A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles
Abstract
:1. Introduction
2. Mechanisms of Solvent for CO2 Desorption
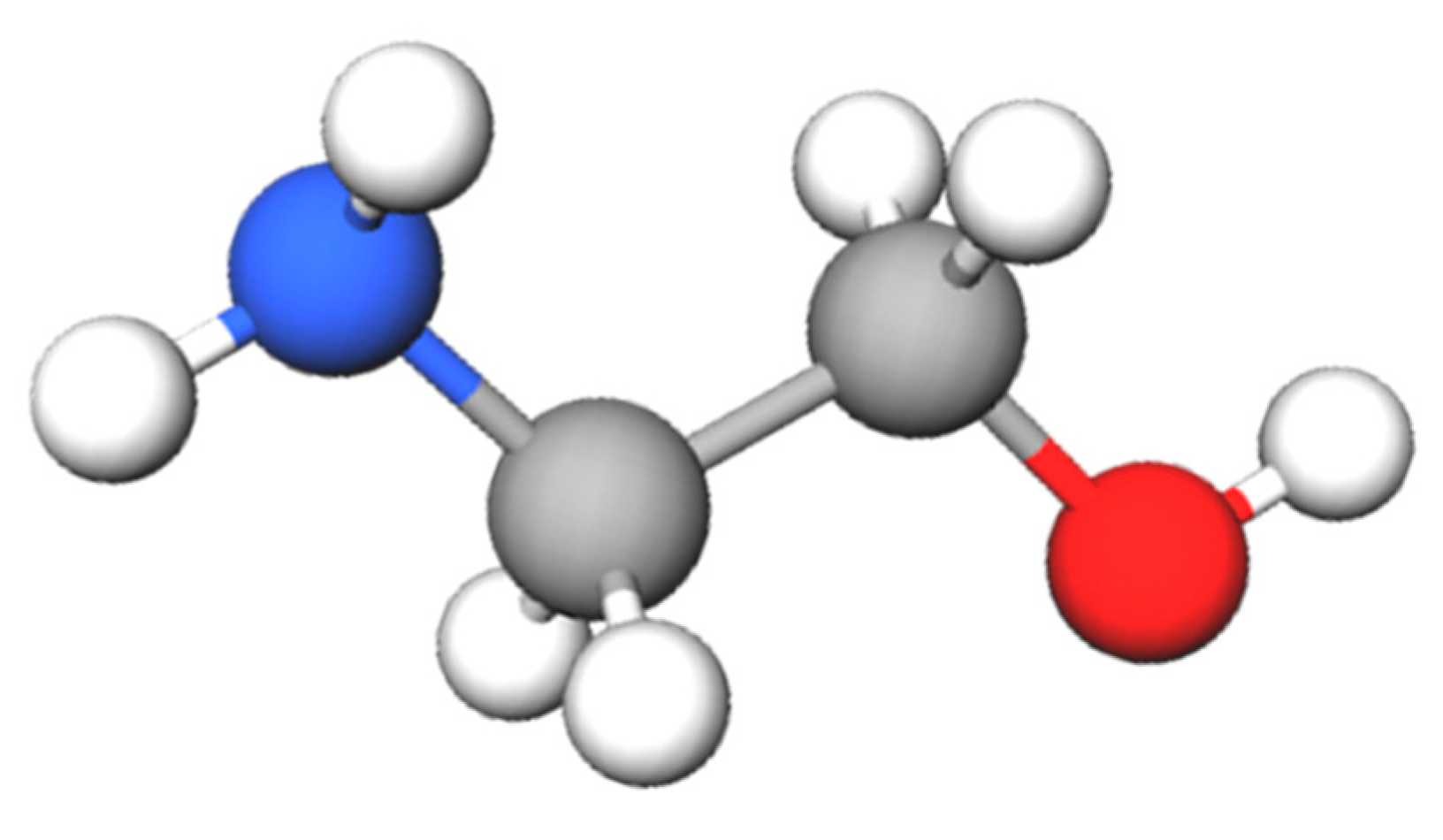
2.1. Reaction Mechanism of Monoethanolamine
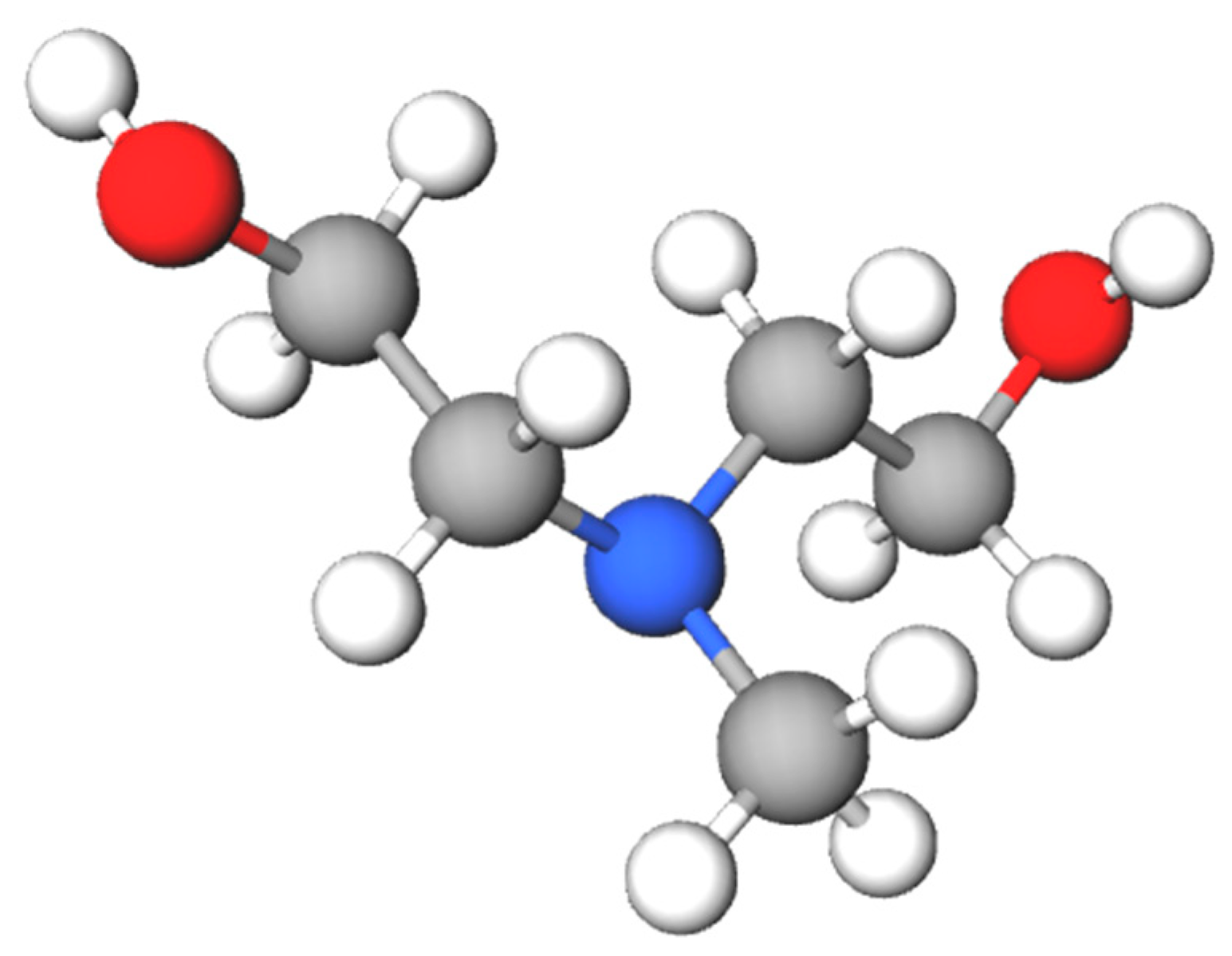
2.2. Reaction Mechanism of Methyldiethanolamine
- Reaction I—CO2 with MDEA
- Reaction II—Bicarbonate formation
- Reaction III—CO2 with water
2.3. Reaction Mechanism of Inorganic Carbonate Solutns
3. Fluid Mechanics and Flow Properties of Nanoparticle-Based Solvents
4. Physical and Chemical Enhancement Mechanism
4.1. Physical Enhancement Mechanism of Nanoparticles
- (1)
- Increase in heat transfer surface area: As reported by Kim et al. [68], at a concentration below the critical concentration, the effective heat transfer surface area increases upon the increase in nanoparticle concentration. However, exceeding the critical concentration can cause a smooth nanoscale surface to form, which reduces the effective heat surface area.
- (2)
- Change in surface roughness: During the boiling process, the nanoparticles are deposited on the heating surface, which causes the change in the microstructure and topography of the heating surface. A porous layer is formed on the boiling surface, which produces a structural effect and increases wettability [67,69,70]. Therefore, more bubbles are more easily generated and desorbed from the surface. This mechanism is supported by Lee et al. [59], who studied the visualization of the CO2 bubble generation when employing SiO2 and Al2O3 nanoparticles to deionized water. The Al2O3 showed better bubble generation and desorption upon adding heat, in comparison to water and SiO2 nanoparticles.
- (3)
- Increase in nucleation site density: In a fluid, bubbles are primarily generated at the small sites on the irregular surface (cavities, scratches, pits and cracks), which is called the “nucleation site”. As nanoparticles are deposited on the boiling surface, more nucleation sites are created. In addition to that, the floating nanoparticles, such as those on the heater surface, can also become bubble generation points. More CO2 can be discharged as more regeneration sites are created. It has also been reported that the nucleation site density increases if the surface roughness is larger than the particle size, and the nucleation site density is reduced if the two values are similar [71].
4.2. Catalytic Enhancement Mechanism of Nanoparticles
5. Nanoparticle Selection Criteria
6. Specific Nanoparticles That Enhance Solvent Regeneration
6.1. Metal Oxides
6.1.1. SiO2
6.1.2. Al2O3
6.1.3. TiO2
6.1.4. Transition Metal Oxides
6.1.5. TiO(OH)2
6.2. Zeolites
6.3. Mesoporous Silica
7. Summary of Nanoparticles According to Selection Criteria
8. Perspective and Future Directions
- (1)
- The existing studies on the regeneration performance of nanoparticles is still limited. The employment of different types of nanoparticles has been widely studied, though many of them do not focus on desorption performance. For instance, the employment of Fe3O4 and CNT nanoparticles has been reported to have a better absorption performance than SiO2 and Al2O3 at lower concentrations [145]. However, the desorption performance has yet to be investigated for these nanoparticles.
- (2)
- The stability of a nanoparticle is an important characteristic in the application of the CO2 separation process. Long term stability for nanoparticles is considered to be an issue for practical applications as different nanoparticles may require different stability methods. Identifying an easy and low-cost method to improve the stability should be considered. It has been reported that the addition of surfactants could further improve the stability, however their effects on the desorption rate should be further investigated. Modifying the surface, for instance, on Fe3O4, is one way to improve its stability [146]. However, its effect on the desorption performance of CO2 capture should be further discussed.
- (3)
- The enhancement factors that affect the regeneration performance of nanoparticles have been explained in terms of the size, concentration and type in the current review. More factors should be considered, such as the gas flow rate and the gas concentration. Apart from that, the physical and chemical properties of transformed nanoparticles should be properly discussed. The density, viscosity and other thermodynamic properties are important to further evaluate the overall performance of CO2 capture.
- (4)
- Since nanoparticles exhibit both catalytic and physical effects on the desorption performance, the relationship between the two mechanisms should be properly discussed. Metal oxide nanoparticles have been widely discussed as having both effects. However, this is not the case for zeolite and mesoporous silica.
- (5)
- Apart from that, the reduction in the heat duty of using nanoparticles has been discussed. Future research should quantitively evaluate the regeneration energy requirement and its feasibility for these nanoparticles to be implemented in large scale applications.
9. Conclusions
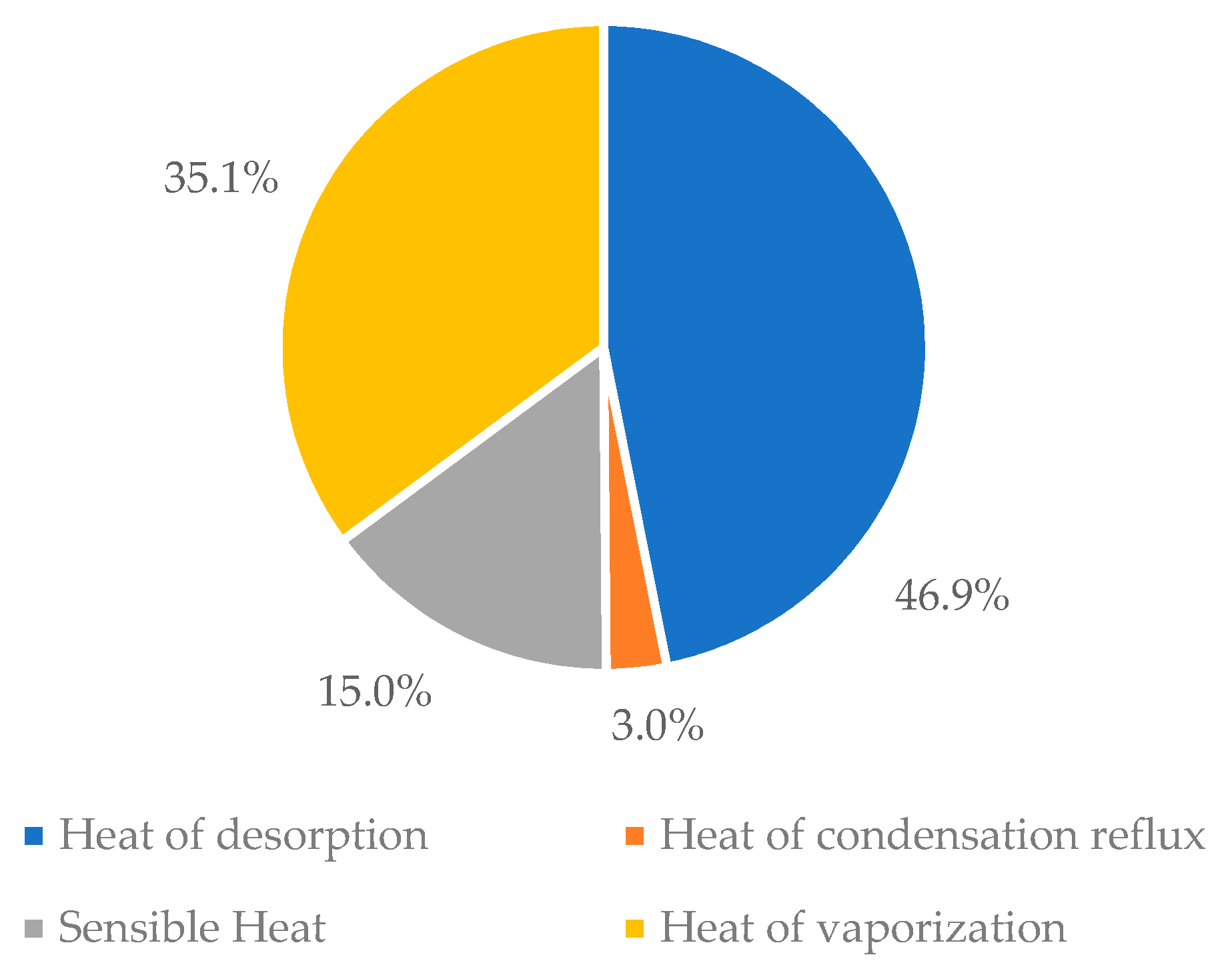
- The high energy requirement in the desorption process is due to the high sensible heat and heat of vaporization. Therefore, to reduce it, the regeneration of the solvent should be achieved at a lower temperature and the higher the amount of CO2 desorbed is desirable so that the cyclic capacity can be higher. It is important to note that this reduction in energy occurs on the assumption that equilibrium is not reached.
- Metal oxides can demonstrate both physical and chemical enhancement mechanisms that improve the heat and mass transfer of the solvent and provide catalytic behavior. However, zeolites and mesoporous silica have only been reported to provide a chemical enhancement mechanism.
- The nanoparticles do not change the thermodynamic properties of the solvent but are able to reduce the energy in a shorter time frame, due to the improvement of the rate of CO2 desorption.
- The physical and chemical properties of the synthesized nanoparticles play a vital role in evaluating the CO2 desorption performance. The acid and basic sites should be evaluated along with the other physical factors, such as MSA, average pore diameter and total surface area.
- The nanoparticle selection criteria have been discussed according to factors that can improve the regeneration of solvent.
- TiO(OH)2 has the highest enhancement ratio among all the nanoparticles in review.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustapa, S.I.; Bekhet, H.A. Analysis of CO2 emissions reduction in the Malaysian transportation sector: An Optimisation Approach. Energy Policy 2016, 89, 171–183. [Google Scholar] [CrossRef]
- United Nations Development Programme. NDC Global Outlook Report 2019. The Heat Is On. Taking Stock of Global Climate Ambition. 2019, p. 5. Available online: https://www.undp.org/content/undp/en/home/librarypage/environment-energy/climate_change/ndc-global-outlook-report-2019.html (accessed on 27 December 2020).
- US EPA. Overview of Greenhouse Gases, Greenhouse Gas (GHG) Emissions|US EPA. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 3 June 2021).
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A Review. Environ. Chem. Lett. 2020, 19, 77–109. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change. The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 14 September 2019).
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Kather, A.; Rafailidis, S.; Hermsdorf, C.; Klostermann, M.; Maschmann, A.; Mieske, K.; Oexmann, J.; Pfaff, I.; Rohloff, K.; Wilken, J. Research and Development Needs for Clean Coal Deployment; IEA Clean Coal Centre: London, UK, 2008. [Google Scholar]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A. Minimising the regeneration heat duty of post-combustion CO2 capture by wet chemical absorption: The misguided focus on low heat of absorption solvents. Int. J. Greenh. Gas Control 2010, 4, 36–43. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot Plant Studies of the CO2 Capture Performance of Aqueous MEA and Mixed MEA/MDEA Solvents at the University of Regina CO2 Capture Technology Development Plant and the Boundary Dam CO2 Capture Demonstration Plant. Ind. Eng. Chem. Res. 2005, 45, 2414–2420. [Google Scholar] [CrossRef]
- Sakwattanapong, R.; Aroonwilas, A.; Veawab, A. Behavior of Reboiler Heat Duty for CO2 Capture Plants Using Regenerable Single and Blended Alkanolamines. Ind. Eng. Chem. Res. 2005, 44, 4465–4473. [Google Scholar] [CrossRef]
- Nwaoho, C.; Idem, R.; Supap, T.; Saiwan, C.; Tontiwachwuthikul, P.; Rongwong, W.; Al-Marri, M.J.; Benamor, A. Heat duty, heat of absorption, sensible heat and heat of vaporization of 2-Amino-2-Methyl-1-Propanol (AMP), Piperazine (PZ) and Monoethanolamine (MEA) tri–solvent blend for carbon dioxide (CO2) capture. Chem. Eng. Sci. 2017, 170, 26–35. [Google Scholar] [CrossRef]
- Notz, R.J.; Tönnies, I.; McCann, N.; Scheffknecht, G.; Hasse, H. CO2 Capture for Fossil Fuel-Fired Power Plants. Chem. Eng. Technol. 2011, 34, 163–172. [Google Scholar] [CrossRef]
- Vega, F.; Moreno, F.M.B.; Fernández, L.M.G.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2019, 260, 114313. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, H.; Lu, Y. Enhanced Stability and Chemical Resistance of a New Nanoscale Biocatalyst for Accelerating CO2 Absorption into a Carbonate Solution. Environ. Sci. Technol. 2013, 47, 13882–13888. [Google Scholar] [CrossRef]
- Cullinane, J.T.; Oyenekan, B.A.; Lu, J.; Rochelle, G.T. Aqueous piperazine/potassium carbonate for enhanced CO2 capture. In Greenhouse Gas Control Technologies 7; Elsevier: Amsterdam, The Netherlands, 2005; pp. 63–71. [Google Scholar] [CrossRef]
- Ramachandran, N.; Aboudheir, A.; Idem, A.R.; Tontiwachwuthikul, P. Kinetics of the Absorption of CO2 into Mixed Aqueous Loaded Solutions of Monoethanolamine and Methyldiethanolamine. Ind. Eng. Chem. Res. 2006, 45, 2608–2616. [Google Scholar] [CrossRef]
- Borhani, N.T.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Park, A.-H.A.; Fang, M. Review of liquid nano-absorbents for enhanced CO2 capture. Nanoscale 2019, 11, 17137–17156. [Google Scholar] [CrossRef] [PubMed]
- Fuskele, V. and Sarviya, R. Recent developments in nanoparticles synthesis, preparation and stability of nanofluids. Mater. Today Proc. 2017, 4, 4049–4060. [Google Scholar] [CrossRef]
- Li, L.; Kang, Y.T. Enhancement mechanisms of mass transfer performance by nanoabsorbents during CO2 absorption process. Int. J. Heat Mass Transf. 2020, 164, 120444. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Bhattacharya, A.P.; Phelan, P.E.; Prasher, R.S. Enhanced Mass Transport in Nanofluids. Nano Lett. 2006, 6, 419–423. [Google Scholar] [CrossRef]
- Lai, Q.; Toan, S.; Assiri, M.A.; Cheng, H.; Russell, A.G.; Adidharma, H.; Radosz, M.; Fan, M. Catalyst-TiO (OH)2 could drastically reduce the energy consumption of CO2 capture. Nat. Commun. 2018, 9, 2672. [Google Scholar] [CrossRef]
- Urusovskaya, A. Defect crystal chemistry and its applications by RJD Tilley. Acta Crystallogr. Sect. Found. Crystallogr. 1987, 43, 840. [Google Scholar] [CrossRef] [Green Version]
- Gates, B.C.; Katzer, J.R.; Schuit, G.C. Chemistry of Catalytic Processes; McGraw-Hill College: NewYork, NY, USA, 1979. [Google Scholar]
- Bhatti, U.H.; Shah, A.K.; Kim, J.N.; You, J.K.; Choi, S.H.; Lim, D.H.; Nam, S.; Park, Y.H.; Baek, I.H. Effects of Transition Metal Oxide Catalysts on MEA Solvent Regeneration for the Post-Combustion Carbon Capture Process. ACS Sustain. Chem. Eng. 2017, 5, 5862–5868. [Google Scholar] [CrossRef]
- Liang, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.Z.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef] [Green Version]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Catalytic Solvent Regeneration for Energy-Efficient CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 18755–18788. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.; Pineda, I.T.; Kang, Y.T. Review of nanoabsorbents for capture enhancement of CO2 and its industrial ap-plications with design criteria. Renew. Sustain. Energy Rev. 2020, 138, 110524. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, J.; Chen, F.; Li, H.; Zhang, W.; Qi, W. Progress in enhancement of CO2 absorption by nanofluids: A Mini Review of Mechanisms and Current Status. Renew. Energy 2018, 118, 527–535. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption Capacity, Cyclic Capacity and pKa. Int. J. Greenh. Gas Control. 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Donaldson, T.L.; Nguyen, Y.N. Carbon Dioxide Reaction Kinetics and Transport in Aqueous Amine Membranes. Ind. Eng. Chem. Fundam. 1980, 19, 260–266. [Google Scholar] [CrossRef]
- Barth, D.; Tondre, C.; Lappai, G.; Delpuech, J.J. Kinetic study of carbon dioxide reaction with tertiary amines in aqueous solutions. J. Phys. Chem. 1981, 85, 3660–3667. [Google Scholar] [CrossRef]
- Elk, N.J.P.-V.; Derks, P.W.; Fradette, S.; Versteeg, G.F. Kinetics of absorption of carbon dioxide in aqueous MDEA solutions with carbonic anhydrase at 298K. Int. J. Greenh. Gas Control 2012, 9, 385–392. [Google Scholar] [CrossRef]
- Ko, J.-J.; Li, M.-H. Kinetics of absorption of carbon dioxide into solutions of N-methyldiethanolamine+water. Chem. Eng. Sci. 2000, 55, 4139–4147. [Google Scholar] [CrossRef]
- Yao, H.; Toan, S.; Huang, L.; Fan, M.; Wang, Y.; Russell, A.G.; Luo, G.; Fei, W. TiO (OH)2–highly effective catalysts for optimizing CO2 desorption kinetics reducing CO2 capture cost: A New Pathway. Sci. Rep. 2017, 7, 2943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, F.; Zou, H.-K.; Chu, G.-W.; Shao, L.; Chen, J.-F. Modeling and experimental studies on absorption of CO2 by Benfield solution in rotating packed bed. Chem. Eng. J. 2009, 145, 377–384. [Google Scholar] [CrossRef]
- Knuutila, H.; Juliussen, O.; Svendsen, H.F. Kinetics of the reaction of carbon dioxide with aqueous sodium and potassium car-bonate solutions. Chem. Eng. Sci. 2010, 65, 6077–6088. [Google Scholar] [CrossRef]
- Hikita, H.; Asai, S.; Takatsuka, T. Absorption of carbon dioxide into aqueous sodium hydroxide and sodium carbonate-bicarbonate solutions. Chem. Eng. J. 1976, 11, 131–141. [Google Scholar] [CrossRef]
- Rauf, A.; Abbas, Z.; Shehzad, S.; Mushtaq, T. Characterization of temperature-dependent fluid properties in compressible viscous fluid flow induced by oscillation of disk. Chaos Solitons Fractals 2019, 132, 109573. [Google Scholar] [CrossRef]
- Van Holst, J.; Kersten, S.R.A.; Hogendoorn, K.J.A. Physiochemical Properties of Several Aqueous Potassium Amino Acid Salts. J. Chem. Eng. Data 2008, 53, 1286–1291. [Google Scholar] [CrossRef]
- Aronu, U.E.; Hessen, E.T.; Haug-Warberg, T.; Hoff, K.A.; Svendsen, H.F. Vapor–liquid equilibrium in amino acid salt system: Experiments and Modeling. Chem. Eng. Sci. 2011, 66, 2191–2198. [Google Scholar] [CrossRef]
- Portugal, A.; Sousa, J.; Magalhães, F.; Mendes, A. Solubility of carbon dioxide in aqueous solutions of amino acid salts. Chem. Eng. Sci. 2009, 64, 1993–2002. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A Review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Kim, S.; Xu, R.; Lee, W.; Kang, Y.T. Mass transfer performance enhancement by nanoabsorbents during CO2 absorption process. Int. J. Heat Mass Transf. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.-N.; Wang, Y.; Ren, S.; Han, J.; Chen, A. CO2 absorption into aqueous potassium salts of lysine and proline: Density, Viscosity and Solubility of CO2. Fluid Phase Equilibria 2015, 399, 40–49. [Google Scholar] [CrossRef]
- Feng, Z.; Jing-Wen, M.; Zheng, Z.; You-Ting, W.; Zhi-Bing, Z. Study on the absorption of carbon dioxide in high concentrated MDEA and ILs solutions. Chem. Eng. J. 2012, 181–182, 222–228. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.; Fang, M.; He, H.; Xiang, Q.; Ma, Q.; Xia, M.; Luo, Z.; Cen, K. Wetted-wall column study on CO2 absorption kinetics enhancement by additive of nanoparticles. Greenh. Gases Sci. Technol. 2015, 5, 682–694. [Google Scholar] [CrossRef]
- Salih, H.A.; Pokhrel, J.; Reinalda, D.; AlNashf, I.; Khaleel, M.; Vega, L.F.; Karanikolos, G.N.; Abu Zahra, M. Hybrid—Slurry/Nanofluid systems as alternative to conventional chemical absorption for carbon dioxide capture: A Review. Int. J. Greenh. Gas Control 2021, 110, 103415. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Esfahany, M.N.; Esmaeili-Faraj, S.H. Investigation of the effects of nanoparticle size on CO2 absorption by silica-water nanofluid. Sep. Purif. Technol. 2018, 195, 208–215. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanopart. Res. 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Esmaeili Faraj, S.H.; Nasr Esfahany, M.; Jafari-Asl, M.; Etesami, N. Hydrogen sulfide bubble absorption enhancement in wa-ter-based nanofluids. Ind. Eng. Chem. Res. 2014, 53, 16851–16858. [Google Scholar] [CrossRef]
- Esmaeili-Faraj, S.H.; Esfahany, M.N. Absorption of Hydrogen Sulfide and Carbon Dioxide in Water Based Nanofluids. Ind. Eng. Chem. Res. 2016, 55, 4682–4690. [Google Scholar] [CrossRef]
- Choi, I.D.; Lee, J.W.; Kang, Y.T. CO2 Capture/Separation Control by SiO2 Nanoparticles and Surfactants. Sep. Sci. Technol. 2014, 50, 772–780. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.W.; Kang, Y.T. CO2 regeneration performance enhancement by nanoabsorbents for energy conversion appli-cation. Appl. Therm. Eng. 2016, 103, 980–988. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.W.; Kang, Y.T. CO2 absorption/regeneration enhancement in DI water with suspended nanoparticles for energy conversion application. Appl. Energy 2015, 143, 119–129. [Google Scholar] [CrossRef]
- Lee, J.W.; Pineda, I.T.; Lee, J.H.; Kang, Y.T. Combined CO2 absorption/regeneration performance enhancement by using nanoabsorbents. Appl. Energy 2016, 178, 164–176. [Google Scholar] [CrossRef]
- Fan, J.; Wang, L. Review of Heat Conduction in Nanofluids. J. Heat Transf. 2011, 133, 040801. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.; Choi, S.U.; Eastman, J. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Pang, C.; Jung, J.-Y.; Lee, J.W.; Kang, Y.T. Thermal conductivity measurement of methanol-based nanofluids with Al2O3 and SiO2 nanoparticles. Int. J. Heat Mass Transf. 2012, 55, 5597–5602. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal Conductivity of Nanoparticle—Fluid Mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Vatani, A.; Woodfield, P.L.; Dao, D.V. A survey of practical equations for prediction of effective thermal conductivity of spherical-particle nanofluids. J. Mol. Liq. 2015, 211, 712–733. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and Comparison of Nanofluid Thermal Conductivity and Heat Transfer Enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Kwark, S.M.; Kumar, R.; Moreno, G.; Yoo, J.; You, S.M. Pool boiling characteristics of low concentration nanofluids. Int. J. Heat Mass Transf. 2010, 53, 972–981. [Google Scholar] [CrossRef]
- Kim, E.S.; Jung, J.-Y.; Kang, Y.T. The effect of surface area on pool boiling heat transfer coefficient and CHF of Al 2 O 3/water nanofluids. J. Mech. Sci. Technol. 2013, 27, 3177–3182. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, J.Y.; Ahn, H.S.; Jo, H.J.; Kim, M.H. Study of Leidenfrost mechanism in droplet impacting on hydrophilic and hydrophobic surfaces. Int. J. Air Cond. Refrig. 2013, 21, 1350028. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- White, S.B. Enhancement of Boiling Surfaces Using Nanofluid Particle Deposition. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 2010. [Google Scholar]
- Zhang, X.; Zhang, R.; Liu, H.; Gao, H.; Liang, Z. Evaluating CO2 desorption performance in CO2-loaded aqueous tri-solvent blend amines with and without solid acid catalysts. Appl. Energy 2018, 218, 417–429. [Google Scholar] [CrossRef]
- Bairq, Z.A.S.; Gao, H.; Huang, Y.; Zhang, H.; Liang, Z. Enhancing CO2 desorption performance in rich MEA solution by addition of SO42−/ZrO2/SiO2 bifunctional catalyst. Appl. Energy 2019, 252, 113440. [Google Scholar] [CrossRef]
- Liang, Z.; Idem, R.; Tontiwachwuthikul, P.; Yu, F.; Liu, H.; Rongwong, W. Experimental study on the solvent regeneration of a CO2-loaded MEA solution using single and hybrid solid acid catalysts. AIChE J. 2015, 62, 753–765. [Google Scholar] [CrossRef]
- Bhatti, U.H.; Sivanesan, D.; Lim, D.H.; Nam, S.C.; Park, S.; Baek, I.H. Metal oxide catalyst-aided solvent regeneration: A promising method to economize post-combustion CO2 capture process. J. Taiwan Inst. Chem. Eng. 2018, 93, 150–157. [Google Scholar] [CrossRef]
- Idem, R.; Shi, H.; Gelowitz, D.; Tontiwachwuthikul, P. Catalytic Method and Apparatus for Separating a Gaseous Component from an Incoming Gas Stream. Google Patents WO2011120138A1, 6 October 2011. [Google Scholar]
- Shi, H.; Naami, A.; Idem, R.; Tontiwachwuthikul, P. Catalytic and non catalytic solvent regeneration during absorption-based CO2 capture with single and blended reactive amine solvents. Int. J. Greenh. Gas Control 2014, 26, 39–50. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Cui, Y.; Huang, J.; Shiko, E.; Zhou, Y.; Zeng, Z.; Liu, Y.; Zhang, R. Toward Solvent Development for Industrial CO2 Capture by Optimizing the Catalyst–Amine Formulation for Lower Energy Consumption in the Solvent Regeneration Process. Energy Fuels 2019, 33, 11507–11515. [Google Scholar] [CrossRef]
- Gates, B.C.; Katzer, J.R.; Olson, J.; Schuit, G. Chemistry of catalytic processes. Chem. Eng. Educ. 1974, 8, 172–175. [Google Scholar]
- Zhang, X.; Zhu, Z.; Sun, X.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Reducing Energy Penalty of CO2 Capture Using Fe Promoted SO42–/ZrO2/MCM-41 Catalyst. Environ. Sci. Technol. 2019, 53, 6094–6102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Gao, H.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Zeolite catalyst-aided tri-solvent blend amine regeneration: An Alternative Pathway to Reduce the Energy Consumption in Amine-Based CO2 Capture Process. Appl. Energy 2019, 240, 827–841. [Google Scholar] [CrossRef]
- Srisang, W.; Pouryousefi, F.; Osei, P.A.; Decardi-Nelson, B.; Akachuku, A.; Tontiwachwuthikul, P.; Idem, R. CO2 capture efficiency and heat duty of solid acid catalyst-aided CO2 desorption using blends of primary-tertiary amines. Int. J. Greenh. Gas Control 2018, 69, 52–59. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, L.; Huang, M.; Zuo, Y.; Kang, S.; Huang, Y.; Idem, R.O.; Tontiwachwuthikul, P. Catalytic-CO2-Desorption Studies of DEA and DEA–MEA Blended Solutions with the Aid of Lewis and Brønsted Acids. Ind. Eng. Chem. Res. 2018, 57, 11505–11516. [Google Scholar] [CrossRef]
- Srisang, W.; Pouryousefi, F.; Osei, P.A.; Decardi-Nelson, B.; Akachuku, A.; Tontiwachwuthikul, P.; Idem, R. Evaluation of the heat duty of catalyst-aided amine-based post combustion CO2 capture. Chem. Eng. Sci. 2017, 170, 48–57. [Google Scholar] [CrossRef]
- Akachuku, A.; Osei, P.A.; Decardi-Nelson, B.; Srisang, W.; Pouryousefi, F.; Ibrahim, H.; Idem, R. Experimental and kinetic study of the catalytic desorption of CO2 from CO2-loaded monoethanolamine (MEA) and blended monoethanolamine—Methyl-diethanolamine (MEA-MDEA) solutions. Energy 2019, 179, 475–489. [Google Scholar] [CrossRef]
- Shi, H.; Fu, J.; Wu, Q.; Huang, M.; Jiang, L.; Cui, M.; Idem, R.; Tontiwachwuthikul, P. Studies of the coordination effect of DEA-MEA blended amines (within 1 + 4 to 2 + 3 M) under heterogeneous catalysis by means of absorption and desorption parameters. Sep. Purif. Technol. 2019, 236, 116179. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, B.; Jiang, H.; Li, L.; Wei, J. Investigation of the regeneration of a CO2-loaded ammonia solution with solid acid catalysts: A Promising Alternative for Reducing Regeneration Energy. Fuel Process. Technol. 2020, 205, 106452. [Google Scholar] [CrossRef]
- Ko, Y.G.; Shin, S.S.; Choi, U.S. Primary, secondary, and tertiary amines for CO2 capture: Designing for Mesoporous CO2 Ad-sorbents. J. Colloid Interface Sci. 2011, 361, 594–602. [Google Scholar] [CrossRef]
- Bhatti, U.H.; Sivanesan, D.; Nam, S.; Park, S.Y.; Baek, I.H. Efficient Ag2O–Ag2CO3 Catalytic Cycle and Its Role in Minimizing the Energy Requirement of Amine Solvent Regeneration for CO2 Capture. ACS Sustain. Chem. Eng. 2019, 7, 10234–10240. [Google Scholar] [CrossRef]
- Bhatti, U.H.; Nam, S.; Park, S.Y.; Baek, I.H. Performance and Mechanism of Metal Oxide Catalyst-Aided Amine Solvent Regeneration. ACS Sustain. Chem. Eng. 2018, 6, 12079–12087. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, J.; Liu, H.; Luo, X.; Olson, W.; Tontiwachwuthikul, P.; Liang, Z. SO42−/ZrO2 supported on γ-Al2O3 as a catalyst for CO2 desorption from CO2-loaded monoethanolamine solutions. AIChE J. 2018, 64, 3988–4001. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Amine-based CO2 capture aided by acid-basic bifunctional catalyst: Advancement of amine regeneration using metal modified MCM-41. Chem. Eng. J. 2019, 383, 123077. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P.; Al-Marri, M.J.; Benamor, A. Reducing energy consumption of CO2 desorption in CO2-loaded aqueous amine solution using Al2O3/HZSM-5 bifunctional catalysts. Appl. Energy 2018, 29, 562–576. [Google Scholar] [CrossRef]
- Walum, E. Acute oral toxicity. Environ. Health Perspect. 1998, 106, 497–503. [Google Scholar]
- Trevan, J.W. The error of determination of toxicity. Proceedings of the Royal Society of London. Ser. B Contain. Pap. A Biol. Character 1927, 101, 483–514. [Google Scholar]
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification; World Health Organization: Geneva, Switzerland, 2019.
- US Fish and Wildlife Service. Acute-toxicity rating scales. Res. Inf. Bull. 1984, 84, 1–23. [Google Scholar]
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, E.M.T.; Lowry, G.V. Nanoparticle aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Yu, W.; Liu, F.; Fang, M.; Farooq, M.; Luo, Z. Enhanced CO2 Absorption and Desorption by Monoethanolamine (MEA)-Based Nanoparticle Suspensions. Ind. Eng. Chem. Res. 2016, 55, 7830–7838. [Google Scholar] [CrossRef]
- Salimi, J.; Salimi, F. CO2 capture by water-based Al2O3 and Al2O3-SiO2 mixture nanofluids in an absorption packed column. Rev. Mex. Ing. Química 2016, 15, 185–192. [Google Scholar]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Kim, J.; Kang, Y. The Effect of Binary Nanofluids and Chemical Surfactants on the Absorption Performance; Purdue University: West Lafayette, IN, USA, 2006. [Google Scholar]
- Yan, Z.; Taylor, M.G.; Mascareno, A.; Mpourmpakis, G. Size-, Shape-, and Composition-Dependent Model for Metal Nanoparticle Stability Prediction. Nano Lett. 2018, 18, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Ono, L.K.; Roldán-Cuenya, B. Effect of interparticle interaction on the low temperature oxidation of CO over size-selected Au nanocatalysts supported on ultrathin TiC films. Catal. Lett. 2007, 113, 86–94. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Liu, H.; Li, W.; Xiao, M.; Gao, H.; Liang, Z. Reduction of energy requirement of CO2 desorption from a rich CO2-loaded MEA solution by using solid acid cat-alysts. Appl. Energy 2017, 202, 673–684. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.; Zhang, X.; Bairq, Z.A.S.; Huang, Y.; Tontiwachwuthikul, P.; Liang, Z. Catalytic performance and mechanism of SO42−/ZrO2/SBA-15 catalyst for CO2 desorption in CO2-loaded monoeth-anolamine solution. Appl. Energy 2020, 259, 114179. [Google Scholar] [CrossRef]
- Pauley, C.R.; Hashemi, R.; Caothien, S. Analysis of foaming mechanisms in amine plants. In Proceedings of the American Institute of Chemical Engineers Summer Meeting, Denver, CO, USA, 21–24 August 1988. [Google Scholar]
- Ross, S. Mechanisms of foam stabilization and antifoaming action. Chem. Eng. Prog. 1967, 63, 41. [Google Scholar]
- Liu, W.; Wu, Y.; Cai, T.; Chen, X.; Liu, D. Use of nanoparticles Cu/TiO (OH)2 for CO2 removal with K2CO3/KHCO3 based so-lution: Enhanced Thermal Conductivity and Reaction Kinetics Enhancing the CO2 Sorption/Desorption Performance of K2CO3/KHCO3. Greenh. Gases: Sci. Technol. 2019, 9, 10–18. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Bula, K.; Sobczak, M.; Jesionowski, T. Influence of Processing Conditions on the Thermal Stability and Mechanical Properties of PP/Silica-Lignin Composites. Int. J. Polym. Sci. 2016, 2016, 1627258. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Zhou, S. Poly(ethylene glycol)-grafted silica nanoparticles for highly hydrophilic acrylic-based polyurethane coatings. Prog. Org. Coat. 2017, 106, 145–154. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhao, B.; Zhuo, Y.; Wang, S. Experimental study of CO2 absorption in aqueous MEA and MDEA solutions enhanced by nanoparticles. Int. J. Greenh. Gas Control 2014, 29, 135–141. [Google Scholar] [CrossRef]
- Zoccal, J.V.M.; Arouca, F.O.; Gonçalves, J.A.S. Synthesis and Characterization of TiO2 Nanoparticles by the Method Pechini. Mater. Sci. Forum 2010, 660–661, 385–390. [Google Scholar] [CrossRef]
- Krishna, A.G.; Ravikumar, R.; Kumar, T.V.; Ephraim, S.D.; Ranjith, B.; Pranoy, M.; Dola, S. Investigation and Comparison of Optical and Raman Bands of Mechanically Synthesised MoO3 Nano Powders. Mater. Today Proc. 2016, 3, 54–63. [Google Scholar] [CrossRef]
- Nagabhushana, G.; Samrat, D.; Chandrappa, G.T. α-MoO3 nanoparticles: Solution Combustion Synthesis, Photocatalytic and Electrochemical Properties. RSC Adv. 2014, 4, 56784–56790. [Google Scholar] [CrossRef]
- Xavier, J.R. High protection performance of vanadium pentoxide-embedded polyfuran/epoxy coatings on mild steel. Polym. Bull. 2020, 78, 5713–5739. [Google Scholar] [CrossRef]
- Jaswal, V.S.; Arora, A.K.; Kinger, M.; Gupta, V.D.; Singh, J. Synthesis and Characterization of Chromium Oxide Nanoparticles. Orient. J. Chem. 2014, 30, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhang, S.; Zhang, W.; Wang, M.; Feng, F. Controllable synthesis of gossamer-like Nb2O5-RGO nanocomposite and its application to supercapacitor. J. Nanopart. Res. 2020, 22, 57. [Google Scholar] [CrossRef]
- Lu, S.; Wang, C.; Wang, H.; Liu, J.; Yan, H. Excellent electrochromic properties of tungsten oxide films with a mesoporous structure. J. Mater. Sci. Mater. Electron. 2017, 28, 10049–10055. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, S.; Yang, J. Preparation of Ag2O/g-C3N4/Fe3O4 composites and the application in the photocatalytic degradation of Rhodamine B under visible light. J. Alloys Compd. 2017, 708, 1141–1149. [Google Scholar] [CrossRef]
- Channu, V.R.; Holze, R.; Rambabu, B. Synthesis and characterization of NiO nanoparticles for electrochemical applications. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 414, 204–208. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Ramli, Z.A.C.; Samad, W.Z.; Yarmo, M.A. Capturing Greenhouse Gas Carbon Dioxide to Form Carbonate Compounds. J. Teknol. 2015, 77. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Bhandari, S.; Khastgir, D. Synthesis of MnO2 nanoparticles and their effective utilization as UV protectors for outdoor high voltage polymeric insulators used in power transmission lines. Phys. Chem. Chem. Phys. 2016, 18, 32876–32890. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Gao, H.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Investigation of CO2 Regeneration in Single and Blended Amine Solvents with and without Catalyst. Ind. Eng. Chem. Res. 2017, 56, 7656–7664. [Google Scholar] [CrossRef]
- Prasongthum, N.; Natewong, P.; Reubroycharoen, P.; Idem, R. Solvent regeneration of a CO2-loaded BEA–AMP Bi-blend amine solvent with the aid of a solid brønsted Ce (SO4)2/ZrO2 superacid catalyst. Energy Fuels 2019, 33, 1334–1343. [Google Scholar] [CrossRef]
- Ordomsky, V.; Ivanova, I.; Knyazeva, E.; Yuschenko, V.; Zaikovskii, V. Cumene disproportionation over micro/mesoporous cat-alysts obtained by recrystallization of mordenite. J. Catal. 2012, 295, 207–216. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.-D.; Park, E.D. A comparative study for gas-phase dehydration of glycerol over H-zeolites. Appl. Catal. A Gen. 2011, 393, 275–287. [Google Scholar] [CrossRef]
- Tobiesen, A.; Mejdell, T.; Svendsen, H.F. A comparative study of experimental and modeling performance results from the CASTOR Esbjerg pilot plant. In Proceedings of the International Conference on Greenhouse Gas Control Technologies, Trondheim, Norway, 19–22 June 2006. [Google Scholar]
- Seo, S.; Simoni, L.D.; Ma, M.; DeSilva, M.A.; Huang, Y.; Stadtherr, M.A.; Brennecke, J.F. Phase-Change Ionic Liquids for Postcombustion CO2 Capture. Energy Fuels 2014, 28, 5968–5977. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous silica nanoparticles: Structural Design and Applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef] [Green Version]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of highly ordered mesoporous materials from a layered polysilicate. J. Chem. Soc. Chem. Commun. 1993, 680–682. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Teng, Y.; Zhang, K.; Cao, Y.; Pan, W.-P. CO2 adsorption properties and thermal stability of different amine-impregnated MCM-41 materials. J. Fuel Chem. Technol. 2013, 41, 469–475. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Liang, Z. CO2 Desorption in Single and Blended Amine Solvents with and without Catalyst. Energy Procedia 2017, 114, 1862–1868. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Abadeer, N.; Haynes, C.L. Stability of small mesoporous silica nanoparticles in biological media. Chem. Commu-Nications 2011, 47, 532–534. [Google Scholar] [CrossRef]
- Kim, J.M.; Kwak, J.H.; Jun, S.; Ryoo, R. Ion Exchange and Thermal Stability of MCM-41. J. Phys. Chem. 1995, 99, 16742–16747. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Li, H.-X.; Davis, M.E. Studies on mesoporous materials: I. Synthesis and Characterization of MCM-41. Microporous Mater. 1993, 2, 17–26. [Google Scholar] [CrossRef]
- Patil, U.; Fihri, A.; Emwas, A.-H.; Polshettiwar, V. Silicon oxynitrides of KCC-1, SBA-15 and MCM-41 for CO2 capture with excellent stability and regenerability. Chem. Sci. 2012, 3, 2224–2229. [Google Scholar] [CrossRef]
- Rahmatmand, B.; Keshavarz, P.; Ayatollahi, S. Study of absorption enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 na-noparticles in water and amine solutions. J. Chem. Eng. Data 2016, 61, 1378–1387. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Zaeri, M.R.; Mehdipour, M.; Keshavarz, P. Synthesis of different modified magnetic nanoparticles for selective physical/chemical absorption of CO2 in a bubble column reactor. J. Environ. Chem. Eng. 2020, 8, 104195. [Google Scholar] [CrossRef]


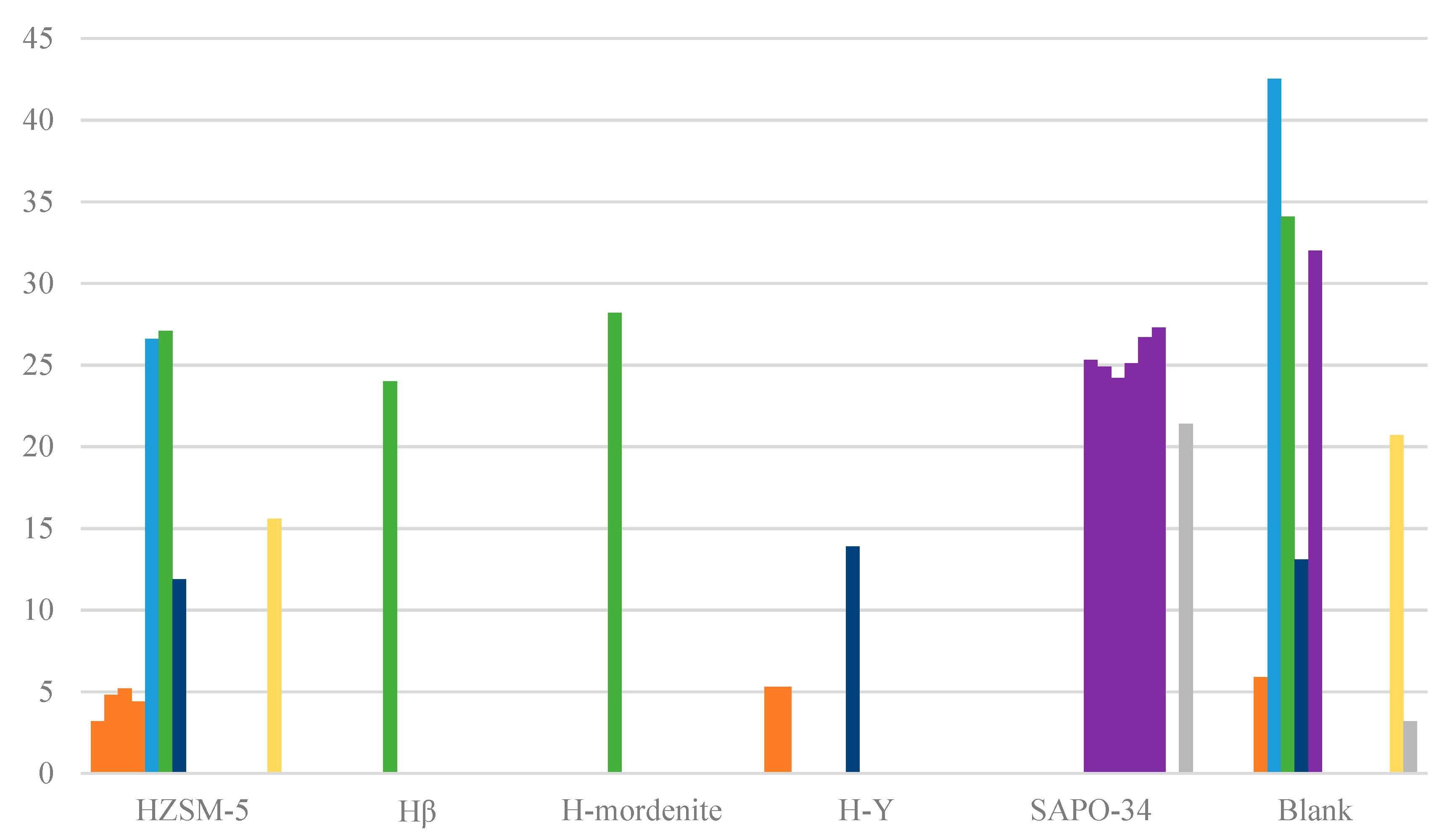
| Class | LD50 for Rat (mg/kg Body Weight) | ||||
|---|---|---|---|---|---|
| Oral | Dermal | ||||
| Solid | Liquid | Solid | Liquid | ||
| Ia | Extremely hazardous | 5 or less | 20 or less | 10 or less | 40 or less |
| Ib | Highly hazardous | 5–50 | 20–200 | 10–100 | 40–400 |
| II | Moderately hazardous | 50–500 | 200–2000 | 100–1000 | 400–4000 |
| III | Slightly hazardous | Over 500 | Over 2000 | Over 1000 | Over 4000 |
| Classification | LC50 (mg/L or ppm) |
|---|---|
| Super toxic | <0.01 |
| Extremely toxic | 0.01–0.1 |
| Highly toxic | 0.1–1.0 |
| Moderately toxic | 1.0–10.0 |
| Slightly toxic | 10.0–100.0 |
| Practically non-toxic | 100.0–1000 |
| Relatively harmless | >1000 |
| Solvent | Nanoparticles | Size and Concentration | Temperature | Enhancement Ratio | Ref. |
|---|---|---|---|---|---|
| Deionized Water | SiO2 | 0.01–0.1 vol% | 20 | 1.078 | [57] |
| Deionized Water | SiO2 | 15 nm 0.0–0.05 vol% | 100 | 1.118 | [59] |
| Al2O3 | 45 nm 0.0–0.05 vol% | 100 | - | ||
| Methanol | Al2O3 | 20 nm, 45 nm 0, 0.01 vol% | 60 | - | [58] |
| Methanol | SiO2 | 15 nm 0.01 vol% | < 65 | 1.22 | [60] |
| Al2O3 | 45 nm 0.01 vol% | < 65 | 1.16 | ||
| MEA | TiO2 | 15 nm 0.1 wt% | 103 | 1.42 | [100] |
| SiO2 | 1.26 | ||||
| Al2O3 | 1.21 | ||||
| MEA | MoO3 | 5 g | 86 | 1.94 | [27] |
| V2O5 | 1.84 | ||||
| Cr2O3 | 1.44 | ||||
| TiO2 | 1.44 | ||||
| WO3 | 1.44 | ||||
| MEA | Ag2O | 10 g | 70–85 | 1.48 | [75] |
| Nb2O5 | 1.46 | ||||
| NiO | 1.23 | ||||
| CuO | 1.30 | ||||
| MnO2 | 1.15 | ||||
| Na2CO3 | TiO(OH)2 | 17.12 Å | 40–70 | 9.00 | [38] |
| MEA | TiO(OH)2 | 17.1 Å 1–3 wt% | 88 | 46.00 | [24] |
| K2CO3 | Cu-TiO(OH)2 | 0.014 vol% | 373 K | - | [110] |
| Solvent | Size and Concentration | Temperature °C | Remarks | Ref. |
|---|---|---|---|---|
| Deionized water | 15 nm 0.01–0.1 vol% | 20 |
| [57] |
| Deionized water | 15 nm 0.0–0.05 vol% | 100 |
| [59] |
| Methanol | 15 nm 0.01 vol% | <65 |
| [60] |
| MEA | 15 nm 0.1 wt% | 103 |
| [100] |
| Solvent | Size and Concentration | Temperature °C | Remarks | Ref. |
|---|---|---|---|---|
| Deionized water | 45 nm 0–0.05 vol% | 100 |
| [59] |
| Methanol | 20, 45 nm 0, 0.01 vol% | 60 |
| [59] |
| Methanol | 45 nm 0.01 vol% | <65 |
| [60] |
| MEA | 15 nm 0.1 wt% | 103 |
| [100] |
| MEA | 250 g | 90 |
| [84] |
| Amine blend | 25 g | 96 |
| [72] |
| Solvent | Concentration | Temperature °C | Remarks | Ref. |
|---|---|---|---|---|
| Sodium Carbonate Na2CO3 | - | 40–70 |
| [38] |
| MEA | 1–3 wt% | 88 |
| [24] |
| K2CO3 | 0.010 vol% 0.014 vol % (For Cu/TiO(OH)2 | 100 |
| [110] |
| Solvent | Quantity | Temperature °C | Remarks | Ref. |
|---|---|---|---|---|
| MEA | 10, 30 and 60 g catalyst | 70–98 |
| [74] |
| MEA | 25 g (catalyst was 3–4 mm in size) | 95 |
| [77] |
| MEA | 12.5 g catalyst | 98 |
| [81] |
| MEA | 250 g | 90 |
| [84] |
| MEA | 10–70 g catalyst | 96 |
| [106] |
| MEA | 25 g catalyst | 98 |
| [129] |
| DEAPA | 25 g catalyst | 90 |
| [78] |
| Solvent | Quantity | Temperature °C | Remarks | Ref. |
|---|---|---|---|---|
| MEA | 25 g | 98 |
| [129] |
| MEA | 25 g | 98 |
| [140] |
| DEAPA | 25 g | 90 |
| [78] |
| MEA | 6.25 ± 0.01 g catalyst | 98.5 |
| [107] |
| Nanomaterial | Agglomeration/Sedimentation | Thermal Stability (°C) | Recycling Ability | Foaming | Toxicity **a | Environmentally Friendly **b | Desorption Enhancement |
|---|---|---|---|---|---|---|---|
| Metal Oxides | |||||||
| SiO2 | Yes | High | Reusable | Yes | Slight | Relatively Harmless | Low |
| Al2O3 | Yes | Medium | Reusable | Yes | Slight | Relatively Harmless | Low |
| TiO2 | Yes | Low | Reusable | Yes | Slight | Relatively Harmless | Medium |
| MoO3 | Yes | Medium | Non-reusable | N/A | Slight | Relatively Harmless | High |
| V2O5 | Yes | Low | Non-reusable | N/A | Moderately Hazardous | Relatively Harmless | Medium |
| Cr2O5 | Yes | High | Reusable | N/A | Slight | N/A | Medium |
| WO3 | Yes | Low | Non-reusable | N/A | Slight | N/A | Medium |
| Ag2O | Yes | Medium | Non-reusable | N/A | Slight | N/A | Medium |
| Nb2O5 | Yes | High | Reusable | N/A | Slight | Relatively Harmless | Medium |
| NiO | Yes | Low | Reusable | N/A | Slight | N/A | Medium |
| CuO | Yes | Low | Non-reusable | Yes | Moderately Hazardous | N/A | Medium |
| MnO2 | Yes | Low | Reusable | N/A | Slight | N/A | Low |
| Ti(OH)2 | Yes | Medium | Reusable | N/A | Slight | Relatively Harmless | Very high |
| Nanomaterial | Agglomeration/Sedimentation | Thermal Stability | Recycling Ability | Foaming | Toxicity **a | Environmentally Friendly **b | Heat Duty (MJ/Kg CO2) |
|---|---|---|---|---|---|---|---|
| Zeolites | |||||||
| HZSM-5 | N/A | High | Reusable | N/A | Slight | N/A | (0.25–5.1) **c |
| Hβ | No | N/A | Reusable | N/A | Slight | N/A | 0.21–0.44 |
| H-mordenite | N/A | N/A | N/A | N/A | Slight | N/A | 0.08–0.26 |
| H-Y | N/A | N/A | N/A | N/A | Slight | N/A | 5.3 |
| SAPO-34 | N/A | N/A | Reusable | N/A | Slight | N/A | 0.34–0.83 |
| Mesoporous Silica | |||||||
| MCM-41 | Yes | High | Reusable | N/A | Slight | N/A | (1.7–3.8) **d |
| SBA-15 | Yes | Low | Reusable | N/A | Moderately Hazardous | N/A | 0.51 |
| Potential Direction | |
|---|---|
| Metal Oxides |
|
| Zeolites |
|
| Mesoporous Silica |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Rozaiddin, S.A.; Lau, K.K. A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles. Sustainability 2022, 14, 4750. https://doi.org/10.3390/su14084750
Mohd Rozaiddin SA, Lau KK. A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles. Sustainability. 2022; 14(8):4750. https://doi.org/10.3390/su14084750
Chicago/Turabian StyleMohd Rozaiddin, Siti Aishah, and Kok Keong Lau. 2022. "A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles" Sustainability 14, no. 8: 4750. https://doi.org/10.3390/su14084750
APA StyleMohd Rozaiddin, S. A., & Lau, K. K. (2022). A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles. Sustainability, 14(8), 4750. https://doi.org/10.3390/su14084750








