic compounds. Nanoscale 2020, 12, 7976–7985. [Google Scholar] [CrossRef]
- Baborová, P.; Möder, M.; Baldrian, P.; Cajthamlová, K.; Cajthaml, T. Purification of a new manganese peroxidase of the white-rot fungus Irpex lacteus, and degradation of polycyclic aromatic hydrocarbons by the enzyme. Res. Microbiol. 2006, 157, 248–253. [Google Scholar] [CrossRef]
- Moon, D.S.; Song, H.G. Degradation of alkylphenols by white rot fungus Irpex lacteus and its manganese peroxidase. Appl. Biochem. Biotechnol. 2012, 168, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, J.; Zhang, X.; Yang, Y. Induction, purification and characterization of a novel manganese peroxidase from Irpex lacteus CD2 and its application in the decolorization of different types of dye. PLoS ONE 2014, 9, e113282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Eugenio, L.I.; Peces-Pérez, R.; Linde, D.; Prieto, A.; Barriuso, J.; Ruiz-Dueñas, F.J.; Martínez, M.J. Characterization of a dye-decolorizing peroxidase from Irpex lacteus expressed in Escherichia coli: An enzyme with wide substrate specificity able to transform lignosulfonates. J. Fungi 2021, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, L.; Jia, R.; Wang, N. Cloning and expression of a new manganese peroxidase from Irpex lacteus F17 and its application in decolorization of reactive black 5. Process Biochem. 2015, 50, 1748–1759. [Google Scholar] [CrossRef]
- Bouws, H.; Wattenberg, A.; Zorn, H. Fungal secretomes—Nature’s toolbox for white biotechnology. Appl. Microbiol. Biotechnol. 2008, 80, 381–388. [Google Scholar] [CrossRef]
- Salvachúa, D.; Martínez, A.T.; Tien, M.; López-Lucendo, M.F.; García, F.; De Los Ríos, V.; Martínez, M.J.; Prieto, A. Differential proteomic analysis of the secretome of Irpex lacteus and other white-rot fungi during wheat straw pretreatment. Biotechnol. Biofuels 2013, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.D.G.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef]
- Brondi, M.G.; Elias, A.M.; Furlan, F.F.; Giordano, R.C.; Farinas, C.S. Performance targets defined by retro-techno-economic analysis for the use of soybean protein as saccharification additive in an integrated biorefinery. Sci. Rep. 2020, 10, 7367. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Process and environmental simulation in the validation of the biotechnological production of nisin from waste. Biochem. Eng. J. 2021, 174, 108105. [Google Scholar] [CrossRef]
- Viere, T.; Amor, B.; Berger, N.; Fanous, R.D.; Arduin, R.H.; Keller, R.; Laurent, A.; Loubet, P.; Strothmann, P.; Weyand, S.; et al. Teaching life cycle assessment in higher education. Int. J. Life Cycle Assess. 2021, 26, 511–527. [Google Scholar] [CrossRef]
- de Lapuente Díaz de Otazu, R.L.; Akizu-Gardoki, O.; de Ulibarri, B.; Iturrondobeitia, M.; Minguez, R.; Lizundia, E. Ecodesign coupled with Life Cycle Assessment to reduce the environmental impacts of an industrial enzymatic cleaner. Sustain. Prod. Consum. 2022, 29, 718–729. [Google Scholar] [CrossRef]
- Arias, A.; Barreiro, D.; Feijoo, G.; Moreira, M.T. Waste biorefinery towards a sustainable biotechnological production of pediocin: Synergy between process simulation and environmental assessment. Environ. Technol. Innov. 2022, 26, 102306. [Google Scholar] [CrossRef]
- Mezule, L.; Civzele, A. Bioprospecting White-Rot Basidiomycete Irpex lacteus for Improved Extraction of Lignocellulose-Degrading Enzymes and Their Further Application. J. Fungi 2020, 6, 256. [Google Scholar] [CrossRef]
- Beisl, S.; Quehenberger, J.; Kamravamanesh, D.; Spadiut, O.; Friedl, A. Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study. Processes 2019, 7, 956. [Google Scholar] [CrossRef] [Green Version]
- Ghaffar, S.H. Wheat straw biorefinery for agricultural waste valorisation. Green Mater. 2019, 8, 60–67. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Sohail, I.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic Biomass: A Sustainable Bioenergy Source for the Future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, X.; Liu, Y. Production of fermentable sugars from wheat straw by formic acid pretreatment. Adv. Mater. Res. 2012, 550–553, 1258–1261. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Sárvári Horváth, I. Sustainable and efficient sugar production from wheat straw by pretreatment with biogas digestate. RSC Adv. 2019, 9, 27692–27701. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Wu, X.; Huang, C.; Huang, C.; Lai, C.; Yong, Q. Enhancing enzymatic digestibility of waste wheat straw by presoaking to reduce the ash-influencing effect on autohydrolysis. Biotechnol. Biofuels 2019, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Jiang, B.; Yang, L.; Jin, Y. Isolation of Lignin from Masson Pine by Liquid-Liquid Extraction Based on Complete Dissolution in NaOH Aqueous Solution. BioResources 2018, 13, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Ginni, G.; Kavitha, S.; Yukesh Kannah, R.; Bhatia, S.K.; Adish Kumar, S.; Rajkumar, M.; Kumar, G.; Pugazhendhi, A.; Chi, N.T.L. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Eisen, A.; Bussa, M.; Röder, H. A review of environmental assessments of biobased against petrochemical adhesives. J. Clean. Prod. 2020, 277, 124277. [Google Scholar] [CrossRef]
- Arias, A.; González-García, S.; Feijoo, G.; Moreira, M.T. Environmental benefits of soy-based bio-adhesives as an alternative to formaldehyde-based options. Environ. Sci. Pollut. Res. 2021, 28, 29781–29794. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management–Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- European Commission. ILCD Handbook: Specific Guide for Life Cycle Inventory Data Sets; EUR 24709 EN; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Xu, D. Life cycle assessment of complex forestry enterprise: A case study of a forest-fiberboard integrated enterprise. Sustainability 2020, 12, 4147. [Google Scholar] [CrossRef]
- Yang, Y.; Ni, J.Q.; Zhu, W.; Xie, G. Life cycle assessment of large-scale compressed bio-natural gas production in China: A case study on manure co-digestion with corn stover. Energies 2019, 12, 429. [Google Scholar] [CrossRef] [Green Version]



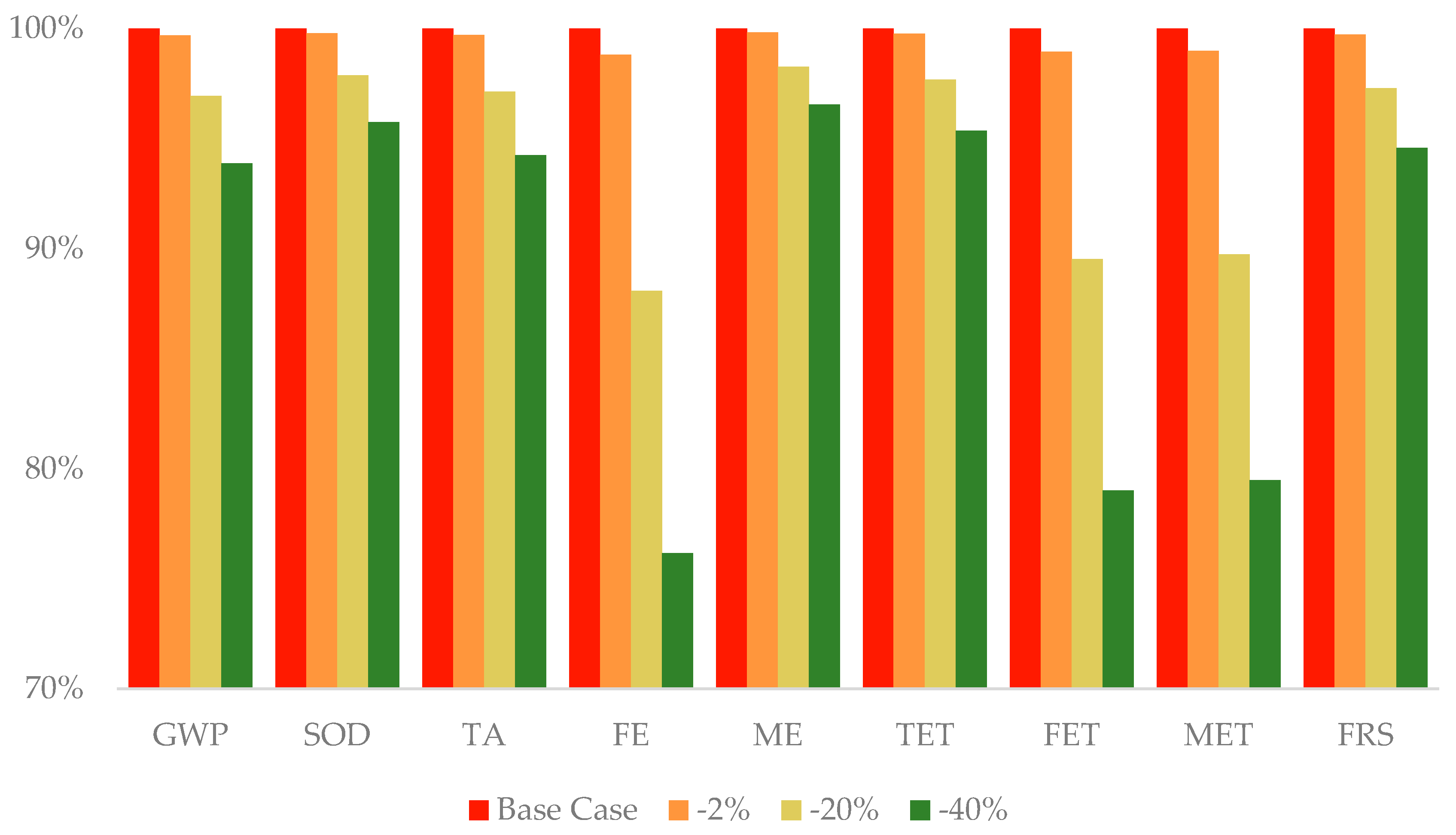
| Composition (% Dry Weight) | |||
|---|---|---|---|
| Cellulose | 30.6 ± 0.1 | Extractives | 9.8 ± 0.3 |
| Hemicellulose | 32.3 ± 0.2 | Saccharides | 5.3 ± 0.7 |
| Xylan | 23.5 ± 0.2 | Glucose | 1.2 ± 0.0 |
| Arabinan | 4.6 ± 0.3 | Protein | 5.4 ± 0.0 |
| Lignin | 16.8 ± 0.2 | Others | 0.1 ± 0.0 |
| Ash | 5.0 ± 0.0 | ||
| Global Process Inventory | |||||
|---|---|---|---|---|---|
| Inputs from Technosphere | Outputs to Technosphere | ||||
| Material | Amount | Unit | Material | Amount | Unit |
| Air | 865.76 | kg/batch | MnP | 0.31 | kg/batch |
| Ammonium sulfate | 2.38 | kg/batch | |||
| Sulfuric acid | 0.15 | kg/batch | |||
| Hydrolase | 61.70 | kg/batch | |||
| Sodium azide | 0.26 | kg/batch | Emissions to air | ||
| Sodium phthalate | 15.95 | kg/batch | Carbon dioxide | 36.86 | kg/batch |
| Water | 1.55 | m3/batch | |||
| Wheat straw | 500 | kg/batch | |||
| Energy | Outputs | ||||
| Steam | 1992 | kg/batch | Biomass | 499.29 | kg/batch |
| Electricity | 354.66 | kWh/batch | Wastewater | 0.55 | m3/batch |
| Water, vapor | 510.73 | kg/batch | |||
| Label | Equipment | Size (Capacity) | Description |
|---|---|---|---|
| Pretreatment of wheat straw (Stage I) | |||
| HX-101 | Heat exchanger | 2.62 m2 | Pre-heat the inlet stream to the autohydrolysis reactor |
| R-101 | Reactor | 1.58 m3 | Autohydrolysis of wheat straw, 210 °C and 10 bar. |
| V-101 | Flash Drum | 0.67 m3 | Release of high temperature water vapor |
| HX-102 | Heat exchanger | 7.45 m2 | Reduce the temperature of the outlet stream from R-101 |
| BF-101 | Belt Filtration | 1.15 m | Separation of solid and liquid fraction |
| R-102 | Reactor | 0.67 m3 | Enzymatic hydrolysis of the solid fraction using hydrolases |
| R-103 | Reactor | 0.39 m3 | Chemical post-hydrolysis with sulfuric acid of the liquid fraction |
| BF-102 | Belt Filtration | 0.39 m | Biomass separation |
| Inoculum preparation (Stage II) | |||
| SFR-102 | Seed Fermenter | 0.12 m3 | Inoculum preparation, 30 °C, 0.5 vvm |
| SFR-101 | Seed Fermenter | 0.62 m3 | Inoculum preparation, 30 °C, 0.5 vvm |
| Fermentation (Stage III) | |||
| ST-101 | Heat Sterilization | 1.16 m3/h | Sterilization of the inoculum, 110 °C, 45 min |
| G-101 | Air Compressor | 1.59 kW | Air compression |
| AF-101 | Air Filtration | 3 m3/h | Filtration of the air inlet stream to the fermenter |
| FR-101 | Fermenter | 1.18 m3 | Enzyme production at 30 °C for 3.3 days. |
| RVF-101 | Rotatory Vacuum Filtration | 3.82 m2 | Separation of the biomass |
| Downstream (Stage IV) | |||
| MF-101 | Microfiltration | 4.32 m2 | Separation of higher molecules, retained in the filter |
| UF-101 | Ultrafiltration | 2.77 m2 | Separation of the enzymatic cocktail, retained in the filter |
| Impact Category | Unit | Total | Stage I. Pretreatment | Stage II. Inoculum | Stage III. Fermentation | Stage IV. Downstream |
|---|---|---|---|---|---|---|
| GWP | kg CO2 eq | 884.27 | 688.89 | 37.91 | 152.59 | 4.88 |
| SOD | kg CFC 1 11 eq | 6.1 × 10−4 | 3.99 × 10−4 | 4.98 × 10−7 | 2.132 × 10−4 | 3.03 × 10−6 |
| TA | kg SO2 eq | 3.46 | 2.51 | 4.09 × 10−3 | 0.93 | 1.89 × 10−2 |
| FE | kg P eq | 0.23 | 8.25 × 10−2 | 1.04 × 10−3 | 0.14 | 5.96 × 10−3 |
| ME | kg N eq | 0.12 | 0.10 | 1.20 × 10−4 | 1.13 × 10−2 | 3.58 × 10−3 |
| TET | kg 1.4-DCB 1 | 706.65 | 604.42 | 0.74 | 98.57 | 2.92 |
| FET | kg 1.4-DCB 1 | 6.85 | 2.03 | 2.77 × 10−2 | 4.66 | 0.13 |
| MET | kg 1.4-DCB 1 | 9.65 | 3.08 | 3.74 × 10−2 | 6.35 | 0.19 |
| FRS | kg oil eq | 267.83 | 228.55 | 0.29 | 37.69 | 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, S.; Arias, A.; Feijoo, G.; Moreira, M.T. Modelling and Environmental Profile Associated with the Valorization of Wheat Straw as Carbon Source in the Biotechnological Production of Manganese Peroxidase. Sustainability 2022, 14, 4842. https://doi.org/10.3390/su14084842
González-Rodríguez S, Arias A, Feijoo G, Moreira MT. Modelling and Environmental Profile Associated with the Valorization of Wheat Straw as Carbon Source in the Biotechnological Production of Manganese Peroxidase. Sustainability. 2022; 14(8):4842. https://doi.org/10.3390/su14084842
Chicago/Turabian StyleGonzález-Rodríguez, Sandra, Ana Arias, Gumersindo Feijoo, and Maria Teresa Moreira. 2022. "Modelling and Environmental Profile Associated with the Valorization of Wheat Straw as Carbon Source in the Biotechnological Production of Manganese Peroxidase" Sustainability 14, no. 8: 4842. https://doi.org/10.3390/su14084842
APA StyleGonzález-Rodríguez, S., Arias, A., Feijoo, G., & Moreira, M. T. (2022). Modelling and Environmental Profile Associated with the Valorization of Wheat Straw as Carbon Source in the Biotechnological Production of Manganese Peroxidase. Sustainability, 14(8), 4842. https://doi.org/10.3390/su14084842








